Abstract
Ca2+/calmodulin dependent protein kinase II (CaMKII) is a broadly distributed metazoan Ser/Thr protein kinase that is important in neuronal and cardiac signaling. CaMKII forms oligomeric assemblies, typically dodecameric, in which the calcium-responsive kinase domains are organized around a central hub. We review the results of crystallographic analyses of CaMKII, including the recently determined structure of a full-length and autoinhibited form of the holoenzyme. These structures, when combined with other data, allow informed speculation about how CaMKII escapes calcium-dependence when calcium spikes exceed threshold frequencies.
Introduction
The pioneering investigations of Bruce Kemp and colleagues introduced the pseudosubstrate hypothesis for how calcium-responsive protein kinases are regulated by Ca2+/calmodulin (Ca2+/CaM) or its paralogs [1]. These Ca2+− dependent kinases have C-terminal autoinhibitory segments that resemble the protein substrates of the enzyme in certain respects, but lack the canonical phosphorylation sites found in true targets. Because these segments are present at high local concentration with respect to the active site, they bind to it and block substrate access. Adjacent to the pseudosubstrate motif, or spanning it, is a recognition element for Ca2+/calmodulin, or paralogs such as S100 and troponin C. When Ca2+ levels rise, the Ca2+/CaM complex (or a related one) binds to the autoinhibitory segment of the kinase and displaces it, thereby activating the enzyme.
Beginning with the structural and biochemical analyses of the giant protein kinase twitchin by Kemp and co-workers [2,3] and followed by the determination of the structure of phosphorylase kinase [4], CaMKI [5] and titin kinase [6], the general features of this mechanism have been validated. The autoinhibitory segments in these different enzymes do not always block the site of phosphate transfer, but in all cases the entrance groove to the catalytic center, as first defined by the structure of cAMP-dependent protein kinase (PKA) bound to a peptide inhibitor (PKI) [7], is blocked by the autoinhibitory segment.
For most of these kinases, such as twitchin and CaMKI, the catalytic activity is directly related to Ca2+ levels, rising and falling as the Ca2+ levels increase and decrease. In contrast, Ca2+/CaM dependent protein kinase II (CaMKII) has the ability to acquire Ca2+ independence, referred to as autonomy, when activated strongly by Ca2+ [8,9]. This step is sensitive to the frequency of the Ca2+ spike trains that activate CaMKII and is due to autophosphorylation [10]. If subjected to short Ca2+ spikes at low frequency (e.g., < 1 Hz), CaMKII reverts to a quiescent state in the absence of Ca2+. If, however, the Ca2+ spike train is at higher frequency (e.g., >10 Hz) with the same total exposure to Ca2+, the enzyme acquires Ca2+ independence and is able to phosphorylate substrates even when Ca2+ levels subside. CaMKIV, a monomeric Ca2+/CaM-dependent protein kinase, also has the ability to acquire some calcium independence upon phosphorylation by Ca2+/CaM-dependent protein kinase kinase (CaMKK) [11]. The mechanism by which CaMKIV is regulated is quite distince from that of CaMKII, and is not discussed further.
The acquisition of autonomy prolongs the active state of CaMKII and is likely to be critical for the generation of long-term potentiation (LTP), a strengthening of synaptic connections that underlies synaptic plasticity in learning and memory [12]. Transgenic mice deficient in neuronal CaMKII or mutated at critical phosphorylation sites within the autoinhibitory segment have limited LTP generation and display impairments in learning and memory [13,14].
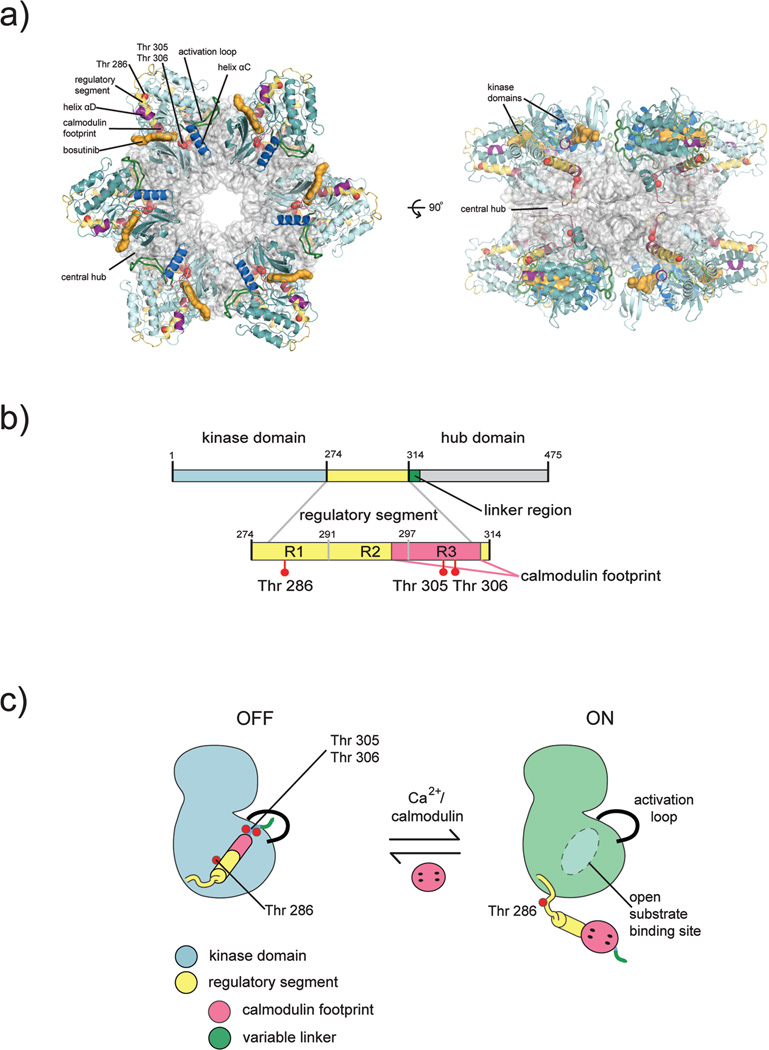
Early electron microscopic images revealed that CaMKII forms symmetric holoenzyme assemblies, usually dodecameric (Fig. 1a) [15–17]. Each subunit of CaMKII within the holoenzyme is comprised of a kinase domain, a regulatory segment, and a hub domain (also referred to as the association domain) (Fig 1b). The hub domain is necessary for oligomerization, and it acts as a central scaffold point for the kinase domains. There exists a diversity of CaMKII isoforms in mammals; four CaMKII genes in humans, termed α, β, δ, and γ, give rise to ~40 isoforms through alternative splicing [18]. The kinase and hub domains of the four human isoforms are closely related in sequence (~95% and ~80% identity, respectively), and the most striking difference between these isoforms is in the length of the linker connecting the kinase domain to the hub domain, which ranges from no residues to ~30 residues. The α and β isoforms are found predominantly in neurons, while the δ and γ isoforms are found throughout the rest of the body.
Figure 1.
Structural organization of CaMKII. a) Crystal structure of an intact CaMKII holoenzyme. The hub assembly is shown as a surface representation (gray) and the kinase domains are shown as cartoons. Each kinase domain is nestled between two hub domains, making contacts with each. The CaM binding region is buried by interactions with the hub. b) Each CaMKII subunit is comprised of a kinase domain (blue), regulatory segment (yellow), variable linker region (green), and a hub domain (gray). The regulatory segment contains three critical phosphorylation sites: Thr 286 is located within the R1 region of the regulatory segment, and Thr 305, Thr 306 are located within the CaM binding region (pink). The CaM binding footprint spans part of the R2 region (docking site for the catalytic domain) and the entire R3 region within the regulatory segment. c) Activation by Ca2+/CaM displaces the regulatory segment from its docking site, thereby freeing the substrate binding site and exposing Thr 286 for trans phosphorylation by an adjacent CaMKII kinase. This figure was adapted from (35).
The importance of CaMKII in neuronal processes is well documented [19–21]. Important insights into how it functions have been obtained by directly visualizing CaMKII in neurons, where it is one of the most highly expressed proteins. One striking example is a study in which fluorescently labeled CaMKII was shown to translocate to the pre-synaptic sites of dendrites upon stimulation by glutamate, which activates specific receptors at the synapse, including the NMDA receptor [12]. Active CaMKII binds the NMDA receptor, which locks CaMKII in an active conformation [22]. A particularly impressive study used laser-pulsing and caged glutamate to induce Ca2+ spikes in dendrites, with simultaneous observation of the activation of CaMKII, using a FRET-based reporter [23]. It is clear from these studies and others, (see, for example, [24–29]) that CaMKII has the ability to respond to the frequency and not just the amplitude of Ca2+ spikes, and this property is likely to be important for its role in LTP.
CaMKII is important for the mechanical and electrical properties of cardiac cells, where the δ isoform is prevalent [30]. Increased CaMKII autophosphorylation, along with increased transcription and expression, is associated with atrial fibrillation, arrhythmias, hypertrophy and heart failure [31–35]
Considering the size of the CaMKII holoenzyme (~700 kDa), the first steps toward understanding its atomic-level structure were to break it down into more manageable pieces. Thus, the crystal structures of the hub domain assembly and kinase domain alone were the first determined separately [36,37]. Recently, the crystal structure of an intact holoenzyme assembly was determined in the autoinhibited form, in the absence of Ca2+/CaM [38]. Electron microscopy (EM) [15–17] and small angle X-ray scattering (SAXS) [36] studies have also been critical for piecing together the properties of the holoenzyme.
Structures of the CaMKII kinase domain
CaMKII has a canonical Ser/Thr kinase domain, but one distinguishing feature is that the activation loop of CaMKII does not contain a phosphorylation site. In contrast to canonical kinases, in which the activation loop is stabilized in an active conformation by phosphorylation, in CaMKII this loop adopts an active conformation without phosphorylation. Instead, phosphorylation control in CaMKII is mediated by the regulatory segment, which occludes the active site in the absence of Ca2+/CaM or when unphosphorylated.
The regulatory segment contains the CaM binding domain as well as three key sites of regulatory autophosphorylation: Thr 286, Thr 305 and Thr 306 (mouse α isoform numbering) (Fig 1b). Thr 286 is autophosphorylated after Ca2+/CaM binding, which results in autonomous Ca2+/CaM-independent activity [39,40] (Fig. 1c). The subsequent dissociation of Ca2+/CaM leads to autophosphorylation of residues 305 and 306, which prevents reassociation of Ca2+/CaM because these residues lie within the CaM binding interface [41]. Interestingly, Met 281 (as well as Met 282 in some isoforms), which lies in the regulatory segment, is sensitive to an oxidation that generates an autonomous kinase with functional implications in cardiac tissue [42,43].
Several crystal structures of autoinhibited forms of CaMKII kinase domains have been determined. Before proceeding to a discussion of these structures, we note that there is some controversy in the literature about the interpretation of the role of dimerization of the CaMKII kinase domain in autoinhibition. Our group determined the first structure, that of C. elegans CaMKII, and showed that the regulatory segments form a coiled-coil dimer in the crystal [36]. We had noted in our original paper that there is no evidence that the isolated autoinhibited kinase domain dimerizes in solution, and so the potential relevance of the crystallographic dimer might manifest itself only in the context of the assembled holoenzyme, where the local concentration of kinase domains is extremely high. Subsequent crystallographic analysis of autoinhibited mammalian CaMKII kinase domains [40] and electron paramagnetic resonance (EPR) analysis of the isolated C. elegans CaMKII kinase domain [44] found no evidence for coiled-coil formation of the regulatory segment, but this may simply be a consequence of the lack of assembly into an intact holoenzyme. The CaMKII assembly is highly dynamic, and we feel that it is premature to conclude that the dimer seen in the C. elegans structure is a crystal artifact, as has been suggested [44]. Resolution of this apparent disparity awaits further dissection of the role of the regulatory segment in intact CaMKII holoenzymes.
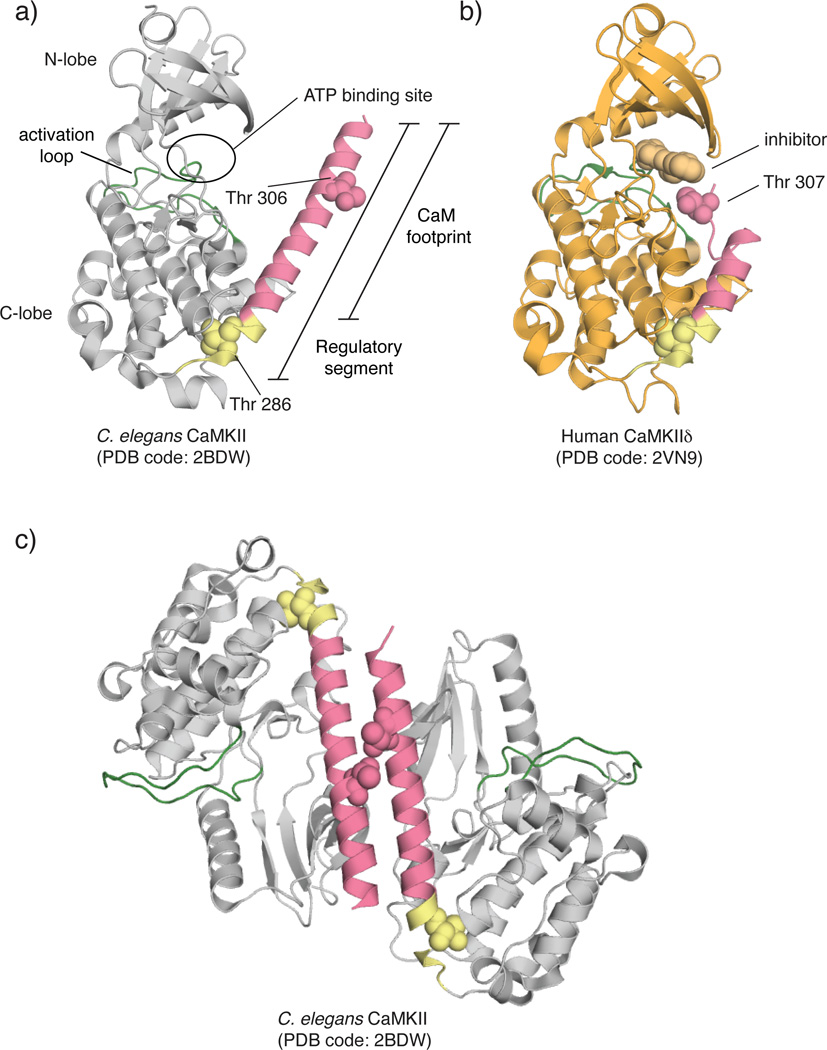
Crystal structures of the isolated kinase domain of CaMKII can be classified into two types: those with the active site blocked by the regulatory segment and those with released regulatory segments [38,40]. In the first category, there is the structure of C. elegans kinase domain with the full-length regulatory segment (Fig. 2a) (PDB: 2BDW). As noted above, the C. elegans kinase domain forms a dimer in the crystal, mediated by a coiled-coil formed by the regulatory segments. There are also several structures of human CaMKII kinase domains bound to small molecule inhibitors in this category, and these do not form similar dimers (isoform crystallized: PDB code, α: 2VZ6, β: 3BHH, δ: 2VN9 (Fig. 2b), γ: 2V7O) [40]. There is also a structure of the C. elegans kinase domain bound to a peptide inhibitor (PDB: 3KL8). In the second category, there is a structure of the human δ isoform crystallized in the presence of Ca2+/CaM (PDB: 2WEL), in which the portion of the regulatory segment spanning Thr 286 of one kinase is bound to the active site of another. Autophosphorylation at Thr 286 in the regulatory segment requires two kinases, one serving as the enzyme and one as the substrate [45]. This structure represents the enzyme-substrate complex that is formed during autophosphorylation, upon release of the regulatory segment by Ca2+/CaM. A similar enzyme-substrate complex has been obtained for C. elegans CaMKII, but without bound Ca2+/CaM (PDB: 3KK8 and 3KK9).
Figure 2.
Crystal structures of kinase domains of CaMKII variants. a) C. elegans CaMKII. The regulatory segment is completely helical and projects Thr 306 far from the active site. PDB code: 2BDW
b) Human CaMKIIδ crystallized bound to a small molecule inhibitor, Bisindolylmaleimide IX. The residues of the CaM binding region form a flexible loop instead of a helix as seen in (a). Colors correspond to those in (a). PDB code: 2V7O
c) Crystallized dimer of C. elegans CaMKII. The regulatory segments of two kinases form a coiled-coil, burying the CaM binding region.
Dimerization of the autoinhibited kinase domain by the coiled-coil formed by the regulatory segment, as seen in the structure of autoinhibited C. elegans CaMKII, is expected to keep the kinase inactive even when the kinases are released from the hub (Fig. 2c) [36]. In particular, such a dimeric arrangement would prevent autophosphorylation of Thr 305 and Thr 306, which could otherwise occur in cis (slow autophosphorylation of Thr 305 does occur under basal conditions [46]). Formation of such a dimer would resist activation because the CaM binding region is occluded in the coiled-coil [5,47]. The physiological relevance of this dimer has yet to be demonstrated, and the importance of the coiled-coil interface is difficult to probe by mutation because it contains residues that are critical for Ca2+/CaM binding. Additionally, the high concentrations achieved in crystallization may mimic what is imposed by the dodecameric structure, a condition that is difficult to achieve in vitro with the kinase domain alone.
The other crystal structures of the CaMKII kinase domain do not reveal dimerization interfaces between kinase domains. Although dimerization of the kinase domains has been shown in vivo in the context of the holoenzyme [48,49] and in vitro with the kinase domain alone [40], evidence for coiled-coil formation is lacking. It should be noted that two of these structures of the autoinhibited kinases (PDB: 2VZ6 and 3BHH) are truncated constructs, which exclude portions of the regulatory domain that would comprise the coiled-coil.
A particularly interesting structure is that of the autoinhibited CaMKIIδ kinase domain (PDB: 2VN9), which does contain the entire regulatory sequence. The CaM binding region is no longer helical and, interestingly, Thr 307 (corresponding to 306 in the standard numbering) in this construct is placed precisely where a threonine residue in a substrate would be located during a phosphorylation reaction (Fig. 2b). This structure resembles the pseudosubstrate model originally proposed by Kemp, and a detailed analysis by EPR of the regulatory segment in the isolated C. elegans kinase domain supports the idea that autoinhibition involves interactions between the regulatory segment and the ATP-binding region of the kinase [44]. It should be noted, however, that in contrast to a strict pseudosubstrate model, the regulatory segment in this structure behaves like a proper substrate, and the adoption of this conformation is likely to result in phosphorylation of Thr 307. Phosphorylation of Thr 307 would block Ca2+/CaM binding, so this configuration presumably occurs at a later stage, after autonomy has been achieved. Conversely, in the autoinhibited dimer structure of the C. elegans enzyme, the corresponding threonine residue (306) is far from the active site and its phosphorylation within the dimer is prevented by the coiled-coil (Fig. 2c). Establishing the importance of these different conformations of the regulatory segment in the context of the complete reaction cycle of CaMKII awaits further study.
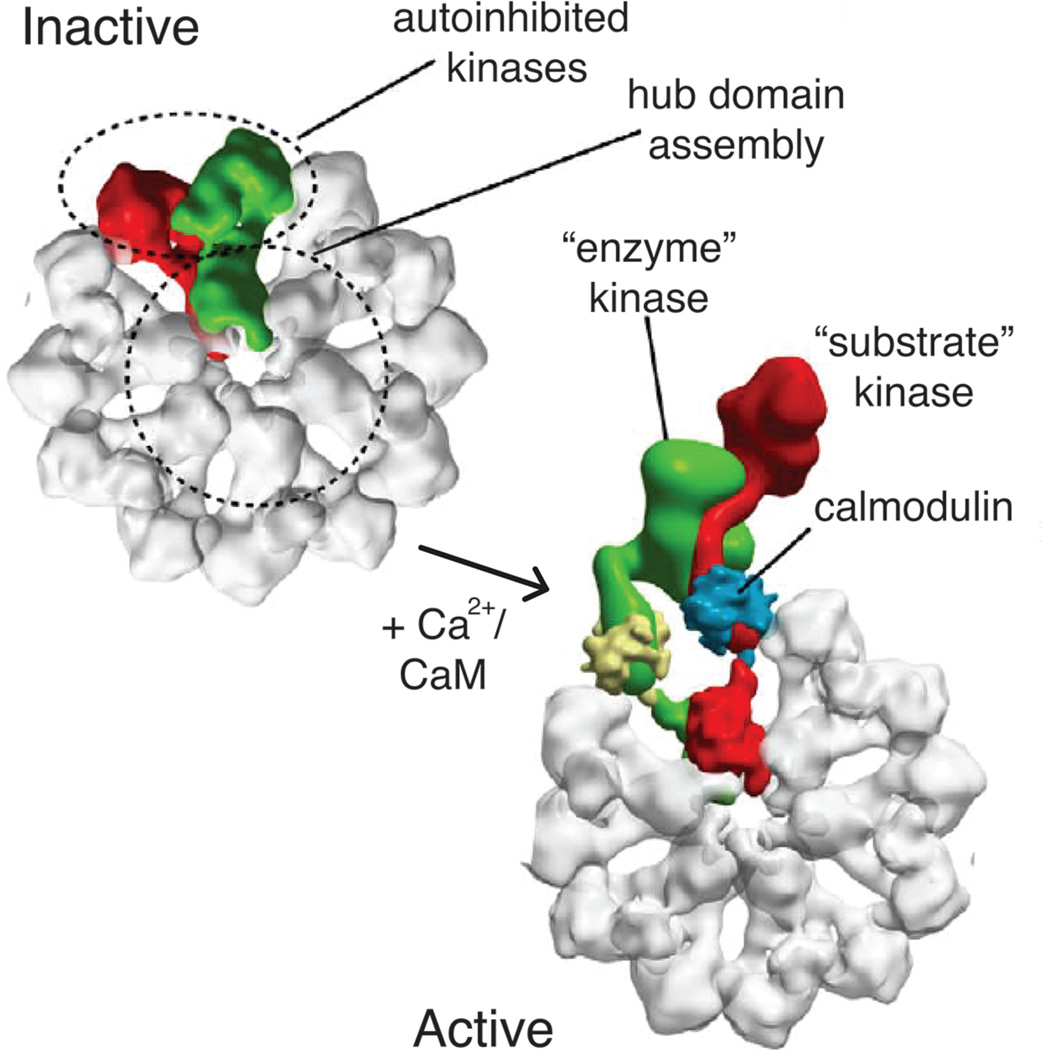
The activation of CaMKII by Ca2+/CaM is highly cooperative [50]. One component of this cooperativity arises from interactions between the regulatory segment in one kinase and the peptide-recognition groove of another [51]. In structures where this interaction is seen (PDB: 2WEL, 3KK8, 3KK9), the portion of the regulatory segment that is upstream of Thr 286 interacts in trans with the substrate-binding groove of another kinase (this groove is occupied by the portion of the regulatory segment that is downstream of Thr 286 in autoinhibited forms of CaMKII). This suggests a substrate-capture mechanism in which the binding of Ca2+/CaM and consequent release of the autoinhibitory segment in one subunit (the “enzyme”) facilitates the capture of the regulatory segment from another subunit to which Ca2+/CaM has not yet bound (the “substrate”) (Fig. 3). This would facilitate binding of Ca2+/CaM to the “substrate” subunit, leading to cooperativity. Biochemical analysis supports the existence of such a mechanism [51].
Figure 3.
Substrate capture during activation within the CaMKII holoenzyme. In the inactive state, each kinase is autoinhibited. Upon Ca2+/CaM binding, activation occurs cooperatively within the holoenzyme via a substrate capture mechanism. An enzyme kinase captures a substrate kinase (both bound to CaM) and results in Thr 286 trans autophosphorylation. This figure was adapted from (39) with permissions.
The central hub
The kinase domains in the CaMKII holoenzyme are presented as protrusions from the central hub, forming a double-layered ring. Such an organization, in which a central hub displays catalytic domains that are splayed outwards, appears to be unusual, although EM reconstructions of the apoptosome display an architecture somewhat similar to that of CaMKII [52,53].
There are several crystal structures of the hub domain of CaMKII, which show two different stoichiometries. Crystallization of mouse CaMKIIα and C. elegans hub domains alone revealed a tetradecameric assembly of two 7-membered rings stacked head to head [37,54]. Importantly, EM images of the full-length mouse CaMKIIα holoenzyme demonstrated that the assembly has sixfold symmetry, consistent with it being a dodecamer [54]. When the kinase domains were released from the hub by proteolytic cleavage, the resulting hub assembly was shown to have seven-fold symmetry. Thus, the hub assembly can convert from a dodecameric assembly to a tetradecameric one when the kinase domains are removed. These findings suggest there is some strain imposed on the hub domain ring by the kinase domains. The human CaMKIIδ isoform hub domain has been crystallized in both dodecameric and tetradecameric forms [40]. Overlaying these two structures reveals that the structure of the hub domains is unchanged, with the conversion from dodecameric to tetradecameric forms involving a small change in the angle between each hub subunit. The physiological significance of this interconversion between oligomeric states is unclear at present.
Holoenzyme structure
Although CaMKII has been shown to adopt different oligomeric states [40,48,54,55], it is likely to be predominantly dodecameric in solution. The first purification of CaMKII in 1983 from rabbit skeletal muscle showed that SDS separation yielded a product of ~58 kDa, while analytical ultracentrifugation yielded a product of 696 kDa, indicating a dodecameric complex [17]. Initial EM studies reported in this early paper captured CaMKII in circular formations with petal-like extensions that have six-fold symmetry.
This dodecameric assembly was later corroborated by negative stain and cryo-EM studies [15,16]. Although both studies concluded that rat neuronal CaMKIIα holoenzyme is comprised of two stacked hexameric rings, the two reconstructions look quite different. The fundamental difference between their resulting reconstructions is the orientation of the kinase domains relative to the hub domains. In one reconstruction, the kinase domains protrude above and below the plane of the hub, resulting in a taller structure when viewed from the side. In the other study, the reconstruction shows the kinase domains extending outwards in the same plane as the hub, resulting in a wider structure. We now appreciate that these two reconstructions of CaMKII may represent alternative configurations of the CaMKII holoenzyme.
A human CaMKIIα holoenzyme with a very short linker was recently crystallized in an inhibited conformation (Fig. 1a) [38]. Three tricks were used to obtain crystals of the holoenzyme, which diffracted X-rays anisotropically to 4.0/3.6 Å. First, a small molecule inhibitor of the c-Abl tyrosine kinase, found previously to inhibit CaMKII adventitiously, helped stabilize the holoenzyme [56]. Second, Thr 306 was mutated to Val, in order to reduce phosphorylation heterogeneity. Finally, the linker connecting the kinase domain to the hub was shortened to the maximal extent to restrict movement of the kinase domain (the construct used for the linker region corresponds to a naturally occurring short linker isoform, β7, of CaMKII) [57].
The crystal structure of the CaMKII holoenzyme is likely to represent a maximally autoinhibited form of the enzyme. This structure reveals a compact state in which each kinase domain is nestled between its own hub domain and the one adjacent to it (Fig. 4a), making them completely inaccessible to peptide substrates. The regulatory domain is not completely helical; instead, the end of the helix leading into the hub melts into a short β turn and random coil. This β turn, termed the β-clip, is comprised of some of the residues required for Ca2+/CaM binding, and makes contacts with a β-sheet of its own hub domain. The β-clip interaction effectively buries the residues corresponding to Thr 305 and Thr 306 and ultimately yields the regulatory segment completely inaccessible to Ca2+/CaM. These and other interactions hold the kinase domains slightly above and below the plane of the central hub.
Figure 4.
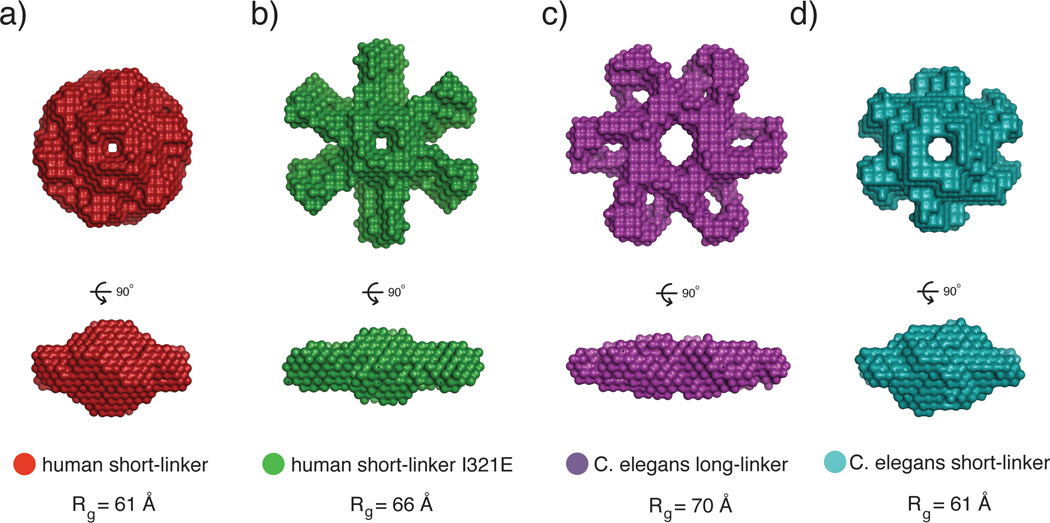
SAXS analysis of the CaMKII holoenzyme. a) The human short-linker construct, used for the crystallization of the full-length holoenzyme, has a compact SAXS envelope. b) A mutation (I321E) introduced to the short-linker construct at the docking site between the kinase and hub domain shows a significantly larger SAXS envelope. This may represent an extended form of the autoinhibited holoenzyme where the kinases are popped out from the hub domain. Additionally, the I321E has a left-shifted EC50 value for CaM binding, which corroborates the model that the extended conformation is more accessible to CaM than the compact conformation observed in the crystal structure. c,d) C. elegans CaMKII also converts from a large to small SAXS envelope when the variable linker is shortened. These may also represent the extended and compact conformations of CaMKII, respectively. This figure was adapted from (35).
The compact nature of the crystallized holoenzyme assembly is very different from the more extended conformations observed for isoforms with longer linkers, using EM [15,16] or SAXS [36,38]. This raises the question as to whether the compact arrangement is specific to isoforms with short linkers. Mutations in the hub domain that are expected to disrupt the docking of the kinase domain onto the hub affect the cooperativity of activation by Ca2+/CaM for both short and long linker isoforms, suggesting that the compact assembly is relevant for both (Fig. 4b). Interestingly, the effects of these mutations on the long linker isoforms are seen only under molecular crowding conditions, suggesting that under dilute conditions the long linker isoforms prefer a more extended conformation in which the kinase domains are not docked onto the hub [38].
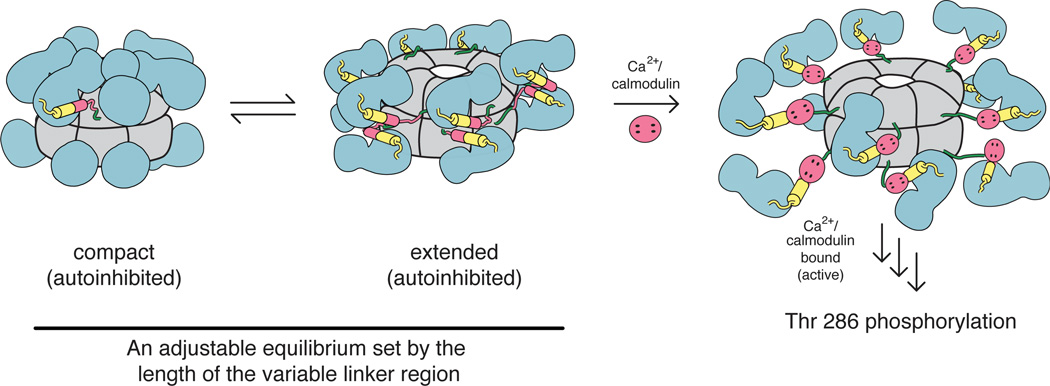
Our current thinking is that the autoinhibited form of the CaMKII holoenzyme undergoes a dynamic equilibrium between compact and extended conformations (Fig. 5). The crystal structure of the holoenzyme provides a clear picture of the compact state, but the extended state remains nebulous due to the lack of direct structural evidence within the context of the holoenzyme. Once the kinase domains are popped out from the hub, they may adopt the coiled-coil inhibited structure or remain as monomers with the regulatory segment blocking the ATP binding site, or they may dimerize in a different conformation altogether. The equilibrium between the compact and extended states is tuned by the linker length. Longer linker lengths favor an extended autoinhibited form, which is more easily activated by Ca2+/CaM, and has a lower frequency threshold for activation by Ca2+ spikes. Such a mechanism provides an easy way for nature to tune the responsiveness of different isoforms without much variation in the sequences of the kinase and hub domains.
Figure 5.
Conformational changes in the holoenzyme. The CaMKII holoenzyme has been shown to adopt both compact (crystallization, SAXS) and extended (SAXS) autoinhibited states. An equilibrium exists between these states, and is sensitive to the length of the variable linker connecting the kinase and hub domains. In the compact autoinhibited conformation, residues comprising the CaM binding region make contacts with the hub domain, thereby making it highly inaccessible to CaM. In the extended autoinhibited conformation, the CaM binding region is more exposed, though the structural details of this extended state have yet to be elucidated. Upon Ca2+/CaM binding, the regulatory segment is released from the kinase domain, thereby relieving inhibition. Active kinases then trans autophosphorylate adjacent kinases and subsequently phosphorylate downstream targets. Colors correspond to those in Fig. 1. This figure was adapted from (35).
The results of SAXS analyses of various CaMKII isoforms are consistent with the linker length controlling the balance between compact and extended assemblies [38]. SAXS reconstructions of a short linker construct reveal a compact structure, while reconstructions of a long linker constructs reveal an extended structure with six knobs protruding from the central hub. The reconstruction for the short linker isoform can be switched from compact to extended by making a point mutation in the hub domain at the docking site for the kinase domain (I321E). Likewise, the reconstruction for the C. elegans holoenzyme, which has a long linker, can be switched from extended to compact by shortening the linker (Fig. 4c, d).
We believe that the extended form promotes Ca2+/CaM binding and thereby facilitates activation. It is clear that Ca2+/CaM binding induces a large conformational change within the kinase that releases inhibition by the regulatory segment. EPR studies have shown that upon activation, there is a transition between an inhibited (closed) state and an activated (released) state as suggested from the crystal structure of the activated truncated kinase [40,44]. This conformational change has been cleverly exploited to create FRET probes for activation [58–60] that have been used in vivo to study CaMKII function [23,28].
Frequency dependent activation must be a consequence of the kinetics of activation within a CaMKII holoenzyme. Simulation studies have shown that this phenomenon could be achieved without holoenzyme formation, if the various rate constants and concentrations are tuned appropriately (see, for example, [61,62]). An important role for the oligomeric structure is probably to bring the enzyme and substrate CaMKII into close proximity so that the rates of transphosphorylation and release of inhibition are matched appropriately to the Ca2+ spike frequency.Importantly, the duration of each Ca2+ pulse will affect the frequency threshold – a shorter pulse duration will necessitate a higher threshold frequency for activation and vice versa. Once some subunits within the holoenzyme have been autophosphorylated at or above the threshold frequency, lower frequencies are sufficient to achieve further activation [10]. Additionally, the Ca2+/CaM dissociation rate is decreased by 10,000-fold after Thr 286 phosphorylation [63], which, in combination with cooperativity, acts to perpetuate activation.
Conclusion
Our knowledge of CaMKII structure and regulation has improved tremendously in the past decade, but various important aspects of the structure still await further clarification. A detailed picture of the extended autoinhibited state is unavailable. At the same time, the development of sophisticated fluorescence-based tools now allows the interrogation of CaMKII function in cells with unprecedented control over experimental parameters such as the frequency of Ca2+ spikes. These advances make it feasible to eventually close the gap between our deepening understanding of the molecular architecture of CaMKII and its many functions in brain, cardiac, and other tissues.
Acknowledgements
This work was partially funded by a Jane Coffin Childs Postdoctoral Fellowship given to M.M.S. We thank Dr. Tiago Barros for help with artwork.
References recommended reading
Papers of interest, published within the period of review, have been highlighted as:
• of special interest
••of outstanding interest
- 1.Kemp BE, Parker MW, Hu S, Tiganis T, House C. Substrate and pseudosubstrate interactions with protein kinases: determinants of specificity. Trends Biochem Sci. 1994;19:440–444. doi: 10.1016/0968-0004(94)90126-0. [DOI] [PubMed] [Google Scholar]
- 2.Heierhorst J, Kobe B, Feil SC, Parker MW, Benian GM, Weiss KR, Kemp BE. Ca2+/S100 regulation of giant protein kinases. Nature. 1996;380:636–639. doi: 10.1038/380636a0. [DOI] [PubMed] [Google Scholar]
- 3.Kobe B, Heierhorst J, Feil SC, Parker MW, Benian GM, Weiss KR, Kemp BE. Giant protein kinases: domain interactions and structural basis of autoregulation. EMBO J. 1996;15:6810–6821. [PMC free article] [PubMed] [Google Scholar]
- 4.Owen DJ, Noble ME, Garman EF, Papageorgiou AC, Johnson LN. Two structures of the catalytic domain of phosphorylase kinase: an active protein kinase complexed with substrate analogue and product. Structure. 1995;3:467–482. doi: 10.1016/s0969-2126(01)00180-0. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg J, Nairn AC, Kuriyan J. Structural basis for the autoinhibition of calcium/calmodulin-dependent protein kinase I. Cell. 1996;84:875–887. doi: 10.1016/s0092-8674(00)81066-1. [DOI] [PubMed] [Google Scholar]
- 6.Mayans O, van der Ven PF, Wilm M, Mues A, Young P, Furst DO, Wilmanns M, Gautel M. Structural basis for activation of the titin kinase domain during myofibrillogenesis. Nature. 1998;395:863–869. doi: 10.1038/27603. [DOI] [PubMed] [Google Scholar]
- 7.Narayana N, Cox S, Shaltiel S, Taylor SS, Xuong N. Crystal structure of a polyhistidine-tagged recombinant catalytic subunit of cAMP-dependent protein kinase complexed with the peptide inhibitor PKI(5-24) and adenosine. Biochemistry. 1997;36:4438–4448. doi: 10.1021/bi961947+. [DOI] [PubMed] [Google Scholar]
- 8.Lai Y, Nairn AC, Greengard P. Autophosphorylation reversibly regulates the Ca2+/calmodulin-dependence of Ca2+/calmodulin-dependent protein kinase II. Proc Natl Acad Sci U S A. 1986;83:4253–4257. doi: 10.1073/pnas.83.12.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller SG, Kennedy MB. Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell. 1986;44:861–870. doi: 10.1016/0092-8674(86)90008-5. [DOI] [PubMed] [Google Scholar]
- 10.De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- 11.Soderling TR. The Ca-calmodulin-dependent protein kinase cascade. Trends Biochem Sci. 1999;24:232–236. doi: 10.1016/s0968-0004(99)01383-3. [DOI] [PubMed] [Google Scholar]
- 12.Shen K, Meyer T. Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science. 1999;284:162–166. doi: 10.1126/science.284.5411.162. [DOI] [PubMed] [Google Scholar]
- 13.Silva AJ, Paylor R, Wehner JM, Tonegawa S. Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257:206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- 14.Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- 15.Kolodziej SJ, Hudmon A, Waxham MN, Stoops JK. Three-dimensional reconstructions of calcium/calmodulin-dependent (CaM) kinase IIalpha and truncated CaM kinase IIalpha reveal a unique organization for its structural core and functional domains. J Biol Chem. 2000;275:14354–14359. doi: 10.1074/jbc.275.19.14354. [DOI] [PubMed] [Google Scholar]
- 16.Morris EP, Torok K. Oligomeric structure of alpha-calmodulin-dependent protein kinase II. J Mol Biol. 2001;308:1–8. doi: 10.1006/jmbi.2001.4584. [DOI] [PubMed] [Google Scholar]
- 17.Woodgett JR, Davison MT, Cohen P. The calmodulin-dependent glycogen synthase kinase from rabbit skeletal muscle. Purification subunit structure and substrate specificity. Eur J Biochem. 1983;136:481–487. doi: 10.1111/j.1432-1033.1983.tb07766.x. [DOI] [PubMed] [Google Scholar]
- 18.Tombes RM, Faison MO, Turbeville JM. Organization and evolution of multifunctional Ca(2+)/CaM-dependent protein kinase genes. Gene. 2003;322:17–31. doi: 10.1016/j.gene.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 19.Liu XB, Murray KD. Neuronal excitability and calcium/calmodulin-dependent protein kinase type II: location, location, location. Epilepsia. 2012;53(Suppl 1):45–52. doi: 10.1111/j.1528-1167.2012.03474.x. [DOI] [PubMed] [Google Scholar]
- 20.Coultrap SJ, Bayer KU. CaMKII regulation in information processing and storage. Trends Neurosci. 2012;35:607–618. doi: 10.1016/j.tins.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci. 2012;13:169–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411:801–805. doi: 10.1038/35081080. [DOI] [PubMed] [Google Scholar]
- 23.Lee SJ, Escobedo-Lozoya Y, Szatmari EM, Yasuda R. Activation of CaMKII in single dendritic spines during long-term potentiation. Nature. 2009;458:299–304. doi: 10.1038/nature07842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemieux M, Labrecque S, Tardif C, Labrie-Dion E, Lebel E, De Koninck P. Translocation of CaMKII to dendritic microtubules supports the plasticity of local synapses. J Cell Biol. 2012;198:1055–1073. doi: 10.1083/jcb.201202058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang CC, Hsu KS. Activation of NMDA receptors reduces metabotropic glutamate receptor-induced long-term depression in the nucleus accumbens via a CaMKII-dependent mechanism. Neuropharmacology. 2012;63:1298–1307. doi: 10.1016/j.neuropharm.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Zhang P, Lisman JE. Activity-dependent regulation of synaptic strength by PSD-95 in CA1 neurons. J Neurophysiol. 2012;107:1058–1066. doi: 10.1152/jn.00526.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gambrill AC, Barria A. NMDA receptor subunit composition controls synaptogenesis and synapse stabilization. Proc Natl Acad Sci U S A. 2011;108:5855–5860. doi: 10.1073/pnas.1012676108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erickson JR, Patel R, Ferguson A, Bossuyt J, Bers DM. Fluorescence resonance energy transfer-based sensor Camui provides new insight into mechanisms of calcium/calmodulin-dependent protein kinase II activation in intact cardiomyocytes. Circ Res. 2011;109:729–738. doi: 10.1161/CIRCRESAHA.111.247148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eshete F, Fields RD. Spike frequency decoding and autonomous activation of Ca2+-calmodulin-dependent protein kinase II in dorsal root ganglion neurons. J Neurosci. 2001;21:6694–6705. doi: 10.1523/JNEUROSCI.21-17-06694.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Swaminathan PD, Purohit A, Hund TJ, Anderson ME. Calmodulin-dependent protein kinase II: linking heart failure and arrhythmias. Circ Res. 2012;110:1661–1677. doi: 10.1161/CIRCRESAHA.111.243956.• CaMKII involvement in heart disease is reviewed. A large body of evidence suggests that the active form of CaMKII leads to myocardial disfunction and electrical instability. Oxidation of susceptible Met residues (281/282) may play a large role in hyper-activation of CaMKII and lead to disease phenotypes.
- 31.Zhang T, Kohlhaas M, Backs J, Mishra S, Phillips W, Dybkova N, Chang S, Ling H, Bers DM, Maier LS, et al. CaMKIIdelta isoforms differentially affect calcium handling but similarly regulate HDAC/MEF2 transcriptional responses. J Biol Chem. 2007;282:35078–35087. doi: 10.1074/jbc.M707083200. [DOI] [PubMed] [Google Scholar]
- 32.Backs J, Backs T, Neef S, Kreusser MM, Lehmann LH, Patrick DM, Grueter CE, Qi X, Richardson JA, Hill JA, et al. The delta isoform of CaM kinase II is required for pathological cardiac hypertrophy and remodeling after pressure overload. Proc Natl Acad Sci U S A. 2009;106:2342–2347. doi: 10.1073/pnas.0813013106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, Li N, Santonastasi M, Muller FU, Schmitz W, et al. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–1951. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neef S, Dybkova N, Sossalla S, Ort KR, Fluschnik N, Neumann K, Seipelt R, Schondube FA, Hasenfuss G, Maier LS. CaMKII-dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ Res. 2010;106:1134–1144. doi: 10.1161/CIRCRESAHA.109.203836. [DOI] [PubMed] [Google Scholar]
- 35.Rokita AG, Anderson ME. New Therapeutic Targets in Cardiology: Arrhythmias and Ca2+/Calmodulin-Dependent Kinase II (CaMKII) Circulation. 2012;126:2125–2139. doi: 10.1161/CIRCULATIONAHA.112.124990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rosenberg OS, Deindl S, Sung RJ, Nairn AC, Kuriyan J. Structure of the autoinhibited kinase domain of CaMKII and SAXS analysis of the holoenzyme. Cell. 2005;123:849–860. doi: 10.1016/j.cell.2005.10.029.•• The crystal structure of C. elegans kinase domain shows an autoinhibited dimeric conformation where the regulatory segments of two kinase domains form a coiled-coil interaction. A model for the extended form of the CaMKII holoenzyme is presented. It was generated by fitting the coiled-coil kinase dimer into the SAXS envelope of the C. elegans holoenzyme.
- 37.Hoelz A, Nairn AC, Kuriyan J. Crystal structure of a tetradecameric assembly of the association domain of Ca2+/calmodulin-dependent kinase II. Mol Cell. 2003;11:1241–1251. doi: 10.1016/s1097-2765(03)00171-0. [DOI] [PubMed] [Google Scholar]
- 38. Chao LH, Stratton MM, Lee IH, Rosenberg OS, Levitz J, Mandell DJ, Kortemme T, Groves JT, Schulman H, Kuriyan J. A mechanism for tunable autoinhibition in the structure of a human Ca2+/calmodulin-dependent kinase II holoenzyme. Cell. 2011;146:732–745. doi: 10.1016/j.cell.2011.07.038.•• A crystal structure of the full-length CaMKII holoenzyme is reported for the human α isoform containing a short variable linker region. This structure shows CaMKII in an autoinhibited conformation where each kinase is nestled between two association domains, effectively burying its regulatory domain and CaM binding domain in interactions with the hub domain. It is proposed that linker length may tune autoinhibition within the holoenzyme assembly, creating CaMKII isoforms that have varying threshold frequencies for activation by calcium spikes.
- 39.Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- 40. Rellos P, Pike AC, Niesen FH, Salah E, Lee WH, von Delft F, Knapp S. Structure of the CaMKIIdelta/calmodulin complex reveals the molecular mechanism of CaMKII kinase activation. PLoS Biol. 2010;8:1000426. doi: 10.1371/journal.pbio.1000426.•• Crystal structures of all human isoforms' (α, β, δ, γ) kinase domains in isolation and two hub domain assemblies (δ, γ) are reported. All kinase domains crystallized as monomers. The CaMKIIδ and γ hub assemblies crystallized as a tetradecameric and dodecameric complexes, respectively. The crystal structure of CaMKIIδ kinase domain was crystallized as a complex with calmodulin, revealing an extended regulatory domain.
- 41.Hanson PI, Schulman H. Inhibitory autophosphorylation of multifunctional Ca2+/calmodulin-dependent protein kinase analyzed by site-directed mutagenesis. J Biol Chem. 1992;267:17216–17224. [PubMed] [Google Scholar]
- 42.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh MV, Swaminathan PD, Luczak ED, Kutschke W, Weiss RM, Anderson ME. MyD88 mediated inflammatory signaling leads to CaMKII oxidation, cardiac hypertrophy and death after myocardial infarction. J Mol Cell Cardiol. 2012;52:1135–1144. doi: 10.1016/j.yjmcc.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hoffman L, Stein RA, Colbran RJ, McHaourab HS. Conformational changes underlying calcium/calmodulin-dependent protein kinase II activation. EMBO J. 2011;30:1251–1262. doi: 10.1038/emboj.2011.40.•• Electron paramagnetic resonance spectroscopic studies of the isolated kinase domain and regulatory segment of C. elegans CaMKII provide evidence for an autoinhibited monomeric kinase where the regulatory domain makes contacts with the catalytic domain, effectively blocking the ATP binding site. Additionally, activation by CaM binding induces a conformational change of the regulatory segment away from the catalytic domain.
- 45.Hanson PI, Meyer T, Stryer L, Schulman H. Dual role of calmodulin in autophosphorylation of multifunctional CaM kinase may underlie decoding of calcium signals. Neuron. 1994;12:943–956. doi: 10.1016/0896-6273(94)90306-9. [DOI] [PubMed] [Google Scholar]
- 46.Colbran RJ. Inactivation of Ca2+/calmodulin-dependent protein kinase II by basal autophosphorylation. J Biol Chem. 1993;268:7163–7170. [PubMed] [Google Scholar]
- 47.Hu SH, Parker MW, Lei JY, Wilce MC, Benian GM, Kemp BE. Insights into autoregulation from the crystal structure of twitchin kinase. Nature. 1994;369:581–584. doi: 10.1038/369581a0. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen TA, Sarkar P, Veetil JV, Koushik SV, Vogel SS. Fluorescence polarization and fluctuation analysis monitors subunit proximity, stoichiometry, and protein complex hydrodynamics. PLoS One. 2012;7:38209. doi: 10.1371/journal.pone.0038209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thaler C, Koushik SV, Puhl HL, 3rd, Blank PS, Vogel SS. Structural rearrangement of CaMKIIalpha catalytic domains encodes activation. Proc Natl Acad Sci U S A. 2009;106:6369–6374. doi: 10.1073/pnas.0901913106.• It is proposed that the CaMKII holoenzyme is inhibited as dimeric units, and that they monomerize upon activation. These experiments were performed in vivo (in both HeLa and hippocampal neuronal cultures) by expressing CaMKII subunits with Venus at the N- or C-terminal end and observing changes in FRET and fluorescence anisotropy in real-time.
- 50.Bradshaw JM, Kubota Y, Meyer T, Schulman H. An ultrasensitive Ca2+/calmodulin-dependent protein kinase II-protein phosphatase 1 switch facilitates specificity in postsynaptic calcium signaling. Proc Natl Acad Sci U S A. 2003;100:10512–10517. doi: 10.1073/pnas.1932759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chao LH, Pellicena P, Deindl S, Barclay LA, Schulman H, Kuriyan J. Intersubunit capture of regulatory segments is a component of cooperative CaMKII activation. Nat Struct Mol Biol. 2010;17:264–272. doi: 10.1038/nsmb.1751.•• A structural model for intersubunit capture is proposed, which explains how trans autophosphorylation occurs cooperatively within the holoenzyme complex of CaMKII after calmodulin binding. Biochemical data in conjunction with crystal structures of an "enzyme" CaMKII and a "substrate" CaMKII corroborate this model.
- 52.Yuan S, Yu X, Topf M, Dorstyn L, Kumar S, Ludtke SJ, Akey CW. Structure of the Drosophila apoptosome at 6.9 a resolution. Structure. 2011;19:128–140. doi: 10.1016/j.str.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuan S, Yu X, Topf M, Ludtke SJ, Wang X, Akey CW. Structure of an apoptosome-procaspase-9 CARD complex. Structure. 2010;18:571–583. doi: 10.1016/j.str.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosenberg OS, Deindl S, Comolli LR, Hoelz A, Downing KH, Nairn AC, Kuriyan J. Oligomerization states of the association domain and the holoenyzme of Ca2+/CaM kinase II. FEBS J. 2006;273:682–694. doi: 10.1111/j.1742-4658.2005.05088.x. [DOI] [PubMed] [Google Scholar]
- 55.Kanaseki T, Ikeuchi Y, Sugiura H, Yamauchi T. Structural features of Ca2+/calmodulin-dependent protein kinase II revealed by electron microscopy. J Cell Biol. 1991;115:1049–1060. doi: 10.1083/jcb.115.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Remsing Rix LL, Rix U, Colinge J, Hantschel O, Bennett KL, Stranzl T, Muller A, Baumgartner C, Valent P, Augustin M, et al. Global target profile of the kinase inhibitor bosutinib in primary chronic myeloid leukemia cells. Leukemia. 2009;23:477–485. doi: 10.1038/leu.2008.334. [DOI] [PubMed] [Google Scholar]
- 57.Wang P, Wu YL, Zhou TH, Sun Y, Pei G. Identification of alternative splicing variants of the beta subunit of human Ca(2+)/calmodulin-dependent protein kinase II with different activities. FEBS Lett. 2000;475:107–110. doi: 10.1016/s0014-5793(00)01634-3. [DOI] [PubMed] [Google Scholar]
- 58.Takao K, Okamoto K, Nakagawa T, Neve RL, Nagai T, Miyawaki A, Hashikawa T, Kobayashi S, Hayashi Y. Visualization of synaptic Ca2+ /calmodulin-dependent protein kinase II activity in living neurons. J Neurosci. 2005;25:3107–3112. doi: 10.1523/JNEUROSCI.0085-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kwok S, Lee C, Sanchez SA, Hazlett TL, Gratton E, Hayashi Y. Genetically encoded probe for fluorescence lifetime imaging of CaMKII activity. Biochem Biophys Res Commun. 2008;369:519–525. doi: 10.1016/j.bbrc.2008.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lam AJ, St-Pierre F, Gong Y, Marshall JD, Cranfill PJ, Baird MA, McKeown MR, Wiedenmann J, Davidson MW, Schnitzer MJ, et al. Improving FRET dynamic range with bright green and red fluorescent proteins. Nat Methods. 2012;9:1005–1012. doi: 10.1038/nmeth.2171.•• A CaMKII variant was constructed, deemed Camui, with a fluorescent protein at the N-terminal end and its FRET partner at the C-terminal end. A decrease in FRET is observed upon activation, which reports on the conformational change within the kinase domain following calmodulin binding. This has allowed in vivo studies of real-time CaMKII activation. In this paper, an improved version of Camui is reported.
- 61.Lucic V, Greif GJ, Kennedy MB. Detailed state model of CaMKII activation and autophosphorylation. Eur Biophys J. 2008;38:83–98. doi: 10.1007/s00249-008-0362-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chiba H, Schneider NS, Matsuoka S, Noma A. A simulation study on the activation of cardiac CaMKII delta-isoform and its regulation by phosphatases. Biophys J. 2008;95:2139–2149. doi: 10.1529/biophysj.107.118505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singla SI, Hudmon A, Goldberg JM, Smith JL, Schulman H. Molecular characterization of calmodulin trapping by calcium/calmodulin-dependent protein kinase II. J Biol chem. 2001;276:29353–29360. doi: 10.1074/jbc.M101744200. [DOI] [PubMed] [Google Scholar]