Abstract
Cladribine has been used in the treatment of hairy cell leukemia for about 30 years. In addition, the number of indications for the application of 2-CdA is constantly increasing. The treatment with cladribine, of younger persons and even children, appears to be a major factor stimulating the more exact recognition of its activities. However, till now, little has been known about the impact of cladribine on the reproductive system. The aim of the study was to evaluate the immunohistochemical expression of cell proliferation and apoptosis markers in ovarian surface epithelial (OSE) cells. In our study, ten rats were placed into two equal groups. The study group received daily subcutaneous injections of cladribine in a dose of 0.10 mg/kg of weight/day for one cycle lasting 7 days. The control group received only saline injections. The rats were sacrificed 24 h after the last injection, and their ovaries were extracted. The sections were immunohistochemically stained with cell proliferation marker Ki-67 and the apoptosis marker caspase 3. The expressions of the markers were evaluated using a light microscope. An analysis was made using an image analysis system and the CellAD software. The results were then statistically explored by way of the Mann–Whitney U test. The proliferative index (Ki-67) of ovarian surface epithelial cells was significantly lower in the study group than in the control group (p < 0.05). These results suggest that cladribine treatment has a potential to inhibit the OSE cell proliferation in rats. The apoptosis marker demonstrated a significant increase after the cladribine treatment. These suggest that cladribine induces apoptosis in OSE cells.
Keywords: Apoptosis, Ki-67, Caspase 3, Ovarian surface epithelium, Cladribine
Introduction
The ovarian surface epithelium (OSE) covers the entire ovary, separating the peritoneal cavity from the ovarian stroma while maintaining a constant contact between these structures. The epithelium rests on the basal membrane, under which there is a collagenous connective tissue layer, the tunica albuginea (Auersperg et al. 2001). Just below the tunica albuginea, in the ovarian cortex, ovarian follicles that are in various stages of maturity can be found. The development stage of a given follicle, connected with the phase of the menstrual cycle and hormonal level, plays a crucial role in the structure and functions of the OSE. As the ovaries prepare to release a mature oocyte, the OSE cells undergo a gradual degeneration under the influence of different factors (Murdoch and McDonnel 2002; Slot et al. 2006). Proteinases (including collagenases) cause the degradation of connective tissue elements while the OSE cells undergo apoptosis allowing the release of the oocyte from the ovary. The site of rupture (wound) of the layer covering the ovary after ovulation is then restored by proliferating OSE cells that migrate to the edges of the wound (Gava et al. 2008; Murdoch and McDonnel 2002).
It has also been demonstrated that OSE cells synthesize various substances present in the extracellular matrix and also exhibit contractile activity (Auersperg et al. 1991). It is believed that the variation of the epithelial cells is not firmly determined, and they may constitute precursors, not only for new OSE cells, but also for other cell types, such as the ovarian stromal cells, as well as the epithelial cells of the oviduct or endometrium. This type of conversion protects against the formation of aggregates of epithelial cells in unusual sites, e.g., the interior of the ovary. However, at the same time, this conversion favours the circumvention of the mechanisms that control the proliferation and differentiation of OSE cells, which leads to the formation of many types of neoplasms derived from the ovary (Singavarapu et al. 2010). Data show that 90 % of human ovarian malignant neoplasms are derived from the OSE (Auersperg et al. 2001). The proliferation of OSE cells is increased after each ovulation in order to repair the interrupted continuity of the epithelium. Different factors can disrupt this process, leading to changes within the tunics covering the ovary. These factors include inflammation in the genital tract, the activity of reactive oxidants, hormonal stimulation of ovulation, and also the use of various therapeutic agents acting systemically. Substances that have been reported to affect both the course of proliferation and other processes related to the cell cycle include immunosuppressive and anticancer drugs, among these, steroids, cytotoxic drugs, and monoclonal antibodies. So far, there has been little data on the detailed effects of the drugs of this kind on the OSE cells (Perez et al. 1993).
The purine nucleoside analogues (PNA), which include cladribine, constitute a group of cytostatic drugs, which result in the induction of apoptosis in both proliferating cells and those remaining at rest. The mechanism of PNA is multidirectional and is still not fully discovered. PNA exhibits the highest activity in lymphoid cells due to the favourable concentrations of active enzymes present in such cells. Active PNA metabolites cause a number of changes in the cell, leading to its destruction (Johnston 2011; Robak et al. 2009). The effect of PNA on cells is partially dependent on the p53 protein, and mutations within the gene of the p53 protein may weaken or remove the impact of PNA (Schlette et al. 2009). However, other ways of activating PNA apoptosis that are independent of the p53 protein in the cells have been described (Robak et al. 2009; Johnston 2011).
There are many known side effects induced by cladribine. These are connected mainly with the suppressive effects of 2-CdA on the bone marrow. The consequences resulting from myelosuppression include inter alia, leukopenia, thrombocytopenia, and anemia. The side effects of cladribine also include bacterial, viral, and fungal infections; disorders of the nervous system (headaches, dizziness, insomnia, anxiety, peripheral neuropathy), cardiac insufficiency, nephropathy, disorders of the gastrointestinal tract (nausea, vomiting, abdominal pain), musculoskeletal disorders, local skin reactions, teratogenicity and infertility, and also secondary malignancies, such as, acute myeloid leukemia (AML), non-Hodgkin lymphoma (NHL) (Chubar and Bennett 2003; Rossini et al. 2004; Hassan et al. 2004; Marczak et al. 2004; Saven et al. 1998; Tawfik and Nasr 2011).
The effect of PNA on the genital tract has not yet been accurately described. This is probably because these have been mostly administered for the treatment of hairy cell leukemia, which occurs mainly in older people. However, currently, PNA administration has become a useful tool in treating young persons and even children. Indeed, positive effects of cladribine in the treatment of chronic lymphocytic leukemia have been described (Chow et al. 2000), as its efficacy in treating NHL [Chow et al. 2000], mantle cell lymphoma (Inwards et al. 2008), AML (Wierzbowska et al. 2008), myelodysplastic syndromes (Laurencet et al. 1997), chronic myeloid leukemia (Martinelli et al. 1996), Waldenström macroglobulinemia (Rourke et al. 2010), histiocytosis, also in children (Mottl et al. 2006), astrocytomas (Ceruti et al. 2000, Ceruti et al. 2003), multiple sclerosis (Hartung 2009; Leist and Weissert 2011; Vosoughi and Freedman 2010), mast cell disease (Aichberger et al. 2008), psoriasis, including psoriatic arthritis (Eibschutz et al. 1995), systemic lupus erythematosus (Bernknopf et al. 2011), angioedema, and anaphylaxis (Dutta et al. 2002).
In this research, we examined the effects of cladribine on the OSE cells of female Wistar rats. In so doing, the expression of caspase 3 and the nuclear antigen Ki-67 in the OSE cells of rats subjected to cladribine to assess apoptosis and proliferation in these cells has been evaluated.
Materials and methods
Experimental model
White female Wistar rats were used for the study. The rats were 3 to 4-month-old females with an average body weight of 275 g. Cladribine was applied subcutaneously, interchangeably in the right and left side skinfolds at the height of the lumbar spine (the study was approved by the Local Bioethics Commission of Medical University in Lublin number 126/2001). During the experiment, the animals were housed in cages with a surface area of 0.5 m2, with a preserved circadian cycle (12-h day, 12-h night), air temperature around 21 °C, and relative humidity around 60 %. The rats received standard LSM feed and water without limitations. Before starting the experiment, a cytological smear of females was analyzed to determine the phase of the estrous cycle of the studied females. In all the studied females, the estrous cycle lasted 4 days and consisted of four phases: proestrus, estrus, metestrus, diestrus. On the first day of the fourth cycle, drug administration was begun.
Research groups
The animals were randomly placed into two groups: one control group (K) and one study group (A), each of which consisted of five individuals. The animals in the study group were administered the drug at a dose of 0.10 mg/kg body weight/24 h, for 7 consecutive days, at exactly the same time. The dose used corresponded to the therapeutic dose used in the treatment of proliferative disorders of the lymphatic system in humans. The control group animals received only food and water without limitations. In order to introduce a stress-inducing factor as to that of the study group, rats in the control group were administered physiological saline subcutaneously in a volume corresponding to the amount of drug administered to rats in the study group. Decapitation of the animals of both groups was conducted 24 h after the last injection, i.e., on the fourth day of the cycle (the final stage of diestrus). Their ovaries were then taken for histomorphological and immunohistochemical tests.
Histological tests (H&E staining, immunohistochemical tests)
After fixing the material in 10 % formalin, the organs were embedded in paraffin blocks. The material was then tailored into sections 5-μm thick. The histomorphological evaluation of tissues stained with hematoxylin and eosin (H&E) was performed with the use of a light microscope, at × 400 and × 1,000 magnification. Immunohistochemical tests were then done using antibodies that were produced by Sigma directed against the antigen Ki-67 and caspase 3. The exposure of the antigenic sites was performed thermally by incubation in citrate buffer solution with pH = 6, in a microwave oven at 800 W, for three cycles lasting 5 min each. To inhibit endogenous peroxidase activity, 0.3 % perhydrol (H202) in methanol was used. Normal Serum was also used to block the nonspecific bindings of antigen. The material was incubated in a primary antibody diluted as recommended by the manufacturer (1:150 for Ki-67, 1:100 for caspase 3) overnight at 4 °C. To visualize the reaction, diaminobenzidine solution and hematoxylin coloration were used. In the negative control, experiments were conducted in a similar manner but omitted the specific primary antibody. The material was evaluated with the use of a light microscope at × 400 magnification.
Subsequently, randomly selected 15 corpora lutea in the process of repair from the animals of study group and the control group underwent an analysis of the expression of individual proteins (on average, 20–75 cells in each corpus luteum). OSE cells with positive expression were counted. After this, the percentage of Ki-67- and caspase 3-positive cells was calculated (the number of protein-positive cells divided by the total number of cells counted multiplied by 100). Moreover, the intensity of expression of individual proteins of OSE cells was compared. The intensity was graded as low (+), intermediate (++) and high (+++), by using the BX4 image analysis system manufactured by Olympus, with a DP 25 digital camera and the Cell D software. In addition, the percentage of cells of the given intensity expression in each tested corpus luteum was calculated. These values were expressed as a mean ± standard deviation. The obtained tests results were subjected to statistical analysis with the use of the Statistica 10.0 software. The chi-square (χ 2) test was applied to compare the mean of Ki-67 and caspase 3 indicating positive and negative cells in both groups. A statistical analysis of the intensity of protein expression in both groups was performed with the use of the Mann–Whitney U test. In this regard, a probability (p) value less than 0.05 was considered statistically significant.
Results
The histomorphological assessment of ovarian surface epithelium in H&E staining
OSE cells of the analyzed groups of animals, in the histomorphological study, showed no discernible pathological changes under the light microscope at × 400 magnification (Figs. l and 2). The epithelium located on the newly formed corpora lutea was cuboidal and fragmentarily simple squamous. Cell nuclei showed no abnormalities within their structure. The basal membrane was also well preserved. The analyzed OSE cells covered the newly formed corpora lutea. Vacuoles were commonly present, particularly in the cells in the center of these large corpora lutea. Fibrous tissue formation was seen in what was previously the central, fluid-filled cavity.
Fig. l.
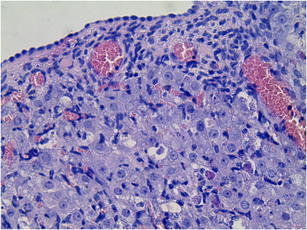
Ovarian surface epithelium of the study group (A). H&E staining. Magnification × 400
Fig. 2.
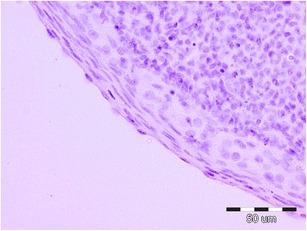
Ovarian surface epithelium of the control group (K). H&E staining. Magnification × 400
Immunohistochemical evaluation of Ki-67 and caspase 3 expressions in OSE cells
The expression of tested proteins has shown statistically significant differences among analyzed groups (Figs. 3 and 4). The existence of positive expression of the nuclear antigen Ki-67 in OSE cells was observed to be definitely rare in the study group, as compared to the control group (p < 0.0001 in the χ 2 test). A positive expression of caspase 3 was statistically more frequently observed in the study group, as compared to the control group (p < 0.0001 in the χ 2 test). The average percentage of cells with positive expression of proteins tested is given in Table 1.
Fig. 3.
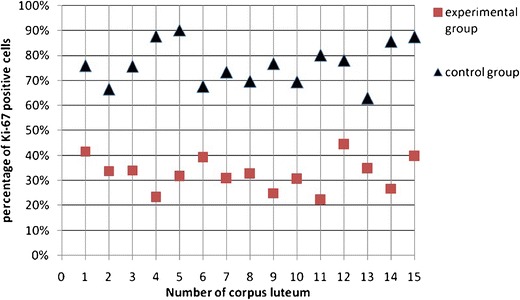
Percentage of positive Ki-67 OSE cells associated with the newly formed corpora lutea
Fig. 4.
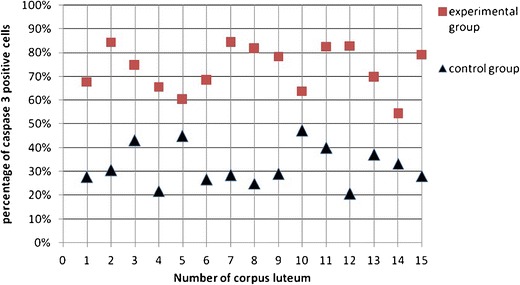
Percentage of positive caspase 3 OSE cells associated with the newly formed corpora lutea
Table 1.
Percentage of OSE cells with positive expression of antigen Ki-67 and caspase 3
| Protein | Group A (study) | Group K (control) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number | Mean | Min | Max | Standard deviation | Number | Mean | Min | Max | Standard deviation | |
| Ki-67 | 15 | 33.40 % | 22.00 % | 44.00 % | 7.16 % | 15 | 76.60 % | 63.00 % | 90.00 % | 8.48 % |
| Casp3 | 15 | 73.17 % | 54.34 % | 84.00 % | 9.60 % | 15 | 32.27 % | 20.68 % | 47.29 % | 8.35 % |
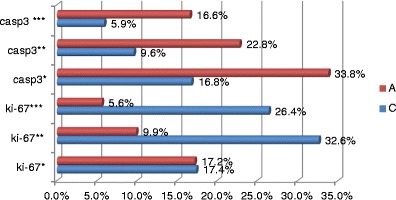
In addition, the intensity of positive immunoprecipitators of the antigen Ki-67 was much stronger in the OSE cells of animals from the control group (Figs. 5 and 6). A statistically significant difference among the groups was not observed only in respect of the frequency of the low intensity of the antigen Ki-67 evaluated as 1 (+) (Fig. 7; Table 2).
Fig. 5.
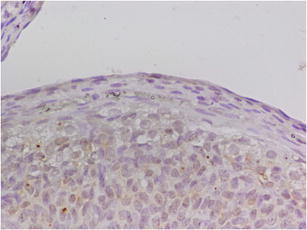
Expression Ki-67 in OSE cells study group (A). Magnification × 400
Fig. 6.
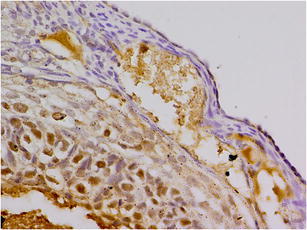
Expression Ki-67 in OSE cells control group (K). Magnification × 400
Fig. 7.
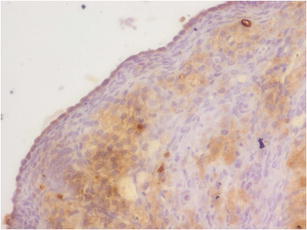
Expression of antigen Ki-67 and caspase 3 of defined intensity in particular research groups. Statistically significant differences in the intensity of protein expression are visible, except for the low expression of the antigen Ki-67 (Ki-67*)
Table 2.
Expression intensity of Ki-67 and caspase 3 in OSE cells of the study (A) and control (K) group
| Expression intensity | Group K (control) | Group A (study) | Statistically significant (p), Mann–Whitney U test | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Mean | Min | Max | Standard deviation | Number | Mean | Min | Max | Standard deviation | ||
| Ki-67 (+) | 15 | 17.45 % | 8.93 % | 25.93 % | 5.38 % | 15 | 17.16 % | 10.53 % | 30.56 % | 5.89 % | 0.708923 |
| Ki-67 (++) | 15 | 32.64 % | 20.00 % | 46.34 % | 8.02 % | 15 | 9.90 % | 5.36 % | 16.22 % | 2.96 % | 0.000003 |
| Ki-67 (+++) | 15 | 26.43 % | 18.52 % | 37.50 % | 5.92 % | 15 | 5.61 % | 1.79 % | 10.64 % | 2.47 % | 0.000003 |
| Casp3 (+) | 15 | 16.77 % | 5.56 % | 32.43 % | 6.81 % | 15 | 33.78 % | 13.21 % | 50.98 % | 10.77 % | 0.000223 |
| Casp3 (++) | 15 | 9.64 % | 6.25 % | 13.89 % | 2.34 % | 15 | 22.83 % | 12.07 % | 47.17 % | 8.90 % | 0.000007 |
| Casp3 (++) | 15 | 5.86 % | 3.13 % | 10.26 % | 2.03 % | 15 | 16.60 % | 9.38 % | 22.22 % | 3.57 % | 0.000004 |
The intensity of observed expression of caspase 3 in the OSE cells of the animals from the study group (Fig. 8) was much stronger than in the control group (Fig. 9). In this regard, the differences in the intensity of expression depending on the research group were statistically significant (Fig. 7; Table 2).
Fig. 8.
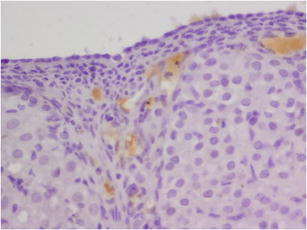
Expression of caspase 3 in OSE cells study group (A). Magnification × 400
Fig. 9.
Expression of caspase 3 in OSE cells control group (K). Magnification × 400
Discussion
Cladribine (2-chlorodeoxyadenosine, 2-CdA) is a synthetic derivative of deoxyadenosine (dA), and belongs to the group of purine nucleoside analogues (PNA). Except for cladribine, the oldest representatives of this group of medicaments are such substances as fludarabin (FA, 2-fluoro-9-β-d-arabinosyl-2-fluoroadenine, F-ara-A) and pentostatin (2´-deoxyformacine). In the 1980s, these medicaments were admitted by the American Food and Drug Administration for the treatment of neoplasms of the hematopoietic system (Robak et al. 2009).
Some new medicaments of this group have become known, such as clofarabine (2-chloro-9-(2´-fluoro-2´-deoxyarabinofuranosilo)adenine, CAFdA), nelarabine (6-metoxy-9-(d-urubinofurunosilo)guanine), and forodesine (immunocyline H, BCX-1777, 7-(3,4-dihydroxy-5-hydrozymethylpyrrolidine-2-yl)-3,5-dihydro-pyrrolo[3,2d]pyrimidin-4-on). These have been found applicable for treatments of disorders resistant to the current therapy (Korycka et al. 2007; Korycka 2008; Robak et al. 2009).
Compounds of this group are of similar chemical structure based on their nucleoside ring. Particles of purine nucleosides were modified in order to change their pharmacological properties and, most of all, in order to increase their resistance to enzymes responsible for their decomposition, such as adenosine deaminase (ADA).
Cladribine was synthesised in the Scripps Research Institute in the 1970's. Through the substitution of its hydrogen atom, with a chlorine atom in position 2 of the deoxyadenosine ring, cladribine is resistant to ADA activity. It may be applied in the form of intravenous infusion, intracutaneous injection or orally taken pills (Tawfik and Nasr 2011). 2-CdA penetrates the cerebrospinal fluid, reaching a concentration of nearly 25 % in the serum. Cladribine is transported to the nuclei of cells by means of nucleoside transporters (Leist and Weissert 2011). In the cell, it is also subordinated to phosphorylation to 2-CdATP being an active form of cladribine. It is extracted from the organism through the kidneys in an unchanged form (21–35 % of a dose) and in the form of a hydrolysed metabolite—2-chloroadenine which is characterised also by apoptosis induction, but in definitely higher concentrations than 2-CdA.
The presence of active metabolites of purine nucleoside analogues in the cell induces numerous enzymatic and structural changes that disturb the balance among damaging factors and reparatory mechanisms, resulting in the cell’s death. The activity of PNA is mostly linked with the inhibition of the synthesis of deoxynucleotides which play an important role in DNA replication and repair. Moreover, they inhibit the activity of ribonucleotide reductase, which is an enzyme conditioning the promptness of diphosphodeoxyribonucleotides (dNDP). The inhibited activity of dNDP disturbs the balance within the nucleoside triphosphates, which activates endonucleases, resulting in a rupture in one of both DNA strands (Johnston 2011; Robak et al. 2009). DNA damages then initiate the reparatory mechanisms and activates the polytransferase enzyme. Induction of reparatory processes results in depletion of NAD energetic reserves, the inhibition of ATP synthesis, a disturbance of all energetic processes and finally, cell death. Cladribine phosphate also acts as a DNA synthesis inhibitor and results in the inhibition of the cell cycle (Leist and Weissert 2011).
The presence of intracellular changes is a signal for increased expression of regulatory protein p53. This p53 protein may block the cell cycle at control points of cell cycle G1/S or G2/M, in order to initiate reparatory mechanisms in a cell. If irreparable damages occur, p53 protein directs a cell to follow the apoptosis pathway (Johnston 2011; Klöpfer et al. 2004; Korycka 2008). The typical course of apoptosis is linked to the activation of caspases. The initiatory caspase 9 plays a crucial role in the endogenous apoptosis pathway, whereas the key stage in the external pathway is the activation of the death receptor and caspase 8.
The mechanism of apoptosis induction through cladribine is the subject of many studies. Numerous publications show the increased activity of caspase 9 (Bastin-Coyette et al. 2008; Conrad et al. 2008; Klöpfer et al. 2004). Individual data also reveal the activation of the FAS receptor and caspase 8 through 2-CdA (Nomura et al. 2000). Moreover, in astrocytoma cells incubated in a cladribine-rich environment, increased expression of caspase 2 was evidenced, that an atypical mechanism may activate the internal apoptosis pathway (Ceruti et al. 2003). Cladribine may also directly activate the apoptotic protease activating factor 1 or induce increased permeability of the mitochondrial membranes for cytochrome c through a caspase-independent mechanism. The intracellular concentration of calcium ions probably also plays an important role in cladribine-induced apoptosis (Conrad et al. 2008). Moreover, the activation of p38 nitrogen-activated protein kinase (MAPK p38) and extracellular signal-regulated kinase 1 and 2 (ERK1/2) are other options to trigger cell death through 2-CdA (Conrad et al. 2008; Smal et al. 2007).
The execution stage relying on the disintegration of the cell and resulting in apoptotic bodies occurs after the initiation of apoptosis. The effector caspases play an important role within this stage, and caspase 3 is one of the most significant executive caspases of the apoptosis process. It is responsible for the proteolysis of intracellular proteins and for morphological changes characteristic of apoptosis (Korzeniewska-Dyl 2007). The measurement of its activity serves for the assessment of the intensity of this process in cells (Chmielewski et al. 2008).
In our study, we have assessed the expression of caspase 3 in OSE cells in female rats treated with cladribine. The results of this analysis demonstrate that 2-CdA induces a cytotoxic effect upon cells of this epithelium. Furthermore, the statistically significant growth in expression of caspase 3 in OSE cells in the study group, as compared to the control group, is evidence for the intensification of the apoptosis process in cells that are influenced by cladribine.
So far, the inductive activity of 2-CdA on programmed cell death in the lymphatic system has been confirmed. Individual publications concern the damage of skin cells and the mucous membrane that was brought about by cladribine (Chubar and Bennett 2003; Rossini et al. 2004). Moreover, several publications also described the cytotoxic activity of cladribine on stem cells (Chow et al. 2002; Lech-Marańda et al. 2000; Petzer et al. 1991). In a study on the impact of cytostatic drugs on cells at various stages of maturity, Chow et al. showed that cladribine induces apoptosis in CD34+/CD38+ progenitor cells (Chow et al. 2002). Lech-Marańda et al. observed the inhibition of granulocyte–macrophage progenitor cell growth, and Petzer et al. observed the inhibition of T lymphocyte colony forming cells subject to cladribine (Lech-Marańda et al. 2000; Petzer et al. 1991).
Cells of a stem cell nature are described among the cells forming the ovarian surface epithelium (Chang et al. 2008). A large amount of data suggests the potential of these cells to differentiate into various forms of progeny cells, such as, ovarian stromal cells, germ cells, or even oocyte-like cells (Nishida and Nishida 2006; Virant-Klun et al. 2011). Disturbances in the proliferation and maturation of these cells are probably linked with the development of ovarian cancer (Chang et al. 2008; Nishida and Nishida 2006). Researchers have shown that the application of gonadotropins to stimulate ovulation results in changes of proliferation activity of these cells. The majority of data reveals that gonadotropins, such as, human chorionic gonadotropin, pregnant mare’s serum gonadotropin, and 17β-estradiol accelerate the proliferation of ovarian cells, including OSE cells (Stewart et al. 2004; Singavarapu et al. 2010). Also, surgically induced injury or ovulation-induced rupture of the ovarian surface increase OSE cells proliferation rate (Stewart et al. 2004). Accelerated proliferation is often linked with the occurrence of irregularities in the genetic material of progeny cells, as well as the inhibition of repair mechanisms. The proliferation of pathologically changed OSE cells induces the development of inclusion cysts and mucous membrane dysplasia, which are potential preneoplastic features (Corakci et al. 2005; Chene et al. 2012).
These data explain the signalled increase in the risk of development of ovarian cancer among persons subject to ovulation induction as well as among women having early menarche and late menopause or nulliparous (Corakci et al. 2005; Gava et al. 2008; King and Burdette 2011). Disturbances in the proliferation process that are linked with ovarian stimulation are also associated with the occurrence of defective embryos and miscarriages in the blastocyst period (Liang et al. 2009).
In our study, we have assessed the impact of cladribine on the proliferation of OSE cells, by way of evaluating the expression of Ki-67 antigen in the ovaries of female rats subject to the activity of 2-CdA. The Ki-67 nuclear antigen is the basic marker of cell proliferation (Gerdes et al. 1984). This protein is present in the nucleus, and also in chromosomes during cell division. The positive expression of this antigen is present in cells at the S, G2, and M stages of the cell cycle, and in G1 after mitosis. The lack of Ki-67 antigen expression is observed only in quiescent cells at G0 stage (Gerdes et al. 1984; Muskhelishvili et al. 2003).
A significantly lower intensity of expression of Ki-67 antigen, as compared to the control group, was observed in OSE cells of ovaries sampled from animals subject to cladribine injections (group A). The percentage of Ki-67 positive OSE cells was definitely higher in the animal tissue of the control group than in ovaries from the animals in the study group.
The inhibition of cell growth and proliferation is one of cladribine’s activity mechanisms described that was confirmed in previous studies of lymphatic cells (Lech-Marańda et al. 2000; Spurgeon et al. 2009). The changes in OSE cells proliferation activity observed in our studies may suggest that cladribine has a similar impact upon ovary epithelium cells.
The inhibition of the proliferation activity of cells facilitates the activation of repair mechanisms, the aim of which is to repair damages in the genetic material of a cell that resulted from various factors (Sznarkowska et al. 2010). As it was shown in many studies, cladribine is one of the factors inhibiting the cell cycle at control point G1/S (Galmarini et al. 2003). Retention of cells between these phases is probably dependent on an efficiently operating p53 protein in cells. This protein is responsible for inter alia, cell proliferation, ageing, and death (Sznarkowska et al. 2010). In another study, we have shown an increase in p53 protein expression in OSE cells of animals subject to cladribine. These results may indicate the initiation of repair mechanisms in these cells. The repair of intracellular damages caused by cladribine requires time. This is because some cells undergo apoptosis, which is indicated by a higher caspase 3 percentage in the group receiving cladribine. Only properly repaired stem OSE cells may be the source of intact and mature OSE cells.
The obtained data confirm the claims that cladribine can be teratogenic and suggest its adverse influence upon the female reproductive system (Wubah et al. 1996; 2001). However, the obtained data need to be expanded, especially with studies on humans, in order to assess the impact of cladribine upon the reproductive function of females and males. Specific information will facilitate providing recommendations on the application of substances protecting the reproductive organs against the harmful activity of cytostatic drugs and ascertaining the safe period of time between the completion of chemotherapy and procreation. These recommendations appear particularly important due to the increasingly broad application of PNA in medicine.
Acknowledgments
Conflict of interest statement
The authors of this manuscript have no conflict of interest to declare.
References
- Aichberger KJ, Sperr WR, Gleixner KV, Kretschmer A, Valent P. Treatment responses to cladribine and dasatinib in rapidly progressing aggressive mastocytosis. Eur J Clin Invest. 2008;38(11):869–873. doi: 10.1111/j.1365-2362.2008.02036.x. [DOI] [PubMed] [Google Scholar]
- Auersperg N, Maclaren IA, Kruk PA. Ovarian surface epithelium: autonomous production of connective tissue-type extracellular matrix. Biol Reprod. 1991;44(4):717–724. doi: 10.1095/biolreprod44.4.717. [DOI] [PubMed] [Google Scholar]
- Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev. 2001;22(2):255–288. doi: 10.1210/er.22.2.255. [DOI] [PubMed] [Google Scholar]
- Bastin-Coyette L, Smal C, Cardoen S, Saussoy P, Van den Neste E, Bontemps F. Mechanisms of cell death induced by 2-chloroadenosine in leukemic B-cells. Biochem Pharmacol. 2008;1;75(7):1451–1460. doi: 10.1016/j.bcp.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Bernknopf A, Rowley K, Bailey T. A review of systemic lupus erythematosus and current treatment options. Formulary. 2011;46:178–194. [Google Scholar]
- Ceruti S, Franceschi C, Barbieri D, Malorni W, Camurri A, Giammarioli AM, Ambrosini A, Racagni G, Cattabeni F, Abbracchio MP. Apoptosis induced by 2-chloro-adenosine and 2-chloro-2'-deoxy-adenosine in a human astrocytoma cell line: differential mechanisms and possible clinical relevance. J Neurosci Res. 2000;1;60(3):388–400. doi: 10.1002/(SICI)1097-4547(20000501)60:3<388::AID-JNR14>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Ceruti S, Beltrami E, Matarrese P, Mazzola A, Cattabeni F, Malorni W, Abbracchio MP. A key role for caspase-2 and caspase-3 in the apoptosis induced by 2-chloro-2'-deoxy-adenosine (cladribine) and 2-chloro-adenosine in human astrocytoma cells. Mol Pharmacol. 2003;63(6):1437–1447. doi: 10.1124/mol.63.6.1437. [DOI] [PubMed] [Google Scholar]
- Chang HL, MacLaughlin DT, Donahoe PK (2008–2009) Somatic stem cells of the ovary and their relationship to human ovarian cancers. StemBook [Internet]. Harvard Stem Cell Institute, Cambridge. doi/10.3824/stembook.1.43.1 [PubMed]
- Chene G, Penault-Llorca F, Tardieu A, Cayre A, Lagarde N, Jaffeux P, Aublet-Cuvelier B, Dechelotte P, Felloni B, Pouly JL, Dauplat J (2012) Is there a relationship between ovarian epithelial dysplasia and infertility? Obstet Gynecol Int. doi:10.1155/2012/429085 [DOI] [PMC free article] [PubMed]
- Chmielewski M, Linke K, Zabel M. Metody wykrywania zjawiska apoptozy w komórkach wątrobowych in situ. [Methods of detecting the phenomenon of apoptosis in liver cells in situ.] [Article in Polish] Nowiny Lekarskie. 2008;3:223–226. [Google Scholar]
- Chow KU, Boehrer S, Bojunga J, Stieler M, Rummel MJ, Fauth F, Schneider B, Martin H, Hoelzer D, Weidmann E, Mitrou PS (2002) Induction of apoptosis by cladribine (2-CdA), gemcitabine and other chemotherapeutic drugs on CD34+/CD38+ and CD34+/CD38– hematopoietic progenitor cells: selective effects of doxorubicin and 2-CdA with protection of immature cells. Leuk Lymphoma 43(2):377–384 [DOI] [PubMed]
- Chow KU, Rummel MJ, Weidmann E, Ries J, Jantschke P, Boehrer S, Pourebrahim F, Napieralski S, Stein J, Martin H, Hoelzer D, Mitrou PS. Induction of apoptosis by 2-chloro-2'deoxyadenosine (2-CdA) alone and in combination with other cytotoxic drugs: synergistic effects on normal and neoplastic lymphocytes by addition of doxorubicin and mitoxantrone. Leuk Lymphoma. 2000;36(5–6):559–567. doi: 10.3109/10428190009148404. [DOI] [PubMed] [Google Scholar]
- Chubar Y, Bennett M. Cutaneous reactions in hairy cell leukaemia treated with 2-chlorodeoxyadenosine and allopurinol. Br J Haematol. 2003;122(5):768–770. doi: 10.1046/j.1365-2141.2003.04506.x. [DOI] [PubMed] [Google Scholar]
- Conrad DM, Robichaud MR, Mader JS, Boudreau RT, Richardson AM, Giacomantonio CA, Hoskin DW. 2-Chloro-2'-deoxyadenosine-induced apoptosis in T leukemia cells is mediated via a caspase-3-dependent mitochondrial feedback amplification loop. Int J Oncol. 2008;32(6):1325–1333. doi: 10.3892/ijo_32_6_1325. [DOI] [PubMed] [Google Scholar]
- Corakci A, Filiz S, Caliskan E, Dalcik C, Ozeren S, Dalcik H. The effects of ovulation induction on ovarian epithelium dysplasia scores and Ki67 expression: an experimental study on rats. Int J Gynecol Cancer. 2005;15(5):866–871. doi: 10.1111/j.1525-1438.2005.00149.x. [DOI] [PubMed] [Google Scholar]
- Dutta EJ, Habermann TM, Hoos GL, Weiler CR. Treatment of idiopathic urticaria, angioedema and anaphylaxis with cladribine. J Allergy Clin Immunol. 2002;109(1):S335. doi: 10.1016/S0091-6749(02)82173-X. [DOI] [Google Scholar]
- Eibschutz B, Baird SM, Weisman MH, Amox DG, Spellman M, Piacquadio D, Carrera CJ, Carson DA. Oral 2-chlorodeoxyadenosine in psoriatic arthritis. A preliminary report. Arthritis Rheum. 1995;38(11):1604–1609. doi: 10.1002/art.1780381112. [DOI] [PubMed] [Google Scholar]
- Galmarini CM, Voorzanger N, Falette N, Jordheim L, Cros E, Puisieux A, Dumontet C. Influence of p53 and p21(WAF1) expression on sensitivity of cancer cells to cladribine. Biochem Pharmacol. 2003;1;65(1):121–129. doi: 10.1016/S0006-2952(02)01448-X. [DOI] [PubMed] [Google Scholar]
- Gava N, Clarke CL, Bye C, Byth K, deFazio A. Global gene expression profiles of ovarian surface epithelial cells in vivo. J Mol Endocrinol. 2008;40(6):281–296. doi: 10.1677/JME-07-0149. [DOI] [PubMed] [Google Scholar]
- Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133(4):1710–1715. [PubMed] [Google Scholar]
- Hartung HP. New oral therapies may offer improved treatment options for patients with multiple sclerosis. Curr Opin Neurol. 2009;22:S10–S14. doi: 10.1097/01.wco.0000347402.08903.87. [DOI] [Google Scholar]
- Hassan R, Gupta M, Kern W, Ozer H. Acute myeloid leukemia following treatment with cladribine for hairy cell leukemia: a case report and review of the literature. Leuk Lymphoma. 2004;45(10):2149–2152. doi: 10.1080/10428190410001714070. [DOI] [PubMed] [Google Scholar]
- Inwards DJ, Fishkin PA, Hillman DW, Brown DW, Ansell SM, Kurtin PJ, Fonseca R, Morton RF, Veeder MH, Witzig TE. Long-term results of the treatment of patients with mantle cell lymphoma with cladribine (2-CDA) alone (95-80-53) or 2-CDA and rituximab (N0189) in the North Central Cancer Treatment Group. Cancer. 2008;1;113(1):108–116. doi: 10.1002/cncr.23537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JB. Mechanism of action of pentostatin and cladribine in hairy cell leukemia. Leuk Lymphoma. 2011;52(Suppl 2):43–45. doi: 10.3109/10428194.2011.570394. [DOI] [PubMed] [Google Scholar]
- King SM, Burdette JE. Evaluating the progenitor cells of ovarian cancer: analysis of current animal models. BMB Rep. 2011;44(7):435–445. doi: 10.5483/BMBRep.2011.44.7.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöpfer A, Hasenjäger A, Belka C, Schulze-Osthoff K, Dörken B, Daniel PT. Adenine deoxynucleotides fludarabine and cladribine induce apoptosis in a CD95/Fas receptor, FADD and caspase-8-independent manner by activation of the mitochondrial cell death pathway. Oncogene. 2004;23(58):9408–9418. doi: 10.1038/sj.onc.1207975. [DOI] [PubMed] [Google Scholar]
- Korycka A. Perspektywy leczniczego zastosowania nowych analogów nukleozydów purynowych [Perspectives of therapeutical application of new purine nucleoside analogues] Article in Polish. Acta Haematol Pol. 2008;39(4):727–741. [Google Scholar]
- Korycka A, Błoński JZ, Robak T. Forodesine (BCX-1777, Immucillin H)—a new purine nucleoside analogue: mechanism of action and potential clinical application. Mini Rev Med Chem. 2007;7(9):976–983. doi: 10.2174/138955707781662636. [DOI] [PubMed] [Google Scholar]
- Korzeniewska-Dyl I. Kaspazy—struktura i funkcja [Caspases—structure and function] Article in Polish. Pol Merkur Lekarski. 2007;23(138):403–407. [PubMed] [Google Scholar]
- Laurencet FM, Zulian GB, Cabrol C, Betticher DC. Myelodysplastic syndrome with biclonal monosomy 7 and trisomy 8 after treatment with cladribine (2-chloro-2-deoxyadenosine) and involved field radiation therapy. Cancer Genet Cytogenet. 1997;99(1):85–89. doi: 10.1016/S0165-4608(96)00432-3. [DOI] [PubMed] [Google Scholar]
- Lech-Marańda E, Korycka A, Robak T. Influence of gemcitabine (2',2'-difluoro-deoxycytidine) and 2-chlorodeoxyadenosine on growth of normal and leukemic cells in vitro. Eur J Haematol. 2000;65(5):317–321. doi: 10.1034/j.1600-0609.2000.065005317.x. [DOI] [PubMed] [Google Scholar]
- Leist TP, Weissert R. Cladribine: mode of action and implications for treatment of multiple sclerosis. Clin Neuropharmacol. 2011;34(1):28–35. doi: 10.1097/WNF.0b013e318204cd90. [DOI] [PubMed] [Google Scholar]
- Liang L, Xu B, Zhu G. Effect of repeated gonadotropin stimulation on ovarian reserves and proliferation of ovarian surface epithelium in mice. Front Med China. 2009;3(2):220–226. doi: 10.1007/s11684-009-0037-2. [DOI] [Google Scholar]
- Marczak A, Łubgan D, Robak T, Jóźwiak Z. Influence of 2-chlorodeoxyadenosine (cladribine) on human erythrocytes. Int J Biochem Cell Biol. 2004;36(8):1645–1654. doi: 10.1016/j.biocel.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Martinelli G, Zinzani PL, Farabegoli P. Purine analogs (fludarabine and 2-chlorodeoxyadenosine) as apoptosis-inducing drugs in CML therapy. Haematologica. 1996;81(3):286–287. [PubMed] [Google Scholar]
- Mottl H, Starý J, Chánová M, Nekolná M, Drahokoupilová E, Smelhaus V. Treatment of recurrent Langerhans cell histiocytosis in children with 2-chlorodeoxyadenosine. Leuk Lymphoma. 2006;47(9):1881–1884. doi: 10.1080/10428190600687281. [DOI] [PubMed] [Google Scholar]
- Murdoch WJ, McDonnel AC. Roles of the ovarian surface epithelium in ovulation and carcinogenesis. Reproduction. 2002;123(6):743–750. doi: 10.1530/rep.0.1230743. [DOI] [PubMed] [Google Scholar]
- Muskhelishvili L, Latendresse JR, Kodell RL, Henderson EB. Evaluation of cell proliferation in rat tissues with BrdU, PCNA, Ki-67(MIB-5) immunohistochemistry and in situ hybridization for histone mRNA. J Histochem Cytochem. 2003;51(12):1681–1688. doi: 10.1177/002215540305101212. [DOI] [PubMed] [Google Scholar]
- Nishida T, Nishida N. Reinstatement of "germinal epithelium" of the ovary. Reprod Biol Endocrinol. 2006;4:42. doi: 10.1186/1477-7827-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura Y, Inanami O, Takahashi K, Matsuda A, Kuwabara M. 2-Chloro-2'-deoxyadenosine induces apoptosis through the Fas/Fas ligand pathway in human leukemia cell line MOLT-4. Leukemia. 2000;14(2):299–306. doi: 10.1038/sj.leu.2401649. [DOI] [PubMed] [Google Scholar]
- Perez RP, Hamaguchi K, Tracey PA, Handel LM, Hamilton TC, Godwin AK. Transformation of rat ovarian epithelial and Rat-1 fibroblast cell lines by RAST24 does not influence cisplatin sensitivity. Cancer Res. 1993;53(16):3771–3775. [PubMed] [Google Scholar]
- Petzer AL, Bilgeri R, Zilian U, Haun M, Geisen FH, Pragnell I, Braunsteiner H, Konwalinka G. Inhibitory effect of 2-chlorodeoxyadenosine on granulocytic, erythroid, and T-lymphocytic colony growth. Blood. 1991;78(10):2583–2587. [PubMed] [Google Scholar]
- Robak T, Korycka A, Lech-Maranda E, Robak P. Current status of older and new purine nucleoside analogues in the treatment of lymphoproliferative diseases. Molecules. 2009;14(3):1183–1226. doi: 10.3390/molecules14031183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini MS, de Souza EM, Cintra ML, Pagnano KB, Chiari AC, Lorand-Metze I. Cutaneous adverse reaction to 2-chlorodeoxyadenosine with histological flame figures in patients with chronic lymphocytic leukaemia. J Eur Acad Dermatol Venereol. 2004;18(5):538–542. doi: 10.1111/j.1468-3083.2004.00969.x. [DOI] [PubMed] [Google Scholar]
- Rourke M, Anderson KC, Ghobrial IM. Review of clinical trials conducted in Waldenstrom macroglobulinemia and recommendations for reporting clinical trial responses in these patients. Leuk Lymphoma. 2010;51(10):1779–1792. doi: 10.3109/10428194.2010.499977. [DOI] [PubMed] [Google Scholar]
- Saven A, Burian C, Koziol JA, Piro LD. Long-term follow-up of patients with hairy cell leukemia after cladribine treatment. Blood. 1998;15;92(6):1918–1926. [PubMed] [Google Scholar]
- Schlette EJ, Admirand J, Wierda W, Abruzzo L, Lin KI, O'Brien S, Lerner S, Keating MJ, Tam C. p53 expression by immunohistochemistry is an important determinant of survival in patients with chronic lymphocytic leukemia receiving frontline chemo-immunotherapy. Leuk Lymphoma. 2009;50(10):1597–1605. doi: 10.1080/10428190903165241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singavarapu R, Buchinsky N, Cheon DJ, Orsulic S. Whole ovary immunohistochemistry for monitoring cell proliferation and ovulatory wound repair in the mouse. Reprod Biol Endocrinol. 2010;8:98. doi: 10.1186/1477-7827-8-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot KA, de Boer-Brouwer M, Voorendt M, Sie-Go DM, Ghahremani M, Dorrington JH, Teerds KJ. Irregularly shaped inclusion cysts display increased expression of Ki67, Fas, Fas ligand, and procaspase-3 but relatively little active caspase-3. Int J Gynecol Cancer. 2006;16(1):231–239. doi: 10.1111/j.1525-1438.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- Smal C, Lisart S, Maerevoet M, Ferrant A, Bontemps F, Van Den Neste E. Pharmacological inhibition of the MAPK/ERK pathway increases sensitivity to 2-chloro-20-deoxyadenosine (CdA) in the B-cell leukemia cell line EHEB. Biochem Pharmacol. 2007;73:351–358. doi: 10.1016/j.bcp.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Spurgeon S, Yu M, Phillips JD, Epner EM. Cladribine: not just another purine analogue? Expert Opin Investig Drugs. 2009;18(8):1169–1181. doi: 10.1517/13543780903071038. [DOI] [PubMed] [Google Scholar]
- Stewart SL, Querec TD, Gruver BN, O'Hare B, Babb JS, Patriotis C. Gonadotropin and steroid hormones stimulate proliferation of the rat ovarian surface epithelium. J Cell Physiol. 2004;198(1):119–124. doi: 10.1002/jcp.10401. [DOI] [PubMed] [Google Scholar]
- Sznarkowska A, Olszewski R, Zawacka-Pankau J. Farmakologiczna aktywacja supresora nowotworu, natywnego białka p53 jako obiecująca strategia zwalczania nowotworów [Pharmacological activation of tumor suppressor, wild-type p53 as a promising strategy to fight cancer] Article in Polish. Postepy Hig Med Dosw (Online) 2010;64:396–407. [PubMed] [Google Scholar]
- Tawfik NM, Nasr AS (2011) Comparative study between intravenous and subcutaneous administration of cladribine in treatment of hairy cell leukemia patients. Comp Clin Pathol, Online First™
- Virant-Klun I, Skutella T, Stimpfel M, Sinkovec J. Ovarian surface epithelium in patients with severe ovarian infertility: a potential source of cells expressing markers of pluripotent/multipotent stem cells. J Biomed Biotechnol. 2011;2011:381928. doi: 10.1155/2011/381928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosoughi R, Freedman MS. Therapy of MS. Clin Neurol Neurosurg. 2010;112(5):365–385. doi: 10.1016/j.clineuro.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Wierzbowska A, Robak T, Pluta A, Wawrzyniak E, Cebula B, Hołowiecki J, Kyrcz-Krzemień S, Grosicki S, Giebel S, Skotnicki AB, Piatkowska-Jakubas B, Kuliczkowski K, Kiełbiński M, Zawilska K, Kłoczko J, Wrzesień-Kuś A, Polish Adult Leukemia Group Cladribine combined with high doses of arabinoside cytosine, mitoxantrone, and G-CSF (CLAG-M) is a highly effective salvage regimen in patients with refractory and relapsed acute myeloid leukemia of the poor risk: a final report of the Polish Adult Leukemia Group. Eur J Haematol. 2008;80(2):115–126. doi: 10.1111/j.1600-0609.2007.00988.x. [DOI] [PubMed] [Google Scholar]
- Wubah JA, Ibrahim MM, Gao X, Nguyen D, Pisano MM, Knudsen TB. Teratogen-induced eye defects mediated by p53-dependent apoptosis. Curr Biol. 1996;1;6(1):60–69. doi: 10.1016/S0960-9822(02)00422-0. [DOI] [PubMed] [Google Scholar]
- Wubah JA, Setzer RW, Lau C, Charlap JH, Knudsen TB. Exposure-disease continuum for 2-chloro-2'-deoxyadenosine, a prototype ocular teratogen. 1. Dose–response analysis. Teratology. 2001;64(3):154–169. doi: 10.1002/tera.1059. [DOI] [PubMed] [Google Scholar]


