Abstract
Radiotherapy plays an essential role in the management of breast cancer. Three-dimensional conformal radiation therapy (3D-CRT) is applied based on 3D image information of anatomy of patients. In 3D-CRT for breast cancer one of the common techniques is tangential technique. In this project, various parameters of tangential and supraclavicular fields are optimized. This project has been done on computed tomography images of 100 patients in Isfahan Milad Hospital. All patients have been simulated and all the important organs have been contoured by radiation oncologist. Two techniques in supraclavicular region are evaluated including: 1-A single field (Anterior Posterior [AP]) with a dose of 200 cGy per fraction with 6 MV energy. This is a common technique. 2-Two parallel opposed fields (AP-Posterior Anterior [PA]). The dose of AP was 150 cGy with 6 MV energy and PA 50 cGy with 18 MV. In the second part of the project, the tangential fields has been optimized with change of normalization point in five points: (1) Isocenter (Confluence of rotation gantry axis and collimator axis) (2) Middle of thickest part of breast or middle of inter field distance (IFD) (3) Border between the lung and chest wall (4) Physician's choice (5) Between IFD and isocenter. Dose distributions have been compared for all patients in different methods of supraclavicular and tangential field. In parallel opposed fields average lung dose was 4% more than a single field and the maximum received heart dose was 21.5% less than a single field. The average dose of planning tumor volume (PTV) in method 2 is 2% more than method 1. In general AP-PA method because of a better coverage of PTV is suggested. In optimization of the tangential field all methods have similar coverage of PTV. Each method has spatial advantages and disadvantages. If it is important for the physician to reduce the dose received by the lung and heart, fifth method is suggested since in this method average and maximum received dose to heart and lung have been reduced few percent in comparison to other methods. If a better coverage of PTV is important for the physician second method can be an optimized method. In this method, average and maximum received dose to PTV have been increased few percent in comparisons of physician's choice method and three other methods. In optimizing of supraclavicular field AP-PA method due to better coverage of PTV is suggested. In optimizing of tangential all methods are similar. Each method has special advantages and disadvantages. The physicians can change the depth of the normalization point in the breast to get the desired average dose.
Keywords: Breast cancer, radiation therapy, treatment planning
INTRODUCTION
Breast cancer is one of the most common cancers in women, with high mortality rate. The incidence of breast cancer is raised year by year, and patients suffered from breast cancer are becoming much younger.[1]
This kind of cancer with the wide-spread use of mammography can be diagnosed in the early stages.[2–6] A comprehensive treatment strategy has been applied for breast cancer patients, which includes radical or breast-conserving surgery, radiotherapy, chemotherapy, endocrine therapy, and immunization therapy.[7,8] For many years, mastectomy was the standard treatment to achieve local control in breast cancer.[9,10,11]
Radiotherapy plays an essential role in the management of breast cancer and many studies have shown better survival of patients after mastectomy followed by radiotherapy.[12,13,14,15,16]
One general goal of radiation therapy is to protect the healthy tissues adjacent to the tumor. Among the side-effects of radiotherapy are secondary cancer and cardiomyopathy. The reduction of side-effects in radiotherapy is more considerable compare to other methods of treatment. [5] Approximately 120, 000 women with breast cancer are treated with radiotherapy annually in the United States.[17,18,19] The proportion of patients with breast cancer treated with radiation therapy has increased substantially during the past two decades.[20,21,22]
Because the local recurrence is a critical factor for patient mortality, radiation therapy plays an significant role in treatment to prevent local recurrence.[9] ,[23,24]
One of the common techniques of radiation therapy is Accelerated Partial Breast Irradiation (APBI). APBI has been studied as a possible alternative to conventional whole breast radiation therapy for patients with early-stage breast cancer treated with breast-conserving therapy.[25]
Several methods to deliver APBI have been developed, including interstitial and intracavitary brachytherapy, external beam radiation therapy, and single-fraction intraoperative treatment.[26]
3 Dimensional-Conformal radiotherapy is a technique for dose delivery based on 3D images of target volume. 3D-conformal is a technique in which the irradiation beams is shaped to conform the tumor in each direction. In the conventional technique, irradiation beam is usually larger than the tumor. However, in 3D-conformal technique the irradiation beams is shaped to conform to the tumor and prevent to irradiate the other organs.[27,28] Radiation therapy for breast cancer and chest wall may come with toxic effects such as skin recurrence, soft-tissue fibrosis.[29,30,31,32,33]
To reduce the risk of radiation toxicity, three-dimensional (3D) treatment planning and dynamic multi leaf collimator (MLC) have been used to modulate the radiotherapy dose in three dimensions across the breast and chest wall. MLC is made of lead plates and this kind of collimator can make various shapes of irradiation beams and it limits irradiation beams to target volume and prevent irradiating other organs. Compare to costume blocks, MLC is very easy to shape and apply to various field shapes.
3D modulation of the radiation beam profile also improves dose homogeneity within the treated breast and it lowers the dose to the contralateral breast and potentially, the heart.[34,35,36,37,38,39,40,41,42,43]
In general, Radiotherapy for breast cancer after mastectomy and breast-conserving surgery include chest wall and for patients with regional lymph node involvement, supraclavicular region regular must irradiated.[44]
There are limited studies about APBI. In 2005 Moon et al. did a dosimetric comparison of three different techniques in APBI and they found that 3D conformal radiation therapy resulted in better coverage of the planning tumor volume (PTV) compared with Mammo Site or interstitial brachytherapy techniques.[45] In this study, they did a dosimetric comparison of two different 3D conformal external beam techniques and they found that 3D conformal external accelerated partial breast irradiation provides excellent normal tissue sparing with adequate coverage of PTV.
General points of the tangential treatment technique are mentioned in various papers and books, but its implementation details are not mentioned. The tangential treatment technique is now being treated by physicians in different ways and there is no exact protocol.[37,46]
The aim of this study is the optimization and comparison of various approaches in supraclavicular and tangential field and finally reaching a proper treatment protocol.
MATERIALS AND METHODS
In radiotherapy for breast cancer usually a dose of 50 Gy is prescribed in 25 fractions.[47,48,49,50,51] So in the standard radiation therapy daily fractions of 2 Gy (200 cGy) to the whole breast and a boost of 10-16 Gy to the tumor bed is delivered. Patients are usually placed in the supine position on an angled breast board with one or both arms stretched above the head. The position of the patient must be similar in treatment and simulation. The patient is placed on an angled breast board because the sternum slope and chest wall slope is modified. Tangential fields must cover the breast and edges of the field are shaped based on patients’ anatomy.
The developments in computer technology allowed the integration of computed tomography (CT)-information in the RT planning system, enabling the design of radiation beams that are based on patient-specific 3D anatomical information. A modern linear accelerator with MLC can deliver these conformal beams to the target. In breast irradiation, the 3D alignment of the tangential beams allows improved coverage of the breast tissue and reduction of the volume of irradiated heart and lung tissue.
Using full CT-data, the location of the match line between the tangential and supraclavicular fields can be matched, depending on the patients’ anatomy.
The tangential technique is illustrated in Figure 1. This technique includes the internal mammary lymph nodes. The tangential technique for the treatment of breast and chest wall includes a small part of the lung in this field.
Figure 1.
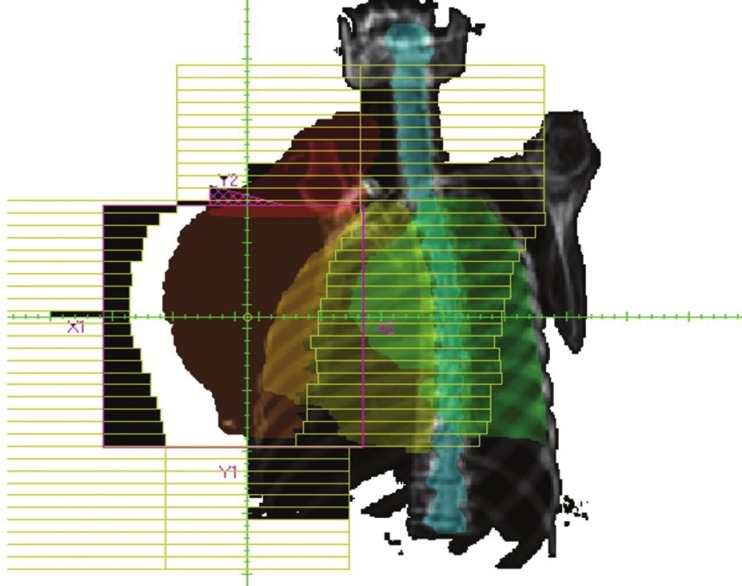
A tangential field in radiotherapy for breast with 15° wedge
In contouring of CT scans, the treatment volume of axillary veins and supraclavicular area is determined. The photon beam energy is usually 6 MV and the gantry angle is between 50 and 60° for right breast. For large chest walls a higher energy might be used.
In the breast tissue, there are different thicknesses of the tissue in the path of the beam and this can causes a hot spot in the thinner regions. In order to avoid hot spots and achieve more homogeneity is isodose for the tangential fields, two wedges with 15-30° angles can be used. In both cases, the thickness of the wedge is placed in the nipple region. Figure 1 illustrates an example of tangential fields for breast and Figure 2 is an example of tangential and supraclavicular fields in the surface of the patient.
Figure 2.
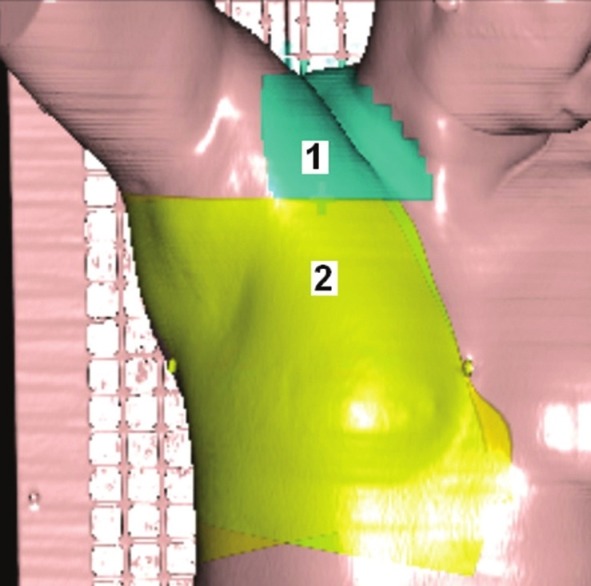
The tangential and supraclavicular areas in radiotherapy for breast
Region 1: Supraclavicular
Region 2: Tangential.
The supraclavicular region can be treated either with single or two fields. The supraclavicular region is treated with single-photon field only in the cases that that target is superficial and dose distribution in the target volume is reasonably uniform.
For example, the prescription dose should be approximately ±5% in the tumor and the maximum received dose to tissues should not be more than ±110% of the prescribed dose.
In this project, the CT images of 100 patients in Isfahan Milad Hospital have been used and all the data is imported in the treatment planning system, TPS (TiGRT form Lina Tech). This software is one of the most important tools in ra diation therapy and it is able to calculate absorbed dose in various points of tissue for each particular patient. The treatment planning system TiGRT has been commissioned based on ONCOR Siemens linear accelerator information.
In contouring, all the important organs such as breast, heart, lung, and spinal have been contoured by radiation oncologist. Contouring procedures is essential for treatment planning and designing the exact direction of the fields and calculation of dose volume histogram (DVH). DVH is the histogram of volume versus dose. In the ideal case of DVH, the tumor volume must be covered by 100% prescription dose and the dose of critical organs has to be kept up to a certain limit. After contouring various treatment plans are designed. This project includes over 400 different planes.
The first step in this study is optimization of supraclavicular field in which two techniques have been used:
A single Anterior Posterior (AP) field (AP) with dose 200 cGy and 6 MV energy
Two parallel opposed AP-Posterior Anterior (PA) fields (AP and PA) in which the dose of the AP is 150 cGy and the energy is 6 MV. The dose of the PA is 50 cGy with 18 MV photons.
Figures 3 and 4 illustrate AP-PA technique and AP technique which are shaped according to gross tumor volume. According to recommendations, the fields are rotated 15° in the opposite direction of the spine to avoid the spine.
Figure 3.
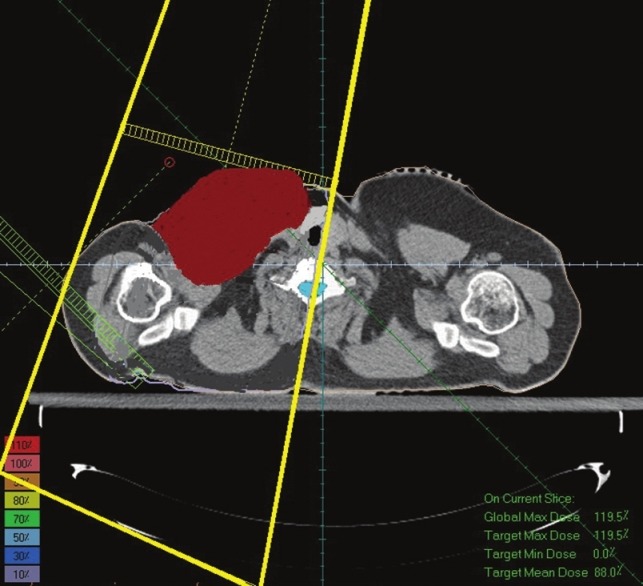
A single field technique (Anterior Posterior) irradiated to gross tumor volume
Figure 4.
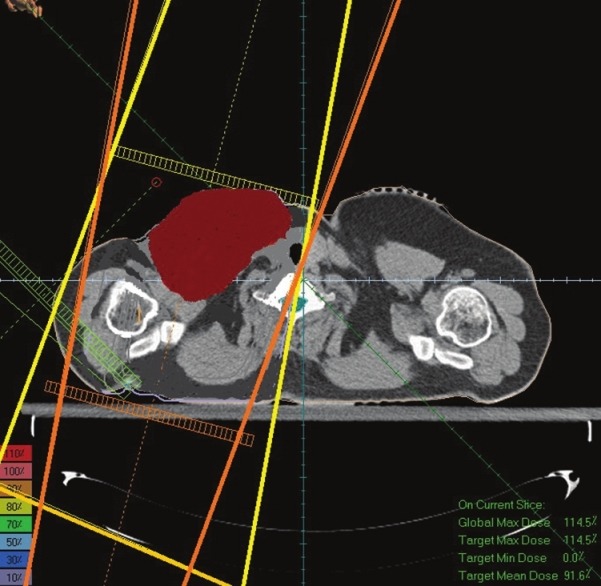
Two parallel opposed fields (Anterior Posterior-Posterior Anterior). Both fields are angled 15° to avoid the irradiation of the spine
The second part of this project is the optimizing of tangential field. One technique in order to change the dose distribution is changing the depth of the normalization point. The normalization point is a point that is supposed to receive 100% of the prescription dose. The TPS increases the amount of radiation until this point receives the prescribed dose. The normalization point is positioned in four various locations, which are illustrated in the Figures 5 and 6.
Figure 5.
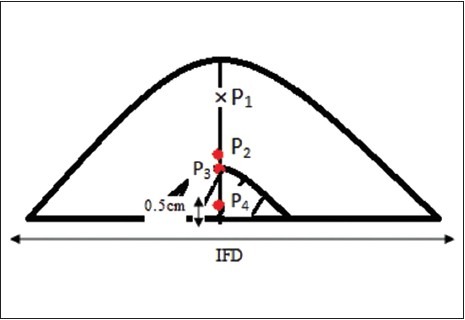
The location of the normalization points P1 is isocenter of the beams. 3 other points are located in the larger depths
Figure 6.
The location of the normalization points
The locations of four points are as follows:
P1: Isocenter (the gantry rotates around this point and the axis of all beams intersects in this point)
P2: Middle of inter field distance (IFD) and isocenter
P3: Border between the lung and chest wall
P4: Middle of the thickest part of breast or middle of IFD.
The dose distributions with these points are compared to the point that the physician has determined. This should be noted that physician choice is the one that is used in reality and the patient is irradiated according to that point. The physician choice could be any of the four points and it is usually in the isocenter (P1). The dose distribution and DVH is calculated for PTV, lung, heart, and spinal each four techniques. With comparing these results, the best point is selected. In this project for data analyzes SPSS 16 statistical software was used.
DISCUSSION AND RESULTS
TPS software after calculating the DVH for each technique calculates automatically the average dose and maximum dose for each organ that have been contoured.
The isodose curves for 100 patients in both supraclavicular and tangential fields were compared and dose distributions for each patient in all techniques were studied.
In optimizing of supraclavicular field in the single field technique the average received supraclavicular dose was 4654 cGy with a standard deviation of 469 and in AP-PA technique average received supraclavicular dose was 4754 cGy with a standard deviation 387. In a single field technique, maximum received supraclavicular dose was 6705 cGy with a standard deviation 1210 and in AP-PA technique Maximum received supraclavicular dose was 6582 cGy with a standard deviation 1206. An example of dose distribution in single and AP-PA technique are illustrated in Figures 7 and 8.
Figure 7.
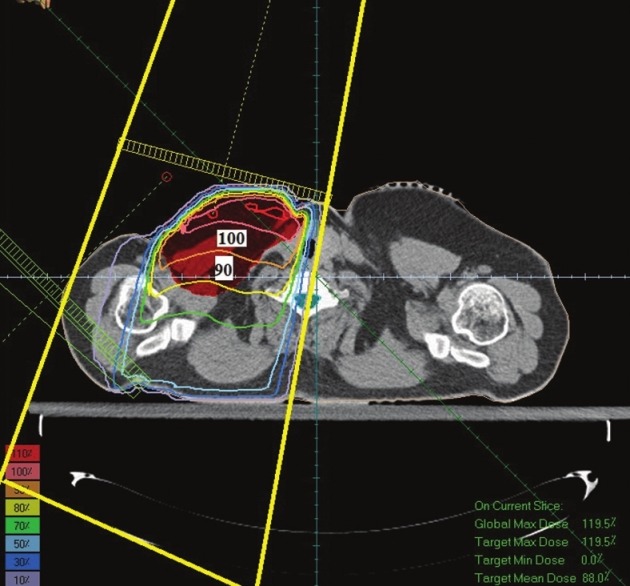
Dose distribution in a single field technique
Figure 8.
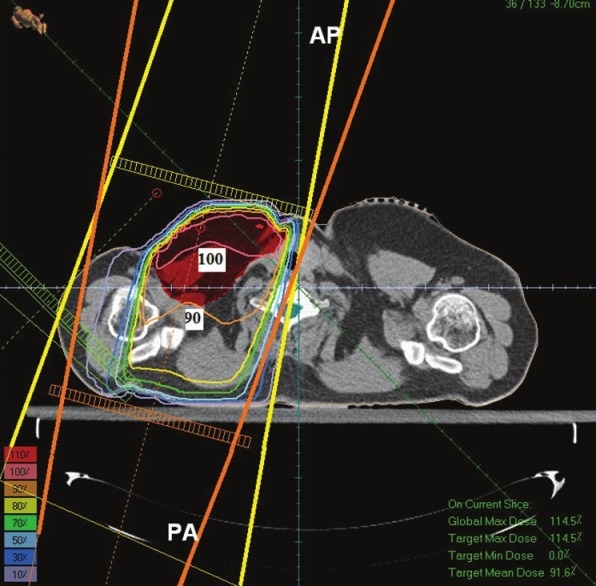
Dose distribution in Anterior Posterior-Posterior Anterior technique
Dose distribution in Figure 8 (AP-PA technique) shows that PTV has been covered with 90%-100% isodose line and this technique has made a better dose uniformity. However, in Figure 7 (single field technique) PTV has been covered with 80%-100% isodose. On the other hand in Figure 7 the 90% isodose line goes across the middle of the tumor; however, in Figure 8 the 90% isodose line covers the PTV completely.
P value for all data is more than 0.05 and just for maximum received skin dose is less than 0.05.
Figures 9–15 illustrates the graphs for all parameters in supraclavicular field techniques. In these figures Technique 1 is a single field, and Technique 2 is AP-PA field.
Figure 9.
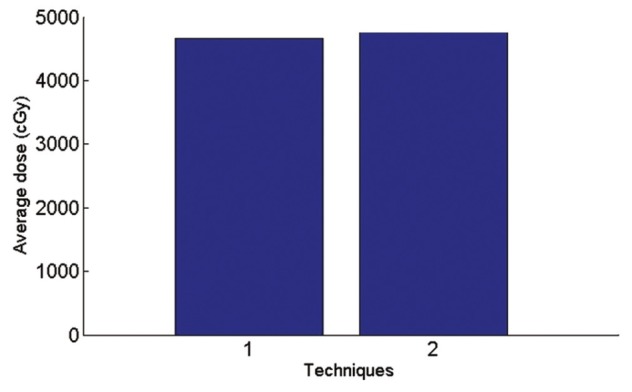
Graph of average received supraclavicular dose in planning tumor volume
Figure 15.
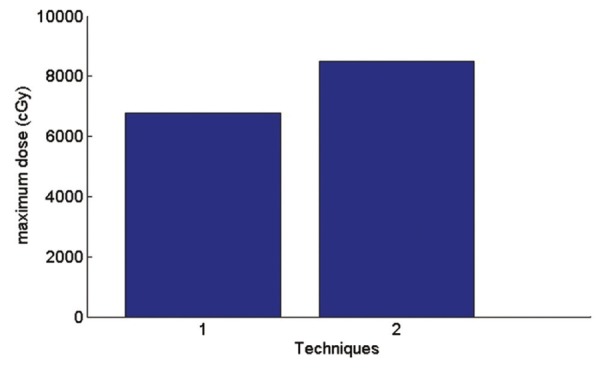
Graph of maximum received skin dose
According to Figure 9, in AP-PA technique the average dose of supraclavicular region is 2.2% more than a single field and maximum received supraclavicular dose is 1.8% less than a single field.
In AP-PA technique average received breast dose is 0.3% more than a single field and maximum received breast dose is 0.14% less than a single field.
Coverage of PTV in AP-PA fields is better than a single field. But according to the Figure 11 average received lung dose in AP-PA technique is 4% more than a single field. This is because of the fact that in AP-PA, the isodose curves moves to the larger depths and therefore are more likely to cover the top of the lung.
Figure 11.
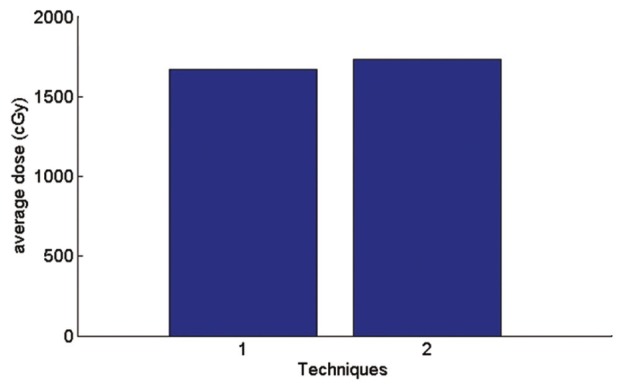
Graph of average received lung dose
In AP-PA technique average received heart dose is 0.4% more than a single field and according to the Figure 10; maximum received heart dose is 21.5% less than a single field.
Figure 10.
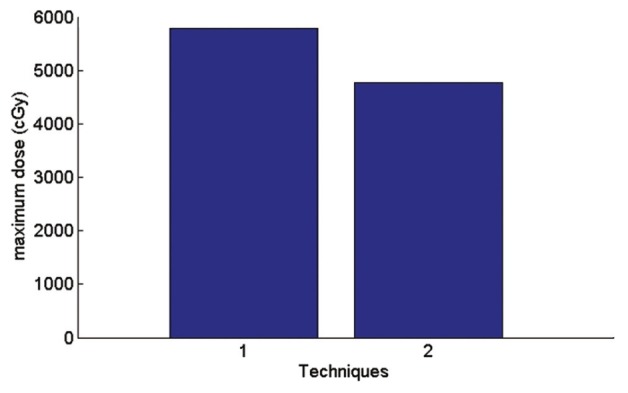
Graph of maximum received heart dose
In AP-PA technique, according to the Figure 12 average received spinal dose is 3% less than a single field and maximum received spinal dose according to the Figure 13 is 1.4% more than a single field.
Figure 12.
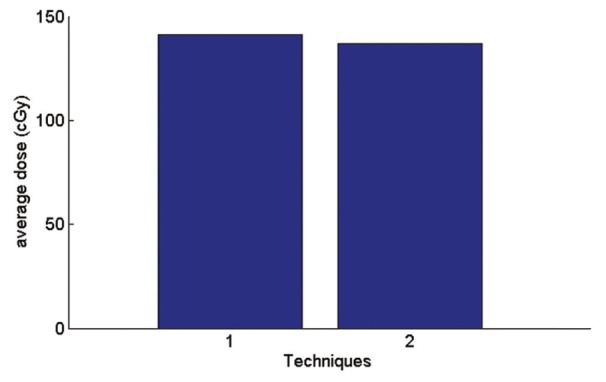
Graph of average received spine dose
Figure 13.
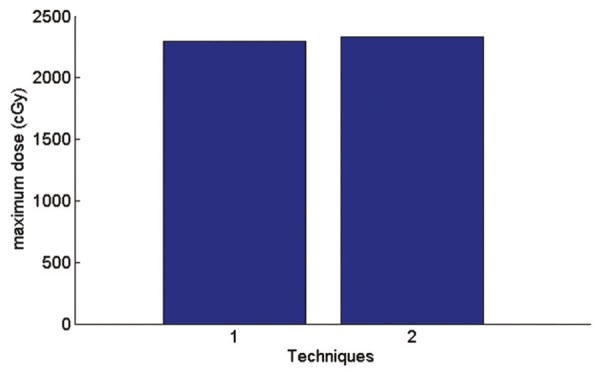
Graph of maximum received spine dose
In AP-PA technique, according to the Figure 14 average received skin dose is 9.8% more than a single field and maximum received skin dose according to the Figure 15 is 25% more than a single field.
Figure 14.
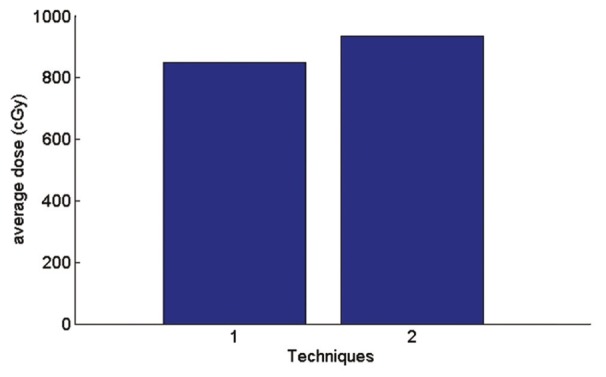
Graph of average received skin dose
Therefore, coverage of PTV in AP-PA technique is better than a single field and maximum received heart dose and average received spinal dose decrease but average and maximum received skin and lung dose increase.
In optimizing of tangential fields, the results are illustrated in Figures 16–20. The number of tangential techniques is named as.
Figure 16.
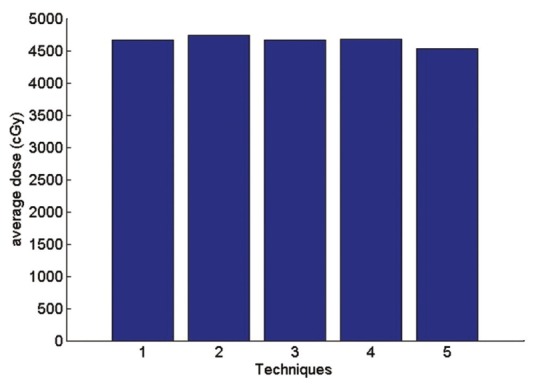
The average received breast dose
Figure 20.
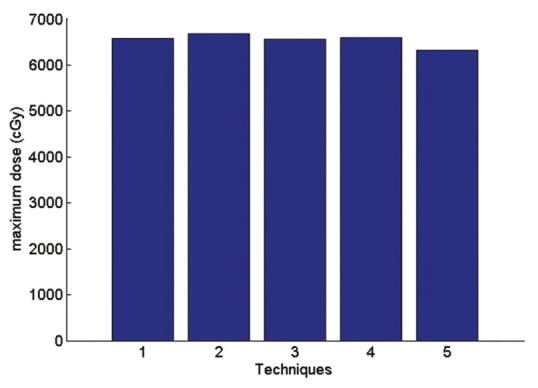
The maximum received lung dose
Figure 18.
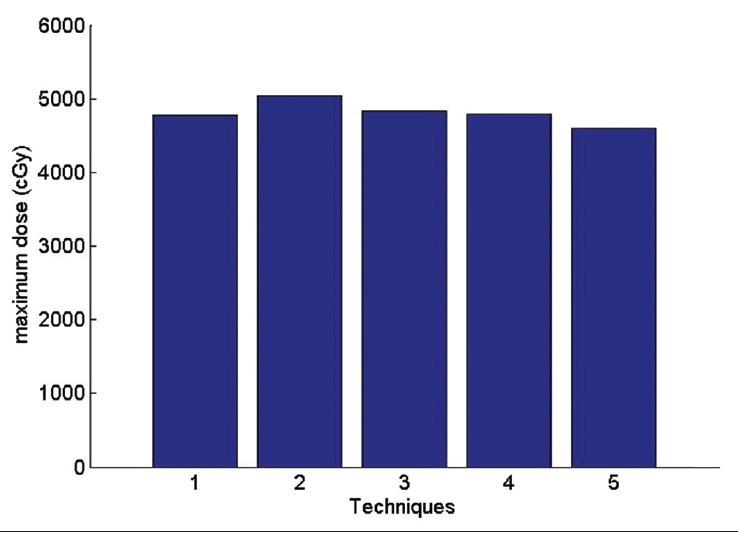
The maximum received heart dose
Technique 1: Isocenter. Technique 2: Middle of the thickest part of breast (IFD). Technique 3: Border between the lung and chest wall Technique 4: physician's choice Technique 5: between IFD and isocenter.
In optimization of tangential field all methods cover PTV very similarly and these methods do not have a big difference.
According to the Figure 16 the average received PTV dose in comparisons of physician's choice method has increased 0.3% in technique 1, 1.4% in technique 2, 0.3% in technique 3. In technique 5 the average received PTV dose has decreased 3.1% in comparisons of physician's choice. The highest dose is related to technique 2 and this is a reasonable result since point 2 has the largest depth. This fact also shows in the maximum dose. For Maximum received a dose of PTV the second method in which the normalization point is in the middle of the IFD has the largest amount compare to other methods.
So in the second method coverage of PTV is better in comparison to other methods. In First and third method, coverage of PTV are similar in comparison of other methods and physician's choice method. In Fifth method, coverage of PTV is less in comparison of other methods and physician's choice method.
According to the Figure 17 average received heart dose in comparisons of physician's choice method has decreased 0.6% in technique1 2.9% in technique3 1.6% in technique 5. In the second method, the dose has increased 5.4%. So in the other side the technique 2 has the maximum average dose to the heart. The same fact is illustrated for lung dose is Figures 19 and 20.
Figure 17.
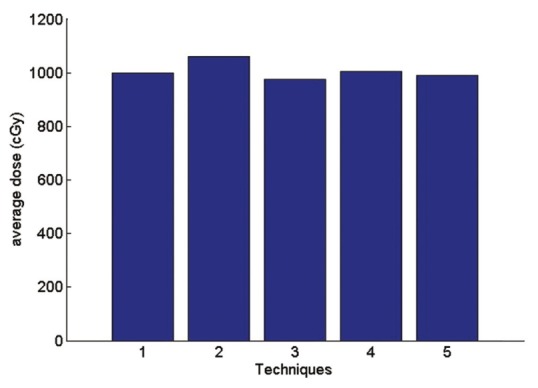
The average received heart dose
Figure 19.
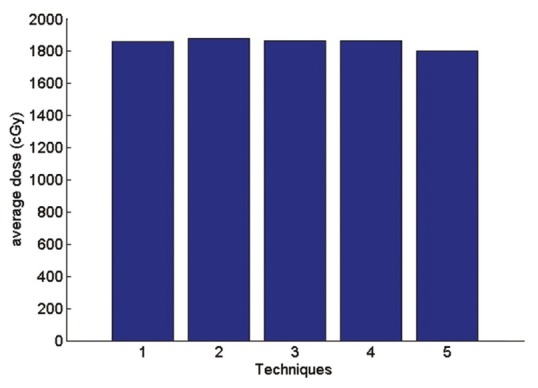
The average received lung dose
In this project, the V20 parameter is also evaluated. V20 is the percentage of lung volume that has received a dose of 20 Gy and more. V20 parameter in comparisons of physician's choice method is similar to technique 1 and it is decreased 0.7% in technique 3 and it is increased 1.6% in technique 2 and 0.3% in technique 5.
Therefore, all methods are similar in comparison of physician's choice method. Each method has special advantages and disadvantages. If it is important for the physician to reduce the dose received by the lung and heart, fifth method can be an optimized method since in this method average and maximum received dose to heart and lung have been reduced few percent in comparisons of physician's choice method and three other methods.
If a better coverage of PTV is important for the physician technique 2 selected. In this method average and maximum received dose to PTV have been increased few percent in comparisons of three other methods. However, in this method we have an increased average and maximum dose of the heart and lung and V20.
CONCLUSION
In this paper, a comparison was done between various tangential and supraclavicular fields techniques.
In supraclavicular field AP-PA technique due to a better coverage of PTV is suggested rather than a single AP. However, in this technique, the average and maximum received lung and skin dose is increased few percent.
In optimizing of tangential field with comparison five techniques with many normalization points were evaluated. If it is important for the physician to reduce the dose received by the lung and heart, the Technique in which the normalization point is located between isocenter and IFD can be selected. In this method average and maximum received dose to heart and lung have been reduced few percent in comparisons three other methods with other depths of normalization points.
If a better coverage of PTV is important for the physician technique that normalization point is located middle of the thickest part of breast or middle of IFD can be chosen. In this method, the average and maximum received dose to PTV have been increased compared with other methods. However, in this method there is increased average and maximum dose for heart and lung and V20. In final, optimizing of tangential showed that each method have spatial advantages and disadvantages.
BIOGRAPHIES

Keyvan Jabbari received B.S. degree in physics from Isfahan University of Technology, Isfahan, IRAN in 1999, M.S. degree in medical radiation engineering from Amir Kabir University, Tehran in 2002, M.S. degree in medical physics from University of Manitoba , Winnipeg, Canada, in 2004. Then he got Ph.D. of Medical Physics from McGill University, Montreal, Canada in 2008.
Since then he is assistant professor in Department of Medical Physics and Medical Engineering , School of Medicine, Isfahan University of Medical Sciences. He is also clinical medical physicist in Isfahan Milad Hospital and Seyed-o-Shohada cancer center. His research interest is fast Monte Carlo in treatment planning, dose calculation and plan optimization
E-mail: jabbari@med.mui.ac.ir

Nazli Azarmahd received B.S. degree in physics from Islamic Azad University Science & Research of Tehran, Iran in 2008, and M. S. degree in medical physics from Isfahan University of Medical Science, Isfahan, Iran in 2013. Her research interest is treatment planning, and plan optimization
E-mail: n.azarmahd@yahoo.com

Shadi Babazadeh was graduated in Medicine from Isfahan University of medical Sciances, Isfahan, Iran in 1991, and Radiation Oncology from Isfahan University of Medical Sciences, Isfahan, Iran in 1995
Since then she was Radiation Oncologist physician and consultant in Seyed-o Shohada Cancer Centre and Isfahan Milad Hospital. She was Cancer Researcher in Cancer research and Treatment Centre in Isfahan University of Medical Sciences(Till July 2012)
She is Clinical Research student in Seneca College, Toronto, Canada, and working as Volunteer in Princes Margaret Hospital, and Sunny Brook Hospital, Toronto, Canada now. She is enthusiastic in Breast Cancer research, and she had experience in that
E-mail: sh_babazade@yahoo.com

Alireza Amouheidari received doctorate degree in General Medicine from Isfahan University of Medical Sciences in 2000 and National Board of Radiation Oncology in 2007. After that he has worked in Sayed-alshohada hospital for about 3 years and since 2009 is working as the chief radiation oncologist and the head of the radiation oncology department in Isfahan Milad Hospital. His research interests are Breast, Prostate and Brain tumors as well as IMRT
E-mail: amouheidari@isfahanmiladhospital.ir
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Nold RJ, Beamer RL, Helmer SD, McBoyle MF. Factors influencing a woman's choice to undergo breast-conserving surgery versus modified radical mastectomy. Am J Surg. 2000;180:413–8. doi: 10.1016/s0002-9610(00)00501-8. [DOI] [PubMed] [Google Scholar]
- 2.Elkin EB, Hudis C, Begg CB, Schrag D. The effect of changes in tumor size on breast carcinoma survival in the U.S.: 1975-1999. Cancer. 2005;104:1149–57. doi: 10.1002/cncr.21285. [DOI] [PubMed] [Google Scholar]
- 3.Anderson WF, Jatoi I, Devesa SS. Assessing the impact of screening mammography: Breast cancer incidence and mortality rates in Connecticut (1943-2002) Breast Cancer Res Treat. 2006;99:333–40. doi: 10.1007/s10549-006-9214-z. [DOI] [PubMed] [Google Scholar]
- 4.Peto R, Boreham J, Clarke M, Davies C, Beral V. UK and USA breast cancer deaths down 25% in year 2000 at ages 20-69 years. Lancet. 2000;355:1822. doi: 10.1016/S0140-6736(00)02277-7. [DOI] [PubMed] [Google Scholar]
- 5.Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: Prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557–65. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 6.Hall EJ. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Oncol Biol Phys. 2006;65:1–7. doi: 10.1016/j.ijrobp.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 7.Siglin J, Champ CE, Vakhnenko Y, Anne PR, Simone NL. Radiation therapy for locally recurrent breast cancer. Int J Breast Cancer. 2012:571946. doi: 10.1155/2012/571946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grossman RA, Pedroso FE, Byrne MM, Koniaris LG, Misra S. Does surgery or radiation therapy impact survival for patients with extrapulmonary small cell cancers? J Surg Oncol. 2011;104:604–12. doi: 10.1002/jso.21976. [DOI] [PubMed] [Google Scholar]
- 9.Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–32. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 10.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–41. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 11.Spooner D, Stocken DD, Jordan S, Bathers S, Dunn JA, Jevons C, et al. A randomised controlled trial to evaluate both the role and the optimal fractionation of radiotherapy in the conservative management of early breast cancer. Clin Oncol (R Coll Radiol) 2012;24:697–706. doi: 10.1016/j.clon.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366:2087–106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 13.Berenson RA. Implementing health care reform – Why Medicare matters. N Engl J Med. 2010;363:101–3. doi: 10.1056/NEJMp1005588. [DOI] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.Bantema-Joppe EJ, de Munck L, Visser O, Willemse PH, Langendijk JA, Siesling S, et al. Early-stage young breast cancer patients: Impact of local treatment on survival. Int J Radiat Oncol Biol Phys. 2011;81:e553–9. doi: 10.1016/j.ijrobp.2011.02.060. [DOI] [PubMed] [Google Scholar]
- 16.Coles CE, Harris EJ, Donovan EM, Bliss P, Evans PM, Fairfoul J, et al. Evaluation of implanted gold seeds for breast radiotherapy planning and on treatment verification: A feasibility study on behalf of the IMPORT trialists. Radiother Oncol. 2011;100:276–81. doi: 10.1016/j.radonc.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Smith BD, Haffty BG, Wilson LD, Smith GL, Patel AN, Buchholz TA. The future of radiation oncology in the United States from 2010 to 2020: Will supply keep pace with demand? J Clin Oncol. 2010;28:5160–5. doi: 10.1200/JCO.2010.31.2520. [DOI] [PubMed] [Google Scholar]
- 18.Menard J, Campana F, Kirov KM, Bollet MA, Dendale R, Fournier-Bidoz N, et al. Radiotherapy for breast cancer and pacemaker. Cancer Radiother. 2011;15:197–201. doi: 10.1016/j.canrad.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Dessena M, Dessi M, Demontis B, Grosso LP, Porru S, Meleddu GF, et al. Exclusive intra-operative radiation therapy (IORT) for early stage breast cancer: Pilot study of the feasibility. G Chir. 2011;32:104–9. [PubMed] [Google Scholar]
- 20.Alford SL, Prassas GN, Vogelesang CR, Leggett HJ, Hamilton CS. Adjuvant breast radiotherapy using a simultaneous integrated boost: Clinical and dosimetric perspectives. J Med Imaging Radiat Oncol. 2013;57:222–9. doi: 10.1111/j.1754-9485.2012.02473.x. [DOI] [PubMed] [Google Scholar]
- 21.Jereczek-Fossa BA, Santoro L, Colangione SP, Morselli L, Fodor C, Vischioni B, et al. Electronic portal imaging registration in breast cancer radiotherapy verification: Analysis of inter-observer agreement among different categories of health practitioners. Neoplasma. 2013;60:302–8. doi: 10.4149/neo_2013_040. [DOI] [PubMed] [Google Scholar]
- 22.Jobsen JJ, van der Palen J, Baum M, Brinkhuis M, Struikmans H. Timing of radiotherapy in breast-conserving therapy: A large prospective cohort study of node-negative breast cancer patients without adjuvant systemic therapy. Br J Cancer. 2013;108:820–5. doi: 10.1038/bjc.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jatoi I, Proschan MA. Randomized trials of breast-conserving therapy versus mastectomy for primary breast cancer: A pooled analysis of updated results. Am J Clin Oncol. 2005;28:289–94. doi: 10.1097/01.coc.0000156922.58631.d7. [DOI] [PubMed] [Google Scholar]
- 24.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–41. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 25.Arthur DW, Vicini FA. Accelerated partial breast irradiation as a part of breast conservation therapy. J Clin Oncol. 2005;23:1726–35. doi: 10.1200/JCO.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 26.Vicini FA, Kestin L, Chen P, Benitez P, Goldstein NS, Martinez A. Limited-field radiation therapy in the management of early-stage breast cancer. J Natl Cancer Inst. 2003;95:1205–10. doi: 10.1093/jnci/djg023. [DOI] [PubMed] [Google Scholar]
- 27.Morris DE, Bourland JD, Rosenman JG, Shaw EG. Three-dimensional conformal radiation treatment planning and delivery for low- and intermediate-grade gliomas. Semin Radiat Oncol. 2001;11:124–37. doi: 10.1053/srao.2001.22060. [DOI] [PubMed] [Google Scholar]
- 28.Tome WA, Meeks SL, Buatti JM, Bova FJ, Friedman WA, Li Z. A high-precision system for conformal intracranial radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:1137–43. doi: 10.1016/s0360-3016(00)00502-2. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro CL, Recht A. Side effects of adjuvant treatment of breast cancer. N Engl J Med. 2001;344:1997–2008. doi: 10.1056/NEJM200106283442607. [DOI] [PubMed] [Google Scholar]
- 30.Fowble B, Hanlon A, Freedman G, Nicolaou N, Anderson P. Second cancers after conservative surgery and radiation for stages I-II breast cancer: Identifying a subset of women at increased risk. Int J Radiat Oncol Biol Phys. 2001;51:679–90. doi: 10.1016/s0360-3016(01)01665-0. [DOI] [PubMed] [Google Scholar]
- 31.White J, Joiner MC. Toxicity from radiation in breast cancer. Cancer Treat Res. 2006;128:65–109. doi: 10.1007/0-387-25354-8_5. [DOI] [PubMed] [Google Scholar]
- 32.Fogliata A, Bolsi A, Cozzi L. Critical appraisal of treatment techniques based on conventional photon beams, intensity modulated photon beams and proton beams for therapy of intact breast. Radiother Oncol. 2002;62:137–45. doi: 10.1016/s0167-8140(01)00476-5. [DOI] [PubMed] [Google Scholar]
- 33.Ahunbay EE, Chen GP, Thatcher S, Jursinic PA, White J, Albano K, et al. Direct aperture optimization-based intensity-modulated radiotherapy for whole breast irradiation. Int J Radiat Oncol Biol Phys. 2007;67:1248–58. doi: 10.1016/j.ijrobp.2006.11.036. [DOI] [PubMed] [Google Scholar]
- 34.Van Limbergen E, Weltens C. New trends in radiotherapy for breast cancer. Curr Opin Oncol. 2006;18:555–62. doi: 10.1097/01.cco.0000245327.42281.9f. [DOI] [PubMed] [Google Scholar]
- 35.Pignol JP, Olivotto I, Rakovitch E, Gardner S, Sixel K, Beckham W, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol. 2008;26:2085–92. doi: 10.1200/JCO.2007.15.2488. [DOI] [PubMed] [Google Scholar]
- 36.de la Torre N, Figueroa CT, Martinez K, Riley S, Chapman J. A comparative study of surface dose and dose distribution for intact breast following irradiation with field-in-field technique vs. the use of conventional wedges. Med Dosim. 2004;29:109–14. doi: 10.1016/j.meddos.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Ercan T, Iðdem S, Alço G, Zengin F, Atilla S, Dinçer M, et al. Dosimetric comparison of field in field intensity-modulated radiotherapy technique with conformal radiotherapy techniques in breast cancer. Jpn J Radiol. 2010;28:283–9. doi: 10.1007/s11604-010-0423-3. [DOI] [PubMed] [Google Scholar]
- 38.Lee JW, Hong S, Choi KS, Kim YL, Park BM, Chung JB, et al. Performance evaluation of field-in-field technique for tangential breast irradiation. Jpn J Clin Oncol. 2008;38:158–63. doi: 10.1093/jjco/hym167. [DOI] [PubMed] [Google Scholar]
- 39.Mihai A, Rakovitch E, Sixel K, Woo T, Cardoso M, Bell C, et al. Inverse vs. forward breast IMRT planning. Med Dosim. 2005;30:149–54. doi: 10.1016/j.meddos.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Murthy KK, Sivakumar SS, Davis CA, Ravichandran R, El Ghamrawy K. Optimization of dose distribution with multi-leaf collimator using field-in-field technique for parallel opposing tangential beams of breast cancers. J Med Phys. 2008;33:60–3. doi: 10.4103/0971-6203.41194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohashi T, Takeda A, Shigematsu N, Fukada J, Sanuki N, Amemiya A, et al. Dose distribution analysis of axillary lymph nodes for three-dimensional conformal radiotherapy with a field-in-field technique for breast cancer. Int J Radiat Oncol Biol Phys. 2009;73:80–7. doi: 10.1016/j.ijrobp.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 42.Prabhakar R, Julka PK, Rath GK. Can field-in-field technique replace wedge filter in radiotherapy treatment planning: A comparative analysis in various treatment sites. Australas Phys Eng Sci Med. 2008;31:317–24. doi: 10.1007/BF03178601. [DOI] [PubMed] [Google Scholar]
- 43.Fisher B, Jeong JH, Anderson S, Bryant J, Fisher ER, Wolmark N. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347:567–75. doi: 10.1056/NEJMoa020128. [DOI] [PubMed] [Google Scholar]
- 44.Kozak KR, Doppke KP, Katz A, Taghian AG. Dosimetric comparison of two different three-dimensional conformal external beam accelerated partial breast irradiation techniques. Int J Radiat Oncol Biol Phys. 2006;65:340–6. doi: 10.1016/j.ijrobp.2005.12.053. [DOI] [PubMed] [Google Scholar]
- 45.Moon SH, Shin KH, Kim TH, Yoon M, Park S, Lee DH, et al. Dosimetric comparison of four different external beam partial breast irradiation techniques: Three-dimensional conformal radiotherapy, intensity-modulated radiotherapy, helical tomotherapy, and proton beam therapy. Radiother Oncol. 2009;90:66–73. doi: 10.1016/j.radonc.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 46.Erven K, Van Limbergen E. Regional lymph node irradiation in breast cancer. Future Oncol. 2007;3:343–52. doi: 10.2217/14796694.3.3.343. [DOI] [PubMed] [Google Scholar]
- 47.Wazer DE, Berle L, Graham R, Chung M, Rothschild J, Graves T, et al. Preliminary results of a phase I/II study of HDR brachytherapy alone for T1/T2 breast cancer. Int J Radiat Oncol Biol Phys. 2002;53:889–97. doi: 10.1016/s0360-3016(02)02824-9. [DOI] [PubMed] [Google Scholar]
- 48.Keisch M, Vicini F, Kuske RR, Hebert M, White J, Quiet C, et al. Initial clinical experience with the MammoSite breast brachytherapy applicator in women with early-stage breast cancer treated with breast-conserving therapy. Int J Radiat Oncol Biol Phys. 2003;55:289–93. doi: 10.1016/s0360-3016(02)04277-3. [DOI] [PubMed] [Google Scholar]
- 49.Pignol JP, Rakovitch E, Keller BM, Sankreacha R, Chartier C. Tolerance and acceptance results of a palladium-103 permanent breast seed implant Phase I/II study. Int J Radiat Oncol Biol Phys. 2009;73:1482–8. doi: 10.1016/j.ijrobp.2008.06.1945. [DOI] [PubMed] [Google Scholar]
- 50.Vaidya JS, Joseph DJ, Tobias JS, Bulsara M, Wenz F, Saunders C, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): An international, prospective, randomised, non-inferiority phase 3 trial. Lancet. 2010;376:91–102. doi: 10.1016/S0140-6736(10)60837-9. [DOI] [PubMed] [Google Scholar]
- 51.Woo TC, Pignol JP, Rakovitch E, Vu T, Hicks D, O’Brien P, et al. Body radiation exposure in breast cancer radiotherapy: Impact of breast IMRT and virtual wedge compensation techniques. Int J Radiat Oncol Biol Phys. 2006;65:52–8. doi: 10.1016/j.ijrobp.2005.11.023. [DOI] [PubMed] [Google Scholar]


