Abstract
Background & objectives:
Japanese encephalitis virus (JEV) infection results in acute encephalitic illness. The affinity of JEV to different regions of brain and temporal changes in viral load have not been studied. This study was conducted to describe localization of JEV to different regions of the brain at different stages of disease in a rat model of Japanese encephalitis (JE).
Methods:
Twelve days old Wistar rats were inoculated intracerebrally with a dose of 3 × 106 pfu/ml of JEV. After 3, 6, 10 and 20 days post-inoculation, brains were dissected out and different regions of brain (cortex, striatum, thalamus and mid brain) were taken. Motor deficit was assessed by the rota rod and JEV RNA copies were evaluated using real-time PCR assay.
Results:
There was a significant increase in motor deficit in rats inoculated with JEV compared to the controls. JEV RNA copies were present in all studied regions of the brain on days 3, 6 and 10 post-inoculation. Maximum number of JEV RNA copies were present in the mid brain on days 3 and 10 post-inoculation. JEV RNA copies were not detected in any of the brain regions on day 20.
Interpretation & conclusions:
This study reports JEV RNA load in different brain regions of rat with higher affinity of JEV virus to thalamus and mid brain compared to other regions.
Keywords: Brain regions, infection, Japanese encephalitis virus (JEV), rat model, real-time PCR, thalamus, viral encephalitis
Japanese encephalitis virus (JEV) is an arthropod borne virus of family Flaviviridae. It is one of the most important causes of viral encephalitis worldwide leading to an estimated 35,000-50,000 encephalitis cases and 10,000-15,000 deaths annually in Asia1,2. In case of central nervous system (CNS) infections, viruses localize to specific regions of the brain and cause neuronal damage. Neurologic invasion can develop, possibly by growth of the virus across vascular endothelial cells, leading to involvement of large areas of the brain, including the thalamus, basal ganglia, brain stem, cerebellum, hippocampus, and cerebral cortex. The capacity of viruses to selectively infect specific tissues depends on an interaction between viral gene, proteins and host factors. There are over 120 viruses which cause encephalitis. These viruses have affinity for different species and in different parts of the brain, e.g. Herpes simplex virus I (HSVI) has affinity for frontotemporal area because of neurochemical and immunological properties3, rabies virus has affinity for acetylcholine receptors, reovirus for beta adrenalin receptors, HIV for CD44 and poliovirus for hPVR and CD155 receptors which belong to the immunoglobulin superfamily5. JEV does not produce encephalitis in pigs and birds, suggesting a genetic resistance6. Mice model has been used since 1960s for the study of pathophysiology and possible treatment of JEV infection. Following the intracerebral inoculation of JEV in mice, 100 per cent mortality and 4.8 days mean survival have been reported7. In JEV infection, cytopathic effect may have temporal sequence and may be influenced by a number of variables such as virus load and genetic susceptibility. The study of such changes is possible in an experimental model with a longer survival. Majority of the studies have used mouse model for reporting histological and immunohistopathological changes in JE8,9,10. Ogata and colleagues11 developed a rat model to study the parkinsonian features in JE and emphasized age related neurotropism. They studied the changes up to 12 wk after JEV inoculation. In our previous radiological study on JE patients, maximum involvement of thalamus was noted12. In the cerebral cortex, tropic and non-tropic areas have also been identified in a mouse model of JE8. However, no effort has been made to evaluate affinity of JEV to different regions of brain and temporal changes in viral load. The limitation of conventional real time quantitative-PCR in the diagnosis of JE has been reported13. Real time PCR assay has emerged as a promising technique because of its high sensitivity, specificity and rapidity14. The usefulness of this rapid diagnostic assay in other viral diseases has been suggested15. Based on the common involvement of thalamus, basal ganglia and midbrain on magnetic resonance imaging (MRI) in JE patients12, it may be worthwhile to evaluate the temporal changes of JEV RNA copies in different regions of the brain in the experimental model of JEV infection. The present study was aimed to document the distribution and quantitation of JEV RNA copies in different regions of the brain of rat infected with JEV and changes over a period of time using real-time PCR assay.
Material & Methods
Virus: GP 78668A strain of JEV (a kind gift from Dr S. Vrati, National Institute of Immunology, New Delhi), a neurovirulent strain was used in this study. Virus was propagated in 3-4 days old suckling mouse brain. After 4 days of infection4 mice were sacrificed. Brain was removed aseptically, homogenized in sterile phosphate buffer saline (PBS) and centrifuged at 15,000 g for 30 min at 80°C. The supernatant was collected, aliquoted and stored at -70°C till further use. Virus titre was determined by the standard plaque assay16. The titre of the virus was determined by the following formula:
![]()
Animal: Suckling pups of Wistar strain rats (12 days old rats with mother) purchased from Central Drug Research Institute, Lucknow were used in the study. The rats were maintained in the animal facility at Sanjay Gandhi Postgraduate Institute of Medical Sciences (SGPGIMS), Lucknow on alternating 12 h light and dark cycle. The study was approved by the ethics committee of SGPGIMS, and experiments were carried out in accordance with the institutional guidelines on the care and use of experimental animals. Rats were divided into two groups: JEV infected group (n=24) and mock infected controls (n=6). In JEV infected group, the rats were inoculated intracerebrally with 3 × 106 pfu/ml of JEV11. Control rats were inoculated with sterile PBS 1X (Sigma, USA). The study period was 20 days. The rats were monitored daily and six rats each were sacrificed on days 3, 6, 10 and 20 post-inoculation. The brains were excised aseptically and regions of the brain (cortex, striatum, thalamus and mid brain) were dissected out and were homogenised in chilled lysis buffer (1 x PBS with 10 μg/ml phenyl nethyl sulphonyl fluoride (PMSF).
Rota rod test: Motor deficit was assessed by the rota rod test one day prior to 3, 6, 10 and 20 days post-inoculation. Six rats from each group were tested on a motorized rota rod consisting of a grooved metal roller. The acceleration rate was set at 0.15 rpm/sec. Rats were placed on the roller, and the time they remained on the roller during rotation, was measured. A maximum of 120 sec was allowed per animal for fixed speed tests.
RNA extraction: Total RNA was extracted from tissue samples using a QIAmp viral RNA kit (QIAGEN, Inc., USA) according to the manufacturer's instructions. RNA was eluted in 50 μl of diethylpyrocarbonate (DEPC) treated distilled water and stored at -80°C till further use. The amount of RNA in each sample was quantified17 by measuring the absorbance at 260 nm using a spectrophotometer (Hitachi, Japan) with the following formula:
RNA concentration (μg/ml) = OD260 × DF × 40
Where, OD260 is the absorbance of the diluted samples at 260 nm and DF is the dilution factor.
The purity of RNA was determined by calculating the ratio of OD 260 to OD 280.
Real-time PCR (RT-PCR): The real-time quantitative PCR assay was performed using Geno-Sen's JEV Real-time PCR Kit (Corbett Research, Australia). The forward and reverse primers hybridize to a specific sequence product. JEV-specific forward primer 391-GCAGAAAGCAAAACAAAAGAG and reverse primer 757-ACGGATCTCCTGCTTCGCTTG were designed from the C-prM region by aligning the available sequences in GenBank accession no. AF075723. An internal control gene was added to the reaction mix which was provided in the kit. RNA was reverse-transcribed using reverse primer at 50°C for 15 min using superscript II reverse transcriptase (Invitrogen, India). The cDNA was PCR amplified using the forward (391) and reverse (757) primers for detection of the JEV. PCR amplification was carried out by denaturing the DNA at 95°C for 10 min, followed by 45 cycles of 95°C for 30 sec, 55°C for 20 sec, and 72°C for 15 sec. An internal control gene was added to the reaction mix which has been provided in the kit. For each step, the temperature transition rate was 20°C/sec. After the thermal cycle a standard curve with the dynamic range of detection in copies/ml was constructed by preparing 10-fold serial dilutions of standard JEV.
Statistical analysis: The comparison among groups were made using one-way analysis of variance (ANOVA) with a post-hoc comparison (Newman Keuls multiple) test. Differences between means were considered significant at P<0.05. All the statistical analysis was done using GraphPad Prism (3.03) software, USA.
Results
There were 24 rats in JEV infected group and 6 in controls. All rats were subjected to daily clinical observation. The animals in JEV group started showing clinical symptoms from day 4 post-inoculation which manifested with huddling and slight hind limb weakness, pelvic elevation, somnolence, sluggishness, and lethargy.
No motor deficit was observed in any rat before JEV infection. Durations of stay on the accelerating rota rod for JEV infected rats were 115.5 ± 3.7, 98.7 ± 3.1, 101.4 ± 2.9 and 104.3 ± 3.2 sec, respectively on days 3, 6, 10 and 20 post-inoculation. There was a significant increase in motor deficit on day 6 compared to day 3 post-inoculation (P<0.001) as well as with mock infected control on day 6. The duration of stay on the rota rod was longer in control rats compared to JEV inoculated rats on day 6 (P<0.001), 10 (P<0.0001) and 20 post-inoculation (P<0.0001).
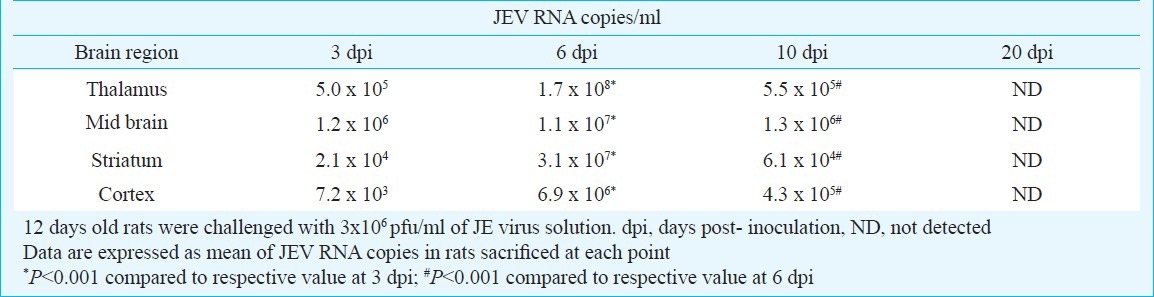
JEV RNA copies were present in all brain regions studied on days 3, 6 and 10 post-inoculation, however, no viral RNA copies were detected on day 20 post- inoculation in any studied brain region. Maximum numbers of JEV RNA copies were present in mid brain on days 3 and 10 post-inoculation as compared to cortex, striatum and thalamus. However, on day 6 post-inoculation, maximum numbers of JEV RNA copies were present in the thalamus (Table). There was a significant increase in JEV RNA copies on day 6 post-inoculation compared to day 3 in all the regions of brain studied (P<0.001); however, JEV RNA copies significantly decreased on day 10 post-inoculation compared to day 6 (P<0.001). No JEV RNA copies were detected in controls.
Table.
Japanese encephalitis virus (RNA copies/ml) in different brain region of rat at various time points
Discussion
The present study revealed that JEV localizes in thalamus, striatum, mid brain and cortex. JEV showed maximum affinity to mid brain and thalamus followed by striatum and cortex. There was a decrease in JEV RNA copies in later phase of the disease and an improvement in motor deficit.
Binding of viral attachment proteins to the host cell receptors is a well-established phenomenon of viral tissue tropism. Using immunohistochemistry, Kim et al8, demonstrated that JEV has neuronal tropism in certain regions of the mice brain such as brain stem, thalamus, striatum and cortex. They have also reported perivascular cuffing in the hippocampus which is considered as non-tropic region, whereas in the tropic cerebral cortex there was disruption of cortical layer and moderate inflammatory changes.
Local replication of JEV occurs at the site of mosquito bite, which is followed by lymphatic or haematogenous spread18. Replication of JEV beyond the primary site leads to secondary viraemia which can result in CNS involvement. In most patients, primary viraemia is terminated by a macrophage response and subsequently by the development of antibodies. JEV infects the endothelial cells of the capillaries of the brain and crosses the blood brain barrier19. Following intracerebral inoculation, the virus may propagate through intracellular or extracellular spaces to lodge into the different areas of central nervous system depending on their tropism. Ogata et al11 demonstrated age dependent neurotropism in a rat model of JE. They reported that JEV antigen started declining in 14 days old rats and was undetectable in any region in 17 days old rats. In thalamus it was undetectable in 14 days old; striatum and brain stem in 17 days old and cerebral cortex in 14 days old rats11. Several studies have detected latent JEV in the mosquitoes using real-time PCR20 and in the serum of humans, pigs and mice21,22. Studies have been conducted for detection of JEV in human samples by real-time RT-PCR assay23, and for detection of WNV and other flaviviruses of the JEV antigenic complex in different wild bird species24. Differential expression of various receptors in different regions of the brain with reference to age could be responsible for difference in JEV RNA copies in different brain regions; however, this has not been investigated in earlier studies. Using immunohistochemistry in autopsy specimen, the highest concentration of antigen was demonstrated in thalamus and brain stem25. These observations are in agreement with the present study. In a study on 56 JE patients, MRI revealed thalamic involvement in 83 per cent, basal ganglia in 46 per cent, mid brain in 35 per cent and cerebral cortex in 23 per cent12. Similar distributions have also been reported in Eastern equine encephalitis, revealing thalamic and basal ganglia involvement in 71 per cent, brain stem in 43 per cent and cortex in 36 per cent patients26.
In this study, the JEV RNA copies were detected on day 3 post-inoculation, highest concentration recorded on day 6, and thereafter progressively declined and were undetectable on day 20. In another study on 12 days old rats, the distribution of JEV was shown in caudate, putamen, substantia nigra, thalamus and amygdaloid nuclei on day 3 post-inoculation using immunohistochemistry11. We observed similar findings using real-time PCR assay. The decline in JEV RNA copies may be due to associated immune response and neutralizing antibodies. Early host defence against JEV infections is mediated by phagocytic cells, followed by a complex mechanism involving B and T effector cells27,28. Motor deficit was more marked on day 6 post-inoculation compared to day 3 in JEV infected rats; however, no significant improvement was observed over the time. In spite of significant reduction in viral load, lack of significant improvement in motor deficit after day 6 post-inoculation may be due to JEV induced neuronal damage. We have earlier reported free radicals generation by neurons in JE rat model. Overproduction of free radicals was observed in acute phase of disease which leads to neuronal damage and abnormal postural reflexes29. Neuronal damage, inflammation, perivascular cuffing and glial nodule have also been reported in histopathological studies in animal and autopsy studies in humans30. Reduction in JEV RNA copies in later phase of the disease corresponds well with our previous findings showing significant decrease in free radicals generation at the later stage of the disease29. We have earlier reported high affinity of JEV to thalamus, mid brain, striatum and cerebral cortex using immunohistochemical and histological techniques31. Cellular infiltration, perivascular cuffing, meningeal disruption, neuronal damage, neuronal shrinkage, and plaque formation were observed in rats from 10 post-inoculation.
In conclusion, thalamus and mid brain were found to be the most affected brain areas after JEV inoculation, indicating region specific affinity of JEV to the brain.
Acknowledgment
We acknowledge the Indian Council of Medical Research, New Delhi for financial support to Ruchi Srivastava as Senior Research Fellow and Council of Science and Technology, Uttar Pradesh for the project on encephalitis research.
References
- 1.Tsai TF. New initiatives for the control of Japanese encephalitis by vaccination: minutes of a WHO/CVI meeting, Bangkok, Thailand, 13-15 October 1998. Vaccine. 2000;18(Suppl 2):1–25. doi: 10.1016/s0264-410x(00)00037-2. [DOI] [PubMed] [Google Scholar]
- 2.Erlanger TE, Weiss S, Keiser J, Utzinger J, Wiedenmayer K. Past, present, and future of Japanese encephalitis. Emerg Infect Dis. 2009;15:1–7. doi: 10.3201/eid1501.080311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demasio AR, Van Hoesen GW. The limbic system and the localisation of herpes simplex encephalitis. J Neurol Neurosurg Psychiatry. 1985;48:297–301. doi: 10.1136/jnnp.48.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klatzmann D, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, et al. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984;312:767–8. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- 5.Bernhardt G, Bibb JA, Bradley J, Wimmer E. Molecular characterization of the cellular receptor for poliovirus. Virology. 1994;199:105–10. doi: 10.1006/viro.1994.1102. [DOI] [PubMed] [Google Scholar]
- 6.Misra UK, Kalita J. Overview: Japanese encephalitis. Prog Neurobiol. 2010;91:108–20. doi: 10.1016/j.pneurobio.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Hase T, Summers PL, Ray P. Entry and replication of Japanese encephalitis virus in cultured neurogenic cells. J Virol Methods. 1990a;30:205–14. doi: 10.1016/0166-0934(90)90021-7. [DOI] [PubMed] [Google Scholar]
- 8.Kim IB, Chae SL, Choi WY, Park C, Joo YR, Cho HW, et al. In vivo study on the Japanese encephalitis: viral localization and histopathology in the mouse brain. Korean J Anat. 2003;36:427–33. [Google Scholar]
- 9.Mathur A, Bharadwaj M, Kulshreshtha R, Rawat S, Jain A, Chaturvedi UC. Immunopathological study of spleen during Japanese encephalitis virus infection in mice. Br J Exp Pathol. 1988;69:423–32. [PMC free article] [PubMed] [Google Scholar]
- 10.Saxena V, Mathur A, Krishnani N, Dhole TN. Kinetics of cytokine profile during intraperitoneal inoculation of Japanese encephalitis virus in BALB/c mice mode. Microbes Infect. 2008;10:1210–7. doi: 10.1016/j.micinf.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 11.Ogata A, Nagashima K, Hall WW, Ichikawa M, Kimura-Kuroda J, Yasui K. Japanese encephalitis virus neurotropism is dependent on the degree of neuronal maturity. J Virol. 1991;65:880–6. doi: 10.1128/jvi.65.2.880-886.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalita J, Misra UK, Pandey S, Dhole TN. A comparison of clinical and Radiological findings in adults and children with Japanese encephalitis. Arch Neurol. 2003;60:1760–4. doi: 10.1001/archneur.60.12.1760. [DOI] [PubMed] [Google Scholar]
- 13.Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res. 1996;6:986–94. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 14.Toriniwa H, Komiya T. Rapid detection and quantitation of Japanese encephalitis virus by real-time reverse transcription loop-mediated isothermal amplification. Microbiol Immunol. 2006;50:379–87. doi: 10.1111/j.1348-0421.2006.tb03804.x. [DOI] [PubMed] [Google Scholar]
- 15.Enomoto Y, Yoshikawa T, Ihira M, Akimoto S, Miyake F, Usui C, et al. Rapid diagnosis of herpes simplex virus infection by a loop-mediated isothermal amplification method. J Clin Microbiol. 2005;43:951–5. doi: 10.1128/JCM.43.2.951-955.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang KD, Yeh WT, Chen RF, Chuon HL, Tsai HP, Yao CW, et al. A model to study neurotropism and persistency of Japanese encephalitis virus infection in human neuroblastoma cells and leukocytes. J Gen Virol. 2004;85:635–42. doi: 10.1099/vir.0.19426-0. [DOI] [PubMed] [Google Scholar]
- 17.Liu RH, Mizuta M, Matsukura S. The expression and functional role of nicotinic acetylcholine receptors in rat adipocytes. J Pharmacol Exp Ther. 2004;310:52–8. doi: 10.1124/jpet.103.065037. [DOI] [PubMed] [Google Scholar]
- 18.Johnston LJ, Halliday GM, King NJ. Langerhans cells migrate to local lymph nodes following cutaneous infection with an arbovirus. J Invest Dermatol. 2000;114:560–8. doi: 10.1046/j.1523-1747.2000.00904.x. [DOI] [PubMed] [Google Scholar]
- 19.Dropulice B, Masters CL. Entry of neurotropic arboviruses into the central nervous system: an in vitro study using mouse brain endothelium. J Infect Dis. 1990;161:685–91. doi: 10.1093/infdis/161.4.685. [DOI] [PubMed] [Google Scholar]
- 20.Pyke AT, Smith IL, van den Hurk AF, Northill JA, Chuan TF, Westacott AJ, et al. Detection of Australasian Flavivirus encephalitic viruses using rapid fluorogenicTaqMan RT-PCR assays. J Virol Methods. 2004;117:161–7. doi: 10.1016/j.jviromet.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Huang JL, Lin HT, Wang YM, Weng MH, Ji DD, Kuo MD, et al. Sensitive and specific detection of strains of Japanese encephalitis virus using a one-step TaqMan RT-PCR technique. J Med Virol. 2004;74:589–96. doi: 10.1002/jmv.20218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang DK, Kweon CH, Kim BH, Lim SI, Kim SH, Kwon JH, et al. TaqMan reverse transcription polymerase chain reaction for the detection of Japanese encephalitis virus. J Vet Sci. 2004;5:345–51. [PubMed] [Google Scholar]
- 23.Cavrini F, Della Pepa ME, Gaibani P, Pierro AM, Rossini G, Landini MP, et al. A rapid and specific real-time RT-PCR assay to identify Usutu virus in human plasma, serum, and cerebrospinal fluid. J Clin Virol. 2011;50:221–3. doi: 10.1016/j.jcv.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 24.García-Bocanegra I, Busquets N, Napp S, Alba A, Zorrilla I, Villalba R, et al. Serosurvey of West Nile virus and other flaviviruses of the Japanese encephalitis antigenic complex in birds from Andalusia, southern Spain. Vector Borne Zoonotic Dis. 2011;11:1107–13. doi: 10.1089/vbz.2009.0237. [DOI] [PubMed] [Google Scholar]
- 25.Johnson RT, Burke DS, Elwell M, Leake CJ, Nisalak A, Hoke CH, et al. Japanese encephalitis immunocytochemical studies of viral antigen and inflammatory cells in fatal cases. Ann Neurol. 1985;18:567–73. doi: 10.1002/ana.410180510. [DOI] [PubMed] [Google Scholar]
- 26.Deresiewicz RL, Thaler SJ, Hsu L, Zamani AA. Clinical and neuroradiographic manifestations of eastern equine encephalitis. N Engl J Med. 1997;336:1867–74. doi: 10.1056/NEJM199706263362604. [DOI] [PubMed] [Google Scholar]
- 27.Mathur A, Arora KL, Chaturvedi UC. Host defense mechanisms against Japanese encephalitis virus infection in mice. J Gen Virol. 1983;64:805–11. doi: 10.1099/0022-1317-64-4-805. [DOI] [PubMed] [Google Scholar]
- 28.Srivastava S, Khanna N, Saxena SK, Singh A, Mathur A, Dhole TN. Degradation of Japanese encephalitis virus by neutrophils. Int J Exp Pathol. 1999;80:17–24. doi: 10.1046/j.1365-2613.1999.00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srivastava R, Kalita J, Khan MY, Misra UK. Free radical generation by neurons in rat model of Japanese encephalitis. Neurochem Res. 2009;34:2141–6. doi: 10.1007/s11064-009-0008-7. [DOI] [PubMed] [Google Scholar]
- 30.German AC, Myint KS, Mai NT, Pomeroy I, Phu NH, Tzartos J, et al. A preliminary neuropathological study of Japanese encephalitis in humans and a mouse model. Trans R Soc Trop Med Hyg. 2006;100:1135–45. doi: 10.1016/j.trstmh.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Kumar S, Kalita J, Saxena V, Khan MY, Khanna VK, Sharma S, et al. Some observations on the tropism of Japanese encephalitis virus in rat brain. Brain Res. 2009;1268:135–41. doi: 10.1016/j.brainres.2009.02.051. [DOI] [PubMed] [Google Scholar]


