Abstract
Background & objectives:
Bagaza virus (BAGV), a flavivirus synonymous with Israel turkey meningoencephalitis virus, has been found to circulate in India. BAGV has recently been held responsible for inducing febrile illness in humans and causing unusually high mortality to wild birds in Spain. A study was therefore, undertaken to determine its replication kinetics in certain mosquitoes and to determine vector competence and potential of the mosquitoes to transmit BAGV experimentally.
Methods:
Aedes aegypti, Culex tritaeniorhynchus and Cx quinquefasciatus mosquitoes were inoculated with BAGV; samples were harvested every day and titrated in BHK-21 cell line. Vector competence and experimental transmission were determined by examining the saliva of infected mosquitoes for virus and induction of sickness in suckling mice, respectively.
Results:
Cx. tritaeniorhynchus and Ae. aegypti mosquitoes yielded 5 log10 and 4.67 log10 TCID50/ml of virus on day 3 post-infection (PI), respectively while Cx. quinquefasciatus yielded a titre of 4 log10 TCID50/ml on day 4 PI. BAGV was detected in saliva of all the infected mosquitoes demonstrating their vector competence. Experimental transmission of BAGV to infant mice as well as transovarial transmission was demonstrated by Cx. tritaeniorhynchus but not by Ae. aegypti and Cx. quinquefasciatus mosquitoes.
Interpretation & conclusions:
Replication of BAGV to high titres and dissemination to saliva in three most prevalent mosquitoes in India is of immense public health importance. Though no major outbreak involving man has been reported yet, BAGV has a potential to cause outbreaks in future.
Keywords: Bagaza virus, Culex tritaeniorhynchus, experimental transmission, transovarial transmission
Bagaza virus (BAGV), an arbovirus belonging to Flaviviridae family has been found to circulate in India as evidenced by the presence of antibodies in human serum and virus isolation from mosquitoes1. BAGV, first isolated from Culex mosquitoes in Bagaza district of Central African Republic during virological investigations in 19662, has been isolated repeatedly from Culicine mosquitoes from West African countries3,4,5. However, no outbreak involving humans or domestic animals due to the virus has been reported from these places. Characterization of the virus demonstrated BAGV as synonymous with Israel turkey meningoencephalitis virus, a pathogen of economic importance in Israel and South Africa6. Recently, BAGV has been held responsible for unusually high mortality of wild birds (Partridges and pheasants) in Spain indicating its potential to cause death in birds7. In India, the virus was isolated from Culex tritaeniorhynchus mosquitoes during investigation of an outbreak of Japanese encephalitis virus (JEV) in Kerala in 19961. Subsequent serological investigations demonstrated the presence of BAGV antibodies in human serum (15%) demonstrating its potential to cause subclinical infection in humans. A preliminary study was, therefore, initiated to determine the replication kinetics of the virus in certain widely prevalent mosquitoes as well as to study their potential to transmit the virus to laboratory mice, and to next generation through the ovary (transovarial transmission).
Material & Methods
The study was carried out at the Microbial Containment Complex, National Institute of Virology (NIV), Pune, India. The original isolate of BAGV (BAG96363), isolated from a pool of Cx. tritaeniorhynchus mosquitoes in 1996 was used in the study1. The isolate has undergone six passages in Baby hamster kidney (BHK-21) cell line before the commencement of the study. Virus stock was prepared in BHK-21 cell line, titrated, aliquoted and used for different experiments. BHK-21 cell line was maintained in minimum essential medium (MEM) supplemented with 10 per cent foetal bovine serum (FBS) and was passaged at every 3-4 days. Both MEM and FBS were procured from Invitrogen, USA.
Cx. tritaeniorhynchus, Cx. quinquefasciatus and Aedes aegypti mosquitoes were procured from the NIV insectary maintained at 28±2°C with 80±5 per cent relative humidity and 12:12 h light:dark cycle. Mosquito larvae were fed on a mixture of yeast powder and dog biscuit (3:1 w/w) while adults were maintained on a diet of 10 per cent glucose. For egg laying, female mosquitoes were provided 5-6 wk old fowls for blood meal on alternate days.
Growth kinetics of BAGV in mosquitoes: Growth kinetic study of BAGV in mosquitoes was carried out in 3-4 day old female mosquitoes which were maintained on 10 per cent glucose. The mosquitoes were immobilized by keeping them in ice for 5-10 min and inoculated intrathoracically (IT) with 100 plaque forming units (pfu) of BAGV inside a BSL-2 laboratory as described by Rosen and Gubler8. The inoculated mosquitoes were secured in plastic mosquito holding jars, allowed to revive at room temperature and transferred to double walled mosquito cages and incubated at 28±2°C with 70-80 per cent humidity. Five mosquitoes were harvested daily from 1st to 14th day post-infection (PI) and stored at -80°C. After completion of the experiment, mosquitoes of each day PI (n=5) were triturated in 1 ml MEM containing 2 per cent FBS using chilled mortar and pestle. The mosquito suspension was centrifuged; Millipore filtered (pore size=0.22 μm), diluted serially (ten-fold) and titrated in BHK-21 cells in quadruplicate. The cultures were observed daily, readings (cells with cytopathic effects) were scored, stained with amido black and virus titre of each day PI was determined as described by Reed and Muench9. The experiment was carried out in duplicate for each species.
Experimental transmission of BAGV in Cx. tritaeniorhynchus mosquitoes: Mosquitoes were infected IT with BAGV and saliva from infected mosquitoes (n=10) was extracted every day PI artificially as described by van den Hurk et al10 with slight modifications. In brief, the proboscis of infected mosquitoes was placed in a capillary tube containing MEM supplemented with 2 per cent FBS for 30 min. The contents of the capillary tube was transferred to 1ml chilled MEM; Millipore filtered (0.22 μm) and titrated in BHK-21 cell line, and the presence of virus in saliva was determined as described above.
BAGV inoculated (IT), seven day old mosquitoes (n=50 each) were allowed to feed on 2-day old Swiss albino mice (n=8 per group) overnight for Culex and during day time for Aedes mosquitoes and observed for the development of sickness. After feeding, the mosquitoes were frozen immediately and titrated in BHK-21 cell line to determine virus titre. Brains of inoculated mice were harvested and titrated in BHK-21 cell line irrespective of whether the mice showed sickness or not after 96 h PI. Animal experiments were conducted after obtaining approval from the Institutional Animal Ethics Committee.
Transovarial transmission (TOT) of BAGV in mosquitoes: BAGV infected seven day old mosquitoes (IT inoculated, n=50) were fed on two day old infant mice (Swiss albino) allowed to lay eggs and hatch in the laboratory. The mosquitoes after oviposition were stored and 20 mosquitoes of each species were titrated to determine the virus titre. A portion of eggs (~400-500 in no.) and adults (n=100) were titrated in BHK-21 cell line to determine the presence of BAGV in eggs and adults of F1 generation (first generation, i.e. the eggs and adults developed from the adults subsequent to the experiment).
Results & Discussion
BAGV, a flavivirus in the family Flaviviridae has gained importance recently due to its ability to cause febrile illness in humans and high mortality in birds7,11. The virus has been identified as an emerging and re-emerging pathogen with a potential to cause infections in humans11. Presence of BAGV antibodies in human serum collected from Kerala, India in 1996 demonstrated the potential of the virus to cause subclinical infection in man1. The virus has been isolated from Cx. univittatus, Cx. thalassius, Cx. perfuscus, Cx. guiarti, Cx. ingrami in Africa and from Cx. tritaeniorhynchus in India demonstrating its potential1,3,12.
BAGV replication kinetics in mosquitoes: Growth kinetics of BAGV in the three mosquito species was found almost identical except for the initial growth phase. Though all mosquitoes were inoculated with the same concentration of virus, virus titre obtained in the three mosquito species at 24 h PI varied. In Cx. tritaeniorhynchus, BAGV growth kinetics was peculiar as a sharp increase in virus titre was observed from day 1 to day 3 PI without having a lag phase. The maximum virus yield was 5 log10 TCID50/ml obtained on days 3 and 4 PI. Titre decreased to 4 log10 (tissue culture infectivity dose 50%) TCID50/ml on day 5 PI but the same titre was maintained for 14 days PI (Figure). A second cycle of virus replication was observed from day 12 PI showing a logarithmic increase in virus titre on subsequent days. However, further results are not available as the study was terminated on day 14 PI in both the replicates.
Fig.
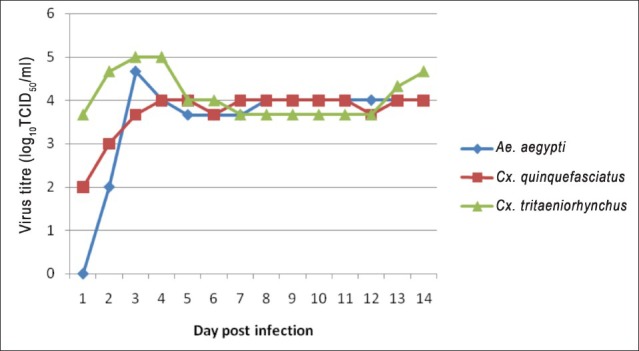
Replication kinetics of BAGV in Ae aegypti, Cx. tritaeniorhynchus and Cx. quinquefasciatus mosquitoes.
Cx quinquefasciatus also replicated BAGV and maintained the virus at a substantially high titre (~4log10) for 14 days. Virus replication could not be observed in the mosquito during the first 24 h PI, but commencement of cytopathic effect (CPE) was observed at 48 h PI. Virus titre increased gradually, reached a peak (4 log10 TCID50/ml) on day 4 PI and maintained the same titre throughout the period of study (Figure).
In Ae. aegypti, the replication kinetics of BAGV differed from the Culex mosquitoes. Virus could not be detected at 24 h; however, a sharp increase in virus titre was detected on subsequent days PI. A maximum titre of 4.7 log10 TCID50/ml was detected on day 3 PI which decreased gradually to 4 log10 TCID50/ml on day 5. The mosquitoes maintained the titre for rest of the study period (Figure).
BAGV transmission potential of different mosquitoes to infant mice: Extraction of saliva from mosquitoes was standardized during the study. Saliva in the form of a small bubble could be seen under a binocular microscope after exerting gentle pressure on the thorax. However, the proboscis was inserted in a sterile capillary tube containing MEM supplemented with 2 per cent FBS for 30 min for extracting saliva. Presence of virus in saliva is indicative of the potential of mosquitoes to transmit virus to susceptible hosts. Both Ae. aegypti and Cx. tritaeniorhynchus mosquito saliva had high virus content (approx 2log10TCID50/ml) on day 5 PI while a lower level was detected in the saliva of Cx. quinquefasciatus (<2 log10TCID50/ml). BAGV infected Cx. tritaeniorhynchus mosquitoes (day 7 PI) induced sickness in two day old infant mice. Acute sickness in mice was observed on day 4 of post feeding and the brains had a titre of 4 log10 TCID50/ml. However, BAGV infected Cx. quinquefasciatus and Ae. aegypti mosquitoes did not induce sickness in mice.
Transovarial transmission (TOT) of BAGV in Cx. tritaeniorhynchus, Cx. quinquefasciatus and Ae. aegypti mosquitoes: BAGV was detected in F1 generation eggs and adults of Cx. tritaeniorhynchus mosquitoes with 2 log10 and 4 log10 TCID50/ml virus titres, respectively. The parent female mosquitoes (F0) had a titre of 4.3 log10 TCID50/ml after oviposition. F1 generation adult mosquitoes induced sickness in mice and the mice brain had a titre of approximately 4 log10 TCID50/ml demonstrating TOT by the mosquito. TOT could not be demonstrated by either Cx. quinquefasciatus or Ae. aegypti mosquitoes. The parent females in both the species had a titre of about 4 log10 TCID50/ml at the time of oviposition. However, virus could not be detected either in the eggs or in adults of F1 generation of both the mosquitoes (Table).
Table.
Presence of BAGV in different stages of mosquitoes in F1 generation
In the present study, the three mosquito species not only replicated BAGV yielding high virus titre but also disseminated the virus to salivary glands and saliva. The virus titre was found to be maintained in all the mosquitoes at higher levels throughout the study period demonstrating their potential to transmit the virus for a substantially longer period of time. Cx. tritaeniorhynchus mosquito, the universal vector of JEV, from which BAGV was isolated in India not only replicated the virus to very high titres but also demonstrated TOT and experimental transmission to infant mice. The mosquito is one of the predominant mosquitoes in India and might be involved in causing an outbreak of BAGV in future13.
BAGV was replicated not only by the two Culex species, but also by Ae. aegypti. BAGV has not been isolated from any Aedes species so far. In this study Ae. aegypti replicated the virus to levels comparable to that of the two Culex mosquitoes. Saliva of infected mosquitoes also showed presence of BAGV from day 5 PI onwards. However, the mosquitoes failed to transmit the virus to infant mice experimentally probably due to the low titre of virus in saliva. TOT also could not be demonstrated by the mosquitoes as virus could not be detected either in eggs or adults of the F1 generation.
The ability to replicate BAGV by Ae aegypti mosquitoes is an important observation as BAGV has not been isolated from the mosquito so far. Though we could not demonstrate experimental transmission, any genetic change/mutation in future may make the mosquitoes a potent vector. It is also noted that these mosquitoes are highly anthropophilic and found in close proximity to humans14. It is well documented that Ae. aegypti is the principal vector for both dengue and chikungunya in India and both the diseases have been reported from almost every part of the subcontinent15. C6/36 cell line, a clonal isolate of Ae albopictus mosquito cell line16 was found to replicate the virus yielding very high titre in comparison to the mosquitoes and vertebrate cell lines viz. BHK-21, VERO CCL81, etc. C6 cell yielded 7log10 TCID50/ml at 108 h PI in comparison to 6 log10 and 5 log10TCID50/ml in BHK-21 and Vero CCL81, respectively (Sudeep unpublished data).
The data presented here were part of a study to understand the role of BAGV in JEV epidemiology in India since both the viruses belong to the same family and are transmitted by the same vector. It was, therefore, felt necessary to understand whether prior infection of the vector mosquitoes with BAGV reduced/inhibited JEV replication. However, looking at the present status of BAGV, it is clear that BAGV has its own potential to cause infection in man and animals. The ability of the virus to replicate in different mosquito species, its potential to cause infection in man and mortality in birds make BAGV an important emerging/re-emerging virus of public health importance.
Acknowledgment
The authors thank Dr A.C. Mishra, Former Director, National Institute of Virology, Pune, for support.
References
- 1.Bondre VP, Sapkal GN, Yergolkar PN, Fulmali PV, Sankararaman V, Ayachit VM, et al. Genetic characterization of Bagaza virus (BAGV) isolated in India and evidence of anti-BAGV antibodies in sera collected from encephalitis patients. J Gen Virol. 2009;90:2644–9. doi: 10.1099/vir.0.012336-0. [DOI] [PubMed] [Google Scholar]
- 2.Digoutte JP. Bagaza (BAG) strain: Dak Ar B 209. Am J Trop Med Hyg. 1978;27:376–7. [Google Scholar]
- 3.Gordon SW, Tammariello RF, Linthicum KJ, Dohm DJ, Digoutte JP, Calvo-Wilson MA. Arbovirus isolations from mosquitoes collected during 1988 in the Senegal river basin. Am J Trop Med Hyg. 1992;47:742–8. doi: 10.4269/ajtmh.1992.47.742. [DOI] [PubMed] [Google Scholar]
- 4.Traore-Lamizana M, Zeller HG, Mondo M, Hervy JP, Adam F, DiGoutte JP. Isolations of West Nile and Bagaza viruses from mosquitoes (Diptera: Culicidae) in Central Senegal (Ferlo) J Med Entomol. 1994;31:934–8. doi: 10.1093/jmedent/31.6.934. [DOI] [PubMed] [Google Scholar]
- 5.Diallo M, Nabeth P, Ba K, Sall AA, Ba Y, Mondo M, et al. Mosquito vectors of the 1998-1999 outbreak of Rift Valley Fever and other arboviruses (Bagaza, Sanar, Wesselsbron and West Nile) in Mauritania and Senegal. Med Vet Entomol. 2005;19:119–26. doi: 10.1111/j.0269-283X.2005.00564.x. [DOI] [PubMed] [Google Scholar]
- 6.Kuno G, Chang GJ. Full-length sequencing and genomic characterization of Bagaza, Kedougou, and Zika viruses. Arch Virol. 2007;152:687–96. doi: 10.1007/s00705-006-0903-z. [DOI] [PubMed] [Google Scholar]
- 7.Agüero M, Fernández-Pinero J, Buitrago D, Sánchez A, Elizalde M, San Miguel E, et al. Bagaza virus in partridges and pheasants, Spain, 2010. Emerg Infect Dis. 2011;17:1498–501. doi: 10.3201/eid1708.110077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosen L, Gubler D. The use of mosquitoes to detect and propagate dengue viruses. Am J Trop Med Hyg. 1974;23:1153–60. doi: 10.4269/ajtmh.1974.23.1153. [DOI] [PubMed] [Google Scholar]
- 9.Reed LJ, Muench H. A simple method for estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–7. [Google Scholar]
- 10.van den Hurk AF, McElroy K, Pyke AT, McGee CE, Hall-Mendelin S, Day A, et al. Vector competence of Australian mosquitoes for yellow fever virus. Am J Trop Med Hyg. 2011;85:446–51. doi: 10.4269/ajtmh.2011.11-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woolhouse M, Gowtage-Sequeria S, Evans B. T16: quantitative analysis of the characteristics of emerging and re-emerging human pathogens. [accessed on February 20, 2012]. Available from: www.foresight.gov.uk .
- 12.Dakar. Senegal: Institut Pasteur; 1988. Institut Pasteur. Rapport Annuel 1988. [Google Scholar]
- 13.Kanojia PC, Paingankar MS, Patil AA, Gokhale MD, Deobagkar DN. Morphometric and allozyme variation in Culex tritaeniorhynchus mosquito populations from India. J Insect Sci. 2010;10:138. doi: 10.1673/031.010.13801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahadev PV. A case study of Aedes aegypti prevalence by settlement types in Dehu town group of Maharashtra State. Indian J Med Res. 1983;78:537–46. [PubMed] [Google Scholar]
- 15.Manimunda SP, Sugunan AP, Rai SK, Vijayachari P, Shriram AN, Sharma S, et al. Outbreak of chikungunya fever, Dakshina Kannada District, South India, 2008. Am J Trop Med Hyg. 2010;83:751–4. doi: 10.4269/ajtmh.2010.09-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Igarashi A. Isolation of Singh's Aedes albopictus cell clone sensitive to dengue and Chikungunya viruses. J Gen Virol. 1978;40:531–44. doi: 10.1099/0022-1317-40-3-531. [DOI] [PubMed] [Google Scholar]


