Abstract
Background:
Prevention of post-spinal hypotension in obstetric patients can be accomplished using intravenous fluid expansion and prophylactic use of sympathomimetic drugs. The affect of combination of colloids and phenylephrine infusion on maternal hemodynamics has not been widely studied and there is no consensus about the dosage required and time of starting its administration.
Materials and Methods:
This prospective, randomized, double-blind study enrolled 90 healthy term parturients undergoing elective Cesarean delivery under lumbar subarachnoid block (0.5% hyperbaric bupivacaine 10 mg with fentanyl 25 μg). Patients in Group A received prophylactic intravenous phenylephrine infusion (60 μg/minute) along with hydroxyl-ethyl-starch cohydration (6% HES 130/0.42;15 ml/kg) immediately after subarachnoid block. In Group B, patients received 6% HES cohydration and intermittent intravenous 50 μg boluses of phenylephrine. The efficacy of these in maintaining maternal SBP at 90-110% of baseline and neonatal well-being was evaluated.
Results:
In Group B, 75.5% of patients required rescue phenylephrine boluses to maintain SBP while maternal hemodynamics were well maintained in Group A and rescue drug was not needed. Reactive hypertension occurred in one patient (2.2%) and bradycardia in two patients (4.4%) in Group A. Six patients complained of nausea in Group B (13.3%) compared to one in Group A. All the newborns had normal Apgar scores and Umbilical arterial pH > 7.2.
Conclusion:
A combination of colloid cohydration and prophylactic phenylephrine infusion initiated at 60 μg/minute maintained maternal hemodynamics and neonatal well-being during Cesarean deliveries requiring minimum interventions by the anesthesiologist.
Keywords: Cesarean delivery, colloid cohydration, phenylephrine infusion, post-spinal hypotension
Introduction
Spinal anesthesia is preferred for Cesarean deliveries because it allows the mother to remain awake, produces a rapid onset of dense neural blockade with minimum amount of intrathecal drug, minimizes problems associated with airway management and avoids possible neonatal depression from drugs used in general anesthesia.[1,2,3] However, these advantages are offset by post-spinal hypotension reported in 50-90% of the parturients, if appropriate preventive measures are not undertaken.[4,5,6] Pregnant females have increased sensitivity to local anesthetics and are more susceptible to the effects of sympathetic blockade. Persistent hypotension can lead to nausea, emesis, dizziness, light headedness, reduced cardiac output, decreased utero-placental blood flow, and impaired fetal oxygenation.[1,5]
A variety of methods have been used to prevent spinal hypotension. These include physical maneuvers (compression stockings, left uterine displacement), intravenous fluid expansion, and prophylactic use of sympathomimetic drugs.[6,7,8] Among the intravenous fluids both crystalloids and colloids are not very effective in preventing post-spinal hypotension when used as a single modality.[9,10] Hence, vasopressors like ephedrine, phenylephrine, methoxamine, dopamine, mephentermine have been used in parturients to maintain hemodynamics after spinal anesthesia.[8,11] Out of these phenylephrine has been found to be the most effective with least side effects.[10,12] Crystalloid loading at the time of spinal injection (co-loading) is known to enhance the hemodynamic control provided by the vasopressors.[13] However, the effect of combination of colloids and phenylephrine infusion on maternal hemodynamics in women undergoing cesarean section has not been widely studied and there is no consensus about the dosage required and when should the administration be started. Hence the present study was planned to evaluate safety and efficacy of a combination of 6% HES (130/0.4) and prophylactic variable rate phenylephrine infusion versus phenylephrine boluses on incidence of maternal hypotension and neonatal outcome. The initial bolus dose of phenylephrine was given to all the participants because it helps to quickly achieve the desirable vasopressor effect and subsequent infusion of phenylephrine facilitates a sustained action of the vasopressor agent used. It was hypothesized that the use of this combination of HES and prophylactic phenylephrine infusion will keep a tight control on maternal hemodynamics and alleviate the need for subsequent intraoperative interventions to manage post-spinal hypotension without compromising on the neonatal well-being.
Materials and Methods
Institutional ethical committee approval was taken and all the participants gave written informed consent for this prospective, randomized, double-blind study. Ninety two, ASA I - II females, aged 18-40 years, with singleton pregnancy, gestational age > 36 weeks and planned for elective lower segment Cesarean section under spinal anesthesia were enrolled. A detailed history regarding physical health, co-existing medical problems, current medications, allergies, previous anesthetic and surgical exposure was taken. Exclusion criteria were patients with extremes of weight (<40 kg or >90 kg) and height (<140 cms or >180 cms), chronic or pregnancy-induced hypertension, associated cardiovascular/cerebral/pulmonary/endocrine disorders, fetal or placental abnormalities, multiple gestations, or any contraindications to spinal anesthesia. Ranitidine (150 mg) and metoclopromide (10 mg) were prescribed per orally a night before and on the morning of surgery. All patients fasted for at least 8 hours prior to surgery. Using computer-generated permuted block randomization, patients were divided into two groups, A and B (n = 45 each) to receive colloid cohydration, along with phenylephrine infusion or phenylephrine bolus. Monitoring consisted of heart rate (HR), arterial oxygen saturation (SpO2), electrocardiography (ECG), and non-invasive blood pressure (NIBP) using a multichannel monitor (Cardiocap, Datex Ohmeda). Heart rate and NIBP readings (left upper arm) were recorded every 2 minutes until they became consistent (three successive measurements of SBP and HR with a difference of not more than 10%). The mean of these three readings was considered baseline maternal SBP and heart rate. Hypotension was defined as SBP < 90% of baseline reading and hypertension was defined as SBP >110% of baseline. A wide bore 16-G intravenous access was secured for HES (130/0.4) infusion. Another 20-G IV cannula was inserted for the study drug infusion. No IV prehydration was given (except at a minimal rate to maintain vein patency). Lumbar subarachnoid block (SAB) was performed in the lateral position with 26-G Quincke's spinal needle and 0.5% hyperbaric bupivacaine 10 mg with fentanyl 25 μg was injected intrathecally. Patients were immediately turned supine with left uterine displacement. Oxygen supplementation was started with Venturi mask (FiO2 0.4). Adequacy of spinal anesthesia was assessed using Bromage scale for motor response and blunt pin prick method for sensory block. Assessment was done every minute for first 10 minutes and every 10 minutes thereafter. Once a sensory block of T4 was achieved surgery was started.
Group A (n = 45) patients received 6% HES (130/0.4); 15 ml/kg and 50 μg intravenous bolus of phenylephrine soon after initiation of spinal anesthesia. This was followed by intravenous infusion of phenylephrine hydrochloride (60 μg/min), initiated @ 60 ml/hr using a syringe pump (Graseby 3500 Anesthesia pump, Graseby Medical Ltd., Watford, Herts, UK). The study phenylephrine solution was prepared in dilutions of 60:1 and diluted to a volume of 20 ml by an anesthesiologist who was not involved in the study. In order to maintain maternal BP at near-baseline levels, a predefined algorithm was used to adjust the infusion rate:[14]
Rate of infusion was maintained at 60 ml/hr, if BP remained within 90-110% of baseline
Rate of infusion was halved (30 ml/hr) if BP increased to 110-120% of baseline
Rescue boluses of 50 μg phenylephrine IV was given if BP decreased to <90% of baseline and repeated every 2 minutes
Infusion was stopped if BP increased to >120% of baseline.
Patients in Group B (n = 45) received 6% HES 130/0.4; 15 ml/kg and 50-μg IV bolus of phenylephrine soon after initiation of spinal anesthesia. This was followed by intermittent boluses of phenylephrine (50-μg IV) to maintain HR and SBP within 10% of the baseline values. In this group placebo infusion of normal saline was initiated at the rate of 60 ml/hr soon after the intrathecal drug administration.
The infusion was discontinued following delivery of the fetus in all the participants. Time of injection of spinal anesthetic, skin incision time, uterine incision time, and uterine incision-delivery time were noted. HES co-loading time, total volume of HES infused and total volume of intravenous fluids administered intraoperatively were noted in all the patients. HR, ECG, SpO2, and end-tidal carbon dioxide (EtCO2) were monitored continuously and NIBP was measured at 1-minute interval till the baby was delivered and at 5-minute interval thereafter till the mother was shifted to the post-operative room. Total volume of study drug infusion along with total number of phenylephrine boluses required were recorded. Rescue medication administered for bradycardia (HR < 50 bpm) associated with SBP less than the baseline value consisted of injection atropine 0.6 mg intravenously. The anesthesiologist involved in the conduct of anesthesia estimated intraoperative blood loss and need for blood transfusion in all the patients.
Patients were evaluated post-operatively for hemodynamic stability (BP and HR + 10% of baseline), regression of spinal anesthesia, pain relief, and post-operative nausea/vomiting. Any episode of nausea or vomiting was recorded and rated using a four point scale: (0 = none, 1 = mild nausea, 2 = nausea requiring treatment, 3 = vomiting). Nausea or vomiting with a score of 2 or 3 was treated with IV metoclopramide if unrelated to hypotension.
Assessment of the neonate included birth weight, umbilical arterial, and venous blood samples from a double-clamped segment of cord for immediate measurement of blood gases using Bayer Rapid LAB 855 gas analyzer (A pH of less than 7.20 was defined as fetal acidosis) and APGAR scores at 1 and 5 minutes after delivery were noted. The primary end point of the study was incidence of spinal hypotension in the two groups. Secondary end points were; need for rescue phenylephrine boluses, maternal side effects (bradycardia, nausea and vomiting, allergic episodes) and neonatal acidosis (pH < 7.2) or low APGAR scores.
The demographic data and other quantitative variables were compared using independent t-test. Chi-square test was used to compare the descriptive data. Variables like heart rate, blood pressure were analyzed using independent t-test. The occurrence of postoperative emetic episodes, rescue antiemetic therapy and rescue analgesic therapy were analyzed with the Chi-square test. Statistical significance was defined as P value (two tailed) ≤0.05. Quantitative data is reported and graphically represented as mean ± SD. Categorical data is shown as number (%).
Statistical power was calculated on the following assumptions: Post-spinal hypotension can occur in 55-90% of the parturients during Cesarean section, if no preventive measures are taken. In a prospective study conducted by Ngan kee et al.[13] a combination of phenylephrine infusion with crystalloid cohydration could reduce the incidence of hypotension to 2%. Thus, assuming an incidence of 2% after treatment with phenylephrine infusion with crystalloid cohydration, with the intention to achieve a decline in the rate of hypotension by 20% (i.e., from 2 to 1.6%) in patients treated with 6% HES cohydration and phenylephrine infusion, 45 patients per group were needed to reach statistical significance, with 80% power and 5% type 1 error (confidence interval 95%).
Results
Ninety-two patients were assessed and found to be eligible for the study. However, two patients were excluded as they underwent emergency LSCS for fetal bradycardia. Remaining 90 parturients were randomized into Group A and B (n = 45 each) [Figure 1]. The demographic, laboratory, and obstetrics characteristics were comparable among the groups. The time for adequate subarachnoid block, uterine incision time, time of delivery of baby and estimated blood loss were all comparable in the two groups. Subarachnoid block with 2 ml of 0.5% hyperbaric bupivacaine and 25 μg fentanyl provided optimum conditions for surgery in all the patients. All patients were co-loaded with 6% HES (130/0.4); 15 ml/kg after the initiation of spinal anesthesia. There was no difference in the total amount of intravenous fluids administered in the two groups [Table 1].
Figure 1.
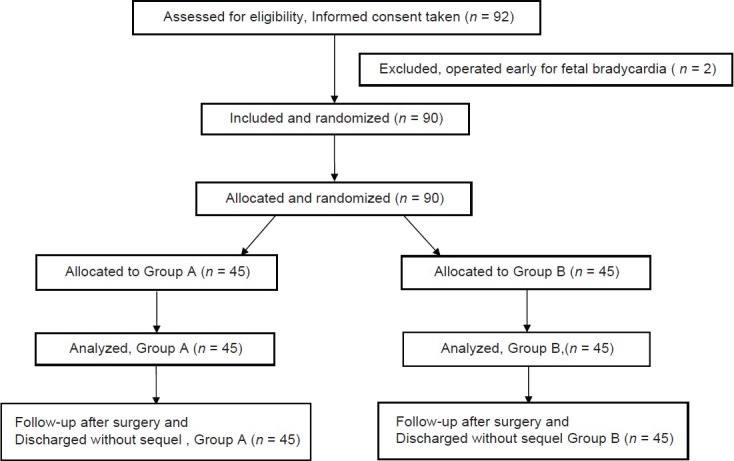
Consort diagram of patients’ recruitment for the study
Table 1.
Demographic profile, peri-operative, obstetric, and neonatal data
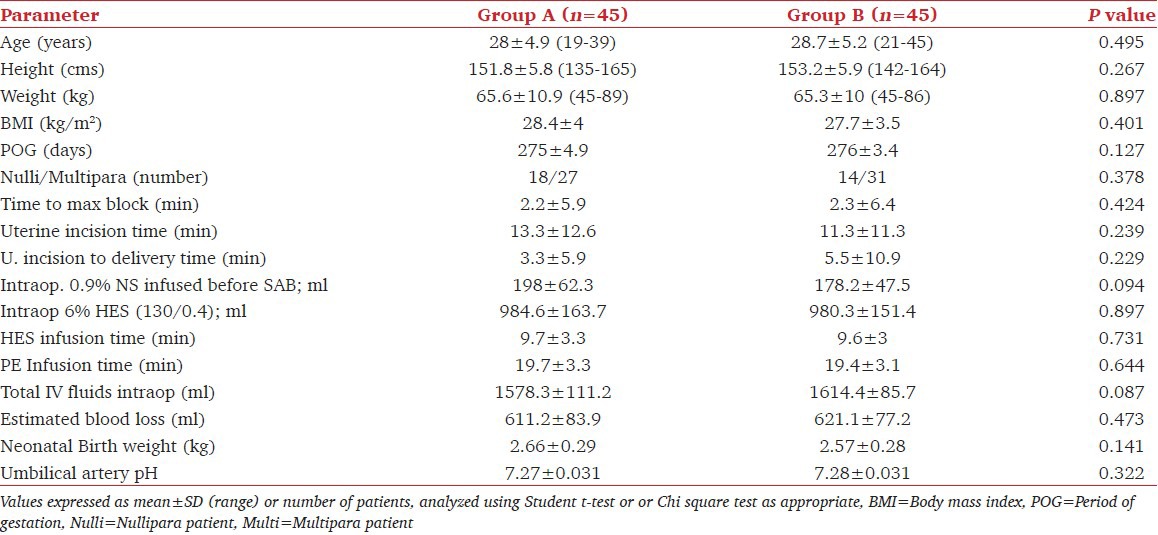
Patients in group A showed a significantly lower heart rate as well as systolic blood pressure from 3 to 12 min after subarachnoid block (P < 0.001) when compared to patients in group B [Figure 2]. Thirty-four out of 45 patients in Group B (75.5%) showed an SBP < 10% of baseline, compared to none in group A. The total number of hypotensive episodes observed was 95 in group B and the frequency of hypotensive events varied from 1 to 6 episodes [Figure 3]. The need for rescue phenylephrine boluses was significantly more in Group B (P = 0.001) and none of the patients who were receiving continuous phenylephrine infusion (Group A) required rescue bolus of the vasopressor agent for hemodynamic stability [Figure 4].
Figure 2.
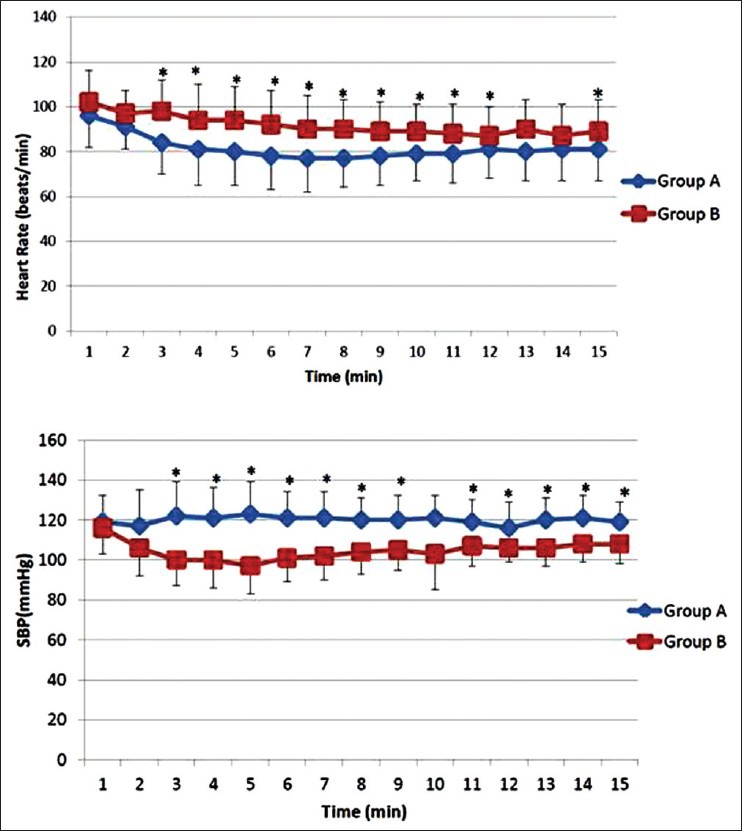
Comparison of mean heart rate and systolic blood pressure between the two groups from the onset of spinal anesthesia
Figure 3.
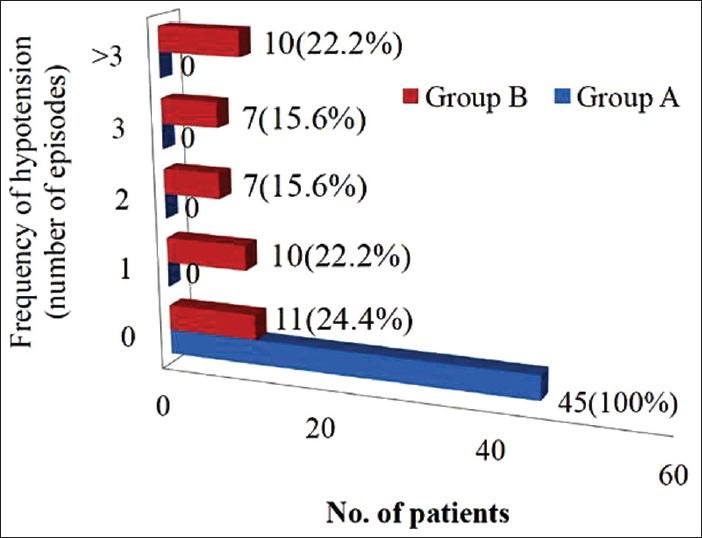
Horizontal bar diagram showing frequency of spinal induced hypotension (SBP < 10% of baseline) in two groups. Values are expressed as number of patients and percentage
Figure 4.
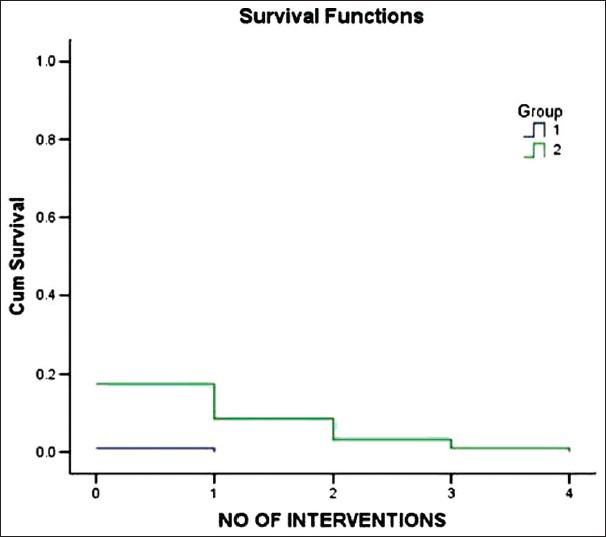
Kaplan Meier survival curve showing the proportion of patients who received intervention in the form of phenylephrine boluses to maintain SBP within 10% of baseline was greater in group B (2) compared to group A (1)
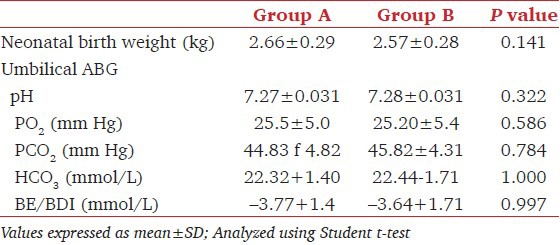
Reactive hypertension defined as SBP > 10% from the baseline values was recorded in one patient in Group A (2.2%) in whom phenylephrine infusion rate was reduced to 30 μg/min and thereafter, SBP was maintained within the required limits with this dose of the drug till the delivery of the baby. Bradycardia was observed in two patients (4.4%) in Group A. This was managed by administering injection atropine 0.6 mg intravenously and the phenylephrine infusion flow was reduced to 30 μg/minute. No further interventions were required in these two patients till the delivery of baby. Six patients in Group B (13.3%) complained of mild nausea compared to one patient in Group A. Emetic episodes did not occur in any of the patients [Table 2]. None of the babies born had evidence of fetal acidosis (Umbilical arterial pH < 7.2) [Table 3]. Apgar scores at 1 and 5 minutes were >7 in all the neonates.
Table 2.
Peri-operative side effects
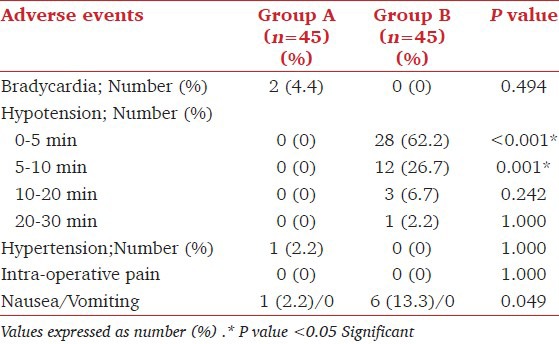
Table 3.
Neonatal outcome
Discussion
The results of the present study showed that patients receiving HES cohydration with phenylephrine infusion did not show any incidence of hypotension compared to an incidence of 75.5% in patients administered HES cohydration and phenylephrine boluses. The need for rescue boluses of phenylephrine was also more in patients in group B compared to group A.
Many authors have shown that uteroplacental perfusion is better maintained when maternal hemodynamics are stabilized close to baseline values.[8,15,16] The present study therefore aimed to maintain SBP within ±10% of baseline by administering colloids and the vasopressor drug soon after the injection of spinal anesthetic. It was hypothesized that the use of this prophylactic strategy will establish a tight control on maternal hemodynamics and alleviate the need for subsequent intraoperative interventions to manage post-spinal hypotension.
A well-balanced volume therapy is known to augment intravascular volume, maintain stable hemodynamics and improve microcirculatory organ perfusion.[17] Thus, different fluid regimens have been tried by many authors to minimize spinal induced hypotension in obstetric patients.[6,8,10,11] Crystalloids alone were found to have inconsistent effect on blood pressure.[9,18] Large volume of crystalloid solution increases venous return, preserves central blood volume, and cardiac output.[8] However, this increase in blood volume is not sustained due to rapid redistribution. It leads to hemodilution and can adversely affect pulmonary functions.[19] Furthermore, return of sympathetic vascular tone before bladder sensation can lead to over distension of urinary bladder while the patient is completely unaware.[6] Better results have been obtained after colloids administration as they stay longer in the vascular compartment, markedly suppress neutrophil influx by decreasing pulmonary capillary permeability and facilitates tissue oxygenation by improving microcirculation.[6,7,19] Karinen et al.,[20] compared 1 liter crystalloid administration with 0.5 liter colloid 6% HES (130/0.42) and found a significant reduction in the incidence of hypotension in colloid group (38%) compared to the crystalloid group (62%). In another study, Ngan et al. reported that during Cesarean deliveries crystalloid prehydration has poor efficacy and cohydration with colloid solutions offers more stable maternal hemodynamics.[7] It was suggested that administration of colloids like 6% HES (130/0.4) either before (preloading) or soon after instillation of spinal anesthetic (coloading) may affect the dose and frequency of vasopressor boluses needed to manage maternal hypotension.[11,16,21,22] Based on a meta-analysis by Banerjee et al. the incidence of hypotension in the coload group was 59.3% compared to 62.4% in the preload group.[9] The authors opined that it is unnecessary to delay the surgery for delivering a fluid preload because regardless of the fluid loading, significant proportion of patients may require therapeutic or prophylactic vasopressor therapy. Therefore, both crystalloids as well as colloids are not very effective in managing post-spinal hypotension when used as a single modality.[7,9] This finding has also been supported in the current publications where the effects of fluid therapy on maternal circulation has been evaluated by measuring cardiac output.[4,10,16] Siddik et al. administered 500-ml colloid either as preload or coload followed by 10 ml/kg/h crystalloids.[21] However, hypotension occurred in 68% in the preload group and 75% in the coload group which was managed using intermittent vasopressor therapy. In our study, colloids were administered using a wide-bore 16-G cannula. It took approximately 10 minutes for the solution to be transfused and most of the cases of fall in maternal BP occurred during this period. Hence, it is proposed that an infusion of colloids should be started soon after the establishment of an intravenous access and infusion rate can be increased as soon as the spinal drug is injected. Thus, any adverse events related to hydroxyethyl starches will also be timely detected and managed though the incidence of these reactions is very low (<0.01%).[6] Our findings are of relevance in high-risk parturients where large volume of crystalloids and hemodynamic instability can adversely affect maternal as well as neonatal well-being.
Among the vasopressors used to manage post-spinal hypotension in obstetric patients phenylephrine hydrochloride, a short acting ά agonist has been found to be the most effective with low risk of complications.[6,7,9,12,23,24,25,26] It constricts the epidural veins and thereby reduces epidural venous engorgement. Intravenous administration of this drug has also been found to prevent rostral spread of spinal anesthesia in pregnant females.[27,28] However, the ideal dose, time, and duration of administration of prophylactic vasopressors for spinal-induced hypotension in parturients are still being defined.[4,6,9,12,26] In the present study, using the technique of colloid cohydration and phenylephrine infusion, none of the patients developed hypotension and in only one patient the SBP rose above 110% but it came back to baseline limits by reducing the flow of phenylephrine infusion. Thus, the number of interventions required was negligible. A significant number of patients in group B had fall in SBP (75.5%) especially in the first 10 minute after the spinal anesthesia though all these patients had also received rapid HES infusion through a wide-bore 16-G cannula and phenylephrine bolus of 50 μg IV soon after the injection of spinal anesthetic. As both the groups were comparable with respect to demographic and hemodynamic parameters, the difference in the number of interventions required to manage spinal-induced hypotension can be attributed to the method of administration of vasopressor agents in optimum dosage since HES was administered in both the groups in a similar fashion. In a study conducted by Allen et al., four fixed rate phenylephrine infusion regimens (25, 50, 75, 100 μg/minute) along with crystalloids infusion were used.[29] The authors observed that though the incidence and severity of hypotension was reduced but the number of physician interventions needed to maintain maternal pre-delivery SBP within 20% of the baseline could not be significantly reduced with phenylephrine infusions. They reported that irrespective of the intravenous fluid used, doses of 75 and 100 μg/minute of phenylephrine were associated with hypertension whereas 25 or 50 μg/minute of phenylephrine infusion maintained better hemodynamic stability. In the present study, a dose of 60-μg/min phenylephrine infusion used in group A significantly reduced the incidence of hypotension. This dose of 60 μg/min was chosen after evaluating results of a previous dose finding study conducted in our institute where 60, 80, and 100 μg/min phenylephrine infusions were compared (unpublished data). It was observed that 60 μg/min phenylephrine infusion provided maximum maternal hemodynamic stability with minimum side effects. Since post-spinal hypotension did not occur after using 60 μg/min of phenylephrine, we believe that this dose would suffice for preventing spinal-induced hypotension in pregnant females undergoing Cesarean section. In the study done by Allen et al., two patients in 100 μg/min group, had bradycardia and were treated with glycopyrrolate. We observed bradycardia, in two patients in group A. Both these patients received atropine with no adverse events. Theoretically, glycopyrrolate might have been a better option as its quaternary structure limits its placental transfer.
In a study by Cooper et al., the incidence of perioperative nausea and vomiting was 66% and 36%, respectively, in the ephedrine versus phenylephrine group.[27] In this study, 17% patients had nausea and no patient had vomiting episodes after the surgery. This low incidence of nausea and zero emesis can be attributed to the use of phenylephrine. Acidotic changes in umbilical arterial pH are a sensitive marker of reduced uteroplacental blood flow. The increased incidence of fetal acidosis may be caused by low maternal arterial blood pressure leading to reduced uteroplacental perfusion or because of direct effect of vasopressor agent on the fetus. Umbilical arterial pH values were well above the cut-off value to define fetal acidosis (pH < 7.2) in all the patients. Hence, our findings support the previous data where phenylephrine has been found to be a safe vasopressor agent.
To conclude, adequately treated maternal hypotension is not associated with maternal or fetal adverse effects. Vasopressors are invariably required to maintain hemodynamics in pregnant females undergoing Cesarean section under spinal anesthesia. Colloid cohydration (6% HES; 130/0.42, 15 ml/kg) along with prophylactic phenylephrine infusion maintains hemodynamic stability and neonatal well being during scheduled Cesarean deliveries under spinal anesthesia with minimum interventions by the anesthesiologist in normal healthy parturients. Further studies can be conducted in high-risk parturients using this regimen to achieve a tight control on maternal hemodynamics.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Kuczkowski KM, Reisner LS, Lin D. Chestnut`s Obstetric Anesthetic Principles and practice; 2006. Anesthesia for caesarean section; pp. 421–36. [Google Scholar]
- 2.Kariya N, Tashiro C, Masvi Spinal anesthesia for caesarean section-safe and effective anesthestic management. Masui. 2010;59:311–8. [PubMed] [Google Scholar]
- 3.Rodgers A, Walker N, Schug S, McKee A, Kehlet H, van Zundert A, et al. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: Results from overview of randomised trials. Br J Anaesth. 2000;321:1493–7. doi: 10.1136/bmj.321.7275.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langesæter E, Dyer RA. Maternal haemodynamic changes during spinal anaesthesia for caesarean section. Curr Opin Anaesthesiol. 2011;24:242–8. doi: 10.1097/ACO.0b013e32834588c5. [DOI] [PubMed] [Google Scholar]
- 5.Doherty A, Ohashi Y, Downey K, Carvalho JC. Non-invasive monitoring based on bioreactance reveals significant hemodynamic instability during elective cesarean delivery under spinal anesthesia. Rev Bras Anestesiol. 2011;61:320–5. doi: 10.1016/S0034-7094(11)70038-1. [DOI] [PubMed] [Google Scholar]
- 6.Cyna AM, Andrew M, Emmett RS, Middleton P, Simmons SW. Techniques for preventing hypotension during spinal anaesthesia for caesarean section. Cochrane Database Syst Rev. 2006;4 doi: 10.1002/14651858.CD002251.pub2. CD002251. [DOI] [PubMed] [Google Scholar]
- 7.Ngan Kee WD. Prevention of maternal hypotension after regional anaesthesia for caesarean section. Curr Opin Anesthesiol. 2010;23:304–9. doi: 10.1097/ACO.0b013e328337ffc6. [DOI] [PubMed] [Google Scholar]
- 8.Mercier FJ, Bonnet MP, De la Dorie A, Moufouki M, Banu F, Hanaf A, et al. Spinal anesthesia for caesarean section: Fluid loading, vasopressors and hypotension. Ann Fr Anesth Reanim. 2007;26:688–93. doi: 10.1016/j.annfar.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee A, Stocche RM, Angle P, Halpern SH. Preload or coload for spinal anaesthesia for elective Caesarean delivery: A meta-analysis. Can J Anesth. 2010;57:24–31. doi: 10.1007/s12630-009-9206-7. [DOI] [PubMed] [Google Scholar]
- 10.McDonald S, Fernando R, Ashpole K, Columb M. Maternal cardiac output changes after crystalloid or colloid coload following spinal anesthesia for elective cesarean delivery: A randomized controlled trial. Anesth Analg. 2011;113:803–10. doi: 10.1213/ANE.0b013e31822c0f08. [DOI] [PubMed] [Google Scholar]
- 11.Dyer RA, Reed AR. Spinal hypotension during regional anaesthesia in elective caesarean delivery: Closer to a solution. Anesth Analg. 2010;111:1093–5. doi: 10.1213/ANE.0b013e3181ea5f77. [DOI] [PubMed] [Google Scholar]
- 12.Ngan Kee WD, Khaw KS. Vasopressors in obstetrics: What should we be using? Curr Opin Anaesthesiol. 2006;19:238–43. doi: 10.1097/01.aco.0000192816.22989.ba. [DOI] [PubMed] [Google Scholar]
- 13.Ngan Kee WD, Khaw KS, Ng FF. Prevention of hypotension during spinal anesthesia for caesarean delivery; an effective technique using combination phenylephrine infusion and crystalloid cohydration. Anesthesiology. 2005;103:744–50. doi: 10.1097/00000542-200510000-00012. [DOI] [PubMed] [Google Scholar]
- 14.George RB, McKeen D, Columb MO, Habib AS. Up-down determination of 90% effective dose of phenylephrine for the treatment of spinal anaesthesia induced hypotension in parturients undergoing caesarean delivery. Anesth Analg. 2010;110:154–8. doi: 10.1213/ANE.0b013e3181c30b72. [DOI] [PubMed] [Google Scholar]
- 15.Klöhr S, Roth R, Hofmann T, Rossaint R, Heesen M. Definitions of hypotension after spinal anaesthesia for caesarean section: literature search and application to parturients. Acta Anaesthesiol Scand. 2010;54:909–21. doi: 10.1111/j.1399-6576.2010.02239.x. [DOI] [PubMed] [Google Scholar]
- 16.Teoh WH, Sia AT. Colloid preload versus coload for spinal anesthesia for caesarean delivery: The effects on maternal cardiac output. Anesth Analg. 2009;108:1592–8. doi: 10.1213/ane.0b013e31819e016d. [DOI] [PubMed] [Google Scholar]
- 17.Chappell D, Jacob M, Hofmann-Kiefer K, Conzen P, Rehm M. A rational approach to peri-operative fluid management. Anesthesiology. 2008;109:723–40. doi: 10.1097/ALN.0b013e3181863117. [DOI] [PubMed] [Google Scholar]
- 18.Udeyama H, He YL, Tanigami H, Mashimo T, Yoshiya I. Effects of crystalloid and colloid preload on blood volume in parturient undergoing spinal anaesthesia for elective caesarean section. Anesthesiology. 1999;91:1571–6. doi: 10.1097/00000542-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Lang K, Boldt J, Suttner S, Haisch G. Colloids versus crystalloid and tissue oxygen tension in patients undergoing major abdominal surgery. Anesth Analg. 2001;93:403–9. doi: 10.1097/00000539-200108000-00034. [DOI] [PubMed] [Google Scholar]
- 20.Karinen J, Rasanen J, Alahuhta S, Jouppila R, Jouppila P. Effect of crystalloid and colloid preloading on uteroplacental and maternal hemodynamic state during spinal anesthesia for caesarean section. Br J Anaesth. 1995;75:531–5. doi: 10.1093/bja/75.5.531. [DOI] [PubMed] [Google Scholar]
- 21.Siddik-Sayyid SM, Nasr VG, Taha SK, Zbeide RA, Shehade JM, Al Alami AA, et al. A randomized controlled trial comparing colloid preload to coload during spinal anesthesia for elective caesarean delivery. Anesth Analg. 2009;109:1219–24. doi: 10.1213/ane.0b013e3181b2bd6b. [DOI] [PubMed] [Google Scholar]
- 22.Carvelho B, Mercier FJ, Riley ET, Brummel C, Cohen SE. Hetastarch coloading as effective as preloading for prevention of hypotension following spinal anaesthesia in caesarean section. Int J Obstet Anaesth. 2009;18:150–5. doi: 10.1016/j.ijoa.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Thiele RH, Nemergut EC, Lynch C. The clinical implications of isolated alpha1 adrenergic stimulation. Anesth Analg. 2011;113:297–304. doi: 10.1213/ANE.0b013e3182120ca5. [DOI] [PubMed] [Google Scholar]
- 24.Prakash S, Pramanik V, Chellani H, Salhan S, Gogia AR. Maternal and neonatal effects of bolus administration of ephedrine and phenylephrine during spinal anaesthesia for caesarean delivery: A randomized study. Int J Obstet Anaesth. 2010;19:24–30. doi: 10.1016/j.ijoa.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Mohta M, Janani SS, Sethi AK, Agarwal D, Tyagi A. Comparison of phenylephrine hydrochloride and mephentermine sulphate for prevention of post spinal hypotension. Anaesthesia. 2010;65:1200–5. doi: 10.1111/j.1365-2044.2010.06559.x. [DOI] [PubMed] [Google Scholar]
- 26.Neves JF, Monterio GA, Almeida JR, Anna RS, Bonin HB, Macedo CF. Phenylephrine for blood pressure control in elective caesarean section: Therapeutic versus prophylactic doses. Rev Bras Anesthesiol. 2010;60:391–8. doi: 10.1016/S0034-7094(10)70048-9. [DOI] [PubMed] [Google Scholar]
- 27.Cooper DW, Jeyaraj L, Hynd R, Thompson R, Meek T, Ryall DM, et al. Evidence that intravenous vasopressors can affect rostral spread of spinal anesthesia in pregnancy. Anesthesiology. 2004;101:28–33. doi: 10.1097/00000542-200407000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Sakum T, Sato M, Yokoi M. Effects of intravenous vasopressors on spread of spinal anaesthesia with 0.5% hyperbaric bupivacaine for caesarean delivery. Masui. 2010;59:691–5. [PubMed] [Google Scholar]
- 29.Allen TK, George RB, White WD, Muir HA, Habib AS. A double-blind, placebo-controlled trial of four fixed rate infusion regimes of phenylephrine for hemodynamic support during spinal anesthesia for caesarean delivery. Anesth Analg. 2010;111:1221–9. doi: 10.1213/ANE.0b013e3181e1db21. [DOI] [PubMed] [Google Scholar]


