Abstract
Background:
Ventilator-associated pneumonia (VAP) is the most common cause of hospital acquired infection and death among patients admitted in ICU. Microorganisms responsible for VAP vary from place to place. Gram-negative bacteria (GNB) have emerged as a major group of pathogen causing VAP and over the years carbapenem group of antibiotics has emerged as one of the important antibiotics used in the critically ill patients. There have been reports of increased occurrence of infection by carbapenem-resistant bacteria in health care settings in recent times.
Aim:
The aim of the study was to assess the incidence of VAP, their microbiological profile with reference to carbapenemase producing GNB in the intensive care unit of a tertiary care hospital, their relation to initial emperical antibiotic therapy, sensitivity patterns, and outcome.
Materials and Methods:
This prospective study was carried out over the period of 1 year (July 2010-June 2011) on 100 randomly selected patients above the age of 18 years admitted in the emergency/ICU and requiring intubation and mechanical ventilation for more than 72 hours. The diagnosis of VAP was established on the basis of clinical and radiological parameters as per Centre of Disease Centres (CDC) guidelines. A baseline sample was obtained after initial endotracheal intubation. Thereafter, the culture sent on the first day of occurrence of clinical sign of VAP. Culture was done on blood agar and MacConkey agar. All imipenem-resistant strains were further confirmed by Modified Hodge test and combined disc for confirmation of respective carbapenemase.
Results:
Incidence of VAP was found to be 51%. GNB mainly Citrobacter 28 (52.83%) and Klebsiella pneumoniae 7 (13.21%), were the most commonly isolated pathogens. The prevalence of carbapenemase-producing GNB was alarmingly high 24/50 (48%). The entire carbapenemase producers showed high degree of cross resistance to antibiotics with some sensitivity to Polymyxin B (94 %) and Tigecycline (96%)
Conclusion:
High incidence of VAP and the potential carbapenemase-producing GNB are real threat in our ICU. The emergence of microorganisms known for its inherent resistance among most of the common first-line antibiotics calls for a alarm in all upcoming tertiary care hospitals.
Keywords: Carbapenemase, multidrug-resistant organisms, nosocomial pneumonia, ventilator-associated pneumonia
Introduction
Ventilator-associated pneumonia (VAP) is the pneumonia occurring in patients within 48 hours following intubation. It is the most common cause of hospital-acquired infections among patients admitted in ICU.[1] The incidence of VAP ranges from 6.8% to 44% and its occurrence is associated with increased length of hospital stay, mortality, and financial burden.[2]
Resistance to b-lactam class of antibiotic is a common occurrence and pan-drug-resistant strains are beginning to emerge. Evolutionary stress such as exposure to antibiotics, bacterial gene transfer is responsible for antibiotic resistant trait.[3] Carbapenem group of antibiotic by virtue of their broad spectrum of antibacterial activity and resistance to most of the β-lactamase enzyme produced by bacteria has emerged as one of the most important group of antibiotic in critically ill patients. There has been a slow but steady emergence of bacteria resistant to this group of antibiotics, playing a significant role in the colonization and infection of patients, especially those in intensive care units. The reason for resistance to carbapenems is due to production of carbapenemase enzyme[4] by the bacteria.
Carbapenem-resistant bacteria were not a common finding in our hospital till 2009 but by 2010 there was an increase in their isolation in our hospital, even as a causative organism of VAP. Understanding the importance of their isolation, we undertook this prospective study among 100 intubated patients admitted in our ICU. We followed them for the occurrence of VAP, bacteriology of organisms, and the incidence of carbapenemase producers among isolated organisms in our setup.
Materials and Methods
The study was carried out as a joint collaboration between Intensive care and Microbiology department of our hospital after obtaining the approval of Institutional Ethic Committee. Patients above the age of 18 years admitted in the emergency/ICU, requiring intubation and mechanical ventilation for more than 72 hours were considered eligible for inclusion. Exclusion criteria included intubation in other areas of hospital, carried tracheotomy or had pneumonia on admission or during first 72 hours of mechanical ventilation. Written informed consent was obtained from nearest relative of the patients.
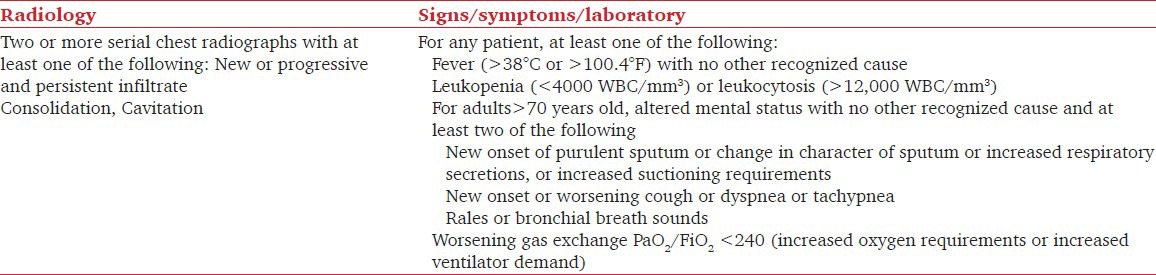
The diagnosis of VAP was established on the basis of clinical and radiological parameters as per Centre of Disease Centres (CDC) guidelines.[5] [Table 1].
Table 1.
Diagnostic criteria for Ventilator-associated pneumonia
The samples for microbiological follow-up were collected by endotracheal aspiration using 12-F suction catheter. A baseline sample was obtained after initial endotracheal intubation. Thereafter the culture sent on the first day of occurrence of clinical sign of VAP. In microbiology laboratory first quality of the sample received was ascertained by counting the composite quality score by making a Gram stain smear of the aspirate received and observing it under high power microscope for presence of Pus cell (more than 25 per high-power field) and absence of epithelial cell. After confirming the sample to be representative of lower respiratory tract secretion, it was cultured on blood agar and MacConkey agar using calibrated loop for quantification of viable count. A colony count of 104 cfu/ml was diagnostic. Bacterial identification was made as per common bacteriological methods.[6,7,8] Sensitivity testing were done as per CLSI guideline.[9,10,11] All imipenem-resistant strains were further confirmed by Modified Hodge test and combined disc for confirmation of respective carbapenemase production. Modified Hodge test was done using an Escherichia coli ATCC 25922 inoculated on the surface of a Mueller Hinton agar (MHA) plate with zinc sulfate and imipenem disk at the center. Test strains were streaked heavily from the edge of the disk to the periphery of the plate and kept for overnight incubation. The presence of a distorted or clover leaf-shaped inhibition zone was interpreted as positive for carbapenemase-producing isolates.[12] The combined disc test was done by inoculating the test strain on MHA with two imipenem disc one of which was impregnated with Ethylenediaminetetraacetic acid (EDTA). After overnight incubation at 37°C the inhibition zones of imipenem and imipenem with EDTA were compared. A zone difference of more than 4 mm between the imipenem and the imipenem plus EDTA zones confirms the isolate to be metallo beta lactamase (MBL), or Klebsiella pneumoniae carbapenemases (KPC) in case of K. pneumoniae.[13]
Results
One hundred intubated patients in the age group of 18-78 years were screened consecutively for appearance for VAP after being found eligible for study, of them 67 were males. All the patients were followed consecutively till patient were either extubated, tracheotomized, expired, or lost in follow-up.
The indication for mechanical ventilation among these patients was different, common indications being head injury (34), respiratory failure (10), neurological disease (28), suicidal poisoning (7), and others (21).
Out of 100 patients followed-up in the study, first sample did not show significant bacterial growth or colonizers. Fifty-three (53%) developed VAP of which 50 cases were of monobacterial origin while three patients had polybacterial VAP that were proved by repeated microbiological follow-up.
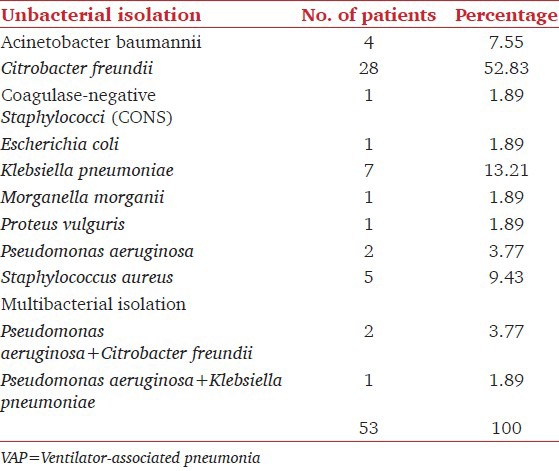
The total numbers of organism isolated were 56 out of which Gram-negative bacteria predominated in 50 (89.29%) cases as shown in Table 2.
Table 2.
Distribution organisms responsible among the patents that developed VAP
Forty-five patients developed VAP within 48 hours to 72 hours of initial ventilation while eight patients developed VAP after 72 hours. It was observed that the entire cases of polymicrobial VAP (n = 3) were cases of delayed onset VAP. Bacterial isolation according to development of VAP is in Figure 1.
Figure 1.
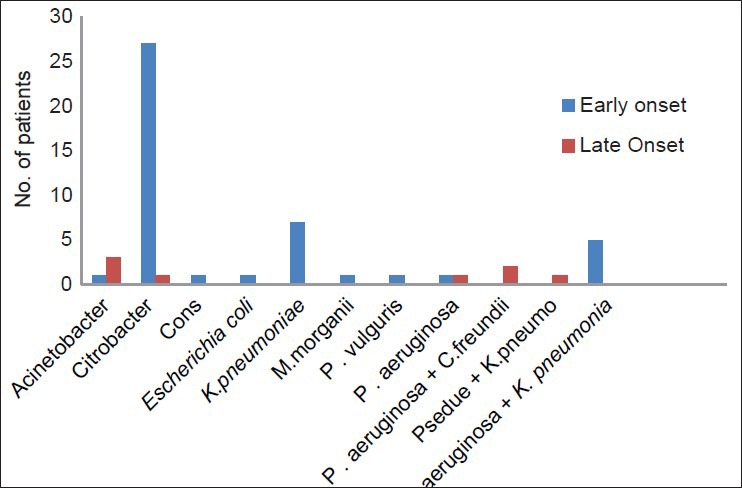
Bacterial distributions among early onset vs. late onset VAP
All imipenem-resistant isolate were subjected to confirmatory test for carbapenemase production and 24 (48%) of them were confirmed to be carbapenemase producing. The prevalence of carbapenemase activity was highest with A. baumannii (100 %), C. freundii (53.33%), and K. pneumoniae (37.5%) [Figure 2].
Figure 2.
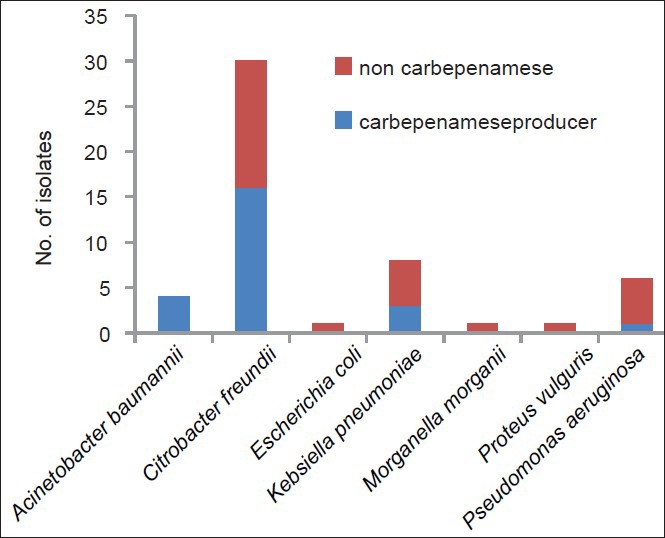
Distribution of Carbepenamase producer among differenct GNB
The sensitivity pattern of those GNB by Kirby Bauer disc diffusion method showed maximum sensitivity for Tigecycline (96%) and Polymyxin B (94%) followed by Levofloxacin (46%). Maximum resistance was seen for cephalosporins [Table 3].
Table 3.
AST Pattern of difference Gram-negative organisms isolated in the study (n=50)
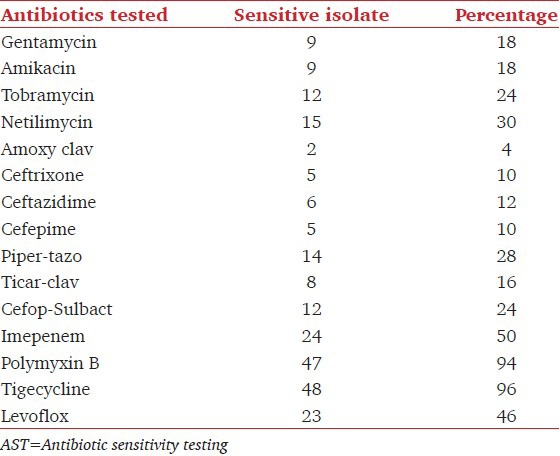
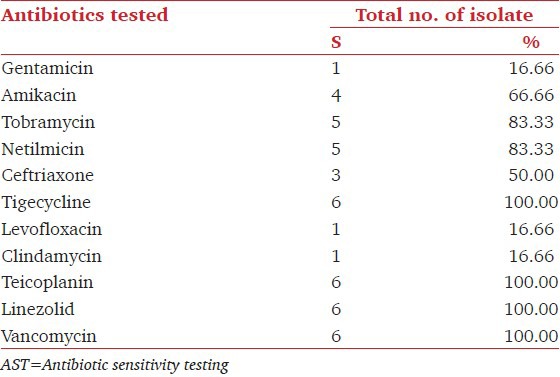
The carbapenemase-producing isolates showed 100% cross resistance to all other antibiotics of Gram-negative penal. The sensitivity pattern of Gram-positive isolates showed highest sensitivity to Linezolid, Tigecycline, Vancomycin, and Teicoplanin 100% each followed by, Tobramycin and Netilmicin 83.33% each. The prevalence of MRS among Staphylococcus was 50%.[Table 4].
Table 4.
AST pattern of Gram-positive organism (n=6)
We also followed the culture report and compared the sensitivity of the organism isolated with the emperical prophylactic/therapeutic antibiotic used after intubation and initiation of mechanical ventilation. The most common empirical antibiotics used were cephalosporin (63), quinolone (34), vancomycin (20) individually or in combination. After microbiological follow-up it was seen that in only 35 (70 %) patients the organism isolated as causative agent of VAP had relevance with the initial empirical antibiotic used. Among those 35 isolates that had relevance only 6 (20%) isolate were having sensitivity to the initial antibiotic advised.
Of the patients who developed VAP 24 were discharged with tracheotomy, 21 died, and 8 were lost to follow-up as attendant shifted the patient out of hospital against medical advice. It was seen that maximum death rate was in those patient infected with S. aureus 4 out of 5 (80%), followed by C. freundii (28.57%). Death rate was nil among isolates of A. baumannii, coagulase-negative Staphylococcus and E. coli. The patients who developed VAP with dual bacteria mortality was 100% (3/3). Gram-negative bacteria were the cause of death in 39% (21/53) cases. MBL and non-MBL producer as cause of death were seen in 29 % (07/24) and 38 % (10/26), respectively.
Discussion
VAP is the most common complication after mechanical ventilation with the incidence estimated to be 3% per day during first 5 days of ventilation,2% per day between days 5 and 10 of ventilation and 1% per day thereafter.[14] Akca et al. in their study have discovered following factors to be responsible for multiresistant bacterial infection of early onset – emergency intubation, aspiration, and Glasgow Coma Scale (GCS) less than 9.[15] Bronchard et al.[16] have demonstrated that loss of consciousness more than tracheal intubation are independent risk factors for early onset VAP. Our study included maximum number of patients needing intubation and mechanical ventilation with the diagnosis of head injury (34%), poisoning (7%), central nervous system disease (28%), and respiratory failure (10%). Association of emergency intubation, micro aspiration and low GCS were all associated in most of our patients and may have been responsible for a high incidence of early onset VAP (48%) compared to 8% incidence of late onset VAP. After this study, VAP prevention bundles have been instituted to decrease the incidence of high early onset VAP.
Risk factors for pneumonia include use of nasogastric tube, continuous enteral feeding, prolonged mechanical ventilation (>1 day), use of H2-receptor antagonist, sucralfate, muscle relaxants, corticosteroids, barbiturates, and inotropic agents, positive end-expiratory pressure, intense sedation, re-intubation, and tracheotomy.
Multidrug-resistant pathogens such as C. freundii, P. aeruginosa, A. baumannii, and S. aureus were found to be the common organisms causing VAP. This highlights the need for treatment of the VAP cases with second-line antibiotics effective against these MDR pathogens. This finding also emphazises the need for stringent preventive measures against VAP, as the treatment of an established VAP becomes very expensive, with case fatality rate.[17] Emergence of C. freundii as a causative organism for VAP many of whom were carbapenemase producing (53.33%) is a new finding in our study. It is much more than expected common trends which cite rate of Citrobacter prevalence is as low as 1%.[18,19] C. freundii, are aerobic Gram-negative bacilli of Enterobacteriaceae family and is known for being an opportunistic pathogen responsible for a number of significant opportunistic infections and possession of various intrinsic drug resistance gene.[20] Although the incidence of Citrobacter is low, Jakribettu[21] et al. in their study showed an isolation of approximately 17 isolates. This signifies that the incidence is on rise though may not have attained significant proportion. Increased incidence in our study needs further evaluation as the cause.
Multidrug resistant and carbapenemase fermenters were chiefly responsible for late onset VAP. In our study the rate of carbapenemase-producing bacteria among all GNB was 48% which is higher than other studies published in recent past,[22] vast majority of these carbapenemase 19/24 (79.16%) were also from Enterobacteriaceae family. Various other studies have from India have shown a rate between 18.75 and 26%.[23,24] Relatively high rate of carbapenemase may be due to increase prevalence of these bacteria as cross colonizer in hospitals, specially in developing countries with poor maintenance of infection control practice. This is a cause for concern as carbapenemase in the past were frequent among non-fermenters like Acinetobacter and Pseudomonas, but this resistance now seems to have transferred to other Enterobacteriaceae also. The increase incidence of carbapenemase production might be as a result of rampant use of carbapenem group of antibiotics and natural selection tool of bacteria like plasmid and chromosomal-mediated gene transfer among species of carbapenemase-producing Enterobacteriaceae. It is fast becoming a major health threat among ICU of developing countries.[25]
The study also indicated that there was less correlation between the initial prophylactic antibiotic and the bacterial sensitivity. The cause maybe multifactorial, common causes being change in microbial flora causing infection from time to time, lack of awareness of causative organism, and their sensitivity pattern, continuation of initial antibiotic being administrated for some other primary infection. A more stringent hospital antibiotic policy is warranted to decrease the misuse of these drugs. Following this study a stringent antibiotic policy was instituted with the collaboration of intensivist, physicians, microbiologists, and hospital infection control team. We had observed that Cephalosporins were the most favoured drug as first-line treatment but its effectivity was found to be poor.[26] A high sensitivity was seen for Tigecycline and Polymyxin B against Gram-negative isolates and Vancomycin and Linezolid for Gram-positive isolates. It may be so because these drugs were reserved as second-line of antibiotic therapy.[27]
A limitation of our study was it being conducted in a resource-limited setting, with small number of patients with VAP and in a single center, few patients being lost as they left against medical advice due to financial constraint and increased cost of treatment. In addition, we recognize that the findings of this study not necessarily reflect the situations in other advanced centers in India. The incidence of VAP in our study was more than other advanced centre though after the study we also have implemented stringent means of control of VAP with our limited resources. We suggest further multi-centric study with larger patient population to confirm our findings, in particular the high incidence of carbapenemase along with other MDR pathogen in Indian ICU.
Conclusions
The emergence of organism with high level of intrinsic resistance like Citrobacter and Klebsiella as causative agents for VAP is a serious concern. The knowledge of ever-changing susceptibility pattern with the local pathogens should guide the choice of antibiotics. Although Polymyxin B and Tigecycline are still effective against most resistant Gram-negative isolate while Vancomycin is still holding the forte against Gram-positive organism caution should be observed against rampant use of these drugs. We should be guided by microbiological follow-up and treatment of each and every patient on ventilator and this has emerged as the need of the hour today.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Wagh H, Acharaya D. Ventilator Associated Pneumonia- an Overview. Br J Med Pract. 2009;2:16–9. [Google Scholar]
- 2.Peter JV, Chacko B, Moran JL. Comparison of closed endotracheal suction versus open endotracheal suction in the development of ventilator associated pneumonia in intensive care patients: An evaluation using meta analytic techniques. Indian J Med Sci. 2007;61:201–11. [PubMed] [Google Scholar]
- 3.Kumar AV, Pillai VS, Dinesh KR, Karim S. The phenotypic detection of Carbapenemase in meropenem resistant Acinetobacter calcoaceticus baumannii complex in a tertiary care hospital in south India. J Clin and Diag Res. 2011;5:223–6. [Google Scholar]
- 4.Anton YP, Harald S, David LP. Acinetobacter baumannii: Emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–82. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horan T, Gaynes R. Survillence of Nosocomial infewction. In: Mayhall C, editor. Hospital epidemiology and infection control. 3rd ed. Philadelphia: Lippincott Williams and Wilkins; 2004. pp. 1659–702. [Google Scholar]
- 6.Colle JG, Fraser AG, Marmion BP, Simmons A, editors. 4th ed. Philadelphia: Churchil Livingstone; 1996. Mackie and McCartney Practical Medical Microbiology; pp. 132–47. [Google Scholar]
- 7.Monical Cheesbrough; Cambridge Low Price Edition. 2006. District Laboratory Practical in Tropical Countries; 2nd ed Part 2; pp. 71–6. [Google Scholar]
- 8.Stokes EJ, Ridgway GL, Wren MW, Miles A, Arnold E, editors. 7th ed. 1993. Clinical Microbiology; pp. 122–45. [Google Scholar]
- 9.James L, Hoppe-Bauer JE. Processing and interpretation of lower respiratory tract specimens. In: Isenberg HD, editor. Clinical Microbiology Procedures Handbook. Washington, DC: ASM Press; 1997. [Google Scholar]
- 10.Camargo LF, De Marco FV, Barbas CS, Hoelz C, Bueno MA, Rodrigues M, Jr, et al. Ventilator associated pneumonia: Comparison between quantitative and qualitative cultures of tracheal aspirates. Critical Care. 2004;8:R422–30. doi: 10.1186/cc2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; 17th informational supplement. CLSI document M100-S17(ISBN 1- 56238-625-5) Clinical and Laboratory Standards Institute USA. 2007 [Google Scholar]
- 12.Lee K, Lim YS, Yong D, Yum JH, Chon Y. Evaluation of the Hodge Test and the Imipenem-EDTA double-disk synergy test for differentiating Metallo β Lactamase-producing isolates of pseudomonas spp. and acinetobacter spp. J Clin Microbiol. 2003;41:4623–9. doi: 10.1128/JCM.41.10.4623-4629.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franklin C, Liolios L, Peleg AY. Phenotypic detection of carbapenem susceptible MBL-producing gram negative bacilli in the clinical laboratory. J Clin Microbiol. 2006;44:3139–44. doi: 10.1128/JCM.00879-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rello J, Torres A, Ricart M, Valles J, Gonzalez J, Artigas A, et al. Ventilator-associated pneumonia by Staphylococcus aureus: Comparison ofmethicillin-resistant and methicillin-sensitive episodes. Am J Respir Crit Care Med. 1994;150:1545–9. doi: 10.1164/ajrccm.150.6.7952612. [DOI] [PubMed] [Google Scholar]
- 15.Akca O, Koltka K, Uzel S, Cakar N, Pembeci K, Sayan MA, et al. risk factors for early onset ventilator associated pneumoniain critical care patients. Anesthesiology. 2000;93:638–45. doi: 10.1097/00000542-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Bronchard R, Albaladejo P, Brezac G, Geffory A, Seince PF, Morris W, et al. Early onset pneumonia risk factors and consequences in head trauma patients. Anesthesiology. 2004;100:234–9. doi: 10.1097/00000542-200402000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Erbay RH, Yalcin AN, Zencir M, Serin S, Atalay H. Costs and risk factors for ventilator-associated pneumonia in a Turkish university hospital's intensive care unit: A case-control study. BMC Pulm Med. 2004;4:3. doi: 10.1186/1471-2466-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 19.Park DR. The Microbiology of Ventilator-Associated Pneumonia. Respir Care. 2005;50:742–63. [PubMed] [Google Scholar]
- 20.Drelichman V, Band JD. Bacteremias due to Citrobacter diversus and Citrobacter freundii. Incidence, risk factors, and clinical outcome. Arch Intern Med. 1985;145:1808–10. [PubMed] [Google Scholar]
- 21.Jakribettu RP, Baloor R. Characterisation of aerobic bacteria isolated from endotracheal aspirate of adult patients suspected ventilator associated pneumonia in a tertiary care centre in Mangalore. Saudi J Anaesth. 2012;6:115–9. doi: 10.4103/1658-354X.97022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dey A, Bairy I. Incidence of multidrug-resistant organisms causing ventilator-associated pneumoniain a tertiary care hospital: A nine months’ prospective study. Ann Thorac Med. 2007;2:52–7. doi: 10.4103/1817-1737.32230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joseph NM, Dutta SS, Badhe AS, Rsistha D, Parija SC. Ventilator associated pneumonia in a tertiary care hospital in India: Role of multidrug resistant pathogen. J Infect Dev Ctries. 2010;4:218–25. doi: 10.3855/jidc.634. [DOI] [PubMed] [Google Scholar]
- 24.Solanke V, Pai C, Urhekar AD. Comparative bacteriological study of community acquired pneumonia. Bombay Hosp J. 2011;53:159–65. [Google Scholar]
- 25.Miriagou V, Cornaglia G, Edelstein M, Galani I, Giske CG, Gniadkowski M, et al. Acquired carbapenemases in Gram-negative bacterial pathogens: Detection and surveillance issues. Clin Microbiol Infect. 2010;16:112–22. doi: 10.1111/j.1469-0691.2009.03116.x. [DOI] [PubMed] [Google Scholar]
- 26.Dancer SJ. The Problem with cephalosporins. J Antimicrob Chemother. 2001;48:463–78. doi: 10.1093/jac/48.4.463. [DOI] [PubMed] [Google Scholar]
- 27.Tsering D, Das S, Adhiakari L, Pal R, Singh T. Extended spectrum beta-lactamase detection in gram-negative bacilli of nosocomial origin. J Glob Infect Dis. 2009;1:87–92. doi: 10.4103/0974-777X.56247. [DOI] [PMC free article] [PubMed] [Google Scholar]


