Abstract
Non-Hodgkin's lymphoma of T-cell types are rare neoplasms. Central nervous system metastasis is unusual. We are reporting a patient with peripheral T-cell lymphoma unspecified who had extra nodal metastasis into the brain that manifested with extrapyramidal dysfunction. The clinical presentation was exceptional in that the course was indolent and patient had no overt extra neural manifestations of malignancy for nearly 3 years after the onset of Parkinsonism. Striking brain imaging late in the disease supported by pathological findings enabled the diagnosis of this rare condition.
Keywords: Brain, hemorrhagic secondaries, neoplastic parkinsonism, peripheral T-cell lymphoma unspecified metastasis
Introduction
Central nervous system (CNS) malignancies present rarely with features of Parkinsonism. Not more than 90 cases of tumor related Parkinsonism have been reported in literature.[1] Peripheral T-cell lymphoma unspecified (PTCLUS) is a rare form of non-Hodgkin's lymphoma with less than 5% of patients developing brain metastasis. To the best of our knowledge this is the first report of PTCLUS wherein the clinical manifestations of cerebral metastatic disease evolved over 3 years, in the form of extrapyramidal dysfunction.
Case Report
A 41-year-old male presented with 3 years history of progressive slowness of gait, stooped posture, and dragging of feet, especially on the left, while walking. He was diagnosed to have Parkinson's disease in the 2nd year of his illness at another center. A non-contrast magnetic resonance imaging (MRI) of the brain showed scanty, ill-defined lesions in the cerebral sub cortex and basal ganglia on T2 weighted (T2 W) images (verified by author LP), which was reported to be nonspecific. He responded poorly to Levodopa therapy which he discontinued within 6 months. In the 3rd year of his illness he become forgetful, had low volume speech and drooling of saliva. Two weeks prior to admission he became irritable, had irrelevant speech, and noticeable deterioration in his gait. On examination the patient was afebrile but disoriented and dysarthric. He had facial hypomimia, bradykinesia, cogwheel rigidity, and festinant gait. Deep tendon reflexes were exaggerated and plantar response was extensor. General examination revealed firm non tender cervical and axillary lymphadenopathy and mild hepatosplenomegaly.
Routine investigations were normal. He was HIV negative. Ultrasound of the abdomen revealed para aortic lympadenopathy with mild ascites. Brain MRI revealed multiple nodular enhancing lesions bilaterally in the corona radiata, centrum semiovale, bilateral basal ganglia, midbrain, pons, and cerebellum [Figure 1a] which were hypointense on T1 weighted (W) and hyperintense on T2 W images. Susceptibility weighted images showed multiple rounded hypointense signals suggestive of hemorrhagic lesions [Figure 1b]. Cerebrospinal fluid examination revealed 15 cells/mm3 (all lymphocytes), protein 156 mg/dl and cytology was normal. Left axillary lymph node and right frontal wedge biopsy of the brain was done. Lymph node biopsy showed effaced architecture, neoplastic cells of intermediate size showing dense T-cell staining and absent B-cell marker consistent with the diagnosis of nodal PTCLUS. Brain biopsy revealed multiple angiocentric, neoplastic T-cell infiltrates [Figure 2a] with micro hemorrhages. Lymphoma was T-cell (CD3) positive [Figure 2b], B-cell [Figure 2c], CD4 and CD8 negative. The Ki-67 proliferation index was <2% in the lymph node biopsy while it was >25% in the brain sections [Figure 2d]. After biopsy, patient received five doses of intravenous methyl prednisolone (1 g/day) followed by a 2 week oral taper. Rigidity and bradykinesia improved remarkably in the immediate period after steroid therapy and the patient was able to walk unaided and became coherent and oriented. At follow-up after 3 months, he was rigid, cognitively declined, and on a wheel chair. Patients’ relatives had declined further treatment and he was subsequently lost to follow-up.
Figure 1.
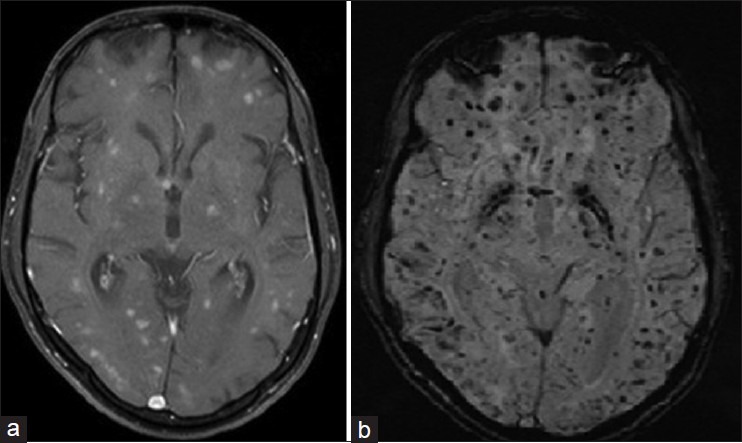
(a) Contrast enhanced magnetic resonance imaging of the brain showing multiple nodular enhancing lesions bilaterally in the corona radiata, centrum semiovale, bilateral basal ganglia, midbrain, pons and cerebellum (b) susceptibility weighted imaging demonstrates extensive signal voids suggestive of hemorrhagic lesions in cerebral hemispheres, brainstem and cerebellum
Figure 2.
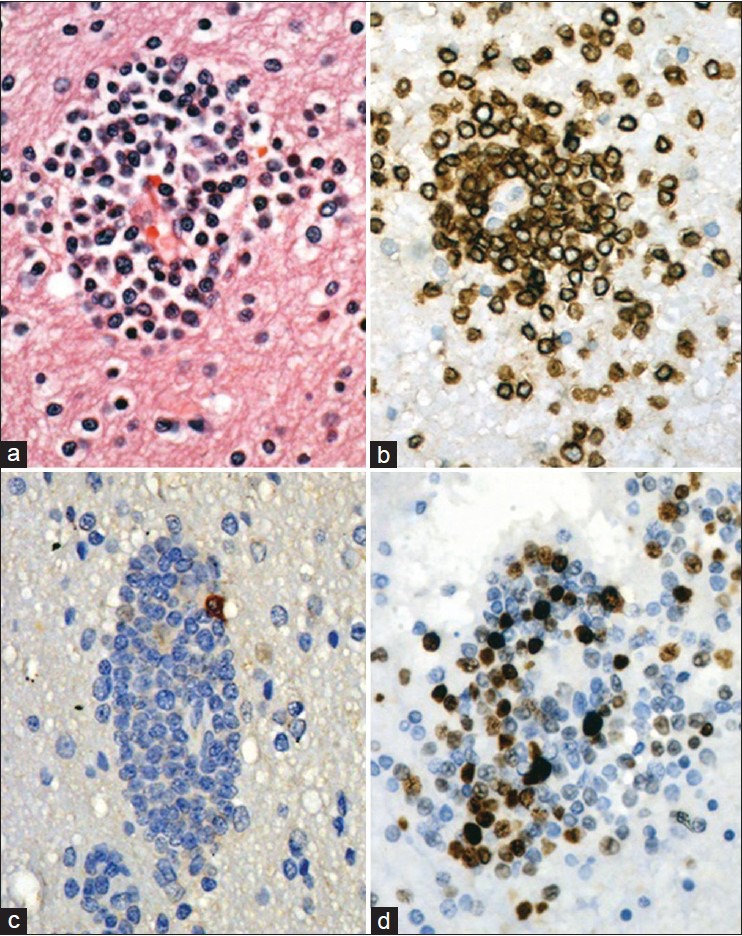
(a) Brain biopsy showing perivascular lymphoma cells, (b) which are T-cell positive, (c) B-cell negative, and (d) focally high Ki-67 labeling
Discussion
Neoplastic Parkinsonism has been noted mostly with primary brain tumors particularly B-cell lymphoma, glioma, and craniopharyngioma. Supratentorial lesions involving the caudate-putamen or the striatonigral tract are the most common lesions described in these reports. Metastatic causes have been previously reported with colorectal cancer patients[1] and rarely with non-killer T-cell type of peripheral T-cell lymphoma.[2]
PTCLUS constitutes >50% of all peripheral T cell lymphoma PTCL that cannot be classified. It is a heterogenous group of conditions that are aggressive and respond poorly to treatment.[3] They may be more commonly seen in patients of Asian and African origin than in Caucasians possibly because of greater prevalence in human T-cell lymphotropic virus-1 endemic areas. Most patients with PTCLUS present with nodal involvement; however, a number of extranodal sites may also be involved (e.g., liver, bone marrow, gastrointestinal, skin). Less than 5% of PTCLUS have CNS metastasis.[4] Most patients have leptomeningeal or spinal epidural metastases. The rate of CNS parenchymal metastases is only 1%. Extensive angiocentric hemorrhagic lesions simulating vasculitis that was seen in our patient has not been previously described. The majority of series report a poor outcome with a 5 year overall survival of approximately 30-35% using standard chemotherapy.
To the best of our knowledge this is the first case report of PTCLUS presenting as neoplastic Parkinsonism, which we are presenting for its rarity and unique clinical course. Our patient presented initially with advanced extra-nodal disease bringing his underlying disease to notice. In the patient the variable tumor proliferation seen in Ki-67 staining favored a more aggressive disease in the brain. It is unlikely that the patient had comorbid Parkinson's disease. There was significant malignant infiltration involving the basal ganglia as seen on MRI. Most importantly there was a short-lived but dramatic response to intravenous steroids when he became ambulant and had significant reduction in rigidity and bradykinesia. Steroid-induced apoptotic death of lymphoma cells resulting in short-term clinical improvement is well known with lympho-proliferative malignancies.[5] In the patient it confirmed the causal relationship between lymphoma and extrapyramidal disorder. No exposure to pesticides or other environmental carcinogens was reported in the patient. Although neoplastic Parkinsonism is uncommon, the presentation may be confused with degenerative disorders of the basal ganglia especially since some patients show response to L-dopa therapy.[6]
Footnotes
Source of Support: Nil
Conflict of Interest: Nil
References
- 1.Hortelano E, Perea C, Uña E, Cebayos A, Diezhandino P, González M. Parkinsonism secondary to metastatic lesions within the central nervous system: A case report. J Med Case Rep. 2010;4:218. doi: 10.1186/1752-1947-4-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishihara S, Kano O, Ikeda K, Shimokawa R, Kawabe K, Iwasaki Y. Clinicoradiological changes of brain NK/T cell lymphoma manifesting pure akinesia: A case report. BMC Neurol. 2011;11:137. doi: 10.1186/1471-2377-11-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agostinelli C, Piccaluga PP, Went P, Rossi M, Gazzola A, Righi S, et al. Peripheral T cell lymphoma, not otherwise specified: The stuff of genes, dreams and therapies. J Clin Pathol. 2008;61:1160–7. doi: 10.1136/jcp.2008.055335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savage KJ, Chhanabhai M, Gascoyne RD, Connors JM. Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Ann Oncol. 2004;15:1467–75. doi: 10.1093/annonc/mdh392. [DOI] [PubMed] [Google Scholar]
- 5.Pandit L, Chickabasaviah Y, Raghothaman A, Mustafa S, Vasudevan A. Lymhomatosis cerebri: A rare cause of leukoencephalopathy. J Neurol Sci. 2010;293:122–4. doi: 10.1016/j.jns.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 6.Pramstaller PP, Salerno A, Bhatia KP, Prugger M, Marsden CD. Primary central nervous system lymphoma presenting with a parkinsonian syndrome of pure akinesia. J Neurol. 1999;246:934–8. doi: 10.1007/s004150050485. [DOI] [PubMed] [Google Scholar]


