Abstract
Hashimoto encephalopathy remains a Rubik's cube for the present generation of clinical research. Myriad presentations have been noted, and observations recorded in few subgroups of patients have gone on only to be trashed by a second group of patients with a completely different clinical profile. Steroids have been traditionally held to be the treatment for this condition, but long-term side effects associated with it limits its use. Although multiple drugs have been tried, yet there exists no data for their long-term efficacy in maintaining remission. No radiological findings have been consistently associated with this condition. We report the use of azathioprine in maintaining long-term remission in one such patient with Hashimoto encephalopathy and the presence of lactate peak in magnetic resonance spectroscopy of the patient, which showed dramatic regression with institution of immunosuppression.
Keywords: Anti-thyreoperoxidase, azathioprine, encephalopathy, lactate peak, methylprednisolone, MRI-spectroscopy
Introduction
About a hundred cases have been reported in adult and pediatric age group. Even fewer with treatment options. Presentation of a patient with myoclonic movements and delirious behavior sets off a train of investigations with several possibilities. However, when the movements persist despite use of anti-convulsants and no organic cause seems to be evident on routine laboratory investigations and imaging studies, one needs to look beyond the obvious findings. Hashimoto encephalopathy has been a controversial, rare neuro-endocrinologic disorder that is often misdiagnosed as a psychiatric problem in view of the patients presenting with confusion and hallucinations. The usual association with a thyroid disorder may not be evident at the outset, and patients are usually found to be clinically euthyroid. Less than 120 reports have been published so far, and varied clinical and laboratory findings have been seen to be associated with it. This raises the barriers to diagnose this challenging yet potentially curable condition. Magnetic resonance spectroscopy and titers of anti-thyroperoxidase antibodies were both seen to correlate with the clinical presentation of this patient with a characteristic lactate peak in the spectroscopy, which disappeared when the patient entered clinical remission. Anti-thyroperoxidase levels peaked during the symptomatic phase of the disease and decreased as the patient entered clinical remission. Azathioprine was seen to induce long-term remission in this patient when oral glucocorticoids were not tolerated.
Case Report
Dev Raj, a 45-year-old healthy male presented with abnormal brief shock-like movements of the body for 20 days with confused behavior for 1 week. The abnormal movements developed insidiously over 2 days without any stimulating factors and did not remit with sleep or anti-epileptics (valproate 1.5 gm and clobazam 40 mg divided doses), which he had been prescribed for approximately 2 weeks before seeking an admission at our institute. They occurred without any periodicity with a frequency of about 8-10 per minute and incapacitated the patient. There was no loss of consciousness or bowel and bladder tone. He gave no history of addictions of any kind.
Patient was obese with a BMI of 34 kg/m2 and was seen to have loss of lateral third of eyebrows with dry and eczematous skin. Blood pressure was 170/100 mmHg. He had severe cognitive impairment on admission, scoring about 4/30 in MMSE. Speech was fluent and articulate but lacked insight and consistency. Cerebellar signs, motor and sensory examination could not be performed consistently, but reflexes were slow with a delayed relaxation phase observed in the ankle jerk. Plantar response was extensor in both limbs. There were no signs of meningeal or cranial nerve involvement.
There were no similar episodes noted in the patient in the past or any history of trauma. He did not suffer from any chronic cardiovascular, pulmonary, or neurologic conditions.
Serum electrolytes, blood sugar, liver and renal function tests, and hematology profile were seen to be within normal range. However, his thyroid hormone levels were abnormal with an initial TSH of 46.04 IU/ml. MRI of brain was normal, but on spectroscopy, there was an evidence of a lactate peak corresponding to 1.3 μm in the right occipital area, suggestive of a focus of anaerobic metabolism in the brain. [Figures 1, 2] There was a corresponding dip of N-acetyl aspartate in the corresponding area. There were no areas of hypoperfusion in the brain. EEG and brain mapping showed a background α activity with a 6-7 Hz slowing in the delta region with frontal predominance β-fast activity. [Figure 3] The Anti-TPO (Thyroid peroxidase) antibody levels were 3195.70 units (normal values below 60.00 units). A lumbar puncture revealed mildly raised protein levels in cerebrospinal fluid at 95 mg%, with a sugar level of 84 mg%, and a total leukocyte count of 5/cumm. CSF sent for serology against common viruses did not reveal any viral titers and involvement, and Gram stain and oligo-clonal bands were negative. He was sero-negative for HIV, HBSAg, and anti-HCV. An ultrasonogram for thyroid showed bilateral lobes of thyroid to be small and atrophic with altered echotexture. The biopsy for thyroid tissue did not reveal any functional thyroid follicles.
Figure 1.
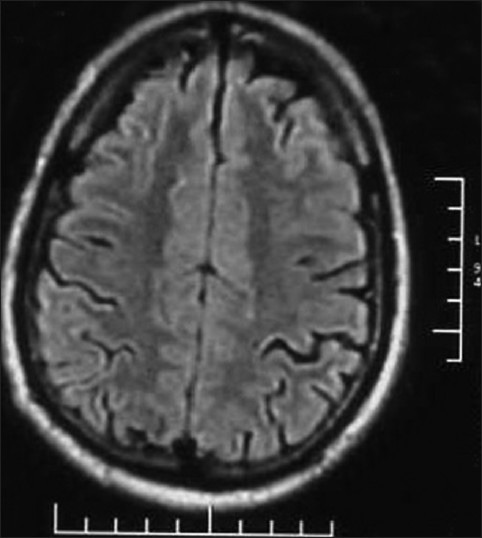
MRI Brain of the patient with Hashimoto encephalopathy showing normal study during the initial presentation
Figure 2.
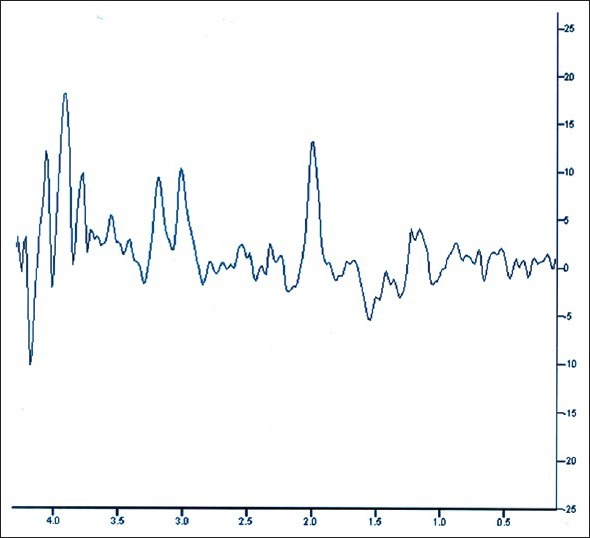
MR spectroscopy of the brain showing lactate peak at 1.3 micrometer during symptomatic stage of the disease with probe placed at right occipital region
Figure 3.
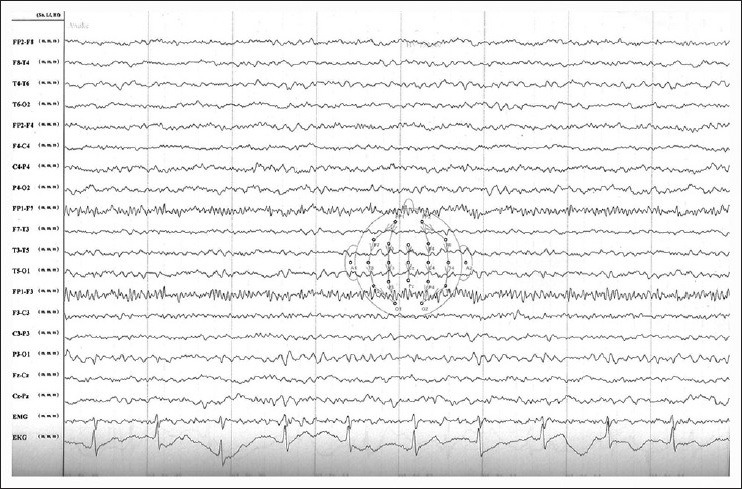
Electroencephalography of the patient during symptomatic stage of the disease showing background alpha activity with a 6-7 Hz slowing in the delta region with frontal predominance and #946;-fast activity
With the provisional diagnosis of Hashimoto Encephalopathy in mind, he was started on levothyroxine 150 μgm/day along with I.V. methylprednisolone. With this treatment, the seizures were controlled completely on the second day itself. Repeat assessments of thyroid hormone levels were done weekly. T3, T4, and TSH levels normalized, with second TSH at 7.52 IU/ml and anti-TPO antibodies were 1457 units after 2 weeks and 40 units at 2 months of treatment. A repeat EEG was normal, and a follow-up MRI showed no abnormality. He was started on oral prednisolone 60 mg/day but he developed one episode of hematemesis, after which oral steroids were stopped and he was started on azathioprine 100 mg in divided doses. He was also prescribed telmisartan for hypertension at a dose of 40 mg per day.
He was in remission for up to 4 months after the initial episode and discontinued his medications. His symptoms recurred, and this time they consisted of vague tingling sensations on the face with slurring of speech and loss of attention. Anti-thyroperoxidase levels were elevated at about 400 U/ml. An MR angiogram was repeated with spectroscopy, which revealed a lactate peak corresponding to 1.3 μm in the left temporal area. However, a probe placed on the right occipital are showed no activity, suggesting new onset activity. [Figure 4] He was not given steroids this time and merely started on azathioprine at 100 mg, and symptoms remitted as before. He was discharged on oral azathioprine with telmisartan, atorvastatin, and oral calcium supplements. Patient continues to stay in remission till today after about one and half years of therapy.
Figure 4.
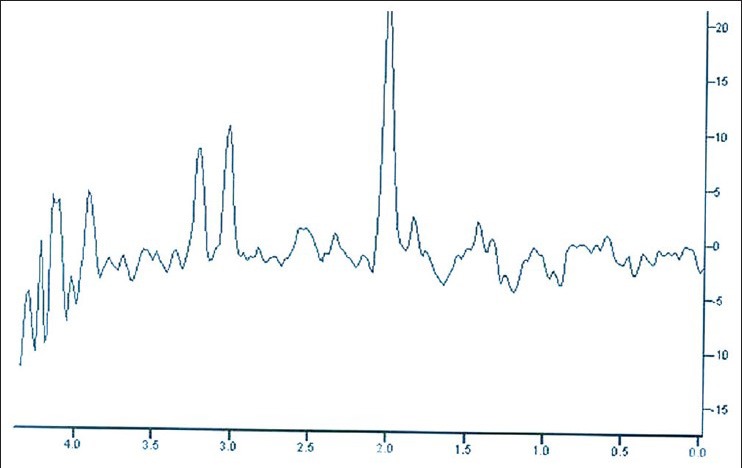
MR spectroscopy of the brain showing a normalized lactate peak during clinical remission of the disease with probe in right occipital region
Discussion
Hashimoto encephalopathy is an autoimmune process that involves formation of antibodies, possibly resulting in an acute encephalomyelitis like picture. Various pathogenic hypotheses, that include autoimmune vasculitis,[1,2,3] autoimmune reaction to antigens shared by thyroid and the CNS,[4] cerebral hypoperfusion,[5] and toxic effects of thyrotropin-releasing hormone.[4] The majority of patients with this condition are euthyroid and do not have a goiter. Anti-thyroid peroxidase antibodies have been found in high titers, although their role in the manifestation of CNS symptoms seems to be limited, with the hypothesis of the formation of a putative antigen complex eliciting an immune response in CNS. A raised level of the antibody may itself be the first clue to diagnosis. There are yet no evidence of any shared antigens between the brain and the thyroid.[4] Also, the antibody titers have generally been found to have no correlation with disease severity.[1]
It has been seen to encompass a broad age group between 14 to 78 years. Two types of clinical presentation have been observed, depending on which Hashimoto Encephalopathy has been subdivided into two classes. However, mostly the disease follows a sub-acute, relapsing-remitting, steroid-responsive encephalopathy characterized by protean neurologic and neuropsychiatric symptoms, diffuse electroencephalographic abnormalities, and increased titers of anti-thyroid antibodies in serum and/or in cerebrospinal fluid. The first type is characterized by acute stroke-like episodes with transient focal neurologic deficits and even epileptic seizures. The second form has a more insidious onset, progressing to dementia, psychosis, and coma over several weeks. No focal neurologic deficits are seen in the latter type, although neuropsychologic testing reveals severe cognitive deficits. Tremors are the most commonly observed symptom, followed by transient aphasia, myoclonus, gait ataxia, seizures, and sleep abnormalities.[6]
Since this condition shows a dramatic response to steroids, it has been renamed as steroid-responsive encephalopathy associated with Hashimoto thyroiditis (SREAT) instead of the conventional Hashimoto encephalopathy (HE).[1,5] However, several cases have been reported when the patients have not responded to steroids and alternative drugs have been tried in them. Several studies have suggested the role of intravenous immunoglobulins in the management of this condition.[7] However, patients have often respond to steroids and stay in remission as long as treatment is continued and relapse once they discontinue the drug. The most common reasons for discontinuation of steroids are intolerance and adverse effects related to steroids. Repeated administration with IVIg in this setting is not a practical and affordable option for this subset of patients, and it is desirable to look for alternative drugs patients can continue to take, which will act as steroid-sparing agents while being as effective in maintenance of remission. Several drugs including aspirin, azathioprine, mycophenolate mofetil have been tried in this setting and found to be variously successful. No long-term data exists on the effectiveness of these drugs in maintaining remission in these patients.[8,9]
Shaw et al. reported 5 cases with Hashimoto encephalopathy, in which response to various treatment options was observed. He treated one patient with a combination of steroids and azathioprine and ventriculo-peritoneal shunt and reported a substantial improvement with medications. The CT-head of that patient showed ventricular dilatation, and EEG showed diffuse slowing. However, the patient relapsed upon discontinuation of the drug.[1]
Spiegel et al. similarly used azathioprine after induction of remission with intravenous methylprednisolone and reported a remission period that lasted 5 months.[10]
Azathioprine is an imidazolyl derivative of 6-mercaptopurine. Following exposure to nucleophiles such as glutathione, azathioprine is cleaved to 6-mercaptopurine, which in turn is converted to additional metabolites that inhibit de novo purine synthesis in the body. 6-Thio-IMP, a fraudulent nucleotide, is converted to 6-thio-GMP and finally to 6-thio-GTP, which is incorporated into DNA. Cell proliferation is thereby inhibited, impairing a variety of lymphocyte functions. The mechanism of azathioprine in Hashimoto encephalopathy is supposed to be the same as that of steroids in suppressing inflammation and auto-reactive antibodies.
Our patient showed a complete remission with the use of azathioprine and continues to remain in remission for the last one and half years. A flare-up of the disease activity upon drug discontinuation was also well controlled with reinstitution of azathioprine. In addition, the follow-up anti-thyroperoxidase levels have shown a consistent decrease with improvement in patient status, an observation that hitherto differs from the published reports where clinical status of the patient has no relation with the levels of anti-thyroperoxidase levels observed.
The spectroscopy findings in our patient showed a lactate peak during both the times the patient was symptomatic and were normal with institution of immunosuppressants and clinical improvement of the patient. Presence of lactate in the areas of the brain usually indicates the areas where anaerobic metabolism is taking place. Lactate has been seen in spectroscopy of patients suffering from post-necrotic encephalopathy and acute necrotizing encephalopathy and in others like HIV encephalopathy. It has also been reported in patients suffering from brain abscesses and vascular tumors. It is usually seen in spectroscopy as a peak corresponding to 1.3 μm, and its presence in one particular area signifies an focus of anaerobic metabolism.[11] It was seen in the right occipital area of the brain during the first presentation of the patient and subsequently in the left temporal area during the relapse and disappeared completely upon treatment when the patient was in remission. Hence, future determination of a lactate peak may prove to be useful in corroborating with clinical picture in patients suffering from this disease.
An increasing number of cases are being diagnosed with Hashimoto encephalopathy due to unknown and multiple genetic susceptibility. Long-term oral steroids have been established as a means to suppress the symptoms and to keep the patient in remission. However, with the passage of time, the side-effects of steroids accumulate and lead to their own set of complications. Azathioprine could be used in this setting as an effective steroid sparing agent, useful not only in induction of remission but also in avoiding the morbidities associated with long-term steroid use. The corroboration observed from the presence of a lactate peak and its disappearance with improvement in disease activity could further enhance our understanding of the disease process that still presents a challenge to the medical community.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil
References
- 1.Shaw PJ, Walls TJ, Newman PK, Cleland PG, Cartlidge NE. Hashimoto's encephalopathy: A steroid-responsive disorder associated with high anti-thyroid antibody titres—report of 5 cases. Neurology. 1991;41:228–33. doi: 10.1212/wnl.41.2_part_1.228. [DOI] [PubMed] [Google Scholar]
- 2.Watemberg N, Willis D, Pellock J. Encephalopathy as the presenting symptom of Hashimoto's thyroiditis. J Child Neurol. 2000;15:66–9. doi: 10.1177/088307380001500116. [DOI] [PubMed] [Google Scholar]
- 3.Shibata N, Yamamoto Y, Sunami N, Suga M, Yamashita Y. Isolated angiitis of the CNS associated with Hashimoto's disease. Rinsho Shinkeigaku. 1992;32:191–8. [PubMed] [Google Scholar]
- 4.Nolte KW, Unbehaun A, Sieker H, Kloss TM, Paulus W. Hashimoto encephalopathy: A brainstem vasculitis? Neurology. 2000;54:769–70. doi: 10.1212/wnl.54.3.769. [DOI] [PubMed] [Google Scholar]
- 5.Latinville D, Bernardi O, Cougoule JP, Bioulac B, Henry P, Loiseau P, et al. Thyroidite d’Hashimoto et encéphalopatie myoclonique: Hypothèses pathogéniques. Rev Neurol (Paris) 1985;141:55–8. [PubMed] [Google Scholar]
- 6.Brain L, Jellinek EH, Ball K. Hashimoto's disease and encephalopathy. Lancet. 1966;2:512–4. doi: 10.1016/s0140-6736(66)92876-5. [DOI] [PubMed] [Google Scholar]
- 7.Jacob S, Rajabally YA. Hashimoto's encephalopathy: Steroid resistance and response to intravenous immunoglobulins. J Neurol Neurosurg Psychiatry. 2005;76:455–6. doi: 10.1136/jnnp.2004.049395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghika-Schmid F, Ghika J, Regli F, Dworak N, Bogousslavsky J, Städler C, et al. Hashimoto's myoclonic encephalopathy: An underdiagnosed treatable condition? Mov Disord. 1996;11:555–62. doi: 10.1002/mds.870110511. [DOI] [PubMed] [Google Scholar]
- 9.Korthbauer-Margreiter I, Sturzenegger M, Komor J, Baumgarter R, Hess CW. Encephalopathy associated with Hashimoto thyroiditis: Diagnosis and treatment. J Neurol. 1996;243:585–93. doi: 10.1007/BF00900946. [DOI] [PubMed] [Google Scholar]
- 10.Spiegel J, Hellwig D, Becker G, Muller M. Progressive dementia caused by Hashimoto's encephalopathy - report of two cases. Eur J Neurol. 2004;11:711–3. doi: 10.1111/j.1468-1331.2004.00909.x. [DOI] [PubMed] [Google Scholar]
- 11.Luiz Ramin S, Tagnola WA, Spotti AR. Proton magnetic resonance spectroscopy: Clinical applications in patients with brain lesions. Sao Paulo Med J. 2003;121:254–9. doi: 10.1590/S1516-31802003000600008. [DOI] [PMC free article] [PubMed] [Google Scholar]


