Abstract
OBJECTIVE
Assess the efficacy of Farabloc for treating chronic phantom limb pain (PLP).
DESIGN
Randomized, double-blind, placebo controlled trial.
SETTING
VA Long Beach Healthcare System Outpatient Amputee Clinic.
PARTICIPANTS
We randomized 57 subjects into two groups: true Farabloc (n=30) and sham Farabloc (n=27). Inclusion criteria included age ≥ 18 years, upper or lower extremity amputation with healed stump, and ≥ 3 episodes of PLP during previous 6 weeks.
INTERVENTIONS
Subjects received two true or sham Farabloc limb covers to be worn over the prosthesis and stumps 24 hours/day for 12 weeks.
MAIN OUTCOME MEASURES
Primary outcome measure was the numerical pain rating scale of PLP level (0-10). Secondary outcomes included overall pain level (0-10), PLP frequency/week, and the Veterans RAND 12-Item Health Survey (VR-12). We collected data at baseline, 6-week, and 12-week follow-up visits.
RESULTS
Demographic and clinical characteristics were not significantly different between groups. The true Farabloc group reported non-significant reductions in PLP from 5.9 (SD = 1.9) at baseline to 3.9 (SD = 1.7) at the 12-week follow-up. The sham Farabloc group also had non-significant reducations in PLP from 6.5 (SD = 1.8) to 4.2 (SD = 2.3). PLP did not differ significantly between the two groups at 6 weeks (mean difference, +0.8 [95% confidence intervals (CI), −1.4 to 3]) or at 12 weeks (mean difference, +0.2 [ 95% CI −1.9 to 2.3]). Similarly, overall pain level, PLP episodes/week, and VR-12 physical and mental health component scores did not differ between two groups at 6 weeks and 12 weeks.
CONCLUSIONS
True Farabloc did not significantly decrease PLP levels or frequency of PLP episodes/week, overall bodily pain levels, or VR-12 physical and mental health component scores compared with sham Farabloc in our Veteran amputee sample.
Keywords: Phantom Limb Pain, Farabloc, VR-12, Health-related Quality of Life
Phantom limb pain (PLP) is a painful sensation perceived in the missing limb after amputation and may be triggered by episodes of stump pain (1). A growing body of literature indicates that 50 to 80% of amputees may have PLP (2-5). Over 1.2 million individuals in the United States (US) suffer a limb loss each year (6, 7) primarily due to vascular disease, traumatic injury, diabetes mellitus, congenital defects, or malignancy (8). In the US, the highest prevalence of limb loss (19.4 per 1000) occurs in the elderly (individuals 65 years of age or older), and the majority of new amputations is caused by peripheral vascular disease (7). In the US military, amputations commonly occur in younger individuals (9, 10) who suffer from blast injuries due to high velocity weapons. Soldiers with amputations who were involved in previous military conflicts had higher mortality, underwent more surgery,(11) and were hospitalized twice as long as non-amputees (12). Therefore, PLP is a major cause of morbidity for the civilian, military, and veteran populations and its cost of care represents a major burden on the health care system.
The vast majority (50-80%) of amputees suffer from PLP (13), yet causes of PLP have not been specifically determined and a clear definition is lacking. PLP has been described as a painful sensation felt in the missing portion of a limb following amputation, although burning, tingling, and electric shock sensations are also experienced. These sensations are purported to decrease over time in frequency and duration, although prevalence remains constant (14). Residual limb pain (pain in the stump) and PLP are associated and may be difficult to differentiate (5, 15). In one study, subjects experiencing residual pain were twice as likely to have PLP and subjects having phantom sensations (any feelings experienced other than pain in the missing limb) were 11 times more at risk of having phantom pain (4). This evidence underscores the importance of residual limb pain and phantom sensations when characterizing PLP.
The etiology and pathophysiology of PLP are poorly understood. Some studies suggest PLP may be associated with somatosensory cortex reorganization, amputation cause, prosthetic use, time since amputation, gender, and age (2, 13, 14, 16). Other theories include spontaneously firing neuromas (17) and reorganization of the primary somatosensory cortex, subcortex, and thalamus (18, 19). Some have postulated that the central nervous system plays an important role in PLP(2). For instance, sensitization of neurons may occur at the spinal cord level as a result of upregulation of N-methyl D-aspartate (NMDA) receptors (20). Increased sodium conductance may play an important role as gallamine injection has been shown to produce PLP while lidocaine blocks it (21). These theories, however, do not completely explain the pathophysiology of PLP because it may also be caused by congenital defects.
Many amputees who suffer from PLP are underdiagnosed and suboptimally treated by their physiatrists, prosthetists, podiatrists, and other providers. For instance, 72% of clinicians believed that less than one-fifth of amputees suffer from severe PLP, whereas the prevalence of PLP is as high as 50-80% (22). While many patients with PLP are treated with pain medications and epidural injections, fewer than 10% of patients reported lasting pain relief from conventional medical treatment (23). Therefore, many amputees with PLP are disabled by their chronic pain. Conventional medical treatment has had limited success in treating phantom pain. Thus many amputees with PLP have turned to Farabloc, a noninvasive limb cover made with proprietary technology, to treat their phantom limb pain. Farabloc has been shown to block out high-frequency electromagnetic fields (24). Previous studies have suggested that Farabloc is efficacious in treating chronic PLP, fibromyalgia, and pain related to delayed-onset muscle soreness (24-26). However, the prior PLP study was limited by its small sample and short intervention time. The purpose of this study was to assess the efficacy of Farabloc for treating chronic phantom limb pain in a larger sample and longer intervention duration in a Veteran amputee population. In a double-blind, randomized, sham-controlled trial, we evaluated the impact of Farabloc for PLP pain and frequency and general health-related quality of life.
MATERIALS AND METHODS
This study was approved by the Long Beach VA Healthcare System Institutional Review Board and the Office of Research Protections for the U.S. Army Medical Research and Material Command. All subjects provided written informed consent prior to any study-related procedures being performed. Fifty-seven patients (56 men and 1woman) routinely treated in the prosthetics/amputee clinic at the VA Long Beach Healthcare System participated in this study. Inclusion criteria included subjects presenting with upper or lower extremity amputation with healed stump that experienced episodes or intermittent PLP, ≥ 3 episodes of PLP during the previous 6 weeks, and had not used Farabloc within the last 6 months. Subjects were excluded from the study if they had stump complications (e.g., cellulites and stump pain caused by new bone spur within past 12 months), previous use of Farabloc within 6 months, or were pregnant. Randomization of subjects was performed by Farabloc Corporation, and both investigators and subjects were blinded to their assignment. The Farabloc Corporation revealed the true assignment of subjects after the completion of data collection.
All subjects were measured for individual fit of the Farabloc limb cover at screening, and received 2 covers four weeks later at the baseline visit. Subjects were asked to wear the cover 24 hours per day, 7 days per week, for the duration of the study. A few subjects reported the cover repeatedly falling off the limb. We attempted to remedy this by using medical tape to tighten the limb cover so that it could be worn more snugly. Surveys were admistered and data collected at baseline, and at 6-week and 12-week follow-ups. Subjects received $50 honorarium for participating. Our primary outcome was the severity of PLP and overall bodily pain measured using the Numerical Pain Rating Scale (NPRS). The NPRS consists of a 10 cm line with numbers ranging from zero (no pain) to ten (worst pain possible). Subjects were asked to indicate the point on the line that represented their pain during an episode of PLP (27, 28). The secondary outcomes included PLP frequency/week, PLP frequency/month, and the Veterans RAND 12-Item Health Survey (VR-12) general health-related quality of life measure (29).
Student’s t-test was used to calculate statistical significance of pain improvement and frequency reduction and VR-12 [hysical and mental health component scores between the true and sham Farabloc groups. Effects were evaluated on an intent-to-treat basis, and participants who did not complete the follow-up visits were considered not to have had any changes in scores. A two-sided P value of less than 0.05 indicated statistical significance and results were presented as between-group differences with 95% confidence intervals. The sample size was calculated to ensure that we would have enough statistical power to conduct analyses for the primary outcome of NPRS. Effective sample size calculations for this study are based on a prior study (26) evaluating the use of Farabloc in patients with PLP with a two-period crossover design. The effect size from the first period was δ= 0.637 while the effect size from the second period of the cross-over was δ= 0.820. Assuming that the overall effect of the treatment is at least the average of the two, δ= 0.729, with N = 30 in each group (N = 60), we will have at least 80% power to detect a difference between the two groups with a two-sided α = 0.05.
RESULTS
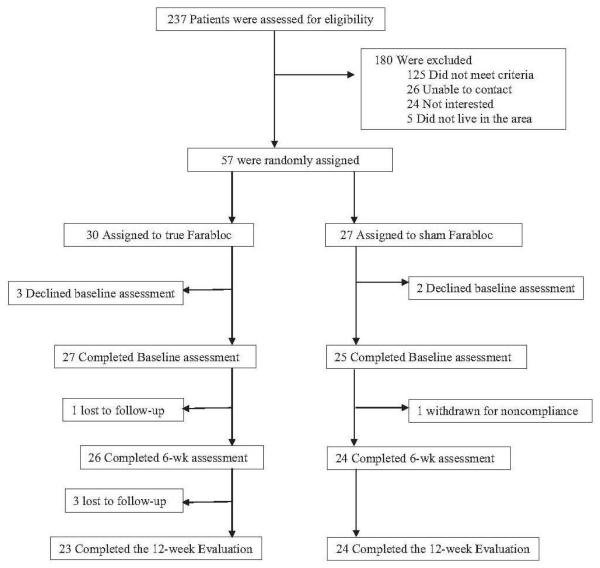
Between January 2009 and April 2010 we screened 237 patients by telephone. We excluded 180 patients for the following reasons: 125 did not meet inclusion criteria, 26 could not be contacted, 24 declined, and 5 lived outside of Long Beach and couldn’t come for follow-up visits. Fifty-Seven eligible participants were randomly assigned to either the true Farabloc (n=30) or sham Farabloc (n=27) group (Fig. 1).
Fig 1.
Participant flowchart.
Baseline Study Population Demographic and Clinical Chracteristics
The demographic and clinical characteristics of the sham and true Farabloc were not significantly different (table 1). The mean age for the sham Farabloc group was 67 and the mean age for the true Farabloc was 62. Both true and sham Farabloc groups had similar rates for causes of amputation. Seventy-three percent of subjects in the true Farabloc group reported cause of amputation as diabetes and peripheral vascular disease versus 70% of the sham Farabloc group. Seventeen percent of the true Farabloc reported the cause of amputation as trauma versus 26% of the sham Farabloc group, and 10% of the true Farabloc reported cause of amupation as other versus 4% in the sham Farabloc group. Fifty-three percent of the true Farabloc group had below knee amputation and 47% had above knee amputation. Similarly, 59% of the sham Farabloc group had below knee amputation and 41% had above knee amputation.
Table 1.
Study Population Demographic and Clinical Characteristics
|
True Farabloc (n=30) |
Sham Farabloc (n=27) |
|
|---|---|---|
|
| ||
| Age | ||
| (mean year, SD) | 61.8, 12.3 | 65.8, 13.4 |
|
| ||
| Gender (%) | ||
| Men | 97% | 100% |
| Women | 3% | 0% |
|
| ||
| Cause of Amputation (%) | ||
| Diabetes/PVD/Infection/Osteomyelitis | 73% | 70% |
| Trauma | 17% | 26% |
| Other | 10% | 4% |
|
| ||
| Type of Amputation (%) | ||
| Below Knee | 53% | 59% |
| Above Knee | 47% | 41% |
|
| ||
| Time Since Amputation | ||
| (mean year, SD) | 10.5, 15.3 | 15.6, 19.5 |
|
| ||
| Baseline Phantom Limb Pain Level | 4.7, 2.4 | 4.7, 2.6 |
| (mean pain level, SD) | ||
|
| ||
| Baseline Overall Pain Levels | 5.9, 1.9 | 4.5, 2.2 |
| (mean pain level, SD) | ||
Table 2 compares baseline, 6 week and 12 weeks outcomes between the true and sham Farabloc groups. The true and sham Farabloc groups had similar levels of PLP (5.9 ± 1.9 vs. 6.5 ± 1.8) and overall bodily pain (4.7 ± 2.4 vs. 4.8 ± 2.6). At 6 week the sham Farabloc had lower PLP levels than did the true Farabloc group (4.3 ± 2.1 vs. 4.5 ± 2.0), whereas the true Farabloc group had lower PLP levels than did the sham Farabloc group at 12 weeks (3.9 ± 1.7 vs. 4.2 ± 2.3). The similar trends are observed in overall pain levels, PLP frequency/week, and PLP frequency/month.
Table 2.
Comparison of True Farabloc and Sham Farabloc Groups on Pain and Health-Related Quality of Life
| True Farabloc (n=30) | Sham Farabloc (n=27) | |||||
|---|---|---|---|---|---|---|
| (Mean, SD) | Baseline | 6 week | 12 week | Baseline | 6 week | 12 week |
|
Phantom
limb pain level |
5.9, 1.9 | 4.5, 2.0 | 3.9, 1.7 | 6. 5, 1.8 | 4.3, 2.1 | 4.2, 2.3 |
|
Overall
Pain level |
4.7, 2.4 | 4.6, 2.5 | 3.4, 2.0 | 4.8, 2.6 | 4.0, 2.6 | 4.7, 2.2 |
|
PLP Fre-
quency per week |
10.7, 15.4 | 6.1, 12.4 | 4.3, 9.8 | 20.0, 27.0 | 11.5, 23.0 | 11.9, 23.4 |
|
PLP Fre-
quency per month |
48.7, 68.9 | 24.1, 49.5 | 17.5, 39.3 | 62.3, 84.2 | 21.2, 37.4 | 21.1, 39.2 |
|
VR-12
Physical Component Score |
31.5, 8.3 | 32.1, 8.4 | 31.8, 7.8 | 33.9, 9.0 | 34.2, 9.5 | 33.7, 7.9 |
|
VR-12
Mental component Score |
50.0, 12.0 | 49.1, 11.7 | 48.0, 10.7 | 47.4, 13.8 | 49.0, 12.5 | 49.2, 12.0 |
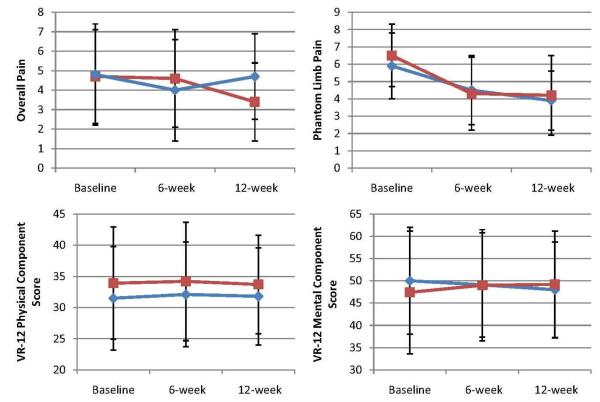
Table 3 show changes from baseline to 6 and 12 weeks for all outcomes. Figure 2 shows changes from basesline to 6 and 12 weeks for overall pain, PLP, VR-12 physical health component score, and VR-12 mental health component score. At 6 weeks, the sham Farabloc group had a greater reduction in PLP than did the true Farabloc group (−2.2 points [95% confidence interval {CI}, −6.2 to 1.8] vs. −1.4 points [95% CI, −6.4 to 3.6]). The mean between group difference was 0.8 point (95% CI, −3.6 to 5.2; p=0.78) but the difference was not significant. Similarly, at 12 weeks the sham Farabloc group had a greater reduction in PLP than did the true Farabloc group (−2.4 points [95% CI, −6.8 to 2.0] vs. −2.2 points [95% CI, −6.4 to 2.0]). The mean between group difference was 0.2 point (95% CI, −4.0 to 4.4; p=0.38) but the difference was not significant.
Table 3.
Mean Changes in Primary and Secondary Outcomes
| Variable | Mean Change from Baseline (95% CI) |
Between-Group Difference (95% CI) |
||
|---|---|---|---|---|
|
| ||||
| True Farabloc | Sham Farabloc | True vs. Sham | P Value | |
| Phantom Limb Pain | ||||
| Week 6 | −1.4 (−6.4 to 3.6) | −2.2 (−6.2 to 1.8) | 0.8 (−3.6 to 5.2) | 0.78 |
| Week 12 | −2.2 (−6.4 to 2.0) | −2.4 (−6.8 to 2.0) | 0.2 (-4.0 to 4.4) | 0.38 |
|
| ||||
| Overall Pain | ||||
| Week 6 | −0.2 (−5.2 to 4.8) | −0.7 (−4.7 to 3.3) | 0.5 (−4.1 to 5.1) | 0.55 |
| Week 12 | −1.4 (−5.2 to 2.4) | −0.2 (−5.2 to 4.8) | −1.2 (−5.6 to 3.2) | 0.22 |
|
| ||||
| PLP Frequency/wk | ||||
| Week 6 | −4.7 (−30.7 to 21.3) | −9.2 (−43.3 to 25.0) | 4.5 (−25.7 to 34.7) | 0.08 |
| Week 12 | −6.6 (−31.6 to 18.4) | −9.5 (−52.3 to 33.3) | 2.9 (−31.1 to 36.9) | 0.06 |
|
| ||||
| PLP Frequency/mo | ||||
| Week 6 | −24.8 (−139 to 89) | −39.9 (−74 to -5.7) | 15.1 (−59.1 to 89.3) | 0.10 |
| Week 12 | −31.7 (−165 to 102) | −40.1 (−211.9 to 131.7) | 8.4 (−144.2 to 161) | 0.09 |
|
| ||||
| VR-12 PCS | ||||
| Week 6 | 0.6 (−10.0 to 11.2) | 0.3 (−12.9 to 13.5) | 0.3 (−9.5 to 10.1) | 0.19 |
| Week 12 | 0.3 (−9.7 to 10.3) | −0.2 (−13.2 to 12.8) | 0.5 (−11.1 to 12.1) | 0.14 |
|
| ||||
| VR-12 MCS | ||||
| Week 6 | −1.0 (−8.0 to 6.0) | 1.6 (−13.0 to 16.2) | −2.6 (−13.4 to 8.2) | 0.58 |
| Week 12 | −2.1 (−10.5 to 6.3) | 1.8 (−14.0 to 17.6) | −3.9 (−16.1 to 8.3) | 0.78 |
Fig 2.
Mean changes in 4 outcomes at 6 and 12 weeks, according to treatment groups. Outcome scores are shown for the true Farabloc group (blue diamonds) and sham Farabloc group (red squares). The values shown are unadjusted means; I bars indicate 95% CIs. Measurements were obtained at baseline, 6 weeks, and 12 weeks. Overall pain and PLP assessments were made on a visual analog scale from 0 (no pain) to 10 (excruciating pain). Summary scores on the physical and mental components of the VR-12 are scored on a T-score metric (mean ± SD: 50± 10), with higher scores indicating better health status.
At 6 weeks, the sham Farabloc had a greater decrease in overall pain than did the true Farabloc group (−0.7 points [ 95% CI, −4.7 to 3.3] vs. −0.2 points [95% CI, −5.2 to 4.8]). The mean between group difference was 0.5 point (95% CI, −4.1 to 5.1; p=0.55) but the difference was not significant. At 12 weeks, the true Farabloc had a greater decrease in overall pain than the sham. The mean between group difference was −1.2 point (95% CI, −5.6 to 3.2; p=0.22) but the difference was not significant. The similar trends are also seen in PLP frequency/ week, PLP frequency/ month, and VR-12 mental component score.
DISCUSSION
This randomized, controlled trial shows that true and sham Farabloc did not differ significantly in change over 6-weeks and 12-weeks in PLP, overall pain, PLP frequency, or general physical and mental health. Our results contradict the findings of a prior study (26), which showed that Farabloc was efficacious in reducing PLP levels. Although the previous study was a randomized, double-blind trial, it employed a crossover design with a “wash-out” or no treatment period to control for possible carry-over effect of treatment. However, it was unclear how long the “wash-out” period was, which may potentially confound the efficacy of the true Farabloc interventions. In addition, the researchers enrolled 52 subjects, but conducted analysis on 34 subjects who completed the trial. Because these investigators choose to not use intent-to-treat analysis, the effect of the true Farabloc group may have been biased. Our study had a much longer follow-up period of 12 weeks compared to the prior study, which better evaluated the true efficacy of Farabloc and its long-term efficacy.
The proposed mechanisms by which Farabloc might affect PLP lie within its ability to shield high frequency electromagnetic fields or to act like a “Faraday Cage.” It is hypothesized that high frequency electromagnetic fileds may cause cellar damage and trigger PLP and the blockade of such stimulus will decrease and prevent PLP. The sham Farabloc used in our study was an adequate placebo control because it used the same nylon fabric and is indistinguishable from the true Farabloc material. Our study provides additonal support for the importance of including a placebo control group in conducting clinical trials to control for potential biases of regression to mean and selection bias. Although clinical trials involving medications commonly use a placebo control group, this occurs less commonly in clinical trials focusing on device intervention or surgical procedures because it is much more difficult to design an adequate placebo control. If we had selected “usual care” as a control group instead of sham Farabloc, we probably would’ve falsely concluded the Farabloc material was more efficacious in reducing PLP compared with usual care. To evaluate the true efficacy of Farabloc, it was essential that we included a sham, placebo-control group.
Our study had some limitations. Our study population had a high proportion of amputations due to diabetes and peripheral disease in contrast to trauma as the main cause of amputation in prior study. It is possible that Farabloc may be efficacious in treating chronic phantom limb pain caused by trauma, but not diabetes. Since only 22% of our population reported trauma as cause of their amputation, we are unable to perform a subgroup analysis to evaluate the effficacy of Farabloc on this subgroup. To appropriately address whether Farabloc is efficacious in treating chronic PLP caused by trauma, one needs to conduct a new study focusing on amputee population with trauma as cause of amputation. Another potential limiation is that our subjects used Farabloc as an insert instead of laminating it into the prosthesis. It is possible that using Farabloc as an insert may have resulted in more discomfort and suboptimal fitting and cause more subjects to drop out in the true Farabloc group. However, the randomization and double-blind design of our study should’ve minimized this potential bias and subjects in both true and sham Farabloc group reported comparable, high level of compliance and minimal levels of discomfort.
CONCLUSION
True Farabloc and sham Farbloc did not significantly decrease phantom limb pain levels, overall pain levels, and frequency of phantom limb pain episodes/week in our veteran amputee sample. Farabloc does not appear to be an efficacious, adjunctive thrapy for chronic phantom limb pain in Veteran amputees whose main cause of amputation is diabetes or peripheral disease.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Alex York for their assistance in study design, Farabloc Corporation (Frieder Kempe and Don Nixdorf) for providing the study materials, and Russell Ward for help with recruitment. This project is supported by the US Army Medical Research and Material Command under Award no. W81XWH-06-1-0279 through the Samueli Institute.
SOURCE OF FUNDING: Hays was also supported in part by NIA grants (P30AG021684 and P30-AG028748) and the NCMHD (P20MD000182).
Footnotes
FINANCIAL DISCLOSURE/CONFLICT OF INTEREST: We certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated and we certify that all financial and material support for this research and work are clearly identified in the title page of the manuscript.
SUPPLIERS Farabloc Development Corporation, contact person: Frieder Kempe, 211-3030 Lincoln Avenue, Coqitlam, British Columbia, Canada V3B 6B4, Tel (604) 941-8201.
REFERENCES
- 1.Sherman R. Phantom limb pain: Mechanism based management. Clin Podiatr Med Surg. 1994;11(1):85–106. [PubMed] [Google Scholar]
- 2.Dijkstra PU, Geertzen JHB, Stewart R, van der Schans CP. Phantom pain and risk factors: A multivariate analysis. J Pain Symp Mgmt. 2002;24(6):578–85. doi: 10.1016/s0885-3924(02)00538-9. [DOI] [PubMed] [Google Scholar]
- 3.Ephraim PL, Wegner ST, MacKenzie EJ, Dillingham TR, Pezzin LE. Phantom pain, residual limb pain, and back pain in amputees: Results of a national survey. Arch Phys Med Rehabil. 2005;86:1910–9. doi: 10.1016/j.apmr.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 4.Kooijman CM, Dijkstra PU, Geertzen JH, Elzinga A, van der Schans CP. Phantom pain and phantom sensations in upper limb amputees: An epidemiological study. Pain. 2000;87(1):33–41. doi: 10.1016/S0304-3959(00)00264-5. [DOI] [PubMed] [Google Scholar]
- 5.Richardson C, Glenn S, Nurmikko T, Horgan M. Incidence of phantom phenomena including phantom limb pain 6 months after major lower limb amputation in patients with peripheral vascular disease. Clin J Pain. 2006;22(4):353–8. doi: 10.1097/01.ajp.0000177793.01415.bd. [DOI] [PubMed] [Google Scholar]
- 6.Adams P, Hendershot G, Marano M. Current estimates from the National Health Interview Survey. National Center for Health Statistics; Bethesda, MD: 1996. [PubMed] [Google Scholar]
- 7.National Limb Loss Information Center 2007 Available from: http://www.amputee-coalition.org/nllic_faq.html.
- 8.Ephraim PL, Dillingham TR, Sector M, Pezzin LE, MacKenzie EJ. Epidemiology of limb loss and congenital limb deficiency: A review of the literature. Arch Phys Med Rehab. 2003;84(5):747–61. doi: 10.1016/s0003-9993(02)04932-8. [DOI] [PubMed] [Google Scholar]
- 9.Lin DL, Kirk KL, Murphy KP, McHale KA, Doukas WC. Evaluation of orthopaedic injuries in Operation Enduring Freedom. J Orthop Trauma. 2004;18(5):300–5. doi: 10.1097/00005131-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Potter BK, Scoville CR. Amputation Is Not Isolated : An Overview of the US Army Amputee Patient Care Program and Associated Amputee Injuries. J Am Acad Orthop Surg. 2006;14:S188–S90. doi: 10.5435/00124635-200600001-00041. [DOI] [PubMed] [Google Scholar]
- 11.Korver AJ. Amputees in a hospital of the International Committee of the Red Cross. Injury. 1993;24(9):607–9. doi: 10.1016/0020-1383(93)90124-o. [DOI] [PubMed] [Google Scholar]
- 12.Lin DL, Kirk KL, Murphy KP, McHale KA, Doukas WC. Evaluation of orthopaedic injuries in Operation Enduring Freedom. J Orthop Trauma. 2004;18(5):300–5. doi: 10.1097/00005131-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Sumitani M, Miyauchi S, Yozu A, Otake Y, Saitoh Y, Yamada Y. Phantom limb pain in the primary motor cortex: Topical review. J Anesth. 2010;24:337–41. doi: 10.1007/s00540-010-0921-6. [DOI] [PubMed] [Google Scholar]
- 14.Bosmans JC, Geertzen JHB, Post WJ, van der Schans CP, Dijkstra PU. Factors associated with phantom limb pain: A 3 ½ -year prospective study. Clin Rehab. 2010;24:444–53. doi: 10.1177/0269215509360645. [DOI] [PubMed] [Google Scholar]
- 15.Wartan SW, Hamann W, Wedley JR, McColl I. Phantom pain and sensation among British veteran amputees. Br J Anaesthesia. 1997;78(6):652–9. doi: 10.1093/bja/78.6.652. [DOI] [PubMed] [Google Scholar]
- 16.Wrigley PJ, Press SR, Gustin SM, Macefield VG, Gandevia SC, Cousins MJ, Middleton JW, Henderson LA, Siddall PJ. Nueropathic pain and primary somatosensory cortex reorganization following spinal cord injury. Pain. 2009;141:52–9. doi: 10.1016/j.pain.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Amir R, Devor M. Ongoing activity in neuroma afferents bearing retrograde sprouts. Brain Res. 1993;630(1-2):283–8. doi: 10.1016/0006-8993(93)90667-c. [DOI] [PubMed] [Google Scholar]
- 18.Flor H, Elbert T, Muhlnickel W, Pantev C, Wienbruch C, Taub E. Cortical reorganization and phantom phenomena in congenital and traumatic upper-extremity amputees. Exp Brain Res. 1998;119(2):205–12. doi: 10.1007/s002210050334. [DOI] [PubMed] [Google Scholar]
- 19.Jain N, Florence SL, Kaas JH. Reorganization of Somatosensory Cortex After Nerve and Spinal Cord Injury. New Physiol Sci. 1998;13:143–9. doi: 10.1152/physiologyonline.1998.13.3.143. [DOI] [PubMed] [Google Scholar]
- 20.Doubell TP, Mannion RJ, Woolf CJ. The dorsal horn: state-dependent sensory processing, plasticity and the generation of pain. In: Wall PD, Melzack R, editors. Textbook of Pain. Churchill Livingstone; Edinburgh: 1999. [Google Scholar]
- 21.Chabal C, Jacobson L, Russell LC, Burchiel KJ. Pain responses to perineuromal injection of normal saline, gallamine, and lidocaine in humans. Pain. 1989;36(3):321–5. doi: 10.1016/0304-3959(89)90091-2. [DOI] [PubMed] [Google Scholar]
- 22.Whyte A, Niven CA. The illusive phantom: does primary care meet patient need following limb loss? Disabil Rehabil. 2004 Jul 22 5;Aug 22 5;26(14-15):894–900. doi: 10.1080/09638280410001708904. [DOI] [PubMed] [Google Scholar]
- 23.Sherman R, Sherman CJ, Parker L. Chronic phantom and stump pain among American veterans: results of a survey. Pain. 1984;18:83–95. doi: 10.1016/0304-3959(84)90128-3. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Clement D, Taunton J. The efficacy of Farabloc, an electromagnetic shield, in attentuating delayed-onset muscle soreness. Clin J Sp Med. 2000;10:15–21. doi: 10.1097/00042752-200001000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Bach GL, Clement DB. Efficacy of Farabloc as an analgesic in primary fibromyalgia. Clin Rheum. 2007;26:405–10. doi: 10.1007/s10067-006-0494-9. [DOI] [PubMed] [Google Scholar]
- 26.Conine TA, Hershler C, Alexander SA, Crisp RT. The efficacy of Farabloc in the treatment of phantom limb pain. Can J Rehab. 1993;6(3):155–61. [Google Scholar]
- 27.Jensen MP, Turner JA, Romano JM. What is the maximum number of levels needed in pain intensity measurement? Pain. 1994;58(3):387–92. doi: 10.1016/0304-3959(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 28.Jensen MP, Turner JA, Romano JM, Fisher LD. Comparative reliability and validity of chronic pain intensity measures. Pain. 1999;83(2):157–62. doi: 10.1016/s0304-3959(99)00101-3. [DOI] [PubMed] [Google Scholar]
- 29.Kazis LE, Ren XS, Lee A. Health status in VA patients: results from the Veterans health study. Am J Med Qual. 1999;14(1):28–38. doi: 10.1177/106286069901400105. al e. [DOI] [PubMed] [Google Scholar]