Abstract
Chronic alcohol consumption is a leading cause of chronic liver disease worldwide, leading to cirrhosis and hepatocellular carcinoma. currently, the most widely used model for alcoholic liver injury is ad libitum feeding with the Lieber-DeCarli liquid diet containing ethanol for 4–6 weeks; however, this model, without the addition of a secondary insult, only induces mild steatosis, slight elevation of serum alanine transaminase (alt) and little or no inflammation. Here we describe a simple mouse model of alcoholic liver injury by chronic ethanol feeding (10-d ad libitum oral feeding with the Lieber-DeCarli ethanol liquid diet) plus a single binge ethanol feeding. this protocol for chronic-plus-single-binge ethanol feeding synergistically induces liver injury, inflammation and fatty liver, which mimics acute-on-chronic alcoholic liver injury in patients. this feeding protocol can also be extended to chronic feeding for longer periods of time up to 8 weeks plus single or multiple binges. chronic-binge ethanol feeding leads to high blood alcohol levels; thus, this simple model will be very useful for the study of alcoholic liver disease (ALD) and of other organs damaged by alcohol consumption.
INTRODUCTION
The NIAAA model
ALD, a major cause of morbidity and mortality worldwide, includes a broad spectrum of disorders, ranging from simple steatosis to severe forms of liver injury such as steatohepatitis, cirrhosis and hepatocellular carcinoma1–5. Almost all heavy drinkers develop fatty liver, but only 20–40% of them develop more severe forms of ALD, and the underlying mechanisms that contribute to disease progression remain largely unknown. Several risk factors for advancement to ALD have been suggested, including sex, obesity, dietary factors, non-sex-linked genetic factors, drinking patterns and smoking1–5. Among these risk factors, one’s drinking pattern has been shown to markedly affect alcoholic liver injury and cause damage to other organs6–8. However, how a drinking pattern affects the progression of ALD, and the underlying mechanisms remain unknown. Recently, we have developed a chronic-plus-binge alcohol feeding model in mice9, which is similar to the drinking pattern in many alcoholic hepatitis patients who have a background of chronic drinking for many years (chronic) and a history of recent excessive alcohol consumption (binge)10–12. Such chronic-plus-binge ethanol feeding synergistically induced steatosis, liver injury and inflammation in mice9. Other laboratories have also used the chronic-plus-binge ethanol feeding model, with modifications, in mice and rats, and produced marked increases in steatosis and liver injury13,14. Because chronic feeding plus a single binge ethanol feeding can achieve markedly higher blood alcohol levels compared with chronic or binge feeding alone9, the protocol described here will be very useful not only for the study of ALD pathogenesis but also for the study of alcohol-related damage to other organs, such as the pancreas, heart, kidney, lung and CNS.
Comparison with human ALD and other animal models
Patients with mild and early ALD (such as simple steatosis and mild steatohepatitis) usually have no obvious clinical symptoms. Serum tests and liver histology analyses often reveal elevation of ALT and aspartate transaminase (AST), existence of steatosis, ballooning of hepatocytes, neutrophilic infiltration and Mallory-Denk hyaline inclusions in the liver. Severe forms of alcoholic hepatitis and ALD are associated with elevation of serum ALT and AST, abdominal pain and tenderness, fatigue, jaundice and so on.
Table 1 lists several models that have been used to study alcoholic liver injury in mice, which represent mild and early stages of human ALD. Among them, the model of ad libitum feeding with the Lieber-DeCarli diet containing ethanol for 4 weeks has been widely used by many laboratories15–20. However, this model only induces mild steatosis and slight elevation of serum ALT, with little or no liver inflammation15–20. Stepwise feeding with the Lieber-DeCarli ethanol diet up to 12 weeks has been shown to induce remarkable fatty liver but only mild elevation of serum ALT21. The Tsukamoto-French model induces severe steatosis, mild liver inflammation and mild fibrosis through continuous intragastric feeding22–25. Although this model is very useful for the study of ALD pathogenesis, it has limited use owing to its technical difficulty, and its requirement for intensive medical care and expensive equipment.
TABLE 1.
Mouse models used for the study of alcoholic liver injury.
| Mouse models | Advantages and disadvantages | Refs. |
|---|---|---|
| Chronic ethanol feeding (10 d or longer) plus a single binge (the NIAAA model) |
Easy to perform Marked elevation of ALT and steatosis Short-term (10 d) feeding with no mortality rate No liver fibrosis |
9 |
| Chronic ethanol feeding (4–6 weeks or longer) plus multiple binges (twice a week during the chronic feeding) (the NIAAA model) |
Easy to perform Mild elevation of ALT and marked steatosis Long-term feeding plus multiple binges with a high mortality rate No liver fibrosis |
a |
| Chronic ethanol feeding (4 weeks) plus three binges during the last 3 d of feeding |
Easy to perform Marked elevation of ALT and steatosis Long-term feeding plus multiple binges with a high mortality rate Fibrosis was not reported |
14 |
| Chronic feeding (4- to 6-week feeding model is widely used; some studies used 12-week feeding) |
Easy to perform Mild elevation of ALT and steatosis Long-term feeding with a low mortality rate No liver fibrosis |
15–21 |
| Intragastric infusion (Tsukamoto-French model) | Difficult to perform Requirement for intensive medical care Marked elevation of ALT and steatosis Long-term feeding with a high mortality rate Mild liver fibrosis |
22–24 |
| Acute gavage | Easy to perform Mild elevation of ALT and steatosis Short-term feeding with no mortality rate No liver fibrosis |
20,26–29 |
| Ad libitum oral alcohol in drinking water | Easy to perform Minimal elevation of ALT and mild steatosis Short- or long-term feeding with no mortality rate No liver fibrosis |
30,31 |
H.W., A.B. and B.G., unpublished data.
Acute gavage of a single dose or multiple doses of ethanol has also been used, but only induces hepatic steatosis and slight elevation of serum ALT and AST20,26–29. Administration of various concentrations of ethanol in drinking water given as the only water source for longer-term periods has been shown to cause many immune abnormalities30,31 and mild steatosis, but has little effects on serum ALT and AST and liver inflammation32,33 (N. Horiguchi and B.G., unpublished data). This ad libitum drinking model alone has not been widely used to study the pathogenesis of ALD, but it has been combined with secondary insults to study alcoholic fibrosis and cancer34,35.
The chronic-binge ethanol feeding model described here is cost and time efficient in addition to being very easy to perform. We have demonstrated that feeding mice ad libitum with a Lieber-DeCarli ethanol diet for 10 d followed by gavage of a single dose of ethanol (5 g kg−1) resulted in substantial elevation of serum ALT and AST levels, with the peak levels 9 h post gavage in C57BL/6 mice9, which are much higher than those from mice with ad libitum chronic feeding alone15–20. In addition, this chronic-plus-binge ethanol feeding also closely reproduces the drinking behaviors and acute-on-chronic liver injury in patients with ALD who are often chronic and binge drinkers10–12.
The chronic-binge ethanol feeding model has also been extended to chronic feeding for longer periods of time plus multiple binges. For example, we have examined chronic ad libitum feeding (4–6 weeks) plus multiple binges (twice a week during the 4–6 week feeding) in 8- to 10-week-old male C57BL/6 mice. This feeding protocol resulted in extensive steatosis, but, surprisingly, we found that serum ALT and AST levels were only slightly elevated (see Anticipated Results) and we observed no obvious liver fibrosis (H.W., unpublished data). The reason why chronic plus multiple binges of ethanol feeding induced lower levels of serum ALT and AST than chronic feeding plus a single binge is not known. In general, serum ALT and AST levels reflect acute liver injury and are usually lower in chronic liver injury than in acute liver injury. This may be why chronic feeding plus multiple binges (multiple binges are considered to be chronic) resulted in lower serum ALT and AST compared with chronic feeding plus a single binge (a single binge is considered to be acute). Recently, Petrasek et al.14 fed mice with an ethanol diet for 4 weeks, followed by three binges during the last 3 d of feeding, which resulted in extensive steatosis and elevation of serum ALT, but was also associated with an increased mortality rate.
Experimental design
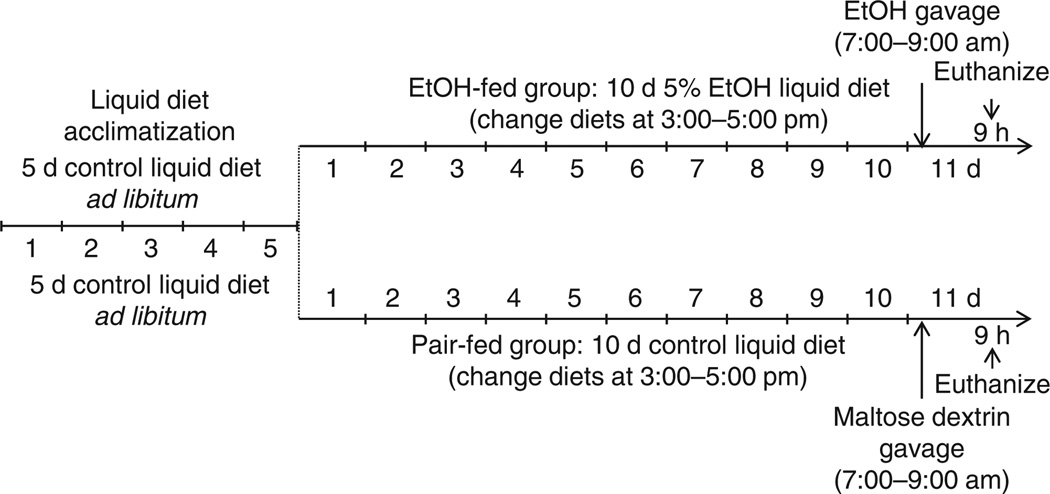
A basic overview of the experimental design routinely used in our laboratory is outlined in Figure 1.
Figure 1.
Overview of the NIAAA model procedure. Mice are initially fed the control Lieber-DeCarli diet ad libitum for 5 d to acclimatize them to liquid diet and tube feeding. Afterward, ethanol (EtOH)-fed groups are allowed free access to the ethanol Lieber-DeCarli diet containing 5% (vol/vol) ethanol for 10 d, and control groups are pair-fed with the isocaloric control diet. At day 11, ethanol-fed and pair-fed mice are gavaged in the early morning with a single dose of ethanol (5 g kg−1 body weight) or isocaloric maltose dextrin, respectively, and euthanized 9 h later. This model can be extended to longer periods of chronic feeding (up to 8 weeks) plus single or multiple binges. All of the animal experiments were approved by the NIAAA Animal Care and Use Committee.
Strains of mice
The C57BL/6 mice are an alcohol-preferring strain and generally the best strain to use for ad libitum ethanol feeding. Many other strains, including BALB/c and DBA, either resist eating the diet with sufficiently high ethanol concentrations to induce damage or are so adversely affected by the 5% alcohol-containing diet that they lose weight and have a high mortality rate before completion of the study36. In our laboratory, when investigating genetically modified mice that are not on a C57BL/6 background, we usually backcross them into the C57BL/6N background for six generations and subsequently breed the heterozygous mice to produce knockout mice and littermate wild-type control mice, or backcross them into the C57BL/6N background for nine or more generations and subsequently breed the homozygous mice to produce knockout mice and use C57BL/6N mice as controls.
Age of mice
Eight- to ten-week-old male mice with body weight more than 20 g or 10- to 12-week-old female mice with body weight more than 19 g are used for the chronic-binge feeding experiments. Younger and lighter mice frequently develop weight loss and hypothermia, resulting in an increased mortality rate. Older mice can be used but are more expensive.
MATERIALS
REAGENTS
Mice
 Animal studies must conform to all relevant ethics and animal welfare regulations and must be reviewed and approved by the appropriate governmental and institutional animal care and use committees.
Animal studies must conform to all relevant ethics and animal welfare regulations and must be reviewed and approved by the appropriate governmental and institutional animal care and use committees.  It is important to note that C57BL/6 mice are generally the best strain for ad libitum ethanol feeding. Other strains may require lengthy acclimatization to the ethanol-containing diet to ensure adequate intake of a 5% (vol/vol) ethanol-containing diet, or may not be able to tolerate the adverse effects of the ethanol-containing diet and have a high mortality rate before completion of the study.
It is important to note that C57BL/6 mice are generally the best strain for ad libitum ethanol feeding. Other strains may require lengthy acclimatization to the ethanol-containing diet to ensure adequate intake of a 5% (vol/vol) ethanol-containing diet, or may not be able to tolerate the adverse effects of the ethanol-containing diet and have a high mortality rate before completion of the study.  For experiments, we recommend using male mice 8–10 weeks of age or older and female mice 10–12 weeks of age or older, weighing at least 20 and 19 g, respectively. Younger and lighter mice may have an increased mortality rate.
For experiments, we recommend using male mice 8–10 weeks of age or older and female mice 10–12 weeks of age or older, weighing at least 20 and 19 g, respectively. Younger and lighter mice may have an increased mortality rate.Kits for serum ALT, AST, triglyceride or cholesterol (Butler Schein Animal Health Supply: ALT, cat. no. 033257; AST, cat. no. 033259; triglyceride cat. no. 033275; cholesterol cat. no. 033262)
Isoflurane
Formalin, 10% (wt/vol) formalin, buffered (Fisher Scientific, cat. no. SF 100-20)
Liquid nitrogen
Diet supplies
Lieber-DeCarli ‘82 Shake and Pour control liquid diet (Bio-Serv, product no. F1259SP; see Reagent Setup)
Lieber-DeCarli ‘82 Shake and Pour ethanol liquid diet (Bio-Serv, product no. F1258SP; see Reagent Setup). The caloric profile of these diets is described in Table 2.
 These Lieber-DeCarli liquid diets are high in monounsaturated fat and low in carbohydrate (see information from Bio-Serv website). Alternatively, low-fat, high–polyunsaturated fat or high–saturated fat Lieber-DeCarli diets (Dyets) can also be used to study the effects of dietary fat on ALD37,38.
These Lieber-DeCarli liquid diets are high in monounsaturated fat and low in carbohydrate (see information from Bio-Serv website). Alternatively, low-fat, high–polyunsaturated fat or high–saturated fat Lieber-DeCarli diets (Dyets) can also be used to study the effects of dietary fat on ALD37,38.Maltose dextrin (BioServ, 10DE, food grade no. 3585)
Ethanol, 100% or 95% (vol/vol; Fisher, cat. no. 04-355-451)
Potable tap water
TABLE 2.
Caloric profile of the diet.
| Control diet (F1259sp) |
Ethanol diet (F1258sp) |
|
|---|---|---|
| Protein (kcal per liter) | 151 | 151 |
| Fat (kcal per liter) | 359 | 359 |
| Carbohydrate (kcal per liter) | 490 | 135 |
| Ethanol + maltose dextrin (kcal per liter) |
0 | 355 |
| Total (kcal per liter) | 1,000 | 1,000 |
EQUIPMENT
A Catalyst Dx chemistry analyzer (IDEXX laboratories) for the measurement of serum ALT, AST, triglyceride and cholesterol. Many other biochemical analyzers can also be used. Alternatively, the mouse sera can be sent to a clinical diagnostic lab to measure ALT, AST, triglyceride and cholesterol.
Diet storage refrigerator
Recirculating water-heating pads with pump (Braintree Scientific)
Diet supplies
Scale
Commercial food blender
Liquid diet feeding tubes with caps, 50 ml (Bio-Serv, product no. 9019)
Storage bottles
Graduated cylinder (1 liter)
Serological pipettes and pipette aid, 50 ml
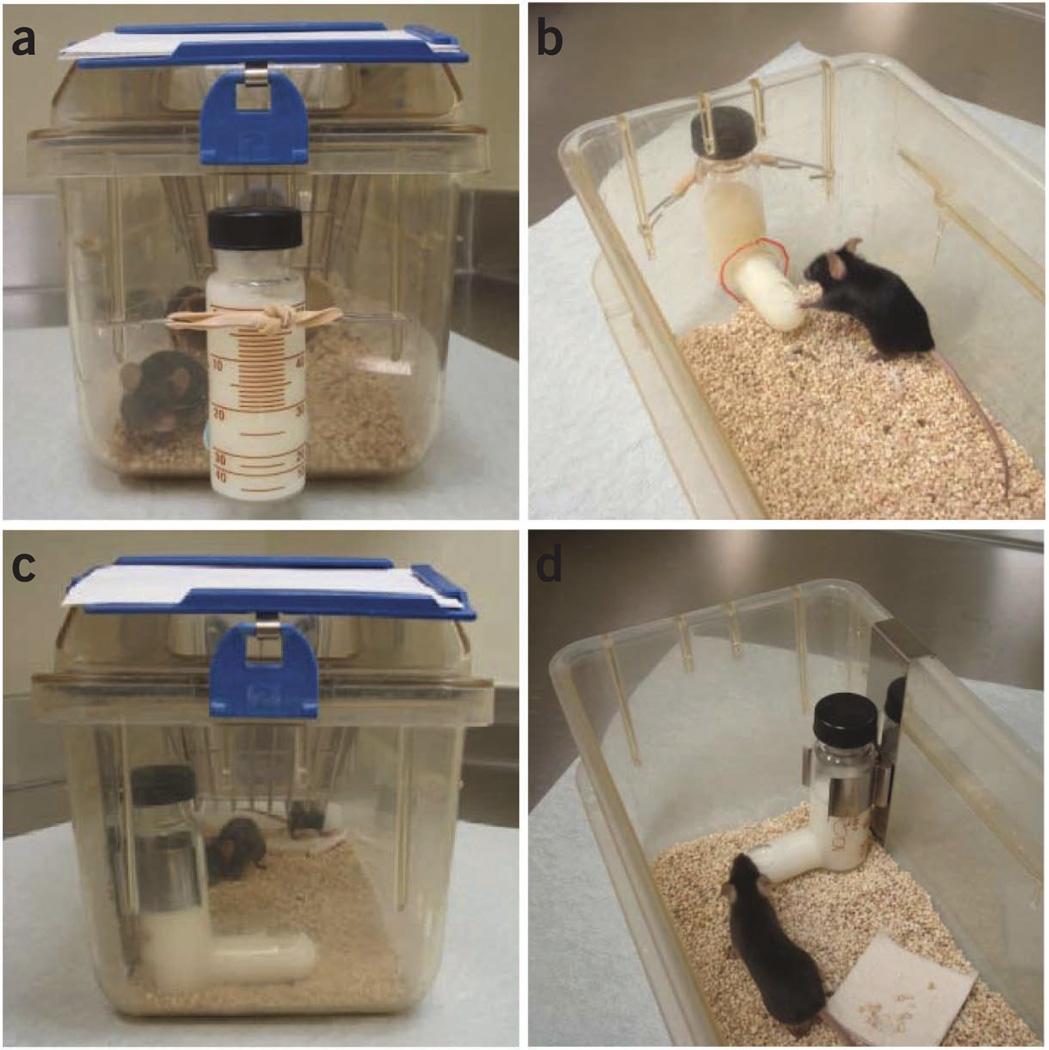
Modified mouse cages with a hole at the bottom of the front side, homemade steel wire hooks and rubber bands; see Figure 2 for photos or regular mouse cages and feeding-tube holders (Bio-Serv, product no. 9015)
Figure 2.
Cage and feeding-tube setup. (a,b) Photos showing modified mouse cages. A hole is drilled at the bottom of the front side of the cage to insert the mouth of the feeding tube into the cage. The feeding tube is held using a rubber band tied to steel wire hooks. (c,d) Photos showing commercial feeding-tube holders designed to hang over the lip of the cage. Note that it is much easier to change the feeding tubes in the modified mouse cages (a,b) than those in tube holders (c,d). Perforations made in cages must be consistent in their location to ensure that the diet in the feeding tube remains accessible after placement.
Gavage supplies
Gavage needle with ball tip. Gauge size and needle length should be determined based on the size and weight of animals. For example, 15–20 g mice: 22-gauge (1–1.5 inches); 20–25 g mice: 20-gauge (1–1.5 inches); 25–30 g mice: 18-gauge (1.5–2 inches).
Syringe (1 ml)
REAGENT SETUP
Control liquid diet
To prepare 1,000 ml of control liquid diet, weigh 225 g of dry mix (Bio-Serv, Product F1259SP). Measure 860 ml of potable tap water. Add the dry mix and tap water into a blender and mix it thoroughly. The final volume should be 1,000 ml. Refrigerate the prepared control liquid diet and use it within 3 d.
Ethanol liquid diet (1–5%, vol/vol)
To prepare 1,000 ml of ethanol liquid diet, add to a blender 133 g of dry mix (Bio-Serv, Product F1258SP) and different amounts of maltose dextrin and a different volume of water to the diet on the basis of the percentage of ethanol required (Table 3). Blend the mixture thoroughly. Refrigerate the prepared liquid diet without ethanol and use it within 3 d. Add a different volume of 95% ethanol immediately before feeding. Table 3 describes the directions for preparing 1,000 ml of diet. You can adjust proportionally the weight of the dry mix and maltose dextrin, as well as the volume of water and 95% ethanol, to prepare the amount of diet you need. A 1–4% ethanol (vol/vol) liquid diet is only required for mice that are not on a C57BL/6 background. The calories from ethanol plus maltose dextrin must be 355 kcal l−1 or 35.5% of total kilocalories.  Add 95% ethanol (see volume in Table 3) immediately before administering the mixture to mice. Mix it thoroughly. Prepare the fresh ethanol liquid diet daily.
Add 95% ethanol (see volume in Table 3) immediately before administering the mixture to mice. Mix it thoroughly. Prepare the fresh ethanol liquid diet daily.  One hundred percent ethanol absorbs water from air, so 95% ethanol (vol/vol) solution should be prepared immediately after opening a new bottle of 100% ethanol.
One hundred percent ethanol absorbs water from air, so 95% ethanol (vol/vol) solution should be prepared immediately after opening a new bottle of 100% ethanol.
TABLE 3.
1–5% (vol/vol) ethanol liquid diet (directions for preparing 1,000 ml).
| Ethanol % in the diet (vol/vol) |
Dry mix (g) | Maltose dextrin (g) |
Watera (ml) |
95% ethanol (ml) |
Calories from maltose dextrin (%) |
Calories from ethanol (%) |
|---|---|---|---|---|---|---|
| 1 | 133 | 77.1 | 900 | 10.5 | 30.0 | 5.5 |
| 2 | 133 | 62.9 | 910 | 21.1 | 24.5 | 11.0 |
| 3 | 133 | 48.7 | 910 | 31.6 | 18.9 | 16.6 |
| 4 | 133 | 34.5 | 920 | 42.1 | 13.5 | 22.0 |
| 5 | 133 | 20.3 | 910 | 52.6 | 7.9 | 27.6 |
Ethanol density = 0.789 g ml−1; 1 g ethanol = 7 kcal; 1 g maltose dextrin (Bio-Serv) = 3.89 kcal.
These water levels are an approximation. The final volume after addition of ethanol should be 1,000 ml.
Ethanol gavage solution (5 g ethanol per kg of body weight)
Prepare a 31.5% (vol/vol) ethanol solution (0.25 g ml−1 ethanol) by mixing 6.6 ml of 95% ethanol with 13.4 ml of water.  This solution must be prepared just before administration to avoid changes in concentration resulting from evaporation.
This solution must be prepared just before administration to avoid changes in concentration resulting from evaporation.
Maltose gavage solution (9 g of maltose dextrin per kg of body weight)
Prepare a 45% (wt/vol) maltose dextrin solution by dissolving 9 g of maltose dextrin in a final volume of 20 ml of water.  We do not filter the solution. Bacterial or fungal contamination may develop even if the solution is stored at 4 °C for a few days. Therefore, the maltose solution should be prepared just before use.
We do not filter the solution. Bacterial or fungal contamination may develop even if the solution is stored at 4 °C for a few days. Therefore, the maltose solution should be prepared just before use.
PROCEDURE
Experimental setup  5 min per mouse
5 min per mouse
-
1
Determine the total number of mice (minimally six to eight mice per group) to be used. Identify and weigh ethanol-fed and pair-fed animals.
-
2
Prepare an adequate number of newly bedded cages, feeding tubes, caps and rubber bands with hooks or tube holders. One or two mice are housed per cage.
stage 1: acclimatization to tube feeding with control liquid diet  5 d
5 d
-
3
Prepare the control liquid diet (Reagent Setup).
-
4
Fill the number of feeding tubes needed with the control liquid diet: 30 ml for one mouse and 50 ml for two mice. By using a gloved hand, place your thumb over the hole in the mouth of the tube while filling it in order to prevent the diet from flowing out of the tube. Tightly cap the feeding tubes.
-
5
Place the feeding tubes containing control liquid diet in cages. Do not add any additional diet or drinking water. We use Modified mouse cages with a hole in the front of the cage, which makes it very easy to change the feeding tubes from outside the cage without having to remove the cage from its holding rack (Fig. 2a,b). Alternatively, commercially available tube holders can be used, but they require removing the cage from its holding rack and opening the cage to remove the used feeder and replace it with a fresh feeder (Fig. 2c,d).
 The liquid diet is the only source of food and water; no additional diet and drinking water are needed.
The liquid diet is the only source of food and water; no additional diet and drinking water are needed.
-
6
Transfer the mice into the cages with feeding tubes. (We usually house one or two mice per cage.)
-
7
Label the cage card with the study group (pair-fed, ethanol-fed) and the date on which feeding was started.
-
8
Change the control liquid diet and feeding tubes daily for the next 4 d to adapt the mice to liquid diet feeding (a total of 5 d of control diet).
 For C57BL/6 mice or genetically modified mice on a C57BL/6 background, it is OK to directly switch from control liquid diet (stage 1) to 5% (vol/vol) ethanol liquid diet (stage 2) on day 6. For other strains of mice, a gradual increase in ethanol concentrations from 1% to 4% (vol/vol) from day 2 to day 5 during the acclimatization stage (stage 1) may be required. In this situation, control groups must be pair-fed with control diet throughout.
For C57BL/6 mice or genetically modified mice on a C57BL/6 background, it is OK to directly switch from control liquid diet (stage 1) to 5% (vol/vol) ethanol liquid diet (stage 2) on day 6. For other strains of mice, a gradual increase in ethanol concentrations from 1% to 4% (vol/vol) from day 2 to day 5 during the acclimatization stage (stage 1) may be required. In this situation, control groups must be pair-fed with control diet throughout.
 Steps 5–8 can be performed at any time for control diet during the day, but we prefer to do it (set up and change the ethanol diet) in the late afternoon closer to the onset of the dark period of the diurnal cycle. This ensures that the animals have fresh diet available at the beginning of their maximal food intake period (i.e., lights-out portion of their diurnal cycle). Replacement of the diet earlier in the day will result in increased soiling of the feeding tube and may allow some evaporation of ethanol from the liquid diet right at the mouth of the feeding tube.
Steps 5–8 can be performed at any time for control diet during the day, but we prefer to do it (set up and change the ethanol diet) in the late afternoon closer to the onset of the dark period of the diurnal cycle. This ensures that the animals have fresh diet available at the beginning of their maximal food intake period (i.e., lights-out portion of their diurnal cycle). Replacement of the diet earlier in the day will result in increased soiling of the feeding tube and may allow some evaporation of ethanol from the liquid diet right at the mouth of the feeding tube.
 During the feeding, the cages should be checked twice (early morning and late afternoon) every day to ensure that the mouths of the feeding tubes are not clogged or leaking diet.
During the feeding, the cages should be checked twice (early morning and late afternoon) every day to ensure that the mouths of the feeding tubes are not clogged or leaking diet.
![]()
Stage 2: chronic administration of ethanol and pair feeding  10 d
10 d
-
9
Prepare 5% (vol/vol) ethanol and control liquid diet (Reagent Setup).
-
10
Fill the number of feeding tubes needed with 5% (vol/vol) ethanol liquid diet—25 ml for one mouse and 40 ml for two mice—and an appropriate volume of control diet. Use a gloved hand to place your thumb over the hole in the mouth of the tube while filling, in order to prevent the diet from flowing out of the tube. Tightly cap the feeding tubes.
-
11
Place the feeding tubes with liquid diets in cages. Do not add any additional food or drinking water. Ensure that the feeder is positioned in the cage in a manner that ensures that the ‘nose’ of the feeder does not tip such that the animals cannot access the diet, especially in the pair-fed groups, which have limited liquid diets.
-
12
Change the feeding tubes with 5% (vol/vol) ethanol or control liquid diets in the late afternoon (between 3:00 and 5:00 pm) on day 6.
 See discussion on timing of feeding in Step 8 above.
See discussion on timing of feeding in Step 8 above.
 The liquid diet is the only source of food and water; no additional diet and drinking water are needed.
The liquid diet is the only source of food and water; no additional diet and drinking water are needed.
-
13
Record the food intake of the ethanol-fed mice and calculate the average daily volume per mouse. Use the calculated volume to adjust the amount of control liquid diet given to pair-fed mice so that ethanol- and pair-fed mice consume equal amounts of diet. The volume of control diets for the first-day pair feeding is based on the pilot experiments or previous experiments. For C57BL/6 mice, we usually give 10 ml of control diet per mouse.
 Because mice consume less ethanol diet than control diet, it is very important to make sure that the control diet is pair-fed (limited) with the same amount of food consumed by ethanol-fed mice.
Because mice consume less ethanol diet than control diet, it is very important to make sure that the control diet is pair-fed (limited) with the same amount of food consumed by ethanol-fed mice.
![]()
-
14
Continue to change the feeding tubes and give fresh 5% (vol/vol) ethanol and control liquid diets in the afternoon for the next 9 d (total 10 d), monitoring the food intake of ethanol diets and adjusting the volume of control diets accordingly each day.
 During the feeding, the cages should be checked twice (early morning and late afternoon) every day to ensure that the mouths of the feeding tubes are not clogged or leaking diet.
During the feeding, the cages should be checked twice (early morning and late afternoon) every day to ensure that the mouths of the feeding tubes are not clogged or leaking diet.
 To prevent the mouse from escaping, manage feeding practices to ensure that cages with perforations are not left without feeders in position.
To prevent the mouse from escaping, manage feeding practices to ensure that cages with perforations are not left without feeders in position.
![]()
Stage 3: acute administration of ethanol by oral gavage  9 h
9 h
-
15
On day 11, prepare ethanol and maltose solutions (Reagent Setup) in the morning just before administration between 7:00 am and 9:00 am.
 Gavage in the early morning is crucial. Mice consume foods and ethanol at night, and thus blood alcohol levels are high in the early morning. An additional gavage of ethanol results in further elevation of blood alcohol levels, which induces substantial liver injury (elevation of serum ALT and AST).
Gavage in the early morning is crucial. Mice consume foods and ethanol at night, and thus blood alcohol levels are high in the early morning. An additional gavage of ethanol results in further elevation of blood alcohol levels, which induces substantial liver injury (elevation of serum ALT and AST).
 The gavage procedure must only be performed by properly trained personnel.
The gavage procedure must only be performed by properly trained personnel.
-
16
Select a gavage needle of appropriate length. This can be determined by measuring the needle against the animal’s body (Fig. 3a). The maximal length should not exceed the distance between the corner of the mouth and the last rib (which is a rough estimate of the stomach’s position).
-
17
Draw the appropriate volume of ethanol or maltose gavage solution into a 1-ml syringe while it is attached to the gavage needle.
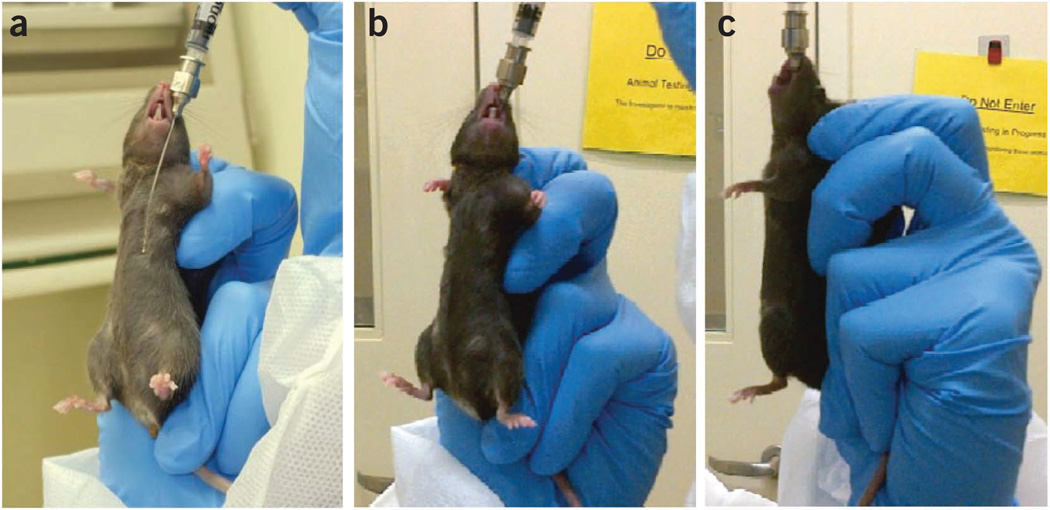
Figure 3.
Oral gavage. (a) Measurement of the length of the gavage needle against the animal’s body. (b,c) Administration of the solution upon verification of proper placement of the gavage needle. All of the animal experiments were approved by the NIAAA Animal Care and Use Committee.
 The volumes to be administered should not be more than 2 ml per 100 g body weight for an aqueous solution. The concentration of ethanol solution for the gavage should not be more than 31.5% (vol/vol) ethanol.
The volumes to be administered should not be more than 2 ml per 100 g body weight for an aqueous solution. The concentration of ethanol solution for the gavage should not be more than 31.5% (vol/vol) ethanol.
 The gavage volume (μl) of 31.5% (vol/vol) ethanol solution for each mouse = mouse body weight in grams × 20; the gavage volume (μl) of 45.0% (wt/vol) maltose dextrin solution for each mouse = mouse body weight in grams × 20.
The gavage volume (μl) of 31.5% (vol/vol) ethanol solution for each mouse = mouse body weight in grams × 20; the gavage volume (μl) of 45.0% (wt/vol) maltose dextrin solution for each mouse = mouse body weight in grams × 20.
 The swallowing reflex must be intact for gavage. Anesthesia is not used.
The swallowing reflex must be intact for gavage. Anesthesia is not used.
-
18
Restrain the mice in a vertical position by tightly pulling up the loose skin on the back or side of the neck so that the spine is fully straight and the head is extended, resulting in a straight vertical alignment of the esophagus (Fig. 3b,c).
-
19
Carefully put the gavage needle into the mouth between the incisors and molars. With the bulb in the mouse mouth, the needle should be softly passed along the roof of the mouth while the head is held in moderate extension.
-
20
Gently extend the head and neck back using the needle as a lever to position the neck and esophagus in a straight line.
-
21
Move the gavage needle smoothly along the upper palate into the esophagus. The mouse usually swallows as the feeding needle approaches the pharynx, facilitating entry into the esophagus.
![]()
-
22
Once the gavage needle is properly positioned, administer the solution.
-
23
After administration, remove the needle gently by following the same angle as insertion.
-
24
Return the animal to the original cage with the liquid diets (control or ethanol diet), place the cage on a heating pad and monitor the mouse for any sign of labored breathing or respiratory distress (aspiration of fluid into the lungs). Any animal with signs of obvious distress after gavage should be promptly euthanized by using standard CO2 inhalation.
 Provide thermal support to minimize loss of body heat. Keep the cages on a heating pad with circulating water maintained at 38 °C to avoid hypothermia.
Provide thermal support to minimize loss of body heat. Keep the cages on a heating pad with circulating water maintained at 38 °C to avoid hypothermia.
-
25
Continue to monitor the mice during recovery. Mice gavaged with ethanol will show signs of inebriation such as staggering gait and ataxia within a few minutes, followed by sedation and loss of consciousness. They usually wake up in the early afternoon. Some mice regain normal behavior in the afternoon but some of them are still slow moving at the time of euthanasia. Mice from neither the pair- nor ethanol-fed groups consume much diet within 9 h after gavage: the ethanol-fed mice usually consume less than 1 ml of liquid diet owing to loss of consciousness, and there is not a substantial amount of control diet remaining for consumption by the pair-fed mice.
-
26
Nine hours after gavage, deeply anesthetize the mice with isoflurane, and collect the blood from the orbital sinus by traumatic avulsion of the globe from the orbit with a pair of tissue forceps. While they are still under general anesthesia, kill the mice by cervical dislocation.
 Timing of blood collection is very important: serum ALT and AST levels reach a peak at 9 h after gavage, and they return to basal levels 24 h after gavage9.
Timing of blood collection is very important: serum ALT and AST levels reach a peak at 9 h after gavage, and they return to basal levels 24 h after gavage9.
-
27
Collect the serum. Measure ALT, AST, triglyceride and cholesterol by using biochemical kits (Butler Schein Animal Health Supply) and a catalyst Dx chemistry analyzer (IDEXX laboratories).
 Because serum levels of ALT in this model are usually lower than 500 IU l−1, the serum should be used directly for the measurement without dilution.
Because serum levels of ALT in this model are usually lower than 500 IU l−1, the serum should be used directly for the measurement without dilution.
![]()
-
28
Collect and weigh liver tissues. Cut two slices in the middle of the left lobe and one slice in the medial lobe, and fix them in 10% (wt/vol) formalin for at least 48 h before processing. Quickly freeze the remaining liver tissues in liquid nitrogen and store them at −80 °C for further analyses9, including Oil Red O staining of frozen sections for steatosis evaluation, RNA extraction for real-time PCR analyses, lipid extraction for measurement of hepatic triglyceride and cholesterol and protein extraction for western blot analyses.
![]()
Steps 8 and 14: changing the feeding tubes
Occasionally, a feeder will develop a small chip or crack near the top, which will allow air under the cap and result in leakage of the liquid diet and soiling of the cage. Do not use a feeder that has chips or cracks on the upper lip where it seals against the lid, and do not use any lids that are cracked and may not make a good seal against the glass feeder. Rarely, the feeder may become so clogged with bedding that the animals will not try to consume their diet. The cages should be checked several times daily, and any crusts over or clogs of the feeding hole should be removed. If you are using an internal tube holder, place the tube as high off the bedding as possible in order to reduce the amount of bedding that gets kicked into the feeder. You can also reduce the amount of bedding to prevent the bedding from getting into the feeder’s opening.
Step 13: pair feeding
If the control diet–fed mice gain more body weight than the ethanol-fed mice, please make sure that the control diet is limited (pair-fed) with the same amount of food consumed by ethanol-fed mice.
Step 21: oral gavage
There are several common trouble areas with the gavage procedure:
Difficulty in getting the gavage needle to pass into the esophagus. The gavage needle needs to be behind the base of the tongue near the center of the back of the mouth. If you get over to the side of the back of the mouth, the animal can get the gavage needle in its teeth and prevent you from being able to pass it down the mouse esophagus. Watch for the swallowing reflex; it will move the tongue forward and the gavage needle will almost ‘fall’ down the esophagus with little or no pressure.
Liquid entering the trachea instead of the esophagus. Again, this is generally avoided by waiting until the animal swallows to gently push or let the gavage needle ‘fall’ down the esophagus. If the needle is in the trachea, the hub of the gavage needle will not go in as far, and the needle hub will be farther from the animal’s nose than when the needle is in its stomach. Before administering the gavage liquid, you should put gentle traction back on the syringe plunger. If you get several bubbles of air, you are inserting the needle in the trachea and should remove the gavage needle and start over.
Pushing the gavage needle too hard and penetrating the back of the mouth, the trachea or the esophagus. If you are using a gavage needle of the proper size, it will require little, if any, force to make the gavage needle descend down the esophagus and into the stomach.
Aspiration of the gavage liquid. This will occur if you administer the gavage liquid in the trachea instead of the esophagus, and it may happen if you administer the gavage liquid in the esophagus before the needle gets to the stomach. Fortunately, the esophagus is fairly distendable, so as long as your gavage volume is small (less than 1 ml) and you do not withdraw your gavage needle too rapidly the mouse will usually move the fluid into its stomach by swallowing after you have removed the gavage needle.
Respiratory distress. If you see frothing at the corners of the mouse’s mouth or nose, or observe difficulty in breathing, the mouse may have aspirated or you may have penetrated the mouse’s trachea or esophagus. If the mouse is in severe distress, or if the distress continues for more than a few minutes, it should be humanely euthanized (e.g., by CO2 inhalation).
Step 27: measurement of serum ALT and AST
Many factors may affect the values of serum ALT and AST, such as blood collection tubes, clotting time, blood hemolysis, serum dilution, the use of different measurement methods and biochemistry analyzers, the time of mouse gavage and euthanization, as well as the mouse strain. To minimize these variations, the pair-fed and ethanol-fed wild-type or genetically modified mice should be fed at the same time, and sera from these mice should be harvested uniformly and analyzed at the same time.
If the serum ALT and AST levels are not markedly elevated in wild-type mice after chronic feeding plus a single binge, the following items should be checked.
The serum ALT and AST levels peak at 9 h and return to basal levels 24 h after gavage9. Thus, the sera should be collected 9 h after gavage.
Dietary fat is known to have an effect on alcoholic liver injury37,38. The Lieber-DeCarli ethanol diet we use is high in monounsaturated fat and low in carbohydrates (Bio-Serv F1258SP). In this model, we have not tested the effects of Lieber-DeCarli ethanol diets that are low in fat or high in polyunsaturated fat or high in saturated fat.
Mice should be gavaged in the early morning and euthanized in the late afternoon. Gavage at a different time may affect serum ALT and AST levels.
Check the strains of mice you used. There are substantial strain differences in alcohol-induced liver injury and steatosis39.
![]()
Steps 1 and 2, (stage 1) weigh mice and prepare cages: 5 min per mouse
Step 3, (stage 1) control diet preparation: 15 min
Steps 4–7, (stage 1) animal housing and feeding setup: 4 min per mouse
Step 8, (stage 1) daily feeding of mice for 5 d: 2 min per mouse per day
Step 9, (stage 2) ethanol liquid diet preparation: 15 min
Steps 10–14, (stage 2) daily feeding of mice for 10 d: 2 min per mouse per day
Step 15, (stage 3) preparation of the ethanol and maltose solutions: 10 min
Steps 16–23, (stage 3) gavage of mice: 3 min per mouse
Steps 24 and 25, (stage 3) monitoring of mice: check mice every 30 min for 9 h
Total for Steps 1–28, acclimatization, chronic feeding and gavage: 15 d
ANTICIPATED RESULTS
Weight gain
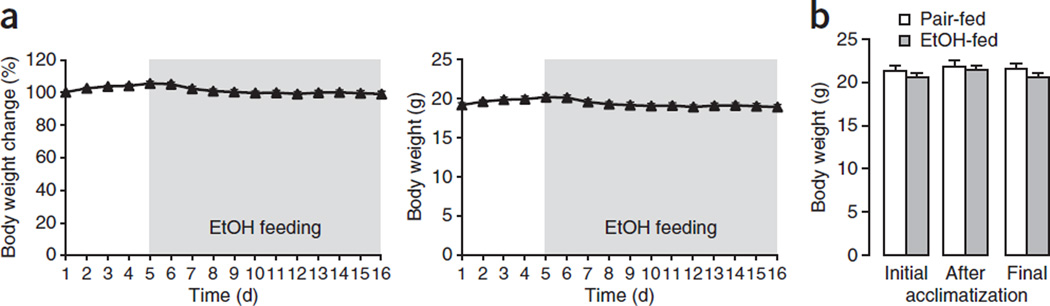
We find that after switching to a 5% (vol/vol) ethanol diet, the mouse’s body weight decreases slightly for the first 2 d, and then recovers and maintains the original body weight (Fig. 4a). Typical body changes for ethanol-fed female C57BL/6J mice during a typical experiment are shown in Figure 4a. We found that the final body weight was comparable between ethanol-fed and pair-fed mice (Fig. 4b).
Figure 4.
Body weight changes during the chronic-plus-binge ethanol feeding model. (a) Evolution of body weight of C57BL/6J female mice (n = 17) during the course of the chronic-plus-single-binge ethanol feeding. (b) Body weight of pair-fed (n = 7) and ethanol-fed (n = 34) C57BL/6 female mice was measured on the first day of feeding (initial), after the 5-d acclimatization period and at day 11 before gavage (final). Data represent means ± s.e.m. All of the animal experiments were approved by the NIAAA Animal Care and Use Committee.
Serum ALT and AST levels
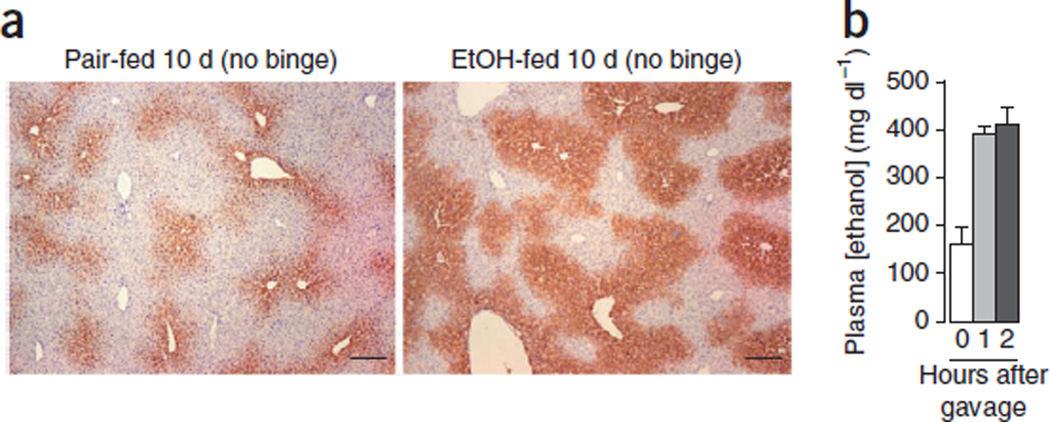
Chronic feeding of mice with a Lieber-DeCarli diet for 4–6 weeks has been widely used in many laboratories; however, this led to only a slight elevation of serum ALT and AST15–20. This chronic (10 d) plus single-binge feeding model yields markedly higher levels of serum ALT and AST9. It would also be interesting to examine whether feeding mice chronically with an ethanol diet for longer periods of time plus single gavage induces even higher levels of serum ALT and AST than those from 10-d chronic feeding plus single gavage. Because feeding mice with an ethanol diet for 10 d markedly upregulated CYP2E1 protein expression in the liver (Fig. 5), which is known to contribute to alcoholic liver injury40,41, we believe that a 10-d chronic feeding regimen is sufficient to increase CYP2E1 activity and to promote a single-binge–induced elevation of serum ALT and AST.
Figure 5.
Hepatic expression of CYP2E1 and plasma ethanol levels post ethanol feeding. (a) Representative immunostaining of CYP2E1 in the liver tissues from 10-d pair-fed and ethanol-fed mice (chronic feeding without binge). Scale bars, 200 µm. (b) Mice were fed the ethanol diet for 10 d. At day 11, blood was collected in the early morning and plasma ethanol concentration was measured (chronic without binge, 0 h after gavage). The mice were then gavaged with 5 g kg−1 ethanol, and plasma ethanol concentration was measured 1 and 2 h after gavage. Data represent mean ± s.e.m. All of the animal experiments were approved by the NIAAA Animal Care and Use Committee.
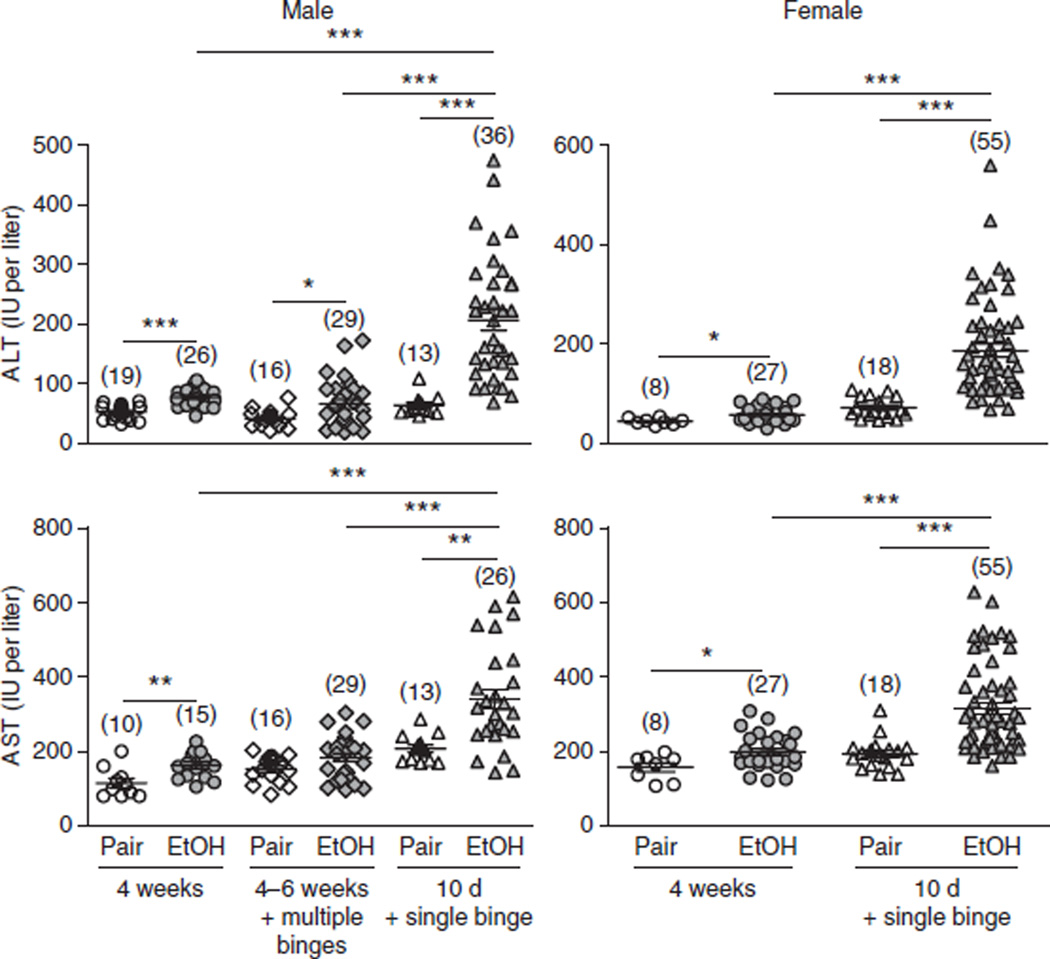
We also examined chronic ad libitum feeding (4–6 weeks) plus multiple binges (twice a week during the 4–6-week feeding) in mice, but surprisingly this model produced significantly lower levels of serum ALT and AST compared with this chronic (10 d)-plus-single-binge model, as shown in Figure 6. Our hypothesis for the lower levels of serum ALT and AST in this model of chronic feeding plus multiple binges is described in the INTRODUCTION.
Figure 6.
Comparison of liver injury induced by the chronic-plus-single-binge ethanol feeding model and other feeding models. C57BL/6 mice were subjected to 4 weeks of chronic feeding alone, 4–6 weeks of chronic feeding plus multiple binges (twice a week during the 4–6 week feeding) and 10 d of chronic feeding plus a single binge. Mice were euthanized 9 h after gavage. Liver injury was assessed by measuring serum ALT and AST levels. Numbers in parentheses in graphs denote the number of mice per group. *P < 0.05, **P < 0.01, ***P < 0.001 (unpaired t test). Note that chronic feeding (4–6 weeks) plus multiple binges (twice a week during the 4–6 week feeding), which was associated with a mortality rate of 10–40% in male C57BL/6N mice, was not tested in female C57BL/6 mice because a higher mortality rate is expected. The values in this figure were obtained from multiple independent experiments. All of the animal experiments were approved by the NIAAA Animal Care and Use Committee.
Figure 6 summarizes the serum ALT and AST levels from many independent experiments after chronic ethanol feeding (4 weeks), chronic feeding (10 d) plus a single binge or chronic feeding (4–6 weeks) plus multiple binges (twice a week during the 4–6-week feeding).
It is well documented that female rodents are more susceptible to ethanol-induced liver injury than male rodents42,43. We have previously demonstrated that the serum levels of ALT and AST were higher in female mice than in male mice after chronic-plus-single-binge ethanol feeding9.
Notably, the average levels of serum ALT and AST from multiple independent experiments were comparable between male and female mice in this 10-d chronic-plus-single-binge model (as shown in Fig. 6). This may be due to interexperiment variations. Further studies on male and female mice should be conducted at the same time to confirm whether female mice are more susceptible to chronic-plus-single-binge–induced elevation of serum ALT and AST.
Plasma ethanol concentration
As shown in Figure 5b, plasma concentration of ethanol reaches ∼180 mg dl−1 after 10-d ethanol feeding (chronic without binge) and reaches ∼400 mg dl−1 1 h and 2 h after gavage in the chronic-binge model.
Liver histology
Liver histology and Oil Red O staining analyses showed obvious microsteatosis in the livers from all ethanol-fed mice9. Macrosteatosis and necrosis were also often observed in some ethanol-fed mice9.
In conclusion
This simple chronic-binge ethanol feeding model achieves high blood alcohol levels, elevation of serum ALT and AST and infiltration of neutrophils. This model can be set up in most laboratories and carried out in a shorter time period compared with previous rodent models of ALD (15 d in this chronic-plus-single-binge model versus 5–7 weeks in previous chronic models), and it is easily applicable to multiple research areas including ALD and other organ damage caused by alcohol consumption. Notably, this model may more closely resemble the pathogenesis of human alcoholic hepatitis.
In this protocol, we described a simple mouse model with 10-d ad libitum feeding plus a single binge of ethanol feeding. It would be interesting to test the effects of different combinations of chronic and binge ethanol feeding on liver injury, such as those caused by chronic feeding for longer periods of time plus a single binge or multiple binges. In addition, in this protocol, we used the Lieber-DeCarli liquid diet, which is high in monounsaturated fat and low in carbohydrates. It would also be of interest to study this model with diets high in polyunsaturated or saturated fats, or low-fat diets with high carbohydrates, which are known to have differential effects on the development of alcoholic liver injury37,44.
ACKNOWLEDGMENTS
We greatly appreciate all current and previous lab members for their technical support and helpful discussions. We also thank L. Chedester, A. Barnes and R. Kechrid from the Office of Laboratory Animal Science for their technical and engineering support, and for their CRITICAL reading of the manuscript. This work is supported by the intramural program of NIAAA at the NIH.
Footnotes
AUTHOR CONTRIBUTIONS
A.B. contributed to generating the data presented, making the figures and tables and drafting the procedures; S.M. contributed to drafting the materials and generating data presented in Figure 4; S.H.K. and H.W. contributed to generating the data presented in Figure 4 and B.G. contributed to drafting and the completion of the manuscript through supervision of others.
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
Published online at http://www.nature.com/doifinder/10.1038/nprot.2013.032. Reprints and permissions information is available online at http://www.nature.com/reprints/index.html.
References
- 1.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsukamoto H, Lu SC. Current concepts in the pathogenesis of alcoholic liver injury. FASEB J. 2001;15:1335–1349. doi: 10.1096/fj.00-0650rev. [DOI] [PubMed] [Google Scholar]
- 3.O’Shea RS, Dasarathy S, McCullough AJ. Practice Guideline Committee of the American Association for the Study of Liver, D. & Practice Parameters Committee of the American College of, G. Alcoholic liver disease. Hepatology. 2010;51:307–328. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 4.Stickel F, Seitz HK. Alcoholic steatohepatitis. Best Pract. Res. Clin. Gastroenterol. 2010;24:683–693. doi: 10.1016/j.bpg.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Beier JI, Arteel GE, McClain CJ. Advances in alcoholic liver disease. Curr. Gastroenterol. Rep. 2011;13:56–64. doi: 10.1007/s11894-010-0157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellentani S, et al. Drinking habits as cofactors of risk for alcohol induced liver damage. The Dionysos Study Group. Gut. 1997;41:845–850. doi: 10.1136/gut.41.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stranges S, et al. Differential effects of alcohol drinking pattern on liver enzymes in men and women. Alcohol. Clin. Exp. Res. 2004;28:949–956. doi: 10.1097/01.alc.0000128229.23396.42. [DOI] [PubMed] [Google Scholar]
- 8.Li TK. Quantifying the risk for alcohol-use and alcohol-attributable health disorders: present findings and future research needs. J. Gastroenterol. Hepatol. 2008;23(suppl. 1):S2–S8. doi: 10.1111/j.1440-1746.2007.05298.x. [DOI] [PubMed] [Google Scholar]
- 9.Ki SH, et al. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–1300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathurin P, Lucey MR. Management of alcoholic hepatitis. J. Hepatol. 2012;56(suppl. 1):S39–S45. doi: 10.1016/S0168-8278(12)60005-1. [DOI] [PubMed] [Google Scholar]
- 11.Choi G, Runyon BA. Alcoholic hepatitis: a clinician’s guide. Clin. Liver Dis. 2012;16:371–385. doi: 10.1016/j.cld.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 12.Altamirano J, Bataller R. Alcoholic liver disease: pathogenesis and new targets for therapy. Nat. Rev. Gastroenterol. and Hepatol. 2011;8:491–501. doi: 10.1038/nrgastro.2011.134. [DOI] [PubMed] [Google Scholar]
- 13.Aroor AR, Jackson DE, Shukla SD. Elevated activation of ERK1 and ERK2 accompany enhanced liver injury following alcohol binge in chronically ethanol-fed rats. Alcohol. Clin. Exp. Res. 2011;35:2128–2138. doi: 10.1111/j.1530-0277.2011.01577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrasek J, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J. Clin. Invest. 2012;122:3476–3489. doi: 10.1172/JCI60777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen JI, Roychowdhury S, McMullen MR, Stavitsky AB, Nagy LE. Complement and alcoholic liver disease: role of C1q in the pathogenesis of ethanol-induced liver injury in mice. Gastroenterology. 2010;139:664–674. doi: 10.1053/j.gastro.2010.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandrekar P, Ambade A, Lim A, Szabo G, Catalano D. An essential role for monocyte chemoattractant protein-1 in alcoholic liver injury: regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology. 2011;54:2185–2197. doi: 10.1002/hep.24599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nath B, et al. Hepatocyte-specific hypoxia-inducible factor-1α is a determinant of lipid accumulation and liver injury in alcohol-induced steatosis in mice. Hepatology. 2011;53:1526–1537. doi: 10.1002/hep.24256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu M, et al. Regulation of hepatic lipin-1 by ethanol: role of AMP-activated protein kinase/sterol regulatory element-binding protein 1 signaling in mice. Hepatology. 2012;55:437–446. doi: 10.1002/hep.24708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liangpunsakul S, et al. Imipramine blocks ethanol-induced ASMase activation, ceramide generation, and PP2A activation, and ameliorates hepatic steatosis in ethanol-fed mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;302:G515–523. doi: 10.1152/ajpgi.00455.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leung TM, et al. Argininosuccinate synthase conditions the response to acute and chronic ethanol-induced liver injury in mice. Hepatology. 2012;55:1596–1609. doi: 10.1002/hep.25543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Z, et al. Zinc supplementation prevents alcoholic liver injury in mice through attenuation of oxidative stress. Am. J. Pathol. 2005;166:1681–1690. doi: 10.1016/S0002-9440(10)62478-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ueno A, et al. Mouse intragastric infusion (iG) model. Nat. Protoc. 2012;7:771–781. doi: 10.1038/nprot.2012.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu J, et al. Synergistic steatohepatitis by moderate obesity and alcohol in mice despite increased adiponectin and p-AMPK. J. Hepatol. 2011;55:673–682. doi: 10.1016/j.jhep.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsukamoto H, et al. Severe and progressive steatosis and focal necrosis in rat liver induced by continuous intragastric infusion of ethanol and low fat diet. Hepatology. 1985;5:224–232. doi: 10.1002/hep.1840050212. [DOI] [PubMed] [Google Scholar]
- 25.Kisseleva T, et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc. Natl. Acad. Sci. USA. 2012;109:9448–9453. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Z, et al. A critical involvement of oxidative stress in acute alcohol-induced hepatic TNF-α production. Am. J. Pathol. 2003;163:1137–1146. doi: 10.1016/s0002-9440(10)63473-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Z, Sun X, James Kang Y. Metallothionein protection against alcoholic liver injury through inhibition of oxidative stress. Exp. Biol. Med. 2002;227:214–222. doi: 10.1177/153537020222700310. [DOI] [PubMed] [Google Scholar]
- 28.Beier JI, Kaiser JP, Guo L, Martinez-Maldonado M, Arteel GE. Plasminogen activator inhibitor-1 deficient mice are protected from angiotensin II-induced fibrosis. Arch. Biochem. Biophys. 2011;510:19–26. doi: 10.1016/j.abb.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kao E, Shinohara M, Feng M, Lau MY, Ji C. Human immunodeficiency virus protease inhibitors modulate Ca2+ homeostasis and potentiate alcoholic stress and injury in mice and primary mouse and human hepatocytes. Hepatology. 2012;56:594–604. doi: 10.1002/hep.25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cook RT, et al. Thymocytes, pre-B cells, and organ changes in a mouse model of chronic ethanol ingestion–absence of subset-specific glucocorticoid-induced immune cell loss. Alcohol. Clin. Exp. Res. 2007;31:1746–1758. doi: 10.1111/j.1530-0277.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meadows GG, Blank SE, Duncan DD. Influence of ethanol consumption on natural killer cell activity in mice. Alcohol. Clin. Exp. Res. 1989;13:476–479. doi: 10.1111/j.1530-0277.1989.tb00359.x. [DOI] [PubMed] [Google Scholar]
- 32.Coleman RA, Young BM, Turner LE, Cook RT. A practical method of chronic ethanol administration in mice. Methods Mol. Biol. 2008;447:49–59. doi: 10.1007/978-1-59745-242-7_4. [DOI] [PubMed] [Google Scholar]
- 33.Brandon-Warner E, Schrum LW, Schmidt CM, McKillop IH. Rodent models of alcoholic liver disease: Of mice and men. Alcohol. 2012;46:715–725. doi: 10.1016/j.alcohol.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brandon-Warner E, Walling TL, Schrum LW, McKillop IH. Chronic ethanol feeding accelerates hepatocellular carcinoma progression in a sex-dependent manner in a mouse model of hepatocarcinogenesis. Alcohol. Clin. Exp. Res. 2012;36:641–653. doi: 10.1111/j.1530-0277.2011.01660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gabele E, et al. A new model of interactive effects of alcohol and high-fat diet on hepatic fibrosis. Alcohol. Clin. Exp. Res. 2011;35:1361–1367. doi: 10.1111/j.1530-0277.2011.01472.x. [DOI] [PubMed] [Google Scholar]
- 36.Wei VL, Singh SM. Genetically determined response of hepatic aldehyde dehydrogenase activity to ethanol exposures may be associated with alcohol sensitivity in mouse genotypes. Alcohol. Clin. Exp. Res. 1988;12:39–45. doi: 10.1111/j.1530-0277.1988.tb00130.x. [DOI] [PubMed] [Google Scholar]
- 37.You M, Considine RV, Leone TC. Kelly, D.P. & Crabb, D.W. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology. 2005;42:568–577. doi: 10.1002/hep.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Everitt H, et al. Ethanol administration exacerbates the abnormalities in hepatic lipid oxidation in genetically obese mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2013;304:G38–G47. doi: 10.1152/ajpgi.00309.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsuchiya M, et al. Interstrain differences in liver injury and one-carbon metabolism in alcohol-fed mice. Hepatology. 2012;56:130–139. doi: 10.1002/hep.25641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Butura A, et al. The impact of CYP2E1 on the development of alcoholic liver disease as studied in a transgenic mouse model. J. Hepatol. 2009;50:572–583. doi: 10.1016/j.jhep.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 41.Lu Y, Zhuge J, Wang X, Bai J, Cederbaum AI. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology. 2008;47:1483–1494. doi: 10.1002/hep.22222. [DOI] [PubMed] [Google Scholar]
- 42.Kono H, et al. Gender differences in early alcohol-induced liver injury: role of CD14, NF-κB, and TNF-α. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;278:G652–G661. doi: 10.1152/ajpgi.2000.278.4.G652. [DOI] [PubMed] [Google Scholar]
- 43.Nanji AA, et al. Increased severity of alcoholic liver injury in female rats: role of oxidative stress, endotoxin, and chemokines. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G1348–G1356. doi: 10.1152/ajpgi.2001.281.6.G1348. [DOI] [PubMed] [Google Scholar]
- 44.Kirpich IA, et al. The type of dietary fat modulates intestinal tight junction integrity, gut permeability, and hepatic toll-like receptor expression in a mouse model of alcoholic liver disease. Alcohol. Clin. Exp. Res. 2012;36:835–846. doi: 10.1111/j.1530-0277.2011.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]