Abstract
Background
Nicotinic signaling in prefrontal layer VI pyramidal neurons is important to the function of mature attention systems. The normal incorporation of α5 subunits into α4β2* nicotinic acetylcholine receptors augments nicotinic signaling in these neurons and is required for normal attention performance in adult mice. However, the role of α5 subunits in the development of the prefrontal cortex is not known.
Methods
We sought to answer this question by examining nicotinic currents and neuronal morphology in layer VI neurons of medial prefrontal cortex of wild-type and α5 subunit knockout (α5−/−) mice during postnatal development and in adulthood.
Results
In wild-type but not in α5−/− mice, there is a developmental peak in nicotinic acetylcholine currents in the third postnatal week. At this juvenile time period, the majority of neurons in all mice have long apical dendrites extending into cortical layer I. Yet, by early adulthood, wild-type but not α5−/− mice show a pronounced shift toward shorter apical dendrites. This cellular difference occurs in the absence of genotype differences in overall cortical morphology.
Conclusions
Normal developmental changes in nicotinic signaling and dendritic morphology in prefrontal cortex depend on α5-comprising nicotinic acetylcholine receptors. It appears that these receptors mediate a specific developmental retraction of apical dendrites in layer VI neurons. This finding provides novel insight into the cellular mechanisms underlying the known attention deficits in α5−/− mice and potentially also into the pathophysiology of developmental neuropsychiatric disorders such as attention-deficit disorder and autism.
Keywords: α5 subunit, CHRNA5, layer VI, neuronal morphology, nicotinic acetylcholine receptor, prefrontal cortex
The brain’s attention system depends on cholinergic neurotransmission within the medial prefrontal cortex (mPFC) for optimal performance (1–3). Layer VI pyramidal neurons of the mPFC likely play an important role within attention pathways because they are directly excited by acetylcholine (ACh) binding to nicotinic acetylcholine receptors (nAChRs) on their cell membrane (4–6) and because these neurons are a major source of feedback projections from the mPFC to the thalamus (7–9). Cholinergic inputs to the cerebral cortex appear early during brain development and are widespread in rat by the third week of postnatal life (10,11). This phenomenon correlates temporally with a developmental peak in the nicotinic current response to ACh in rodent mPFC layer VI neurons (4,5). Since this peak occurs during a critical period of cortical circuit refinement (12,13) and nicotinic stimulation can modulate the retraction and maturation of neuronal processes (14–16), it is important to investigate the role of developmental nicotinic signaling in mPFC layer VI neurons because this may have a significant impact on the function and morphology of attention systems later in life. The nAChR α5 accessory subunit plays an important role in the function of mature mPFC layer VI neurons. We have reported previously that it normally incorporates into α4β2* nAChRs of mature mPFC layer VI neurons to dramatically increase their excitatory response to nicotinic stimulation and that expression of the α5 subunit is required for normal attention behavior in adult mice (6). The role of nAChR α5 subunits in the development of these neurons, however, is not known. We sought to answer this question by examining nicotinic currents and dendritic morphology in wild-type mice compared with mice carrying a null mutation for the α5 subunit (α5−/−) (17) during postnatal development.
Methods and Materials
Experimental Animals
Homozygous wild-type (α5+/+) and α5−/− mice (17) were bred in separate lines that were less than five generations removed from shared heterozygous (α5+/−) parents. For a subset of experiments, a double transgenic line was also generated by crossing α5−/− mice with a knockin line in which all nAChR α4 subunits have been labeled with the yellow fluorescent protein (YFP) motif (18) to generate experimental animals having YFP-labeled nAChR α4 subunits that were either homozygous wild-type for the α5 subunit or homozygous deficient for the α5 subunit. Both of the original transgenic mouse lines were on the C57BL/6 background and male mice were used exclusively for this study. Experiments examining neuron morphology were performed using approximately equal numbers of mice from the original α5−/− line and from the new double-transgenic line. It has been shown previously that the presence of the YFP motif in α4* nAChRs does not affect their expression or function (18), and we also found no effects of the YFP motif on any measure in this study, including nAChR function and neuronal morphology.
Mice were weaned after the third postnatal week (postnatal day [P]21), separated according to sex, and housed in groups of two to four per cage at an ambient temperature of 22°C with a 12-hour light/dark cycle with lights on at 7:00 am. Mice were given ad libitum access to food and water throughout this study. All experimental animals were cared for according to the principles and guidelines of the Canadian Council on Animal Care and the experimental protocol was approved by the University of Toronto Animal Care Committee.
Brain Slice Preparation and Electrophysiology
Whole cell recordings were made in coronal slices 400 μm thick of the mPFC prepared from mice at four distinct postnatal developmental ages: week 2 (P11–13), week 3 (P14–20), week 4 (P21–27), and young adulthood (P60–115). Please see Supplement 1 for a more detailed methodology.
Neuron Morphology
Individual mPFC layer VI pyramidal neurons were patched and electrophysiological recordings were made in acute brain slice as described in Supplement 1, with the exception that the patch electrodes contained .5% (wt/vol) Neurobiotin Tracer (Vector Laboratories, Burlington, Ontario, Canada). Neurons were held for at least 30 minutes to allow the Neurobiotin Tracer to diffuse into the cell, and patch electrodes were then withdrawn slowly to allow resealing of the neurons. Slices were fixed overnight in a solution containing 4% (wt/vol) paraformaldehyde in .1 mol/L phosphate buffer (pH 7.5) and then incubated in a solution containing a 1:500 dilution of streptavidin conjugated with Alexa Fluor 594 (Invitrogen, Burlington, Ontario, Canada) in .1 mol/L phosphate buffer (pH 7.5) for 2 hours at room temperature, washed, mounted onto microscope slides, and cover-slipped using Fluoromount G (SouthernBiotech, Birmingham, Alabama) (protocol adapted from [19]).
Multiphoton imaging of labeled neurons within mounted sections was performed using a Ti:sapphire laser (Mai Tai, Spectra Physics, Mountain View, California) tuned to wavelength 780 nm and an Olympus Fluoview FV1000 microscope with an Olympus XLPlan N 25×, 1.05 NA water-immersion objective (Olympus, Richmond Hill, Ontario, Canada). The inherent z-sectioning in multiphoton imaging allowed us to capture z axis image stacks that were overlapping in the x and y axes and, when stitched together in three dimensions, contained the entire labeled neuron. These image stacks measured 500 μm (x axis) by 500 μm (y axis) with a z axis depth depending on the location each neuron and its dendrites within the slice. Red fluorescence was isolated using a dichroic mirror at 570 nm and filtered with a red light filter (BA570-625; Olympus). Captured image stacks were stitched together using Neurolucida software (MicroBrightField Inc., Williston, Vermont) and neurons were traced and analyzed using Neurolucida AutoNeuron in the interactive mode. Pyramidal neurons included in the analysis had distinguishable apical dendrites and were fully contained within the fixed brain slice.
Immunohistochemistry
Immunohistochemistry for YFP-labeled nAChR α4 subunits located within 400 μm thick coronal slices of the mPFC was performed as described in Supplement 1 and in a previous study (5).
Statistical Analysis
All data are presented as mean ± SEM. Electrophysiology experiments, quantitative dendrite measurements, Sholl analyses, and immunohistochemical experiments were analyzed using two-way analysis of variance (ANOVA) followed by Bonferroni post hoc analysis to test for effects of age and α5 subunit genotype. Contingency analysis of the laminar position of the termination of apical dendrites was performed using the Fisher’s exact test. Statistical analyses were performed using GraphPad Prism 5 (GraphPad Software Inc., San Jose, California) and a level of p < .05 was required to indicate statistical significance.
Results
The α5 Subunit Drives the Developmental Peak in Layer VI Nicotinic Currents
To examine the role of nAChR α5 accessory subunits on electrophysiological responses in mPFC layer VI neurons during postnatal development, we prepared acute brain slices from male mice that were either wild-type (α5+/+) or constitutively deleted for the α5 subunit (α5−/−) (17). We elicited nicotinic currents in response to 1 mmol/L ACh (in the presence of 200 nmol/L atropine) at four postnatal ages (Figure 1). Presence of the nAChR α5 subunit had striking consequences on the magnitude of the nicotinic currents elicited at each age and also on the developmental peak in nicotinic currents in mPFC layer VI neurons [Figure 1A; F(1,166) = 80.72, p < .0001 for the genotype effect]. While neurons from wild-type mice showed a developmental peak during week 3 that was significantly greater than each of the other ages examined for wild-type mice (p < .05 for each comparison), neurons from α5−/− mice showed consistently lower nicotinic currents with negligible developmental changes. Exemplary nicotinic inward current traces for each experimental group are shown in Figure 1B.
Figure 1.
Changes in the excitation of medial prefrontal layer VI pyramidal neurons by acetylcholine during postnatal development are dependent on the nicotinic receptor α5 subunit. (A) The peak inward current response to 10-second bath application of 1 mmol/L acetylcholine (in the presence of 200 nmol/L atropine) was measured in neurons from wild-type (WT) mice expressing the α5 subunit (closed bars) and in neurons from mice in which the α5 subunit has been genetically deleted (α5−/−, open bars) in four postnatal age groups (week 2, week 3, week 4, and adult). Two-way analysis of variance identified an effect of genotype [F(1,166) = 80.72, p < .0001], where the current response in WT neurons was greater than that in α5−/− neurons at each age (Bonferroni post hoc test, *p < .05 at each age), and also a significant interaction between the effects of genotype and age [F(3,166) = 2.98, p = .03]. There was a significant effect of age in WT neurons only, where the acetylcholine response at week 3 was significantly greater than each of the other ages [one-way analysis of variance, F(3,86) = 2.92, p = .04; Newman-Keuls post hoc test, ‡p < .05 compared with each other age]. Typical voltage-clamp traces in response to acetylcholine application (dark gray lines) are shown in (B) for neurons from each experimental group.
The α5 Subunit Underlies Normal Developmental Changes in Layer VI Neuron Morphology
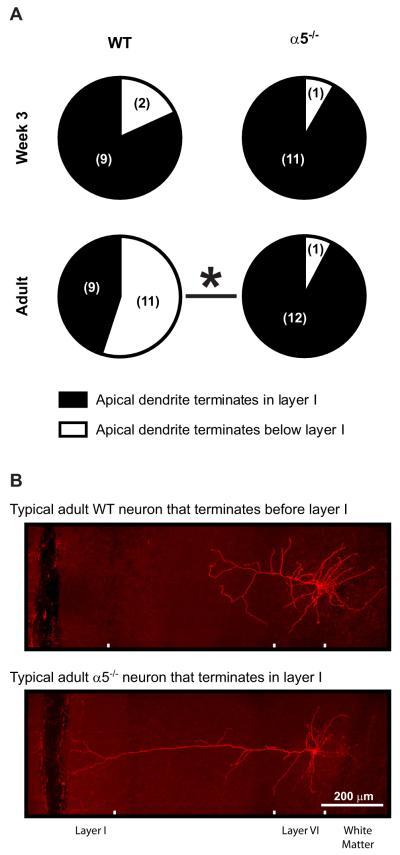
The timing of the developmental peak in mPFC layer VI neuron nicotinic currents is of great interest because nicotinic stimulation can modulate the growth and retraction of neurites for cultured neurons (14,15) and because the observed peak coincides temporally with a critical period of circuit refinement for the developing rodent cerebral cortex (12,13). We next tested whether nicotinic signaling influences the in vivo development of mPFC layer VI neurons by examining dendritic morphology in wild-type and α5−/− mice at postnatal week 3 (near the beginning of the critical developmental period) and in young adulthood. For these experiments, we included Neurobiotin in the patch pipettes, which diffused throughout the neurons while we performed electrophysiological recordings, and then used multiphoton imaging to visualize the filled neurons in situ within fixed brain slices. As demonstrated in Figure 2A, in week 3, almost all layer VI neurons from mice of both genotypes had long apical dendrites that reached into layer I of the mPFC (9 of 11 neurons for wild-type mice and 11 of 12 neurons for α5−/− mice, p = .6). This finding in the mPFC of young mice (mean age of P17 ± 1 day) contrasts greatly with previous morphological studies in sensory and motor cortices across development, where layer VI neuron apical dendrites predominantly terminate within the midlayers of the cortex (reviewed recently in [20]). More consistent with this morphological work in other cortical areas, we found in our young adult wild-type mice (mean age of P77 ± 4 days) that the majority of mPFC layer VI neurons also terminate before layer I (11 of 20 neurons, p = .07 compared with week 3 wild-type neurons), suggesting that a retraction of apical dendrites normally occurs for a subset of mPFC layer VI neurons during postnatal brain maturation. Importantly, we did not observe this maturational change in layer VI neurons of young adult α5−/− mice (mean age of P87 ± 5 days); almost all neurons (12 of 13) retained the immature phenotype with apical dendrites reaching into layer I (Figure 2A; p = 1.0 compared with week 3 α5−/− neurons; p = .009 compared with adult wild-type neurons). These results suggest that ontogenic changes in the morphology of mPFC layer VI neuron apical dendrites during postnatal development depend on the presence of the nicotinic α5 subunit. Exemplary z-projection photomicrographs of a typical adult wild-type neuron and a typical adult α5−/− neuron are shown in Figure 2B.
Figure 2.
Qualitative analysis of medial prefrontal layer VI pyramidal neuron apical dendrites. Pie charts in (A) show the proportion of neurons from wild-type (WT) mice and nicotinic receptor α5 subunit knockout (α5−/−) mice at postnatal week 3 and in adulthood with apical dendrite trees that either terminate within layer I (black portion) or terminate below layer I (white portion) of the medial prefrontal cortex. The absolute number of neurons identified within each group is shown in brackets. At week 3, the majority of neurons of both genotypes had long apical dendrites extending into layer I, whereas in adulthood, the majority of WT neurons had shorter apical dendrites that terminated below layer I (Fisher’s exact test, p = .07 comparing adult WT and week 3 WT neurons; *p = .009 comparing adult WT and adult α5−/− neurons). Representative z-projection photomicrographs of Neurobiotin-filled neurons are shown in (B) for a typical adult WT neuron that terminates below layer I and a typical adult α5−/− neuron that terminates within layer I.
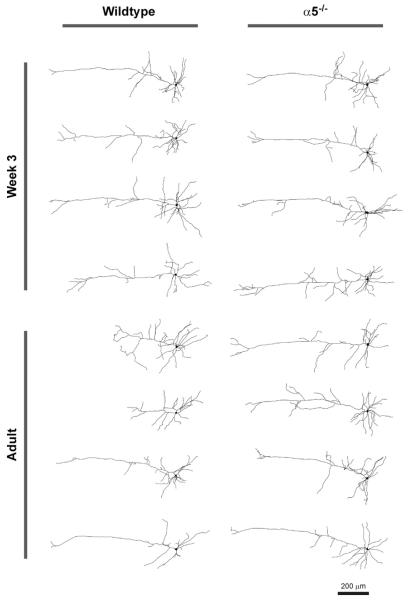
To investigate the developmental changes occurring in wild-type neurons and the striking genotype effect in adulthood, we performed a detailed quantitative analysis of neuronal morphology by tracing and analyzing each neuron in three dimensions using Neurolucida software. Representative z-projections of traced neurons are shown in Figure 3. Results from three-dimensional Sholl analyses are shown in Figure 4A–D, where we found significant effects of genotype on neuronal complexity not only in adulthood (Figure 4B; p < .0001) but also at week 3 (Figure 4A; p = .01), suggesting that the influence of α5-containing nicotinic receptors on apical dendrite morphology is already in progress at this age. Analysis of wild-type neurons only found a significant effect of age on apical dendrite complexity, with the main difference appearing within approximately the distal 300 μm (500–800 μm) from the soma (Figure 4C; p = .01), whereas comparison of Sholl plots for α5−/− neurons only found no effect of age (Figure 4D; p = .6), providing further evidence that developmental changes in apical dendrite morphology are dependent on the α5 nAChR subunit. Further analysis of traced neurons has also found genotype effects on total apical dendrite length (Figure 4E; p = .02), the distance from the soma to the most distal apical dendrite terminal (Figure 4F; p = .03), and the total number of apical dendrite terminals (Figure 4G; p = .03).
Figure 3.
Representative z-projections of traced neurons are shown for wild-type and nicotinic receptor α5 subunit knockout (α5−/−) mice at postnatal week 3 and in adulthood.
Figure 4.
Quantitative analysis of medial prefrontal layer VI pyramidal neuron apical dendrites. (A–D) Three-dimensional Sholl analysis measuring the number of dendrite intersections at concentric spheres of varying distance from the soma for neurons from wild-type (WT) and nicotinic receptor α5 subunit knockout (α5−/−) mice. Two-way analysis of variance found effects of genotype both at week 3 (A) [F(1,578) = 5.97, p = .01] and in adulthood (B) [F(1,1044) = 40.88, p < .0001]. Comparison of the Sholl analyses for WT mice at each age (C) identified a significant effect of age [F(1,850) = 6.65, p = .01] with the difference between ages appearing at the most distal 300 μm from the soma (marked by an arrow), whereas the same comparison in α5−/− mice (D) found no effect of age [F(1,756) = 0.3, p = .6]. Two-way analysis of variance identified significant effects of α5 subunit genotype on total apical dendrite length (E) [F(1,46) = 5.81, p = .02], the distance from the soma to the most distal apical dendrite terminal (F) [F(1,46) = 5.15, p = .03], and the total number of apical dendrite terminals (G) [F(1,46) = 4.85, p = .03]. However, there were no significant effects of age or significant interactions between the effects of genotype and age on these three measures (all p > .05). *p < .05 for the difference between WT and α5−/− neurons in adulthood (Bonferroni post hoc test).
The proposed α5 subunit-dependent retraction appears to be specific for the apical dendrite tree. In these same neurons, the distance between the soma and the most distal basal dendrite terminal was longer in wild-type neurons compared with α5−/− neurons: at week 3, 285 ± 34 μm (n = 8) for wild-type neurons and 206 ± 10 μm (n = 9) for α5−/− neurons; at adulthood, 289 ± 27 μm (n = 18) for wild-type neurons and 241 ± 21 (n = 12) for α5−/− neurons; F(1,43) = 5.27, p = .03 for effect of genotype. Yet, the mean number of basal dendrite trees per neuron was significantly lower in wild-type neurons compared with α5−/− neurons: at week 3, 4.9 ± .4 (n = 8) for wild-type neurons and 5.6 ± .4 (n = 9) for α5−/− neurons; at adulthood, 4.2 ± .2 (n = 18) for wild-type neurons and 5.3 ± .3 (n = 12) for α5−/− neurons; F(1,43) = 8.89, p = .005 for effect of genotype.
We thoroughly compared electrophysiological and morphological properties of traced neurons in this study and found that neuronal input resistance correlated negatively with a number of measures for neuronal size. For all traced neurons, there were significant correlations between input resistance and: soma volume (r = −.45, p = .002), total dendrite volume (r = −.41, p = .006), overall neuron volume (soma + dendrites, r = −.35, p = .02), and total dendrite length (r = −.35, p = .02). Spike amplitude in these neurons correlated positively with soma volume (r = .37, p = .01); however, there were no other significant correlations between spike amplitude or resting membrane potential and any measure of neuronal morphology.
Loss of the α5 Subunit Does Not Alter the Morphology of the Medial Prefrontal Cortex
We next performed a control experiment to test whether genetic deletion of the nAChR α5 subunit in our mouse model alters the morphology of the mPFC or the distribution pattern for neurons expressing α4* nAChRs (presumably α4β2* nAChRs) within layer VI of the mPFC. We performed this experiment by crossbreeding α5−/− mice with a knockin mouse line in which all nAChR α4 subunits are tagged with the YFP motif (18) to create experimental animals having YFP-labeled α4 subunits that were either homozygous wild-type for the α5 subunit or null for the α5 nAChR subunit. Immunohistochemistry for the exogenous YFP motif and immunostaining analysis were performed in 400 μm thick coronal sections as described in Supplement 1 and in a previous study (5).
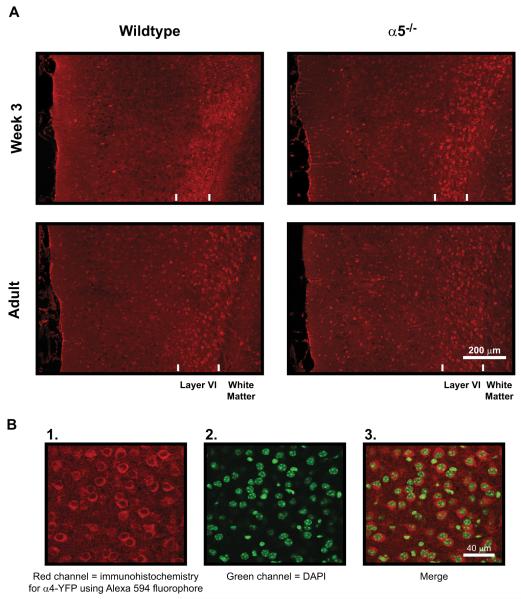
As shown in Figure 5, there was an intense band of immunostaining for α4-YFP within layer VI of the mPFC that was more prominent at week 3 compared with adulthood. However, there were no effects of α5 subunit genotype on mPFC morphology or α4-YFP immunostaining pattern at either age. The mean width of the mPFC, as measured from the layer VI/white matter boundary and the pial surface medial to layer I, was not affected by age [F(1,29) = .00; p = 1.00] or by α5 genotype [F(1,29) = .03, p = .87]. Values at week 3 were 871 ± 20 μm (n = 11) for wild-type and 841 ± 16 μm (n = 11) for α5−/− mice and at adulthood were 837 ± 35 μm (n = 5) for wild-type and 874 ± 18 (n = 6) for α5−/− mice. The width of the layer VI immunoreactive band was lower at week 3 compared with adulthood: at week 3, 209 ± 11 μm (n = 11) for wild-type and 188±10 μm (n=11) for α5−/− mice; at adulthood, 237±11 μm (n=5) for wild-type and 239 ± 5 μm (n = 6) for α5−/− mice; F(1,29) = 12.66, p = .001, but was not affected by genotype [F(1,29) = .68, p = .42]. The percentage of neurons within layer VI expressing α4-YFP was slightly greater at week 3 compared with adulthood: at week 3,75.9 ± 1.3% (n = 11) for wild-type and 74.3±2.3% (n = 11) for α5−/− mice; at adulthood, 70.3±1.8% (n = 5) for wild-type and 69.2±3.0% (n = 6) for α5−/− mice; F(1,29) = 5.52, p = .03), but again was not affected by α5 genotype [F(1,29) = .34, p = .57]. We also examined the expression of α4-YFP positive neurons throughout the remainder of the mPFC (layers I–V) and found no effect of age or genotype (data not shown). These results demonstrate that the observed α5 subunit-dependent developmental changes in mPFC layer VI neuron apical dendrite morphology occur in the absence of overt changes to the morphology of the mPFC itself or to the distribution pattern of this α4* nAChR-expressing neuronal population.
Figure 5.
Immunohistochemistry for α4* nicotinic acetylcholine receptors within the medial prefrontal cortex. (A) Low magnification photomicrographs within medial prefrontal slices showing immunohistochemical staining for nicotinic receptor α4 subunits tagged with the yellow fluorescent protein (YFP) motif in wild-type and nicotinic receptor α5 subunit knockout (α5−/−) mice at postnatal week 3 and in adulthood. The pial (medial) surface is located on the left and the white matter is located on the right of each photomicrograph. Immunostaining in all mice identified a distinct band of bright red neuronal cell bodies expressing α4* nicotinic receptors within layer VI (presumably α4β2* receptors). This band was thinner and more dense at week 3 compared with adulthood. However, there were no effects of α5 subunit genotype on the width of the layer VI immunoreactive band, the proportion of neurons within the band expressing α4* nicotinic receptors, or the width of the medial prefrontal cortex (see Results for more detail). (B) Example high-resolution photomicro-graphs are shown within layer VI for 1) α4-YFP immunostained neurons, 2) 4 ,6-diamidino-2-phenylindole (DAPI) counterstained cell nuclei, and 3) the merge of α4-YPP and DAPI staining from 1) and 2).
Discussion
This study reveals several novel findings about the development of prefrontal attention systems. First, we found that nAChRs comprising the α5 subunit underlie the developmental peak in the nicotinic excitation of mPFC layer VI neurons that occurs during the third week of postnatal life (4,5). Next, we observed what appears to be a normal developmental pruning of apical dendrites in these same neurons between the third week of postnatal life (a juvenile stage of development) and young adulthood that is also driven by the presence of nAChR α5 subunits. These combined results implicate the α5 subunit, most likely via increased nicotinic signaling during the critical period of brain circuit refinement, in the maturation of prefrontal attention systems. Moreover, since α5−/− mice are characterized by mPFC layer VI neurons that retain their long immature phenotype and also display attention deficits in adulthood (6), this mouse model may provide novel insight into the roles for nAChRs, developmental nicotinic signaling, and prefrontal neuronal morphology in the pathophysiology of developmental brain disorders involving deficits in attention.
Role of Nicotinic α5 Subunits Within Developing Prefrontal Layer VI Neurons
The most straightforward explanation for the finding that the developmental peak in mPFC layer VI neuron nicotinic currents depends on the α5 nAChR subunit would be a concurrent developmental peak in its expression, leading to a peak in the total number and/or the proportion of α4/β2/α5 nAChRs. While messenger RNA for the α4 and β2 subunits is not changed in rat cerebral cortex during postnatal development (21), messenger RNA for the α5 subunit is developmentally regulated and peaks in layer VI during the first 2 to 3 weeks of postnatal development (22). The number of epibatidine binding sites (consisting mostly of α4β2* nAChRs[23,24]) is increased in whole cerebral cortex (21) and specifically in layer VI (25) during postnatal week 3, suggesting that developmental changes in α5 subunit expression lead to an increased number of α4/β2/α5 nAChRs during the postnatal current peak. In reduced preparations, incorporation of α5 subunits has been shown to enhance the function of activated α4β2* nAChRs via increased cation flux (26–30), which in neurons leads to an increased excitatory response to nicotinic stimulation, as observed in this study for wild-type neurons compared with α5−/− neurons throughout postnatal development.
Increased nicotinic stimulation during early postnatal life is physiologically relevant in mPFC and is likely to influence the normal morphological development of layer VI pyramidal neurons. The mPFC likely receives significant cholinergic input during this time. Afferent axon terminals containing the ACh-synthesizing enzyme choline acetyltransferase are present at birth and rapidly increase in density during the first 2 postnatal weeks to reach adult levels throughout the rat cerebral cortex near P16–P32 (11). Moreover, immunoreactivity for the ACh-metabolizing enzyme acetylcholinesterase is also present by P16, most predominantly in layer VI where the majority of cortical α4β2* nAChRs are expressed (10). Two elegant studies have demonstrated that direct nicotinic stimulation can lead to shorter neuronal processes for cultured neurons containing nAChRs: Lipton et al. (14) found that nicotinic antagonists can increase neurite outgrowth in cultured rat retinal ganglion neurons, and Pugh and Berg (15) have demonstrated that nicotinic stimulation can lead to neurite retraction in cultured chick ciliary ganglion neurons via calcium-dependent mechanisms. Since the timing of the developmental peak in nicotinic currents for wild-type mice corresponds temporally with the start of the critical period of normal cortical circuit pruning and refinement (reviewed in [12,13]), we hypothesize that increased nicotinic signaling in mPFC layer VI neurons during this time, via α5-containing nAChRs, can lead to increased calcium entry (either directly through the receptor itself [26,27] or through voltage-gated calcium channels present in deep cortical layers at this time [31]) to initiate apical dendrite retraction for a subset of mPFC layer VI neurons. Since the α5 subunit is expressed in relatively few brain regions (17,32), its presence in layer VI neurons themselves would be the simplest mechanism underling the retraction of their apical dendrites. However, it is possible that α5 nAChRs on dopaminergic neurons (17,32) could influence the morphology of mPFC layer VI neurons.
Medial Prefrontal Layer VI Neuron Morphology and Its Functional Implications
The sixth cortical layer is the most complex and least understood (33). The majority of studies examining layer VI neuron morphology have focused on sensory and motor cortices (reviewed recently in [20]), and to our knowledge, this study represents the first morphological analysis of mPFC layer VI pyramidal neurons. In this regard, we observe a stark difference in apical dendrite morphology between the mPFC and other cortical regions, where the vast majority of layer VI pyramidal neurons have relatively short apical dendrites that do not extend beyond layer IV (which is lacking in mPFC but represents the midpoint in cortical depth). Indeed, the predominantly short apical dendrites of layer VI neurons have been identified in rodent cortex consistently across postnatal development: in week 2 to 3 (rat [34,35], mouse [34,36]), in week 4 to 7 (rat [37,38]), and in adulthood (rat [34,39,40], mouse [33,34]).
The terminating layer for the apical dendrite of an mPFC layer VI neuron will have a significant influence on the type and amount of input received, as excitatory input near the distal terminal of layer VI neurons can be detected at the soma (37) and can initiate action potential firing (38). The increased proportion of long mPFC layer VI neurons (terminating within layer I) in adult α5−/− mice would allow this neuronal population to receive distinct and additional input not normally received in adult wild-type mice, potentially affecting the ability of this mPFC output layer to provide appropriate feedback to the thalamus. It is tempting to speculate that apical dendritic retraction may be a part of the normally prolonged maturation of prefrontal attention circuitry (41,42).
We have shown previously that α5−/− mice exhibit decreased performance on a demanding attention task. Indeed, attentional performance, a key behavior mediated by the mPFC, is compromised in adult α5−/− mice compared with wild-type mice (6). It remains possible that α5 subunits expressed in other discrete brain regions (17,22,32) could influence attention performance, although the mPFC is the most likely of these regions to directly influence attention in the five-choice serial reaction time test (43,44). Importantly, a recent study has demonstrated that β2 subunit-containing nAChRs located specifically within mPFC layer VI neurons (which are the focus of our study and contain α5 subunits) are directly involved in attention performance in this task (45).
Implications for the Aberrant Maturation of Prefrontal Attention Circuitry
Several human neurodevelopmental disorders involve nicotinic signaling, prefrontal dysfunction, and attention deficits. For example, inappropriate function of the frontal cortex has been observed in attention-deficit disorder (46), and selectively activating α4β2* nAChRs has shown promise in ameliorating behavioral symptoms in both rodent models (47,48) and human patients (49,50). Similarly, attention deficits in autism spectrum disorder (51) are accompanied by decreased cortical α4β2* nAChR expression (52,53) and dysregulated frontal cortex function (54,55). Interestingly, there is evidence suggesting a delayed maturation of dendritic pruning in these developmental neuropsychiatric disorders (56,57). Finally, a number of single nucleotide polymorphisms (SNPs) have been identified within the α5 subunit gene CHRNA5, which can alter nicotinic currents and influence behavior (58–61). For example, the nonsynonymous SNP D398N decreases the function of α4/β2/α5 nAChRs in cell culture (60,62). Consistent with our findings in the α5−/− mice, this SNP can decrease the connectivity of a cortical output circuit (63) and is linked with a cognitive profile that includes deficits in attention (64).
In summary, our results suggest that developmental nicotinic signaling through α4/β2/α5 nAChRs likely plays an important role in the maturation of mPFC layer VI pyramidal neurons to determine the function of prefrontal attention systems later in life. These broad findings are important for the understanding of the normal development of attention systems and may provide insight into the pathophysiology underlying specific developmental brain disorders involving nAChRs and deficits in attention, such as attention-deficit disorder and autism.
Supplementary Material
Acknowledgments
This work was supported by grants to EKL from the Canadian Institutes of Health Research (MOP 89825), the Canada Research Chairs Program, and the Canadian Foundation for Innovation.
Footnotes
All authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online.
References
- 1.Passetti F, Dalley JW, O’Connell MT, Everitt BJ, Robbins TW. Increased acetylcholine release in the rat medial prefrontal cortex during performance of a visual attentional task. Eur J Neurosci. 2000;12:3051–3058. doi: 10.1046/j.1460-9568.2000.00183.x. [DOI] [PubMed] [Google Scholar]
- 2.Dalley JW, Theobald DE, Bouger P, Chudasama Y, Cardinal RN, Robbins TW. Cortical cholinergic function and deficits in visual attentional performance in rats following 192 IgG-saporin-induced lesions of the medial prefrontal cortex. Cereb Cortex. 2004;14:922–932. doi: 10.1093/cercor/bhh052. [DOI] [PubMed] [Google Scholar]
- 3.Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56:141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kassam SM, Herman PM, Goodfellow NM, Alves NC, Lambe EK. Developmental excitation of corticothalamic neurons by nicotinic acetylcholine receptors. J Neurosci. 2008;28:8756–8764. doi: 10.1523/JNEUROSCI.2645-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alves NC, Bailey CDC, Nashmi R, Lambe EK. Developmental sex differences in nicotinic currents of prefrontal layer VI neurons in mice and rats. PLoS One. 2010;5:e9261. doi: 10.1371/journal.pone.0009261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey CDC, De Biasi M, Fletcher PJ, Lambe EK. The nicotinic acetylcholine receptor α5 subunit plays a key role in attention circuitry and accuracy. J Neurosci. 2010;30:9241–9252. doi: 10.1523/JNEUROSCI.2258-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alitto HJ, Usrey WM. Corticothalamic feedback and sensory processing. Curr Opin Neurobiol. 2003;13:440–445. doi: 10.1016/s0959-4388(03)00096-5. [DOI] [PubMed] [Google Scholar]
- 8.Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: Projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- 9.Zikopoulos B, Barbas H. Prefrontal projections to the thalamic reticular nucleus form a unique circuit for attentional mechanisms. J Neurosci. 2006;26:7348–7361. doi: 10.1523/JNEUROSCI.5511-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kristt DA. Acetylcholinesterase-containing neurons of layer VIb in immature neocortex: Possible component of an early formed intrinsic cortical circuit. Anat Embryol (Berl) 1979;157:217–226. doi: 10.1007/BF00305161. [DOI] [PubMed] [Google Scholar]
- 11.Mechawar N, Descarries L. The cholinergic innervation develops early and rapidly in the rat cerebral cortex: A quantitative immunocyto-chemical study. Neuroscience. 2001;108:555–567. doi: 10.1016/s0306-4522(01)00389-x. [DOI] [PubMed] [Google Scholar]
- 12.Berardi N, Pizzorusso T, Maffei L. Critical periods during sensory development. Curr Opin Neurobiol. 2000;10:138–145. doi: 10.1016/s0959-4388(99)00047-1. [DOI] [PubMed] [Google Scholar]
- 13.Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- 14.Lipton SA, Frosch MP, Phillips MD, Tauck DL, Aizenman E. Nicotinic antagonists enhance process outgrowth by rat retinal ganglion cells in culture. Science. 1988;239:1293–1296. doi: 10.1126/science.3344435. [DOI] [PubMed] [Google Scholar]
- 15.Pugh PC, Berg DK. Neuronal acetylcholine receptors that bind α-bungarotoxin mediate neurite retraction in a calcium-dependent manner. J Neurosci. 1994;14:889–896. doi: 10.1523/JNEUROSCI.14-02-00889.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Z, Neff RA, Berg DK. Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science. 2006;314:1610–1613. doi: 10.1126/science.1134246. [DOI] [PubMed] [Google Scholar]
- 17.Salas R, Orr-Urtreger A, Broide RS, Beaudet A, Paylor R, De Biasi M. The nicotinic acetylcholine receptor subunit α5 mediates short-term effects of nicotine in vivo. Mol Pharmacol. 2003;63:1059–1066. doi: 10.1124/mol.63.5.1059. [DOI] [PubMed] [Google Scholar]
- 18.Nashmi R, Xiao C, Deshpande P, McKinney S, Grady SR, Whiteaker P, et al. Chronic nicotine cell specifically upregulates functional α4* nicotinic receptors: Basis for both tolerance in midbrain and enhanced long-term potentiation in perforant path. J Neurosci. 2007;27:8202–8218. doi: 10.1523/JNEUROSCI.2199-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu RJ, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: Role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci U S A. 2008;105:359–364. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomson AM. Neocortical layer 6, a review. Front Neuroanat. 2010;4:13. doi: 10.3389/fnana.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Liu C, Miao H, Gong ZH, Nordberg A. Postnatal changes of nicotinic acetylcholine receptor α2, α3, α4, α7 and β2 subunits genes expression in rat brain. Int J Dev Neurosci. 1998;16:507–518. doi: 10.1016/s0736-5748(98)00044-6. [DOI] [PubMed] [Google Scholar]
- 22.Winzer-Serhan UH, Leslie FM. Expression of α5 nicotinic acetylcholine receptor subunit mRNA during hippocampal and cortical development. J Comp Neurol. 2005;481:19–30. doi: 10.1002/cne.20357. [DOI] [PubMed] [Google Scholar]
- 23.Mao D, Perry DC, Yasuda RP, Wolfe BB, Kellar KJ. The α4β2α5 nicotinic cholinergic receptor in rat brain is resistant to up-regulation by nicotine in vivo. J Neurochem. 2008;104:446–456. doi: 10.1111/j.1471-4159.2007.05011.x. [DOI] [PubMed] [Google Scholar]
- 24.Gotti C, Moretti M, Meinerz NM, Clementi F, Gaimarri A, Collins AC, Marks MJ. Partial deletion of the nicotinic cholinergic receptor α4 or β2 subunit genes changes the acetylcholine sensitivity of receptor-mediated 86Rb+ efflux in cortex and thalamus and alters relative expression of α4 and β2 subunits. Mol Pharmacol. 2008;73:1796–1807. doi: 10.1124/mol.108.045203. [DOI] [PubMed] [Google Scholar]
- 25.Tribollet E, Bertrand D, Marguerat A, Raggenbass M. Comparative distribution of nicotinic receptor subtypes during development, adulthood and aging: An autoradiographic study in the rat brain. Neuroscience. 2004;124:405–420. doi: 10.1016/j.neuroscience.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 26.Tapia L, Kuryatov A, Lindstrom J. Ca2+ permeability of the (α4)3(β2)2 stoichiometry greatly exceeds that of (α4)2(β2)3 human acetylcholine receptors. Mol Pharmacol. 2007;71:769–776. doi: 10.1124/mol.106.030445. [DOI] [PubMed] [Google Scholar]
- 27.Kuryatov A, Onksen J, Lindstrom J. Roles of accessory subunits in α4β2(*) nicotinic receptors. Mol Pharmacol. 2008;74:132–143. doi: 10.1124/mol.108.046789. [DOI] [PubMed] [Google Scholar]
- 28.Brown RW, Collins AC, Lindstrom JM, Whiteaker P. Nicotinic α5 subunit deletion locally reduces high-affinity agonist activation without altering nicotinic receptor numbers. J Neurochem. 2007;103:204–215. doi: 10.1111/j.1471-4159.2007.04700.x. [DOI] [PubMed] [Google Scholar]
- 29.Grady SR, Salminen O, McIntosh JM, Marks MJ, Collins AC. Mouse striatal dopamine nerve terminals express α4α5β2 and two stoichiometric forms of α4β2*-nicotinic acetylcholine receptors. J Mol Neurosci. 2010;40:91–95. doi: 10.1007/s12031-009-9263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, Role L. Functional contributions of α5 subunit to neuronal acetylcholine receptor channels. Nature. 1996;380:347–351. doi: 10.1038/380347a0. [DOI] [PubMed] [Google Scholar]
- 31.Mize RR, Graham SK, Cork RJ. Expression of the L-type calcium channel in the developing mouse visual system by use of immunocyto-chemistry. Brain Res Dev Brain Res. 2002;136:185–195. doi: 10.1016/s0165-3806(02)00350-4. [DOI] [PubMed] [Google Scholar]
- 32.Wada E, McKinnon D, Heinemann S, Patrick J, Swanson LW. The distribution of mRNA encoded by a new member of the neuronal nicotinic acetylcholine receptor gene family (α5) in the rat central nervous system. Brain Res. 1990;526:45–53. doi: 10.1016/0006-8993(90)90248-a. [DOI] [PubMed] [Google Scholar]
- 33.Chen CC, Abrams S, Pinhas A, Brumberg JC. Morphological heterogeneity of layer VI neurons in mouse barrel cortex. J Comp Neurol. 2009;512:726–746. doi: 10.1002/cne.21926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrer I, Fabregues I, Condom E. A Golgi study of the sixth layer of the cerebral cortex. I. The lissencephalic brain of Rodentia, Lagomorpha, Insectivora and Chiroptera. J Anat. 1986;145:217–234. [PMC free article] [PubMed] [Google Scholar]
- 35.McGann JP, Moyer JR, Jr, Brown TH. Predominance of late-spiking neurons in layer VI of rat perirhinal cortex. J Neurosci. 2001;21:4969–4976. doi: 10.1523/JNEUROSCI.21-14-04969.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brumberg JC, Hamzei-Sichani F, Yuste R. Morphological and physiological characterization of layer VI corticofugal neurons of mouse primary visual cortex. J Neurophysiol. 2003;89:2854–2867. doi: 10.1152/jn.01051.2002. [DOI] [PubMed] [Google Scholar]
- 37.Zarrinpar A, Callaway EM. Local connections to specific types of layer 6 neurons in the rat visual cortex. J Neurophysiol. 2006;95:1751–1761. doi: 10.1152/jn.00974.2005. [DOI] [PubMed] [Google Scholar]
- 38.Ledergerber D, Larkum ME. Properties of layer 6 pyramidal neuron apical dendrites. J Neurosci. 2010;30:13031–13044. doi: 10.1523/JNEUROSCI.2254-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mercer A, West DC, Morris OT, Kirchhecker S, Kerkhoff JE, Thomson AM. Excitatory connections made by presynaptic cortico-cortical pyramidal cells in layer 6 of the neocortex. Cereb Cortex. 2005;15:1485–1496. doi: 10.1093/cercor/bhi027. [DOI] [PubMed] [Google Scholar]
- 40.Zhang ZW, Deschenes M. Intracortical axonal projections of lamina VI cells of the primary somatosensory cortex in the rat: A single-cell labeling study. J Neurosci. 1997;17:6365–6379. doi: 10.1523/JNEUROSCI.17-16-06365.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spencer-Smith M, Anderson V. Healthy and abnormal development of the prefrontal cortex. Dev Neurorehabil. 2009;12:279–297. doi: 10.3109/17518420903090701. [DOI] [PubMed] [Google Scholar]
- 43.Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: Differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 44.Muir JL, Everitt BJ, Robbins TW. The cerebral cortex of the rat and visual attentional function: Dissociable effects of mediofrontal, cingu-late, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cereb Cortex. 1996;6:470–481. doi: 10.1093/cercor/6.3.470. [DOI] [PubMed] [Google Scholar]
- 45.Guillem K, Bloem B, Poorthuis RB, Loos M, Smit AB, Maskos U, et al. Nicotinic acetylcholine receptor β2 subunits in the medial pre-frontal cortex control attention. Science. 2011;333:888–891. doi: 10.1126/science.1207079. [DOI] [PubMed] [Google Scholar]
- 46.Fassbender C, Zhang H, Buzy WM, Cortes CR, Mizuiri D, Beckett L, Schweitzer JB. A lack of default network suppression is linked to increased distractibility in ADHD. Brain Res. 2009;1273:114–128. doi: 10.1016/j.brainres.2009.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ueno K, Togashi H, Matsumoto M, Ohashi S, Saito H, Yoshioka M. α4β2 nicotinic acetylcholine receptor activation ameliorates impairment of spontaneous alternation behavior in stroke-prone spontaneously hypertensive rats, an animal model of attention deficit hyperactivity disorder. J Pharmacol Exp Ther. 2002;302:95–100. doi: 10.1124/jpet.302.1.95. [DOI] [PubMed] [Google Scholar]
- 48.Granon S, Changeux JP. Attention-deficit/hyperactivity disorder: A plausible mouse model? Acta Paediatr. 2006;95:645–649. doi: 10.1080/08035250600719747. [DOI] [PubMed] [Google Scholar]
- 49.Wilens TE, Biederman J, Spencer TJ, Bostic J, Prince J, Monuteaux MC, et al. A pilot controlled clinical trial of ABT-418, a cholinergic agonist, in the treatment of adults with attention deficit hyperactivity disorder. Am J Psychiatry. 1999;156:1931–1937. doi: 10.1176/ajp.156.12.1931. [DOI] [PubMed] [Google Scholar]
- 50.Taly A, Corringer PJ, Guedin D, Lestage P, Changeux JP. Nicotinic receptors: Allosteric transitions and therapeutic targets in the nervous system. Nat Rev Drug Discov. 2009;8:733–750. doi: 10.1038/nrd2927. [DOI] [PubMed] [Google Scholar]
- 51.Allen G, Courchesne E. Attention function and dysfunction in autism. Front Biosci. 2001;6:D105–D119. doi: 10.2741/allen. [DOI] [PubMed] [Google Scholar]
- 52.Martin-Ruiz CM, Lee M, Perry RH, Baumann M, Court JA, Perry EK. Molecular analysis of nicotinic receptor expression in autism. Brain Res Mol Brain Res. 2004;123:81–90. doi: 10.1016/j.molbrainres.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 53.Perry EK, Lee ML, Martin-Ruiz CM, Court JA, Volsen SG, Merrit J, et al. Cholinergic activity in autism: Abnormalities in the cerebral cortex and basal forebrain. Am J Psychiatry. 2001;158:1058–1066. doi: 10.1176/appi.ajp.158.7.1058. [DOI] [PubMed] [Google Scholar]
- 54.Gilbert SJ, Bird G, Brindley R, Frith CD, Burgess PW. Atypical recruitment of medial prefrontal cortex in autism spectrum disorders: An fMRI study of two executive function tasks. Neuropsychologia. 2008;46:2281–2291. doi: 10.1016/j.neuropsychologia.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vaidya CJ, Foss-Feig J, Shook D, Kaplan L, Kenworthy L, Gaillard WD. Controlling attention to gaze and arrows in childhood: An fMRI study of typical development and autism spectrum disorders. Dev Sci. 2011;14:911–924. doi: 10.1111/j.1467-7687.2011.01041.x. [DOI] [PubMed] [Google Scholar]
- 56.Raznahan A, Toro R, Daly E, Robertson D, Murphy C, Deeley Q, et al. Cortical anatomy in autism spectrum disorder: An in vivo MRI study on the effect of age. Cereb Cortex. 2010;20:1332–1340. doi: 10.1093/cercor/bhp198. [DOI] [PubMed] [Google Scholar]
- 57.Shaw P, Gogtay N, Rapoport J. Childhood psychiatric disorders as anomalies in neurodevelopmental trajectories. Hum Brain Mapp. 2010;31:917–925. doi: 10.1002/hbm.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16:36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saccone NL, Saccone SF, Hinrichs AL, Stitzel JA, Duan W, Pergadia ML, et al. Multiple distinct risk loci for nicotine dependence identified by dense coverage of the complete family of nicotinic receptor subunit (CHRN) genes. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:453–466. doi: 10.1002/ajmg.b.30828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, et al. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165:1163–1171. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stevens VL, Bierut LJ, Talbot JT, Wang JC, Sun J, Hinrichs AL, et al. Nicotinic receptor gene variants influence susceptibility to heavy smoking. Cancer Epidemiol Biomarkers Prev. 2008;17:3517–3525. doi: 10.1158/1055-9965.EPI-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuryatov A, Berrettini W, Lindstrom J. Acetylcholine receptor (AChR) α5 subunit variant associated with risk for nicotine dependence and lung cancer reduces (α4β2)2α5 AChR function. Mol Pharmacol. 2011;79:119–125. doi: 10.1124/mol.110.066357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hong LE, Hodgkinson CA, Yang Y, Sampath H, Ross TJ, Buchholz B, et al. A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proc Natl Acad Sci U S A. 2010;107:13509–13514. doi: 10.1073/pnas.1004745107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Winterer G, Mittelstrass K, Giegling I, Lamina C, Fehr C, Brenner H, et al. Risk gene variants for nicotine dependence in the CHRNA5-CHRNA3-CHRNB4 cluster are associated with cognitive performance. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:1448–1458. doi: 10.1002/ajmg.b.31126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.