Abstract
Persistent androgen receptor signaling despite low levels of serum androgens has been identified as a critical target for drug discovery in castration-resistant prostate cancer. As proof of principle that the androgen receptor remains relevant in castration-resistant prostate cancer, two recently FDA-approved androgen receptor–targeted agents—abiraterone and enzalutamide—have increased overall survival for patients with castration-resistant prostate cancer in the setting of prior chemotherapy. This review focuses on the androgen receptor and two direct antagonists, enzalutamide and ARN-509. These next-generation androgen receptor antagonists offer great promise for patients with advanced disease. Relative to conventional antiandrogens such as bicalutamide, they bind to the receptor with higher affinity, prevent nuclear translocation and DNA binding, and induce apoptosis without agonist activity in preclinical models. The success of these androgen receptor–targeted agents in the clinic has changed the landscape of therapy for patients with castration-resistant prostate cancer, and further therapeutic options building on this platform are currently in development.
Keywords: prostate cancer, androgen receptor, androgen receptor antagonists, antiandrogens, enzalutamide, ARN-509
In 1941, Huggins and Hodges demonstrated that prostate cancer is an androgen-dependent disease.1 Patients with metastatic prostate cancer treated with medical or surgical castration demonstrated significant tumor regressions and palliation of symptoms, but Huggins himself noted in 1966,when he accepted the Nobel Prize in Physiology or Medicine, that endocrine therapy often fails to control the disease.2 Despite an initial response to androgen deprivation therapy, patients invariably progress to a castration-resistant state where the cancer will grow despite low levels of serum testosterone.3 Castration-resistant prostate cancer (CRPC) represents the lethal form of the disease and carries a poor prognosis with, until recently, few treatment options and a median survival of less than 2 years for those with metastatic disease.(Figure 1).4
FIGURE1.
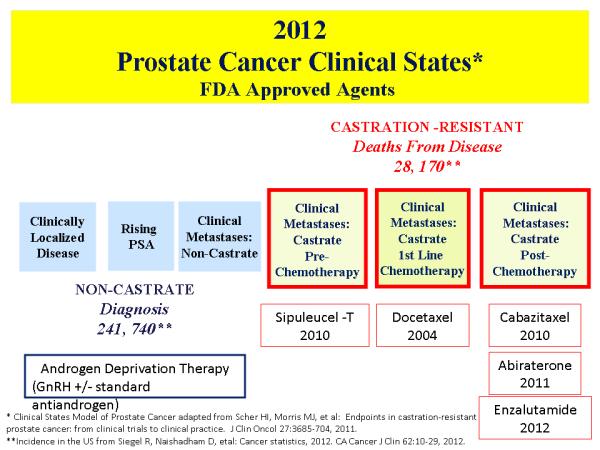
Prostate cancer clinical states and FDA approved agents for each state.
For decades, patients with CRPC were thought to have “hormone-refractory” tumors based on the assumption that clinical progression in the setting of low serum testosterone levels would render additional hormonal manipulations ineffective. Until recently, options for medical management in these patients beyond palliation have been limited primarily to relatively weak second-line hormonal agents such as ketoconazole and cytotoxic agents such as mitoxantrone, (which has shown a palliative benefit versus prednisone alone),5 docetaxel (which demonstrated an overall survival benefit of 2–3 months compared with mitoxantrone),6, 7 and more recently cabazitaxel (which has shown a survival benefit of 2–3 months in the second-line setting for patients who have already received docetaxel).8
Fortunately, the outlook for patients with progressive CRPC has changed dramatically over the past several years as drug development has shifted focus from standard chemotherapy to the rational development of targeted approaches based on a fundamental understanding of disease biology. Since traditional androgen deprivation therapy does not completely deplete intratumoral androgens or the expression of androgen receptor (AR) target genes,9 one of the most successful targeted approaches to date is abiraterone acetate, a drug that inhibits extragonadal and intratumoral synthesis of androgens that contribute to prostate cancer growth. Importantly, abiraterone acetate, an irreversible inhibitor of cytochrome P450-17 (CYP17),10was the first hormonal agent to be FDA approved based on an overall survival benefit in patients with CRPC.11
Enzalutamide (formerly MDV3100) is an AR antagonist that was developed around the same time as abiraterone, and its clinical development is an important validation of the significance of targeting oncogenic alterations associated with resistance to conventional antiandrogens. Molecular profiling studies identified AR overexpression as a frequent genomic alteration in CRPC.12 Laboratory studies showed that tumor latency in castrate mice was shorter and growth was more rapid in LNCaP cells engineered to overexpress AR (LNCaP-AR) relative to those without overexpression. Furthermore, knockdown of the AR with a shRNA inhibited growth. The cells were also stimulated by bicalutamide, suggesting a potential role for AR overexpression in the partial agonist activity of standard antiandrogens. These same cells were then used as a screen to select compounds that optimized growth inhibition without agonist activity, which ultimately led to the identification of enzalutamide for clinical development.
In the clinic, antitumor activity with enzalutamide was observed at the lowest dose level studied, and the drug progressed rapidly through phase 1/2 testing into phase 3, where the final results of the registration trial showed a 37% reduction in the risk of death for patients treated with enzalutamide relative to placebo.13,14 In 2012, enzalutamide was the first next-generation AR antagonist to be FDA-approved for the treatment of patients with CRPC who had received prior docetaxel.14 These results dramatically changed the treatment landscape for patients with CRPC, and additional treatments building on this platform are in development.
AR FORM AND FUNCTION
The effects of androgens on prostate cancer growth are mediated through the AR signaling pathway. Over the past decade, mounting evidence has shown that the AR remains a critical target in CRPC despite low serum androgen levels. Molecular profiling and laboratory models have confirmed the observation that the AR remains relevant in CRPC by demonstrating diverse mechanisms of continued AR signaling despite castrate levels of serum androgen. Clinically, continued dependence on AR signaling is clearly evident by the fact that rising levels of PSA, an AR target gene, occur in virtually all patients with progressive disease.
The human AR gene is located on chromosome Xq11-12 and is a steroid hormone receptor member of the larger nuclear receptor family that includes the estrogen, progesterone, and glucocorticoid receptors. The AR is found in benign epithelial cells as well as in all grades and stages of prostate cancer. Functionally, the AR is a 110-kDa ligand-activated transcription factor consisting of 917 amino acids that harbors3 distinct domains of importance.15 These functional domains include an N-terminal transactivation domain (NTD, exon 1), a DNA-binding domain (DBD, exons 2 and 3), and a C-terminal ligand-binding domain (LBD, exons 5-8). Although most steroid hormone receptors rely on activation factor (AF)-2 for transcriptional activity, for the AR it is the AF-1 region in the NTD that drives AR function.16
In the absence of androgen, the AR associates with a heat shock protein 90 complex that acts as a chaperone to prevent the AR from degradation and maintain the ligand-binding conformation. Since testosterone is a relatively weak ligand, within the prostate it is converted by 5-alpha reductase to the more potent form dihydrotestosterone (DHT).17When DHT binds to the C-terminal LBD of the AR, there is dissociation from the chaperone proteins, resulting in a conformational change and homodimerization of the receptor, translocation into the nucleus, DNA binding, recruitment of cofactors, and ultimately gene transcription of androgen-dependent genes such as PSA, which is mediated in part by androgen-response elements located on target DNA.18, 19
Not surprisingly, there are multiple mechanisms that contribute to continued AR signaling in CRPC that contribute to cell growth and can be exploited to improve upon the current treatment of advanced prostate cancer (Figure 2). These mechanisms include genomic amplification and overexpression of AR,12,20 gain of function mutations allowing AR to be activated by promiscuous ligands such as steroids or antiandrogens,21 upregulation of AR enhancer elements,22 alterations in androgen transport,23,24 increased synthesis of extragonadal androgens,25 abnormalities in AR coactivators and coregulators,26 ligand-independent transactivation of AR by growth factors or cytokines, and AR splice variants that encode for LBD-deficient receptors and are constitutively active but may be dependent upon a full-length receptor for functionality.27-29 This review will focus on direct AR antagonism through inhibition of the AR LBD.
FIGURE 2.

Therapeutic targets of the androgen receptor signaling pathway
FIRST-GENERATION ANTIANDROGENS
The mainstay of treatment for patients with newly diagnosed advanced disease remains suppression of gonadal androgens using a gonadotropin-releasing hormone (GnRH) analog alone or in combination with a conventional antiandrogen such as flutamide, bicalutamide, or nilutamide, which bind to and inhibit the AR.30, 31 These nonsteroidal antiandrogens are reversible inhibitors that bind to the LBD of AR with low affinity relative to androgens and offer incomplete transcriptional inhibition.32
Although clinical testing with antiandrogens with a higher affinity for the AR seemed appealing in vitro, the Achilles heel of this class of agents has been the development of AR agonist potential in patients with CRPC.33 In the setting of AR overexpression, approximately 15%–30% of patients treated with conventional antiandrogens develop mutations that allow for tumor growth.21, 34 One proposed mutation is the W741 AR mutation.35 The agonist potential of drugs such as bicalutamide has been demonstrated in the clinic by the so-called “antiandrogen withdrawal syndrome,” in which withdrawal of conventional antiandrogens results in a paradoxical decline in PSA that is indicative of their agonist activity.36, 37
These limitations of conventional antiandrogens (agonist potential and weak affinity for AR), as well as the discovery that AR gene amplification and overexpression enables prostate cancer to thrive in environments with low levels of circulating androgens, have formed the preclinical basis for developing novel, potent, and pure second-generation AR antagonists that directly target the AR LBD and impair nuclear translocation of the receptor complex.20, 38
SECOND-GENERATION AR ANTAGONISTS
Enzalutamide
Preclinical Development
Enzalutamide (formerly MDV3100) is the first novel antiandrogen targeting the C-terminal LBD to emerge from a chemical structure-activity relationship program designed to develop more potent antiandrogens.39 The nonsteroidal hydantoin agonist RU59063 was used as a chemical scaffold to develop a new class of antiandrogens due to its relatively high affinity for AR and its selectivity for AR over other nuclear hormone receptors. The structural units of RU59063 were systematically modified and the binding affinity, agonism, and antagonism of more than 200 compounds were measured in human prostate cancer cells engineered to overexpress AR. In this way, a new structural class of antiandrogens was developed and the diarylthiohydantoins RD162 and MDV3100 were selected for further biologic study.40
Preclinical testing of RD162 and MDV3100 in castration-resistant LNCaP/AR human prostate cells demonstrated that they could bind with a 5- to 8-fold greater affinity to AR compared with bicalutamide. Furthermore, treatment with RD162 and MDV3100 did not result in increased AR target gene activity indicative of agonist potential, even in the setting of mutant AR protein expression (W741C) contributing to acquired bicalutamide resistance.40, 41
Ultimately, MDV3100 was selected to move forward into clinical testing based on its favorable activity and drug-like properties. MDV3100 was shown to impair nuclear translocation, co-activator peptide recruitment, and DNA binding of the AR by inducing a conformational change in the AR distinct from bicalutamide.MDV3100 also caused poly (ADP-ribose) polymerase cleavage indicative of an apoptotic effect, which is not seen with bicalutamide. And in xenograft models, MDV3100 was associated with a statistically significant reduction in tumor volume relative to bicalutamide, which only had minimal effects and no evidence of tumor regressions.40
Phase 1/2 Trial
Based on promising preclinical results, the first-in-man phase 1/2 trial of the oral next-generation AR antagonist MDV3100 in patients with CRPC was conducted by the Prostate Cancer Clinical Trials Consortium. This trial demonstrated substantial antitumor activity for patients treated with MDV3100 both pre- and post-chemotherapy, credentialing the cell-based screen used to identify the compound and other preclinical models.13 There was evidence of activity based on PSA declines at all dose levels tested, with a plateau between 150 mg and 240 mg. Two-thirds of patients achieved partial remissions or stable disease based on conventional imaging with computed tomography (CT) and bone scan.
Experimental biomarkers incorporated into the trial included 18F-fluoro-5alpha-dihydrotestosterone (FDHT)-PET imaging, which was performed on 22 patients. Analysis of FDHT uptake offers the potential to capture the biologic diversity of metastatic sites through visualization of AR binding and therefore has implications for determining activity and assessing response.42 FDHT-PET imaging showed the on-target effect of MDV3100 through a reduction in FDHT uptake at 4 weeks post treatment with MDV3100 relative to baseline; this appeared to plateau at a dose of 150 mg despite higher plasma concentrations of MDV3100 at higher doses.
The most common grade 3 or 4 toxicity was dose-dependent fatigue (11%).43 There were 2 witnessed seizures, at 300 mg and 600 mg, respectively, and one possible seizure at 480 mg. Although seizure activity is a known potential adverse event for antiandrogens as a class, and is thought to be mediated by off-target inhibition of γ-aminobutyric acid type A (GABA-A) currents,44 it is notable that these patients had concomitant conditions and medications that could have contributed to seizure activity as well. Ultimately, a dose of 160 mg MDV3100 per day was selected to move forward into phase 3 study, based on favorable activity of the drug as demonstrated by PSA, conventional imaging, and FDHT responses at approximately 150 mg relative to higher dose levels, which harbored the potential for greater toxicity.
Phase 3 Trials
The promising phase 1/2 results with MDV3100 in CRPC set the stage for the landmark AFFIRM trial, a phase 3 double-blind study of 1199 men with CRPC following docetaxel-based chemotherapy who were randomized 2:1to receive MDV3100 or placebo. The trial accrued rapidly between September 2009 and November 2010, involving156 centers in15 countries on5 continents. The study was unblended by an independent data safety monitoring committee in November 2011, when a planned interim analysis at 520 events showed that treatment withMDV3100resulted in a 37% reduction in the risk of death as compared with placebo (hazard ratio for death 0.63; 95% CI, 0.53 to 0.75; p<0.001).14
The AFFIRM trial demonstrated a median overall survival of18.4 months in the enzalutamide arm (n=800) versus 13.6 months in the placebo arm (n=399), and this survival advantage was maintained across all patient subgroups. The PSA response rate was equally impressive for a post-docetaxel population, with a ≥50% PSA reduction in 54% of patients taking enzalutamide versus 2% of patients on placebo (p<0.001). Enzalutamide was superior in all secondary endpoints including radiographic progression-free survival, time to PSA progression, soft tissue response rate, and patients’ responses to the Functional Assessment of Cancer Therapy-Prostate (FACT-P) quality-of-life questionnaire. The treatment was well-tolerated, with common side effects including fatigue, diarrhea, and hot flashes. Seizure activity was seen in less than 1% of patients receiving enzalutamide (n=5).14
The ongoing phase 3 PREVAIL trial using enzalutamide versus placebo in men with chemotherapy-naïve CRPC (NCT01212991) has completed accrual, with results of the planned interim analysis being eagerly awaited. Since overall survival in a pre-chemotherapy population can be variable, this study has been designed with a co-primary endpoint of overall survival and radiographic progression-free survival, similar to the COU-AA-302 study of abiraterone in a chemotherapy-naïve CRPC population (NCT00887198).45
ARN-509
Preclinical Development
ARN-509 is a next-generation antiandrogen that also directly binds to the LBD of AR with high affinity, inhibiting its nuclear import and DNA binding capacity. Notably, ARN-509 has several preclinical properties that distinguish it from MDV3100 and support a higher therapeutic index: 1) ARN-509 achieves greater antitumor activity at a lower dose and exposure than MDV3100; 2) steady-state levels of ARN-509 are 2- to 4-fold lower than MDV3100 at equivalent doses, whereas intratumoral levels are equivalent, indicative of a higher tumor/plasma ratio for ARN-509; and 3) brain levels ofARN-509 are 4-fold lower than MDV3100, suggesting a reduced risk of seizure activity.46
Phase 1/2 Trial
Ongoing phase 1/2 analysis of ARN-509 in patients with CRPC has demonstrated that the drug is safe and well tolerated, with linear pharmacokinetics consistent with preclinical models.47 There has been evidence of antitumor activity based on PSA and imaging. To ensure the most accurate dosing of ARN-509, the phase 1 trial used FDHT-PET imaging to confirm the recommended phase 2 dose of 240 mg daily. There was no appreciable difference in uptake at higher doses, suggesting that the maximal efficacy of the drug might be reached before reaching the maximum tolerated dose.
The phase 2studyof ARN-509 is ongoing and was designed to evaluate the activity of ARN-509 in 3 distinct patient populations of men with CRPC: 1) patients with nonmetastatic CRPC who are treatment-naïve (without prior chemotherapy or second-generation antiandrogen exposure), 2) patients with metastatic CRPC who are treatment-naïve, and 3) patients with metastatic CRPC previously treated with abiraterone acetate who are chemotherapy-naïve. Preliminary results for the nonmetastatic and metastatic populations were presented in2012in two separate abstracts at the European Society of Medical Oncology Congress.48, 49The primary endpoint was PSA response rate at 12 weeks according to the Prostate Cancer Working Group 2 (PCWG2) criteria50 in each of the 3 treatment groups. Secondary endpoints included safety, time to PSA progression, and objective response rates. All patients received prior treatment with a luteinizing hormone-releasing hormone analog, with or without a first-generation antiandrogen. The most common treatment-related adverse events were fatigue and gastrointestinal events. At 12 weeks, the proportion of patients in each of the 3 subgroups demonstrating ≥50% decline in PSA from baseline was 91% (nonmetastatic, treatment-naïve), 88% (metastatic, treatment-naïve) and 29% (metastatic, post-abiraterone), respectively. Final analysis is ongoing, with evidence of promising preliminary activity in patients with high-risk nonmetastatic and metastatic, chemotherapy-naïve CRPC both before and after treatment with abiraterone.
FUTURE DIRECTIONS
Given the fulfilled promise of AR-targeted agents for patients with CRPC, a number of new AR-directed therapies capitalizing on this success are in early clinical development (Table 1). ODM-201is a second-generation antiandrogen similar in mechanism to enzalutamide and ARN-509 but it does not cross the blood-brain barrier in preclinical models.51 This has significant implications for next-generation AR antagonists, since seizure activity, albeit at a low frequency, is a concern for antiandrogens as a possible class effect at higher exposures.44 Another small molecule inhibitor of AR that binds to the AR LBD is AZD3514. In addition to inhibition of AR translocation into the nucleus and subsequent transcription of AR-regulated genes, AZD3514 demonstrates a reduction in AR protein levels in vitro, which is a mechanism distinct from that of bicalutamide and other second-generation antiandrogens.52 Additional potential AR antagonists in clinical development include EZN-4176, a novel, locked, nucleic acid-based antisense oligonucleotide against AR that has demonstrated selective and specific down-modulation of AR mRNA and protein in preclinical models,53 and TOK-001, a selective CYP17 lyase inhibitor that has been shown to downregulate and antagonize AR in xenograft models.54
Table 1.
Next-Generation Androgen Receptor Antagonists in Clinical Trials
| Agent | Mechanism | Phase |
|---|---|---|
| MDV3100 (enzalutamide) | • Second-generation antiandrogen |
FDA-approved following docetaxel* |
| ARN-509 (NCT01171898) |
• Second-generation antiandrogen |
Phase 1/2 completed accrual |
| AZD-3514 (NCT01162395) |
• Second-generation antiandrogen • AR downregulation |
Phase 1 |
| EZN-4176 (NCT01337518) |
• AR mRNA antagonist | Phase 1 |
| ODM-201 (NCT01317641 and NCT01429064) |
• Second-generation antiandrogen |
Phase 1/2 |
| TOK-001 (Galeterone) (NCT00959959) |
• Second-generation antiandrogen • Selective CYP 17 lyase inhibition • AR degradation |
Phase 1 completed accrual |
Additional studies with MDV3100 in varied disease states are ongoing
Unfortunately, although AR antagonists have provided benefit to many, not all patients respond to therapy, and among those who do, the durability of response is often limited. In the clinic we have observed the following patterns of response to AR-targeted therapy with enzalutamide: dramatic declines in PSA with durable radiographic control, an intermediate response characterized by a slowly rising PSA, and a proportion of patients who do not respond (Figure 3). Recognizing that PSA is not a surrogate for survival and that patients with a slowly rising PSA might still derive benefit from continuing treatment, the PCWG2 guidelines were incorporated into the phase 3 trials of enzalutamide and abiraterone to ensure that patients could remain on study treatment until radiographic (as opposed to PSA) progression.50 These varied patterns of response to AR-targeted treatment will require an increased understanding of the mechanisms contributing to sensitivity and resistance in order to advance the field and provide maximal benefit to patients with CRPC who will otherwise succumb to their disease.
FIGURE 3.

PSA changes following treatment with enzalutamide: 3 consecutive PSA declines following treatment (A) is associated with prolonged radiographic progression-free survival (median not reached) relative to no sequential declines (B, C), median 23 weeks.
In the laboratory, resistance to enzalutamide has been recapitulated in xenograft models, suggesting both non-AR mechanisms and AR splice forms which lack the LBD, as mediators of progression.55 Approximately 25 variants of post-transcriptional AR splice variants have been identified, the most significant of which appear to be those in which the AR ligand binding domain (LBD) is lost, resulting in ligand-independent constitutive AR activation.56 In a subset of CRPC xenograft models treated with enzalutamide, there is an adaptive shift away from the full length AR towards the AR splice variant, suggesting castration as an important mechanism contributing to drug resistance in prostate cancer progression.57 Since first- and second-generation antiandrogens bind to the C-terminal LBD, newer methods of targeting the AR include targeting the regulatory N-terminal domain, which is essential for transcription in the presence or absence of ligand.58 EPI-001 is a novel small molecule peptide that binds to the N-terminus and disrupts transcription and protein-protein interactions. EPI-001 does not reduce levels of AR protein, but since it is not dependent on ligand, it may have activity in the setting of AR splice variants.16 In LNCaP xeno graft models, treatment with EPI-001 has caused decreased AR gene expression and inhibited tumor growth,59 suggesting that this class of agents might have activity against AR splice variants.
Another avenue of exploration focusing on resistance to AR-targeted therapy is the inhibition of multiple signaling pathways such as the PI3K pathway through reciprocal feedback inhibition. Using xeno graft models and genetically engineered mouse models, it has been shown that prostate tumors with loss of the tumor suppressor Pten are resistant to next-generation antiandrogens. Notably, this resistance can be overcome by combined AR and PI3K/mTOR (mammalian target of rapamycin) pathway inhibition.60, 61 The mechanism of synergy is through reciprocal negative feedback: inhibition of either single pathway activates the other, thereby protecting the tumor cells from death. The most immediate implication of these data is that combinatorial AR + PI3K/mTOR inhibitor therapy could be highly effective in CRPC. The concept of combining hormonal therapy with PI3K/mTOR pathway inhibition has recently been validated in breast cancer in the BOLERO-2 trial.62 This phase 3 study demonstrated superiority of an mTOR inhibitor (everolimus) combined with an aromatase inhibitor (exemestane) in extending progression-free survival of women with metastatic breast cancer when compared to exemestane alone and is readily applicable to the population of men with CRPC.
CONCLUSION
Agents targeting specific molecular alterations in human prostate tumors have changed the landscape of treatment for prostate cancer by validating the importance of the AR across all clinical states in the management of the disease. Next generation AR antagonists such as enzalutamide and ARN509, both of which impair nuclear translocation of the AR and subsequent transcription of AR target genes, represent a giant leap forward in understanding the underlying molecular mechanisms of disease progression and have been applied in a meaningful way to benefit patients in the clinic. Additional exploration of treatments building on the increasing understanding of the AR signaling pathway are underway and offer great hope for patients with CRPC who would otherwise succumb to their disease.
Footnotes
Conflicts of Interest and Source of Funding: None declared.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1:293–297. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 2.Huggins CB. [Accessed December 4, 2012];Nobel Lecture: “Endocrine-Induced Regression of Cancers.”. 1966 http://www.nobelprize.org/nobel_prizes/medicine/laureates/1966/huggins-lecture.html.
- 3.Scher HI, Heller G. Clinical states in prostate cancer: towards a dynamic model of disease progression. Urology. 2000;55:323–327. doi: 10.1016/s0090-4295(99)00471-9. [DOI] [PubMed] [Google Scholar]
- 4.Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232–7. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 5.Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol. 1996;14:1756–64. doi: 10.1200/JCO.1996.14.6.1756. [DOI] [PubMed] [Google Scholar]
- 6.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 7.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 8.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 9.Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–54. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reid AH, Attard G, Barrie E, et al. CYP17 inhibition as a hormonal strategy for prostate cancer. Nat Clin Pract Urol. 2008;5:610–20. doi: 10.1038/ncpuro1237. [DOI] [PubMed] [Google Scholar]
- 11.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 13.Scher HI, Beer TM, Higano CS, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet. 2010;375:1437–46. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 15.Friedlander TW, Ryan CJ. Targeting the androgen receptor. Urol Clin North Am. 2012;39:453–64. doi: 10.1016/j.ucl.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Sadar MD. Small molecule inhibitors targeting the “achilles’ heel” of androgen receptor activity. Cancer Res. 2011;71:1208–13. doi: 10.1158/0008-5472.CAN_10-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Askew EB, Gampe RT, Jr., Stanley TB, et al. Modulation of androgen receptor activation function 2 by testosterone and dihydrotestosterone. J Biol Chem. 2007;282:25801–16. doi: 10.1074/jbc.M703268200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balk SP. Androgen receptor as a target in androgen-independent prostate cancer. Urology. 2002;60:132–8. doi: 10.1016/s0090-4295(02)01593-5. discussion 138-9. [DOI] [PubMed] [Google Scholar]
- 19.Knudsen KE, Kelly WK. Outsmarting androgen receptor: creative approaches for targeting aberrant androgen signaling in advanced prostate cancer. Expert Rev Endocrinol Metab. 2011;6:483–493. doi: 10.1586/eem.11.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scher HI, Sawyers C. Biology of progressive castration resistant prostate cancer: directed therapies targeting the androgen receptor signaling axis. J Clin Oncol. 2005;23:8253–61. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 21.Taplin ME, Bubley GJ, Shuster TD, et al. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med. 1995;332:1393–8. doi: 10.1056/NEJM199505253322101. [DOI] [PubMed] [Google Scholar]
- 22.Cai C, He HH, Chen S, et al. Androgen receptor gene expression in prostate cancer is directly suppressed by the androgen receptor through recruitment of lysine-specific demethylase 1. Cancer Cell. 2011;20:457–71. doi: 10.1016/j.ccr.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright JL, Kwon EM, Ostrander EA, et al. Expression of SLCO transport genes in castration-resistant prostate cancer and impact of genetic variation in SLCO1B3 and SLCO2B1 on prostate cancer outcomes. Cancer Epidemiol Biomarkers Prev. 2011;20:619–27. doi: 10.1158/1055-9965.EPI-10-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang M, Xie W, Mostaghel E, et al. SLCO2B1 and SLCO1B3 may determine time to progression for patients receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2011;29:2565–73. doi: 10.1200/JCO.2010.31.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Locke JA, Guns ES, Lehman ML, et al. Arachidonic acid activation of intratumoral steroid synthesis during prostate cancer progression to castration resistance. Prostate. 2010;70:239–51. doi: 10.1002/pros.21057. [DOI] [PubMed] [Google Scholar]
- 26.Taylor BS, Schultz N, Hieronymus H, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haile S, Sadar MD. Androgen receptor and its splice variants in prostate cancer. Cell Mol Life Sci. 2011;68:3971–81. doi: 10.1007/s00018-011-0766-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu R, Isaacs WB, Luo J. A snapshot of the expression signature of androgen receptor splicing variants and their distinctive transcriptional activities. Prostate. 2011;71:1656–67. doi: 10.1002/pros.21382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watson PA, Chen YF, Balbas MD, et al. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci USA. 2010;107:16759–65. doi: 10.1073/pnas.1012443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akaza H, Hinotsu S, Usami M, et al. Combined androgen blockade with bicalutamide for advanced prostate cancer: long-term follow-up of a phase 3, double-blind, randomized study for survival. Cancer. 2009;115:3437–45. doi: 10.1002/cncr.24395. [DOI] [PubMed] [Google Scholar]
- 31.Crawford ED, Eisenberger MA, McLeod DG, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med. 1989;321:419–24. doi: 10.1056/NEJM198908173210702. [DOI] [PubMed] [Google Scholar]
- 32.Bluemn EG, Nelson PS. The androgen/androgen receptor axis in prostate cancer. Curr Opin Oncol. 2012;24:251–7. doi: 10.1097/CCO.0b013e32835105b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rathkopf D, Liu G, Carducci MA, et al. Phase I dose-escalation study of the novel antiandrogen BMS-641988 in patients with castration-resistant prostate cancer. Clin Cancer Res. 2011;17:880–7. doi: 10.1158/1078-0432.CCR-10-2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Culig Z, Hoffmann J, Erdel M, et al. Switch from antagonist to agonist of the androgen receptor bicalutamide is associated with prostate tumour progression in a new model system. Br J Cancer. 1999;81:242–51. doi: 10.1038/sj.bjc.6690684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hara T, Miyazaki J, Araki H, et al. Novel mutations of androgen receptor: a possible mechanism of bicalutamide withdrawal syndrome. Cancer Res. 2003;63:149–53. [PubMed] [Google Scholar]
- 36.Sartor AO, Tangen CM, Hussain MH, et al. Antiandrogen withdrawal in castrate-refractory prostate cancer: a Southwest Oncology Group trial (SWOG 9426) Cancer. 2008;112:2393–400. doi: 10.1002/cncr.23473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Small EJ, Carroll PR. Prostate-specific antigen decline after casodex withdrawal: evidence for an antiandrogen withdrawal syndrome. Urology. 1994;43:408–10. doi: 10.1016/0090-4295(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 38.Ferraldeschi R, Pezaro C, Karavasilis V, et al. Abiraterone and Novel Antiandrogens: Overcoming Castration Resistance in Prostate Cancer. Annu Rev Med. 2012 doi: 10.1146/annurev-med-121211-091605. [DOI] [PubMed] [Google Scholar]
- 39.Jung ME, Ouk S, Yoo D, et al. Structure-activity relationship for thiohydantoin androgen receptor antagonists for castration-resistant prostate cancer (CRPC) J Med Chem. 2010;53:2779–96. doi: 10.1021/jm901488g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–90. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niraula S, Chi K, Joshua AM. Beyond castration-defining future directions in the hormonal treatment of prostate cancer. Horm Cancer. 2012;3:3–13. doi: 10.1007/s12672-011-0096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beattie BJ, Smith-Jones PM, Jhanwar YS, et al. Pharmacokinetic assessment of the uptake of 16beta-18F-fluoro-5alpha-dihydrotestosterone (FDHT) in prostate tumors as measured by PET. J Nucl Med. 2010;51:183–92. doi: 10.2967/jnumed.109.066159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.National Cancer Institute [Accessed December 5, 2012];Common Terminology Criteria for Adverse Events. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40.
- 44.Foster WR, Car BD, Shi H, et al. Drug safety is a barrier to the discovery and development of new androgen receptor antagonists. Prostate. 2011;71:480–8. doi: 10.1002/pros.21263. [DOI] [PubMed] [Google Scholar]
- 45.Ryan CJ, Smith MR, De Bono JS, et al. Interim analysis (IA) results of COU-AA-302, a randomized, phase III study of abiraterone acetate (AA) in chemotherapy-naive patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). 2012 ASCO Annual Meeting; Chicago, IL. June 1-5, 2012; Abstract LBA4518. [Google Scholar]
- 46.Clegg NJ, Wongvipat J, Joseph JD, et al. ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res. 2012;72:1494–503. doi: 10.1158/0008-5472.CAN-11-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rathkopf DE, Shore N, Antonarakis ES, et al. A phase II study of the androgen signaling inhibitor ARN-509 in patients with castration-resistant prostate cancer (CRPC) J Clin Oncol (Meeting Abstracts) 2012;30 Abstract TPS4697. [Google Scholar]
- 48.Rathkopf D, Antonarakis ES, Shore ND, et al. ARN-509 in men with metastatic castration-resistant prostate cancer (CRPC) Ann Oncol. 2012;23:ix317. Abstract 984TiP. [Google Scholar]
- 49.Smith MR, Antonarakis ES, Ryan CJ, et al. ARN-509 in men with high-risk non metastatic castration-resistant prostate cancer. Ann Oncol. 2012;23:ix303. Abstract 920P. [Google Scholar]
- 50.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Massard C, James ND, Culine S, et al. ARADES Trial: a first-in-man, open-label, phase I/II safety, pharmacokinetic, and proof-of-concept study of ODM-201 in patients (pts) with progressive metastatic castration-resistant prostate cancer (mCRPC) Ann Oncol. 2012;23(suppl 9):ixe1–ixe30. abstr LBA25. [Google Scholar]
- 52.Loddick SA, Bradbury R, Broadbent N, et al. Preclinical profile of AZD3514: a small molecule-targeting androgen receptor function with a novel mechanism of action and the potential to treat castration-resistant prostate cancer. Cancer Res. 2012;72 abstr 3848. [Google Scholar]
- 53.Zhang Y, Castaneda S, Dumble M, et al. Reduced expression of the androgen receptor by third generation of antisense shows antitumor activity in models of prostate cancer. Mol Cancer Ther. 2011;10:2309–19. doi: 10.1158/1535-7163.MCT-11-0329. [DOI] [PubMed] [Google Scholar]
- 54.Montgomery RB, Eisenberger MA, Rettig M, et al. Phase I clinical trial of galeterone (TOK-001), a multifunctional antiandrogen and CYP17 inhibitor in castration-resistant prostate cancer (CRPC) J Clin Oncol. 2012;30(suppl):15. abstr 4665. [Google Scholar]
- 55.Kuruma H, Matsumoto H, Zoubeidi A, et al. Use of MDV3100 to establish androgen-receptor antagonist resistant LNCaP cells for modelling castrate-resistant progression. Cancer Res. 2011;71 Abstract 1595. doi:10.1158/1538-7445.AM2011-1595. [Google Scholar]
- 56.Mostaghel EA, Plymate S. New hormonal therapies for castration-resistant prostate cancer. Endocrinol Metab Clin North Am. 2011;40:625–42. x. doi: 10.1016/j.ecl.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu R, Lu C, Mostaghel EA, et al. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res. 2012;72:3457–62. doi: 10.1158/0008-5472.CAN-11-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jenster G, van der Korput HA, van Vroonhoven C, et al. Domains of the human androgen receptor involved in steroid binding, transcriptional activation, and subcellular localization. Mol Endocrinol. 1991;5:1396–404. doi: 10.1210/mend-5-10-1396. [DOI] [PubMed] [Google Scholar]
- 59.Andersen RJ, Mawji NR, Wang J, et al. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell. 2010;17:535–46. doi: 10.1016/j.ccr.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 60.Carver BS, Chapinski C, Wongvipat J, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19:575–86. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mulholland DJ, Tran LM, Li Y, et al. Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell. 2011;19:792–804. doi: 10.1016/j.ccr.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–9. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]


