Abstract
Celiac Disease (CD) is an autoimmune enteropathy which occurs in genetically susceptible individuals carrying the prerequisite genetic markers HLA DQ2 or DQ8. These genetic markers are present in approximately 30% of the population, and the worldwide prevalence of CD is estimated to be approximately 1-2%. Currently a gluten-free diet is the only treatment for CD, but novel therapies aimed at gluten modification are underway. This review will discuss gluten based therapies including wheat alternatives and wheat selection, enzymatic alteration of wheat, oral enzyme supplements, and polymeric binders as exciting new therapies for treatment of Celiac Disease.
II. Background
When gluten is ingested, gastric and pancreatic proteases cleave gluten into peptide fragments, some of which are immunogenic in CD. Gluten is a group of wheat proteins comprised of gliadins, high molecular weight glutenin subunits (HMW-GS), and low molecular weight glutenin subunits (LMW-GS) [1]. The gliadins tend to be more immunogenic in CD and are further subdivided into α, β, γ and omega fractions. Within these subfractions, the α gliadin has been shown to be the most toxic, with β, γ, and ω having decreasing toxicity [2-4].
Glutamine residues in α-gliadin undergo deamidation to glutamic acid via tissue transglutaminase, thereby increasing the immunogenicity of some gluten derived peptides. Presentation of the immunogenic deamidated gliadin peptides by HLA DQ2 or HLA DQ8 molecules on antigen presenting cells (APCs) to T cells results in the activation of T cells and the production of inflammatory cytokines (Figure 1). The subsequent inflammation ultimately causes small intestinal injury that is characterized by villous atrophy, crypt hyperplasia, and increased intraepithelial leukocytosis [4]. The severity of disease is highly variable and ranges from asymptomatic to severe malnutrition. Complications of continued gluten exposure include increased risk of lymphoma, osteoporosis [5], anemia, infertility [6-7], neurological problems, and cancer [7].
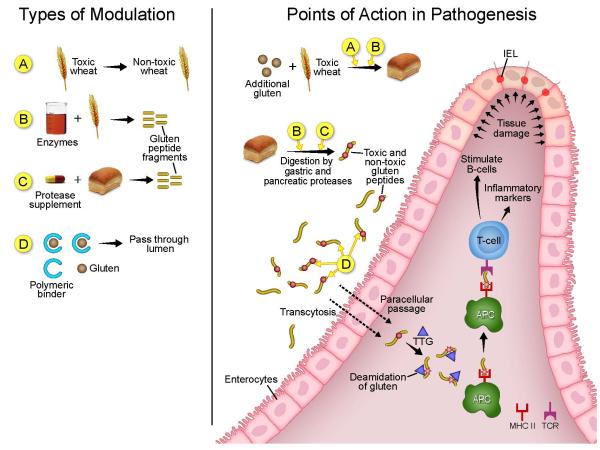
Figure 1.
Depicted on the left side are the four categories in which gluten can be modified: A) gluten removal via wheat mutagenesis B) Enzymatic Pretreatment of Wheat Flour C) Oral Enzyme Supplements and D) Polymeric Binders. Depicted on the right are the points in the pathogenesis of celiac disease that these four categories would act.
The current mainstay of treatment for celiac disease is a gluten free diet (GFD); however, as many as 50% of patients with CD do not achieve histologic remission with a GFD [5]. Daily consumption of as little as 50 mg of gluten, the equivalence of 1/100TH of a slice of bread, may contribute to the persistence of mucosal damage [5,8]. It is estimated that patients who are adherent to a GFD will consume on average 5 to 50 mg of gluten each day as a result of gluten contamination [8]. These concerns and observations have given way to increased interest in therapeutics which may either replace or act in conjunction with a gluten free diet to help diminish the accidental gluten exposures that may account for continued disease activity.
III. What are the findings?
A.) Wheat alternatives and Generating Celiac-Safe Wheat
Gluten replacement
One gluten based therapeutic approach is to use grains that do not have the immunogenic proteins found in wheat derived gluten (Figure 1). One such grain that has garnered some interest as a wheat substitute is sorghum, which is a cereal grain related to maize. It has been consumed for thousands of years in Africa and Asia. More recently, sorghum has been used to make multiple wheat-free products, including breads, tortillas, cookies, and flatbreads that do not have discolorations or odd tastes. In vitro organ cultures do not show an increased production of inflammatory markers indicative of the activation of the innate or adaptive immune systems. In vivo challenge for 5 days in two patients with CD did not show onset of gastrointestinal symptoms or changes in serum anti-tissue transglutaminase levels [9]. Further studies assessing morphologic changes and larger populations will be needed before further recommendations can be made on the safety of sorghum for patients with CD. Gluten contamination of sorghum used commercially has been an issue.
Gluten removal
Another approach is to use breeding and hybridization of different wheat species to remove the immunogenic gluten derived proteins. There are significant differences in the levels of T-cell-stimulatory epitopes in the gluten of the different wheat species and cultivars [4], and T-cell assays have suggested that there may be a way to alter or breed new varieties of wheat which contain fewer toxic peptides. However, one of the obstacles to rendering it gluten-free is the role that gluten plays in the visco-elasticity and polymerization of the bread [1].
Genes encoding the various components of gluten are predominantly contained on chromosomes 1 and 6 of the three homologous genomes of hexaploid bread wheat (Triticum aestivum) (AABBDD). Genes located within the Gli-2 locus of the short arm of chromosome 6D encode most of the α-gliadins [1, 10]. Removal of this locus in the Chinese Spring cultivar of Triticum aestivum results in the removal of T-cell stimulatory epitopes, but also changes the mechanical properties of wheat. Removal of the ω-gliadin, γ-gliadin, and LMW-GS loci from chromosome 1 of the D-genome removed T-cell stimulatory epitopes, but does not appear to negatively affect mechanical properties of wheat [1].
C173 is an experimental wheat line derived from breeding two mutant plants with spontaneous deletions of several gliadins and glutenins; specifically Gli-A2, Gli-D1, and Gli-D3. In vitro studies using duodenal mucosal biopsies from treated and healed CD patients show that C173 does not decrease the villous to crypt ratio but does increase the release of the inflammatory cytokines IFN-gamma and IL-2 as well as the production of IL-10, and anti-tTg antibodies in the collected supernatant [3]. The lack of morphological changes would suggest then that deleting several gliadins and glutenins reduces the toxicity of this wheat; however, the continued production of inflammatory cytokines is of great concern. Therefore C173 may not be appropriate for celiac patients.
B) Enzymatic Pretreatment of Wheat Flour
Fermentation of wheat with sourdough lactobacilli and fungal proteases is another method of rendering wheat less toxic (Figure 1). Specific combinations of lactobacilli and fungal proteases result in complete hydrolyzation of gluten in wheat flour [11]. When treated with this hydrolyzed wheat flour, duodenal mucosa from CD patients do not produce IFNγ mRNA greater than the level produced from duodenal mucosa from healthy controls [12]. The lactobacillus treated wheat sourdough can be mixed with nontoxic flours (ie: oat, millet, and buckwheat flours) to produce a bread that has texture similar to wheat sourdough breads. This fermented bread does not appear to increase intestinal permeability when consumed by patients with CD [11]. When mixed with buckwheat to form pasta, this fermented wheat had lower stickiness and firmness in comparison to standard durum semolina wheat; however these differences did not prohibit its manufacture and did not affect odor or taste [13]. In a randomized trial of 6 patients, those who ingested 200 grams per day of fully hydrolyzed baked goods (containing 8ppm residual gluten) did not experience symptoms, morphologic or serologic changes after 60 days [14]. Hydrolyzed wheat flour still maintains its mechanical properties so that it can be used to make bread, pasta, and sweets [13-14]. A similar study involving ingestion of 200 grams per day of fully hydrolyzed sweet baked goods did not show a change in clinical complaints, serology, or intestinal permeability [12].
Another enzymatic pretreatment method uses transamidation to render the α-gliadin derived peptides non-immunogenic. This enzymatic reaction can be achieved via incubation of commercial wheat flour with microbial transglutaminase and lysine methyl ester. When this altered flour is incubated for 48 hours with T cell lines derived from duodenal biopsies from 12 adults with CD, interferon-gamma expression is less than non-altered flour and binding of immunogenic peptides to DQ2 is decreased [15].
Ultimately, it may not be feasible to alter or select a variety of wheat completely devoid of gluten without compromising its mechanical properties; however current research suggests that less toxic wheat could be designed and may be tolerated better in patients with CD. Conceivably, this less toxic wheat could be used in conjunction with the oral enzyme supplements and polymeric binders to be discussed in further detail below. Also unlikely is that these special varieties of wheat could be a financially sound alternative to industrial wheat.
C.) Oral Enzyme Supplements
Gluten is rich in glutamine and proline residues, the latter of which plays a crucial role in directing tTG-mediated deamidation of the glutamine residues and thereby increasing their affinity to bind to HLA DQ2 and DQ8. These residues are also highly resistant to degradation by gastric and pancreatic proteases [5-6, 16-18]. Given these attributes, they make for are an ideal target for gluten modification. Glutenases are endopeptidases that are designed to target and destroy these proline and glutamine residues and thereby decrease the immunogenecity of gluten (Figure 1) [5-6, 16]. Glutenases are currently intended as oral enzyme supplements which would be used in conjunction with a gluten free diet to diminish the toxicity of accidental gluten exposure.
Prolyl oligopeptidases derived from Flavobacterium mengosepticum (FM-POP), Sphingomonas capsulate, and Myxococus xanthus are capable of cleaving proline residues; however these enzymes do not function optimally at the acidic pH of the stomach and are susceptible to digestion by pepsin [17-18]. Three glutenases which may show promise as oral enzyme supplements include ALV003, AN-PEP, and Stan-1.
ALV003 is a study drug which contains two glutenases – a glutamine-specific cysteine endoprotease derived from germinating barley seeds (EP-B2) and a prolyl endoprotease from Sphingomonas capsulate (SC-PEP). SC-PEP specifically cleaves the proline residues [5]. In vitro and in vivo evaluation of ALV003 in rats demonstrates its ability to retain its enzymatic activity in acidic environments comparable to the stomach and duodenum [6].
Pre-treatment of gluten with ALV003 decreases the immune response, as determined by the presence of polyclonal gluten-specific intestinally derived T cells. This response is seen with the ingestion of 16 grams per day of pre-treated gluten (four slices of bread) over three days. Despite the diminished immune response, ALV003 does not change the occurrence of symptoms such as nausea, bloating, abdominal pain, lethargy, vomiting, headache, and diarrhea [5]. A prospective observational trial is underway to further define the sensitivity of a patient reported outcome instrument to characterize the changes in symptoms as reported by patients after 8 and 12 weeks of taking ALV003 [19].
Phase 1 clinical trials evaluating the safety, tolerability and activity of ALV003 have shown that in healthy and Celiac patients, a dose of 300 mg of ALV003 is safe and effective at degrading 88 ± 5% of 1 gram of gluten [16]. A phase 2A clinical trial of 41 patients showed that patients with CD who consumed 2 grams of gluten daily with ALV003 for 6 weeks reported fewer symptoms, less morphological changes, and no changes in serologic markers in contrast to their placebo counterparts [20]. Encouraging results from this study have prompted plans for a phase 2b study.
AN-PEP is a prolyl endopeptidase derived from Aspergillus Niger. In vitro studies have shown that ANPEP is active at a pH of 2-8 with optimal activity at 4-5, resists digestion by pepsin, and degrades all tested gluten peptides with a t1/2 value ranging between 2.4 and 6.2 minutes [17]. Use of a multi-compartmental in-vitro system has simulated the conditions of the stomach, duodenum, jejunum and ileum. This in vitro system showed that when bread and commercial fast food is “ingested,” AN-PEP cleaves gluten peptides in an acidic setting simulating the environment of the stomach and also within the span of time that food is undergoing mechanical and chemical digestion in the stomach. In vitro studies have also shown that ANPEP eliminates the ability of gluten to stimulate T cells [18].
Building upon these in vitro findings, a number of in vivo studies are underway. One is a randomized, double-blind crossover study which aims to evaluate the effect of caloric density on the ability of AN-PEP to breakdown gluten [21]. Another is a randomized double-blind control trial investigating the efficacy of AN-PEP to detoxify 8 grams of gluten in a commercial product. This study will use histopathological changes and the production of serologic antibodies specific for gliadin, endomysium, or tissue transglutaminase as primary outcomes [22].
Aspergillus Niger has also been harnessed for use of its aspergillopepsin (ASP) in conjunction with dipeptidyl peptidase IV (DPPIV), an exopeptidase, in another glutenase cocktail. ASP is not specific for immunotoxic gluten epitopes and therefore on its own is an insufficient glutenase; however, it may be effective at degrading larger proteins into smaller peptides, thereby exposing the target resides faster to more specific endopeptidases or exopeptidases. DPPIV markedly enhances ASP’s ability to degrade gluten; however it is inactive at a pH < 4 and therefore its use would necessitate concomitant use of antacids [7]. ASP may still have a role in conjunction with endopeptidases such as ALV003 and EP-B2.
STAN 1 is a cocktail of microbial enzymes commonly used in food supplements and has demonstrated good gluten degrading properties in the lab. A randomized, double-blind placebo controlled study of 35 patients with CD on a GFD but with persistent seropositivity for TTG evaluated the effect of STAN-1 when ingested with 1 gram of gluten per day for a total of 12 weeks. This study found no difference in serology between the two arms [23].
D). Polymeric Binders
Just as cholestyramine is used to bind bile acids, another approach to detoxifying gluten is creating a substance which can bind gluten in the gut. Polymeric binders are high molecular weight polymers designed to bind to gluten in the gastrointestinal tract and thereby prevent degradation, absorption, and triggering of an immunologic response.
Poly (hydroxythylmethacrylate-co-styrene sodium sulfonate) [P(HEMA-co-SS)] is a polymeric binder which has been shown to effectively bind α-gliadin at pHs representative of both the stomach and the duodenum (Figure 1). In vitro studies have shown that the addition of P(HEMA-co-SS) to gliadin appears to prevent typical gliadin induced cell permeability changes including cytoskeleton changes and restoration of ZO-1 immunoreactivity at the apex of the lateral cell membrane. P(HEMA-co-SS) was also effective at hindering the enzymatic degradation of gliadin [24].
IV. What are the roadblocks and/or limitations?
Roadblocks and limitations to the clinical application of the aforementioned products (genetically altered wheat, enzymatically altered wheat, oral enzyme supplements, and, polymeric binders) are the tests to measure their safety and efficacy. The ideal gold standard would be to perform serology and histology on all patients; however, the problems with this approach include cost, patient time/comfort, and availability of resources (ie: endoscopy suites and staffing, available pathologists) [5]. Using surrogate markers for disease activity such as fecal fat collection and xylose malabsorption studies are unfortunately not specific to CD [5] and do not adequately assess early stages of intestinal damage in CD [14]. A number of the current clinical studies have shown promising results, but these have been of a short duration and low power; therefore, studies with longer duration periods and greater power will be needed to assess both efficacy and long term safety of each approach including the possibility that patients using such treatments may be exposed to the moral hazard of temptation to cheat. Recruitment of sufficient numbers of participants for large clinical trials to ensure sufficient power will be necessary and will by their nature be quite expensive; however as the prevalence of CD is 1% it should be possible to feasible to recruit patients. As with any novel therapy the projected costs of the new therapies which are likely adjunctive will need to be weighed on the value and benefit provided to patients compared to that of a GFD alone. Ultimately health plans would need to reimburse these costs to permit patients to afford such medications as they already face considerable costs to adhere to a GFD. Equity would suggest that patients with CD should have similar access to therapies to ameliorate symptoms and reduce complications while improving quality of life as are afforded to many other diseases such as allergic rhinitis or type 2 diabetes. Drug development in an area that has not been the focus of drug therapies provides many challenges in translating our now extensive understanding of CD requires long term investments, but for which there is a clear commercial opportunity for a much needed therapy.
V. Conclusions
Celiac Disease is a common immune enteropathy affecting approximately 1% of the world’s population. Currently, the only treatment available is a gluten-free diet which is wrought with multiple issues including inconsistent definitions in different countries, gluten contamination, cost, and availability. As many as 50% of patients adherent to a gluten free diet will not achieve histologic remission; this is likely due to hidden gluten contamination. Further advances in genetically altering wheat varieties may make a gluten free diet easier. In addition, the use of glutenases and polymeric binders could also help prevent toxicity associated with accidental gluten exposure. Larger clinical studies are underway and will be essential in determining the safety and efficacy of these dietary adjuvants. These and other approaches directed at later parts of the pathogenetic pathway hold great promise of changing how we manage celiac disease.
Acknowledgement and Grant Support (Funding)
This work was supported in part by grants DK 57892 and DK071003 from the National Institute of Health (JA Murray)
Abbreviations used in this article
- CD
Celiac Disease
- GFD
Gluten free diet
- ppm
parts per million
- HMWGS
high molecular weight glutenin subunits
- LMW-GS
low molecular weight glutenin subunits
- IFN
Interferon
- tTg
tissue transglutaminase
- APCs
Antigen presenting cells
- IEL
intraepithelial lymphocyte
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest/Disclosure requirement JAM has been a consultant to Alvine Inc
Bibliography
- 1.van den Broeck HC, et al. Removing celiac disease-related gluten proteins from bread wheat while retaining technological properties: a study with Chinese Spring deletion lines. BMC plant biology. 2009;9:41. doi: 10.1186/1471-2229-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frisoni M, et al. Wheat deficient in gliadins: promising tool for treatment of coeliac disease. Gut. 1995;36(3):375–8. doi: 10.1136/gut.36.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carroccio A, et al. Searching for wheat plants with low toxicity in celiac disease: Between direct toxicity and immunologic activation. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2011;43(1):34–9. doi: 10.1016/j.dld.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Spaenij-Dekking L, et al. Natural variation in toxicity of wheat: potential for selection of nontoxic varieties for celiac disease patients. Gastroenterology. 2005;129(3):797–806. doi: 10.1053/j.gastro.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 5.Tye-Din JA, et al. The effects of ALV003 pre-digestion of gluten on immune response and symptoms in celiac disease in vivo. Clinical immunology. 2010;134(3):289–95. doi: 10.1016/j.clim.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Gass J, et al. Combination enzyme therapy for gastric digestion of dietary gluten in patients with celiac sprue. Gastroenterology. 2007;133(2):472–80. doi: 10.1053/j.gastro.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 7.Ehren J, et al. A food-grade enzyme preparation with modest gluten detoxification properties. PloS one. 2009;4(7):e6313. doi: 10.1371/journal.pone.0006313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catassi C, et al. A prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. The American journal of clinical nutrition. 2007;85(1):160–6. doi: 10.1093/ajcn/85.1.160. [DOI] [PubMed] [Google Scholar]
- 9.Ciacci C, et al. Celiac disease: in vitro and in vivo safety and palatability of wheat-free sorghum food products. Clinical nutrition. 2007;26(6):799–805. doi: 10.1016/j.clnu.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Molberg O, et al. Mapping of gluten T-cell epitopes in the bread wheat ancestors: implications for celiac disease. Gastroenterology. 2005;128(2):393–401. doi: 10.1053/j.gastro.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Di Cagno R, et al. Sourdough bread made from wheat and nontoxic flours and started with selected lactobacilli is tolerated in celiac sprue patients. Applied and environmental microbiology. 2004;70(2):1088–96. doi: 10.1128/AEM.70.2.1088-1096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Cagno R, et al. Gluten-free sourdough wheat baked goods appear safe for young celiac patients: a pilot study. Journal of pediatric gastroenterology and nutrition. 2010;51(6):777–83. doi: 10.1097/MPG.0b013e3181f22ba4. [DOI] [PubMed] [Google Scholar]
- 13.di Cagno R, et al. Pasta made from durum wheat semolina fermented with selected lactobacilli as a tool for a potential decrease of the gluten intolerance. Journal of agricultural and food chemistry. 2005;53(11):4393–402. doi: 10.1021/jf048341+. [DOI] [PubMed] [Google Scholar]
- 14.Greco L, et al. Safety for patients with celiac disease of baked goods made of wheat flour hydrolyzed during food processing. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2011;9(1):24–9. doi: 10.1016/j.cgh.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 15.Gianfrani C, et al. Transamidation of wheat flour inhibits the response to gliadin of intestinal T cells in celiac disease. Gastroenterology. 2007;133(3):780–9. doi: 10.1053/j.gastro.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 16.Siegel M, et al. Safety, tolerability, and activity of ALV003: results from two phase 1 single, escalating-dose clinical trials. Digestive diseases and sciences. 2012;57(2):440–50. doi: 10.1007/s10620-011-1906-5. [DOI] [PubMed] [Google Scholar]
- 17.Stepniak D, et al. Highly efficient gluten degradation with a newly identified prolyl endoprotease: implications for celiac disease. American journal of physiology. Gastrointestinal and liver physiology. 2006;291(4):G621–9. doi: 10.1152/ajpgi.00034.2006. [DOI] [PubMed] [Google Scholar]
- 18.Mitea C, et al. Efficient degradation of gluten by a prolyl endoprotease in a gastrointestinal model: implications for coeliac disease. Gut. 2008;57(1):25–32. doi: 10.1136/gut.2006.111609. [DOI] [PubMed] [Google Scholar]
- 19.ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): [cited 2012 Apr 16]. 2000. Alvine Pharmaceuticals. Evaluation of Patient Reported Outcome Instruments in Celiac Disease Patients. Available from: http://clinicaltrials.gov/show/NCT01560169 NLM Identifier: NCT01560169. [Google Scholar]
- 20.Lahdeaho M, et al. ALV003, a novel glutenase, attenautes gluten-induced small intestinal mucosal injurty in Celiac Disease patients: a randomized controlled phase 2A clinical trial. Gut. 2011;60(A12) [Google Scholar]
- 21.DSM Food Specialties . ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): [cited 2012 Apr 16]. 2000. Effect of AN-PEP Enzyme on Gluten Digestion. Available from: http://clinicaltrials.gov/show/NCT01335503 NLM Identifier: NCT01335503. [Google Scholar]
- 22.VU University Medical Center . ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): [cited 2012 Apr 16]. 2000. Effect of Aspergillus Niger Prolyl Endoprotease (AN-PEP) Enzyme on the Effects of Gluten Ingestion in Patients with Coeliac Disease. Available from: http://clinicaltrials.gov/show/NCT00810654 NLM Identifier: NCT00810654. [Google Scholar]
- 23.Korponay-Szabo IR, et al. Food-grade gluten degrading enzymes to treat dietary transgressions in coeliac adolescents. Journal of Pediatric Gastroenterology and Nutrition; 43th Annual Meeting of ESPGHAN; Istanbul. 2010. p. E68. 2010. [Google Scholar]
- 24.Pinier M, et al. The copolymer P(HEMA-co-SS) binds gluten and reduces immune response in gluten-sensitized mice and human tissues. Gastroenterology. 2012;142(2):316–25. e1–12. doi: 10.1053/j.gastro.2011.10.038. [DOI] [PubMed] [Google Scholar]