Abstract
Bacterial vaginosis (BV) and Trichomonas vaginalis (TV) infection are both very common and are associated with increased risk of sexual transmission of HIV. There are several mechanisms by which BV and TV could affect susceptibility including inducing pro-inflammatory cytokines and disrupting mucosal barrier function. This review highlights recent advances in our understanding of how these genital conditions lead to an increased risk of HIV infection in women.
Keywords: Bacterial vaginosis, HIV, Inflammation, Trichomonas vaginalis
Bacterial Vaginosis (BV) and HIV
The lower genital tract microbiota is a dynamic bacterial community that is influenced by many factors including age, sexual activity, hormones, infection, the immune system and individual hygiene practices. The microbiota of many women is composed predominantly of lactobacilli, which are believed to contribute to the health of the vagina by producing lactic acid and by out-competing pathogenic organisms. Lactobacilli can also produce H2O2, which is also thought to promote vaginal health [1-3]. Bacterial vaginosis (BV), in contrast, is a condition in which the types and the relative proportions of bacteria are altered in the lower genital tract. BV is common with 8-23% of women of reproductive age affected. Interestingly, more than half of BV+ women report no symptoms [4].
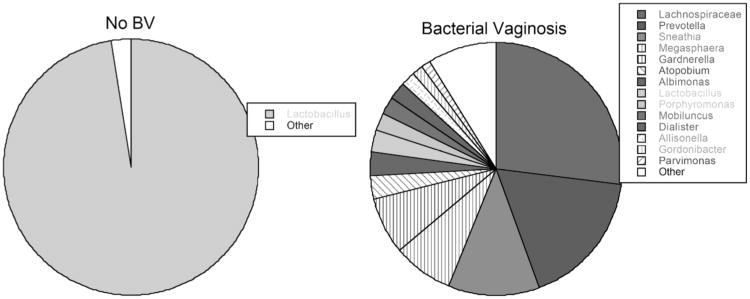
The bacterial communities comprising BV can vary substantially from individual to individual. However, studies using conventional culture methods, as well molecular techniques (i.e. direct sequencing or PCR of the 16S rRNA genes) show that the microbiota in BV mostly consist of bacteria in the order Clostridiales, Gardnerella vaginalis, Mycoplasma hominis, Prevotella sp., Atopobium, and Mobiluncus sp. ([3, 5, 6] and Fig. 1). Antibiotics such as metronidazole and clindamycin are used to treat BV, but unfortunately, as many as 30% of women experience relapse within the first 3 months after treatment [4].
Fig. (1). Lower Genital Tract Bacterial Microbiota of a group of US Women.
Samples were obtained by cervicovaginal lavage from 10 women without BV (left) and 10 with BV (right). Note that BV tends to be polymicrobial while microbiota of non-BV tends to be nearly all lactobacilli. Microbiota was determined by pyrosequencing of the 16S rRNA gene and inputting the sequences into Ribosomal Database 10.
BV can be identified by a variety of methods [3]. Clinically, the Amsel test is commonly used where four criteria are assessed: vaginal fluid pH >4.5, presence of clue cells (bacteria-coated epithelial cells), homogenous thin discharge, and a whiff test that yields an amine odor when KOH is added to vaginal fluid [7]. If three of these tests are positive, BV is diagnosed. BV can also be determined by the Nugent scoring system, where a score is applied to Gram stains of vaginal smears to visually estimate the numbers of lactobacilli and BV-associated bacteria. According to this system, a Nugent score of 0-3 is considered healthy, a score of 4-6 is intermediate and a score of 7-10 signifies BV [8].
In most women, a healthy microbiota consists predominantly of lactobacilli such as L. crispatus, L. jensenii, L. gasseri or L. iners, although several recent cross-sectional studies have shown that Lactobacillus sp. are not the predominant bacteria in a significant number of “healthy” women [3, 9-12]. A recent study sequenced the genital microbiota of 396 asymptomatic North American women and found there were generally five types of bacterial communities, dominated by either L. iners, L. crispatus, L. gasseri, or L. jensenii, as well as a fifth group that was not dominated by lactobacilli but instead consisted of mostly anaerobes and G. vaginalis [12]. In a deep sequencing analysis of the microbiota of a group of HIV+ African women, L. crispatus and L. iners were found to strongly associate with a normal vaginal microbiota [13].
Studies Showing an Association between BV and HIV Infection
BV is associated with the acquisition and transmission of the human immunodeficiency virus (HIV), as well as other sexually transmitted pathogens [4, 14-19]. Given BV's prevalence, especially in areas most afflicted by heterosexual transmission of HIV [20], BV is recognized as one of the most important factors affecting HIV susceptibility in women.
The association between BV and the risk of HIV infection in women has been demonstrated in several cross-sectional [21-23] and longitudinal studies [24, 25] where HIV was more frequent in BV+ women than those without BV. Moreover, sub-Saharan African women, whose vaginal microbiota was not dominated by lactobacilli, were found to be 2-3 times more likely to be infected with HIV, even when other HIV risk factors were taken into account [20, 24-26]. A 2008 meta-analysis of twenty three studies found that BV increased the risk of HIV infection in women by 60% [15]. Several groups have also demonstrated that among HIV sero-positive women, those with BV or with low levels of lactobacilli shed more HIV viral particles in their vaginal secretions [27-29]. Moreover, studies show that vaginal fluids collected from BV+ women can stimulate HIV expression in vitro [30].
Interestingly, within the bacterial communities that can comprise BV, there may be some that are even more strongly associated with increased levels of HIV expression in genital fluids than others. In our own studies, we found there was a significant positive correlation between levels of M. hominis and genital HIV shedding in a multivariate analysis controlling for plasma viral loads [29]. In fact, our data showed that women with low lactobacilli and high M. hominis had 100-fold higher levels of genital HIV than those with high lactobacilli and low M. hominis. However, we found no significant relationship between G. vaginalis and genital tract HIV. These findings suggest that BV in general, and certain types of BV-associated bacteria in particular, can have a profound effect on HIV expression in the genital tract. This is important because the level of expression of HIV in the genital fluid is a critical factor in female to male transmission of HIV.
The studies above clearly establish a link between BV and rates of HIV infection and expression. However, the mechanisms by which BV contributes to increased susceptibility and shedding remain to be determined. An animal model could be helpful in elucidating the relationship between HIV and BV. The vaginal infection of rhesus and pigtailed macaques with the simian immunodeficiency virus (SIV) or the simian-human immunodeficiency virus (SHIV) is used extensively as a model to investigate HIV sexual transmission. Recent studies have addressed the factors that affect macaques' lower genital tract microbiota and their metabolic products. Those studies show that in macaques, similar to women with BV, the lower genital tract is not dominated by lactobacilli but is polymicrobial ([31-33] and Fig. 2). In addition, like BV+ women, macaques' vaginal fluids have low levels of lactic acid and glycogen [34], and a relatively high pH [31, 32, 35]. Recently, Lagenaur et al. showed that introduction of L. jensenii expressing cyanovirin-N (an anti-HIV protein) into the macaques lower genital tract significantly lowered the infection rate of macaques vaginally challenged with SHIV [36], although it remains to be determined how much of this protection is due solely to colonization by lactobacilli.
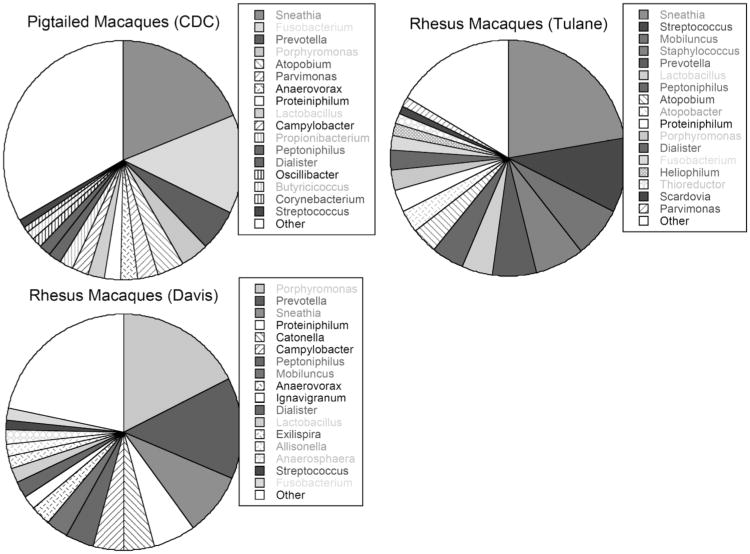
Fig. (2). Lower Genital Tract Bacterial Microbiota of Female Macaques.
Samples were provided by Ellen Kersh from ten pigtailed macaques at the Centers for Disease Control and Prevention, by Ron Veazey from nine rhesus macaques from the Tulane National Primate Research Center and by Chris Miller from 11 rhesus macaques at the California National Primate Research Center at UC Davis. Note that the macaque microbiota at these three primate centers has similarities to BV in humans. Microbiota was determined by pyrosequencing of the 16S rRNA gene and inputting the sequencing into Ribosomal Database 10.
Interestingly, in our analysis of the macaques' lower genital tract microbiota by direct sequencing, we also found some differences and similarities in the types and the frequencies of bacteria colonizing the lower genital tract of rhesus and pigtailed macaques, and some suggestion that the microbiota could be dependent on the facility in which they were housed (Fig. 2). Such variations may affect susceptibility to retrovirus infection and indicate that further studies are needed.
Possible Mechanisms of BV'S Effects on HIV
In this section, we highlight some recent studies that enhance our understanding of the mechanisms by which BV might affect HIV acquisition and transmission.
It is widely believed that the acidic environment of the vagina in women afforded by lactobacilli contributes to the protection against HIV infection [37, 38]. Indeed, while a healthy vagina is characterized as having a pH of less than 4.5, BV is marked by its relatively higher pH (pH > 4.5). It has also been suggested that a decrease in H2O2-producing lactobacilli in BV might contribute to increased rates of HIV infection in BV+ women [14, 39, 40]. However, this view is challenged by recent studies unable to detect H2O2 production by lactobacilli under the hypoxic conditions of the vagina, calling into question the relevance of H2O2 in vivo [41, 42].
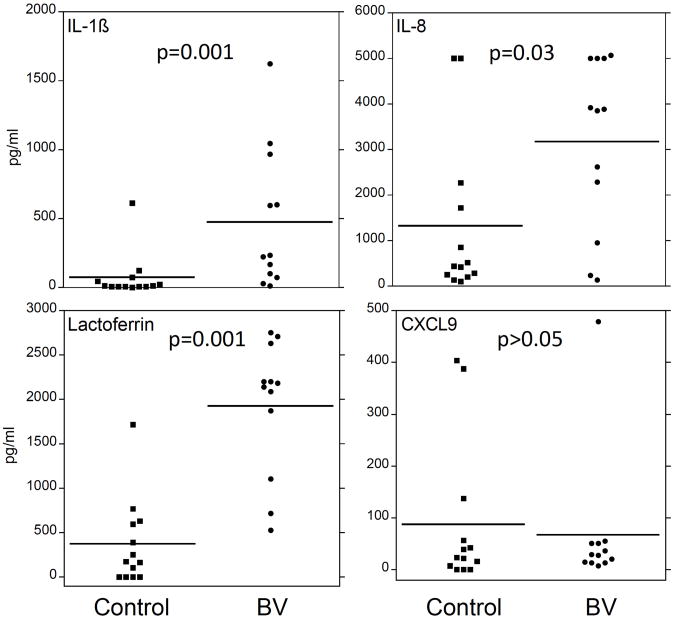
The multimicrobial nature of BV is also believed to induce a pro-inflammatory environment, consisting of cytokines and toll-like receptor (TLR) ligands, that is conducive to HIV propagation ([43] and Fig. 3). Several groups have reported that the levels of pro-inflammatory cytokines are elevated in BV+ vaginal fluids (reviewed in [43]. Many of these studies show an increase in IL-1β levels in the vaginal fluids collected from BV+ women, while others also report an increase in IL-8 and IL-1α levels though the latter cytokines are not consistently observed to be elevated [44-49]. BV+ vaginal fluids also tend to have more RANTES and IL-6, although in most cases the level of these inflammatory mediators fails to reach statistical significance when compared to samples collected from BV-negative women [43]. In addition, genital secretions from women with BV can stimulate cells through TLR2 [50], suggesting that gram positive bacterial products such as peptidoglycan may be involved in inducing the proinflammatory cytokines in BV.
Fig. (3). Bacterial Vaginosis Increases Levels of Proinflammatory Cytokines in the Lower Genital Tract.
Cervicovaginal lavage samples were obtained from women with no STDs and women with BV and no other STDs. IL-β, IL-8, and CXCL9 levels were measured utilizing Cytometric Bead Arrays (BD Biosciences). To detect lactoferrin, custom cytometric bead arrays were made by coupling blank beads with rabbit antibody to lactoferrin. All bead arrays were assayed on a FacsCalibur and levels of cytokines calculated using BD CBA software (BD Biosciences [106]).
It has been hypothesized that pro-inflammatory cytokines may increase susceptibility to HIV infection by causing damage to the vaginal mucosa, thereby allowing HIV to breach the epithelial barrier [17, 20]. Once HIV gains entry to the underlying tissue, it likely infects CD4+ lymphocytes, macrophages, and dendritic cells, which are recruited to the site of inflammation [20]. Recently, Ballweber et al. showed that Langerhans cells in the vaginal epithelium could pick up infectious HIV and shuttle it to CD4+ T cells without the Langerhans cells becoming infected [51]. In addition, both TLR ligands and pro-inflammatory cytokines can activate NF-κB, which has been shown to increase HIV expression in cells [52, 53]. These data support the hypothesis that BV provides an inflammatory environment that can promote HIV infection, and increase viral expression. It has therefore been suggested that inhibiting inflammatory innate immune responses might be important in preventing HIV dissemination [54].
BV-associated anaerobes have also been shown to produce large quantities of short chain fatty acids (SCFAs), including acetic and butyric acids. These small compounds are found at millimolar concentrations in the vaginal fluids of BV+ women (reviewed in [55], and can cross the cell membrane by non-ionic diffusion [56]. SCFAs, particularly butyric acid, may also be transported into cells by a class of transporters known as monocarboxylate transporters or MCTs, that have a wide tissue distribution that includes epithelial cells and leukocytes [57-62]. GPR43, a member of the G-protein-coupled receptor family that is expressed on the surface of monocytes and neutrophils, has also been shown to bind SCFAs and induce signaling [63-65].
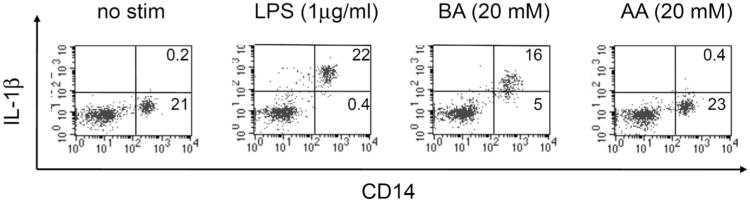
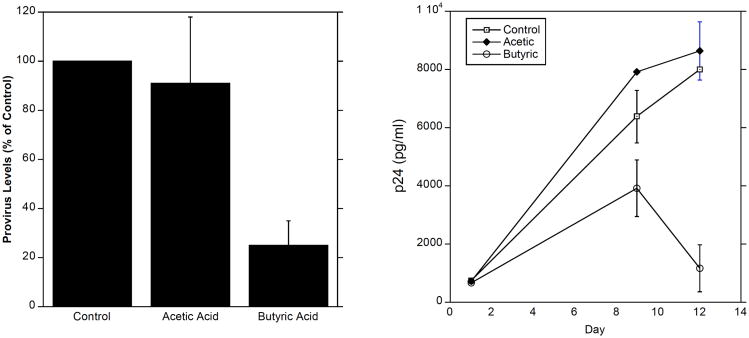
SCFAs can regulate a myriad of immune responses, including cell migration, cytokine production, phagocytosis, and apoptosis in a type- and concentration-dependent manner (reviewed in [55]. Using concentrations of these compounds that are present in BV+ vaginal fluids, we found that high levels of SCFAs (20 mM) induce the production of IL-8, IL-6 and IL-β by peripheral blood mononuclear cells (PBMCs) in vitro (Fig. 4 and [66]). In addition, we found that even at low levels, SCFAs enhanced the production of IL-8 and TNFa when combined with TLR2 and TLR7 ligands, but not TLR4 ligands [66]. Because of their far-reaching effects on immune responses and gene expression [67-72], it will be important to learn how SCFAs might affect HIV infection and expression in target cells. Indeed we found that butyric acid decreased both the formation of HIV provirus and the production of HIV viral protein p24 by macrophages (Fig. 5). These findings suggest that SCFAs may play opposing roles in HIV susceptibility: On the one hand they may induce a pro-inflammatory milieu in the vaginal mucosa, and thereby increase the rate of HIV infection. On the other hand, SCFAs may directly reduce the infection of target cells. It remains to be determined why different SCFAs have such a wide array of effects on immune cell responses.
Fig. (4). Butyric Acid Induces Production of IL-1β.
Peripheral blood mononuclear cells (PBMCs) obtained from healthy donors were incubated with either LPS (1 μg/ml), butyric acid (BA, 20 mM), acetic acid (AA, 20 mM) or medium only (no stim) for 4 hours in the presence of GolgiPlug (BD Biosciences). Cells were washed and stained with an antibody to CD14. They were then fixed, permeablized, and stained with an antibody to IL-1β. Events were collected on a FacsCalibur and analyzed using CellQuest software (BD Biosciences). Total PBMCs were gated by light scatter. This figure shows that the CD14-positive monocyte population in PBMCs, but not CD14-negative lymphocytes, are induced by butyric acid to produce IL-1β.
Fig. (5). Butyric Acid, But Not Acetic Acid, Suppresses HIV Infection of Macrophages.
Monocytes were obtained by adherence from PBMCs and differentiated into macrophages by culture in Monocyte Colony Stimulating Factor (M-CSF) for seven days. Left panel: Cells were treated with SCFAs for 16 hours, washed, and infected with HIVBaL for 16 hours. Levels of HIV provirus in cells relative to GAPDH were then determined. Right panel: Cells were infected for 4 hours, washed and treated with SCFAs for 16 hours. After washing, cells were cultured 12 days and culture supernatant HIV p24 levels measured.
In addition to inducing pro-inflammatory cytokines, BV-associated microbiota produce a number of enzymes and byproducts such as sialidases, glycosidases, and proteases [14]. These microbial compounds can compromise the host immunity to pathogens by 1) breaking down mucin, a component of the protective mucous lining the genital tract, 2) by degrading innate anti-microbial factors present in mucous such as secretory leukoprotease inhibitor (SLPI), β-defensins, and soluble IgA, or 3) by inactivating immune cells [73, 74]. Additionally, these enzymatic activities, which facilitate microbial attachment to the mucosal surfaces and provide nutrition for the bacteria, may disrupt the integrity of the vaginal epithelium. This, in turn, can allow HIV to gain entry into the underlying parenchyma and increase susceptibility to HIV infection. Interestingly, the presence of some pro-inflammatory cytokines, such as IL-1β and IL-8, has also been associated with an increase in the levels of BV-associated hydrolytic enzymes [75].
Trichomonas Vaginalis (TV) and HIV
Trichomonas vaginalis (TV) is the most common non-viral sexually transmitted infection with the World Health Organization estimating 173 million annual cases in 1999 [76]. A study of US women between the ages of 14 and 49 from 2001 to 2004 estimated the prevalence of trichomoniasis at 3.2% [77]. Strikingly, over 80% of cases were asymptomatic at the time of testing [77]. TV infection has been previously regarded by some as a mere nuisance, possibly in part due to the fact that it can be asymptomatic in so many. But more recent awareness that TV is associated with increased susceptibility to HIV infection and other severe problems such as preterm birth, as well as the frequency of TV infection, have led to the realization that TV needs a stronger public health response [76]. The immunology, epidemiology and biology of TV infection have been reviewed recently [78, 79].
Studies Showing an Association between TV and HIV Infection
A number of studies have shown an increase in HIV sero-conversion associated with TV infection. In a longitudinal study, Laga et al. [80] found increased HIV sero-conversion was associated with TV in 531 HIV-1 negative female sex workers in Kinshasa, Zaire that were screened monthly. A prospective study of 1,335 Kenyan women that were monitored monthly for a median of 566 days showed in multivariate analysis that infection with TV was associated with a 1.52 fold increased risk of HIV-1 seroconversion [81]. In a case control study, Van Der Pol et al. [82] used PCR to detect TV in a general population cohort of women from Uganda and Zimbabwe. Multivariate analysis showed an adjusted odds ratio for HIV acquisition of 2.74. Mavedzenge et al. showed that TV increased the risk of HIV sero-conversion in women from South Africa and Zimbabwe (odds ratio of 2.6 [83]).
Studies Showing an Association between TV and HIV Shedding
Infection with TV has also been associated with increased shedding of HIV in co-infected women. In a group of US women HIV shedding was more frequent in TV-infected women and treatment of TV resulted in reduced shedding at a 3-month visit [84]. Similarly, a study in Kenya found decreased HIV-shedding after treatment of TV [85]. In HSV/HIV-coinfected women in Tanzania, TV was also associated with increased HIV shedding [86].
Epithelial Barrier Disruption by TV
While TV infection can be asymptomatic in some women, in others it can induce dramatic changes in the epithelium. Thus, the vagina and/or cervix can be inflamed and swollen, with erosion of the cervical epithelium and punctate hemorrhages that are referred to as strawberry cervix [87]. The mechanism for these extreme changes in the epithelium have not been fully determined. However, TV binds to epithelial cells via adhesins, breaks down the mucous layer and causes epithelial cell apoptosis. TV also promotes inflammatory changes that could contribute to epithelial disruption (see section below).
Adherence of TV to epithelial cells is an important step in causing damage to the epithelium and may involve the lipophosphoglycan and proteins of the parasite membrane as well as host cell lectins [88-91]. TV also secretes proteases and other factors that can be directly cytotoxic to cells that may degrade both the extracellular matrix and anti-microbial proteins [79, 92]. Some of the proteases have been found to cause apoptosis of epithelial cells in vitro [93].
To determine possible mechanisms by which TV can increase susceptibility to HIV infection, Guenthner et al. [94] studied the effects of TV on polarized epithelium in vitro. Addition of TV to the apical side of polarized epithelial cell monolayers disrupted epithelial integrity as measured by electrical resistance. This disruption was enough to allow passage of HIV to the basolateral side of the monolayers.
While TV may affect the barrier function provided by epithelial cells, mucins also provide a protective layer that potentially trap HIV and prevent infection. A TV isolate was shown to secrete several proteases [95] and since mucins have a protein backbone these proteases had the capacity to degraded mucins.
Immunity/Inflammation and TV
TV infection can lead to changes in innate and adaptive immunity in the female genital tract that may contribute to increased HIV susceptibility. The interactions of the immune system and TV were recently reviewed [78]. TV infection results in recruitment of immune cells to the vaginal mucosa and in inflammation. Lymphocytes, including those that express CD4, are increased in swabs taken from mucosa of TV infected women [81, 96, 97]. Neutrophil numbers are also increased in women with TV although this is not know to affect HIV susceptibility [44, 79].
TV infection also results in increased levels of proinflammatory cytokines in genital secretions that could affect HIV susceptibility and shedding in multiple ways (see BV section above). For example, in one study genital IL-1β levels were increased in HIV-infected women with TV infection [98]. In pregnant women with BV, IL-1β and IL-8 levels were increased in women co-infected with TV [44]. Incubation of resting HIV-infected peripheral blood mononuclear cells with TV induced HIV expression and this was dependent on TNFβ production [94]. Cervical vaginal lavage samples, collected from women with TV, induced the production of IL-8 by cells that express TLR4 suggesting that a TLR4-dependent ligand was present in the genital tract of infected women [99]. Vaginal secretions collected after clearance of infection had less of a stimulatory activity. On the other hand, TV has been reported to be immunosuppressive in some respects as it can induce apoptosis of macrophages by activating them, and can also suppress production of pro-inflammatory cytokines by these cells [100, 101].
Alterations of Bacterial Microbiota Associated with TV
While the above studies suggest that TV by itself could alter susceptibility to HIV infection by disrupting the epithelium or by inducing inflammation, co-incidence of TV and BV is common. In the 2001–2004 National Health and Examination Survey approximately half of TV infected women also had BV [102]. This suggests an additional reason for a TV association with increased susceptibility to HIV; that is, TV could be associated with alterations in bacterial microbiota that in turn influence HIV susceptibility. While most of the studies showing an association between TV and HIV controlled for the presence of BV [81, 82], they did not assess more subtle changes in microbiota. For example, women with low levels of H2O2-producing lactobacilli, while not having BV, could be more susceptible to both HIV and TV infection. In fact, in a study of Kenyan women, TV infection was associated with isolation of a lower number of H2O2-producing lactobacilli [103]. A study in the US found an association between TV and lower levels of total vaginal lactobacilli as estimated from gram stains [104]. Another possibility is that TV has a direct influence on genital microbiota. In a study of women attending an STD clinic in South Africa, the incidence of TV was positively associated with Nugent score with an incidence of 12% in subjects with a low score of (3 or less) to 33% in those with higher scores [105]. The authors of that study hypothesized that TV may actually lead to greater susceptibility to BV. This makes mechanistic sense since TV alters the vaginal pH which in turn is known to lead to changes in microbiota. By this mechanism, TV could lead to crowding out of H2O2-producing lactobacilli.
Concluding Remarks
The female genital tract is protected against pathogenic organisms by the innate and adaptive arms of the immune system, the genital tract microbiota and the epithelium. The perturbations of the vaginal environment caused by BV and/or TV bring about the sorts of immunological and physiological changes that render the lower genital tract more conducive to pathogenic infections such as HIV. These changes include the presence of microbial products (e.g. TLR ligands, SCFAs, and sialidases), the induction of proinflammatory cytokines (e.g. IL-1β), the recruitment of target cells to the site of inflammation (e.g. CD4+ cells), and the loss of vaginal epithelium integrity. Given the heterogeneity of microbiota in different individuals, and the diversity in host responses to microorganisms, more work is needed to determine how these BV- and TV-associated alterations may contribute to HIV susceptibility.
Acknowledgments
This work was supported by NIH Grants U19 AI076981 and P01 AI082971.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Eschenbach DA, Davick PR, Williams BL, Klebanoff SJ, Young-Smith K, Critchlow CM, et al. Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J Clin Microbiol. 1989;27(2):251–6. doi: 10.1128/jcm.27.2.251-256.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrons R, Tassone D. Use of Lactobacillus probiotics for bacterial genitourinary infections in women: a review. Clin Ther. 2008;30(3):453–68. doi: 10.1016/j.clinthera.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Lamont RF, Sobel JD, Akins RA, et al. The vaginal microbiome: new information about genital tract flora using molecular based techniques. BJOG. 2011;118(5):533–49. doi: 10.1111/j.1471-0528.2010.02840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marrazzo JM. Interpreting the epidemiology and natural history of bacterial vaginosis: are we still confused? Anaerobe. 2011;17(4):186–90. doi: 10.1016/j.anaerobe.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oakley BB, Fiedler TL, Marrazzo JM, Fredricks DN. Diversity of human vaginal bacterial communities and associations with clinically defined bacterial vaginosis. Appl Environ Microbiol. 2008;74(15):4898–909. doi: 10.1128/AEM.02884-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005;353(18):1899–911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 7.Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74(1):14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 8.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29(2):297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spear GT, Gilbert D, Landay AL, et al. Pyrosequencing of the genital microbiotas of HIV-seropositive and -seronegative women reveals Lactobacillus iners as the predominant Lactobacillus Species. Appl Environ Microbiol. 2011;77(1):378–81. doi: 10.1128/AEM.00973-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anukam KC, Osazuwa EO, Ahonkhai I, Reid G. Lactobacillus vaginal microbiota of women attending a reproductive health care service in Benin city, Nigeria. Sex Transm Dis. 2006;33(1):59–62. doi: 10.1097/01.olq.0000175367.15559.c4. [DOI] [PubMed] [Google Scholar]
- 11.Zhou X, Brown CJ, Abdo Z, et al. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J. 2007;1(2):121–33. doi: 10.1038/ismej.2007.12. [DOI] [PubMed] [Google Scholar]
- 12.Ravel J, Gajer P, Abdo Z, et al. Microbes and Health Sackler Colloquium: Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2010 Aug 19; doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hummelen R, Fernandes AD, Macklaim JM, et al. Deep sequencing of the vaginal microbiota of women with HIV. PLoS One. 2010;5(8):e12078. doi: 10.1371/journal.pone.0012078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hillier SL. The vaginal microbial ecosystem and resistance to HIV. AIDS Res Hum Retroviruses. 1998;14(1):S17–21. [PubMed] [Google Scholar]
- 15.Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS. 2008;22(12):1493–501. doi: 10.1097/QAD.0b013e3283021a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaul R, Nagelkerke NJ, Kimani J, et al. Prevalent herpes simplex virus type 2 infection is associated with altered vaginal flora and an increased susceptibility to multiple sexually transmitted infections. J Infect Dis. 2007;196(11):1692–7. doi: 10.1086/522006. [DOI] [PubMed] [Google Scholar]
- 17.Kaul R, Pettengell C, Sheth PM, et al. The genital tract immune milieu: an important determinant of HIV susceptibility and secondary transmission. J Reprod Immunol. 2008;77(1):32–40. doi: 10.1016/j.jri.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Cherpes TL, Melan MA, Kant JA, Cosentino LA, Meyn LA, Hillier SL. Genital tract shedding of herpes simplex virus type 2 in women: effects of hormonal contraception, bacterial vaginosis, and vaginal group B Streptococcus colonization. Clin Infect Dis. 2005;40(10):1422–8. doi: 10.1086/429622. [DOI] [PubMed] [Google Scholar]
- 19.Allsworth JE, Lewis VA, Peipert JF. Viral sexually transmitted infections and bacterial vaginosis: 2001-2004 National Health and Nutrition Examination Survey data. Sex Transm Dis. 2008;35(9):791–6. doi: 10.1097/OLQ.0b013e3181788301. [DOI] [PubMed] [Google Scholar]
- 20.Thurman AR, Doncel GF. Innate immunity and inflammatory response to Trichomonas vaginalis and bacterial vaginosis: relationship to HIV acquisition. Am J Reprod Immunol. 2011;65(2):89–98. doi: 10.1111/j.1600-0897.2010.00902.x. [DOI] [PubMed] [Google Scholar]
- 21.van De Wijgert JH, Mason PR, et al. Intravaginal practices, vaginal flora disturbances, and acquisition of sexually transmitted diseases in Zimbabwean women. J Infect Dis. 2000;181(2):587–94. doi: 10.1086/315227. [DOI] [PubMed] [Google Scholar]
- 22.Cohen CR, Duerr A, Pruithithada N, et al. Bacterial vaginosis and HIV seroprevalence among female commercial sex workers in Chiang Mai, Thailand. Aids. 1995;9(9):1093–7. doi: 10.1097/00002030-199509000-00017. [DOI] [PubMed] [Google Scholar]
- 23.Sewankambo N, Gray RH, Wawer MJ, et al. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet. 1997;350(9077):546–50. doi: 10.1016/s0140-6736(97)01063-5. (see comments) (published erratum appears in Lancet 1997 Oct 4;350(9083):1036) [DOI] [PubMed] [Google Scholar]
- 24.Martin HL, Richardson BA, Nyange PM, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis. 1999;180(6):1863–8. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 25.Taha TE, Hoover DR, Dallabetta GA, Kumwenda NI, Mtimavalye LA, Yang LP, et al. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. Aids. 1998;12(13):1699–706. doi: 10.1097/00002030-199813000-00019. [DOI] [PubMed] [Google Scholar]
- 26.Myer L, Denny L, Telerant R, Souza M, Wright TC, Jr, Kuhn L. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. J Infect Dis. 2005;192(8):1372–80. doi: 10.1086/462427. [DOI] [PubMed] [Google Scholar]
- 27.Cu-Uvin S, Hogan JW, Caliendo AM, Harwell J, Mayer KH, Carpenter CC. Association between bacterial vaginosis and expression of human immunodeficiency virus type 1 RNA in the female genital tract. Clin Infect Di. 2001;33(6):894–6. doi: 10.1086/322613. [DOI] [PubMed] [Google Scholar]
- 28.Coleman JS, Hitti J, Bukusi EA, et al. Infectious correlates of HIV-1 shedding in the female upper and lower genital tracts. Aids. 2007;21(6):755–9. doi: 10.1097/QAD.0b013e328012b838. [DOI] [PubMed] [Google Scholar]
- 29.Sha BE, Zariffard MR, Wang QJ, et al. Female genital-tract HIV load correlates inversely with Lactobacillus species but positively with bacterial vaginosis and Mycoplasma hominis. J Infect Dis. 2005;191(1):25–32. doi: 10.1086/426394. [DOI] [PubMed] [Google Scholar]
- 30.Al-Harthi L, Roebuck KA, Olinger GG, et al. Bacterial vaginosis-associated microflora isolated from the female genital tract activates HIV-1 expression. J Acquir Immune Defic Syndr. 1999;21(3):194–202. doi: 10.1097/00126334-199907010-00003. [DOI] [PubMed] [Google Scholar]
- 31.Patton DL, Sweeney YC, Cummings PK, Meyn L, Rabe LK, Hillier SL. Safety and efficacy evaluations for vaginal and rectal use of BufferGel in the macaque model. Sex Transm Dis. 2004;31(5):290–6. doi: 10.1097/01.olq.0000124614.91448.d4. [DOI] [PubMed] [Google Scholar]
- 32.Spear GT, Gilbert D, Sikaroodi M, et al. Identification of rhesus macaque genital microbiota by 16S pyrosequencing shows similarities to human bacterial vaginosis: implications for use as an animal model for HIV vaginal infection. AIDS Res Hum Retroviruses. 2010;26(2):193–200. doi: 10.1089/aid.2009.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doyle L, Young CL, Jang SS, Hillier SL. Normal vaginal aerobic and anaerobic bacterial flora of the rhesus macaque (Macaca mulatta) J Med Primatol. 1991;20(8):409–13. [PubMed] [Google Scholar]
- 34.Mirmonsef P, Gilbert D, Veazey RS, Wang J, Kendrick SR, Spear GT. A Comparison of Lower Genital Tract Glycogen and Lactic Acid Levels in Women and Macaques: Implications for HIV and SIV Susceptibility. AIDS Res Hum Retroviruses. 2011 Jun 28; doi: 10.1089/aid.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patton DL, Sweeney YT, Balkus JE, et al. Preclinical safety assessments of UC781 anti-human immunodeficiency virus topical microbicide formulations. Antimicrob Agents Chemother. 2007;51(5):1608–15. doi: 10.1128/AAC.00984-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lagenaur LA, Sanders-Beer BE, Brichacek B, et al. Prevention of vaginal SHIV transmission in macaques by a live recombinant Lactobacillus. Mucosal Immunol. 2011;4(6):648–57. doi: 10.1038/mi.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pybus V, Onderdonk AB. Microbial interactions in the vaginal ecosystem, with emphasis on the pathogenesis of bacterial vaginosis. Microbes Infect. 1999;1(4):285–92. doi: 10.1016/s1286-4579(99)80024-0. [DOI] [PubMed] [Google Scholar]
- 38.Paavonen J. Physiology and ecology of the vagina. Scand J Infect Dis Suppl. 1983;40:31–5. [PubMed] [Google Scholar]
- 39.Hillier SL, Krohn MA, Klebanoff SJ, Eschenbach DA. The relationship of hydrogen peroxide-producing lactobacilli to bacterial vaginosis and genital microflora in pregnant women. Obstet Gynecol. 1992;79(3):369–73. doi: 10.1097/00006250-199203000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Hillier SL, Krohn MA, Rabe LK, Klebanoff SJ, Eschenbach DA. The normal vaginal flora, H2O2-producing lactobacilli, and bacterial vaginosis in pregnant women. Clin Infect Dis. 1993;16(4):S273–81. doi: 10.1093/clinids/16.supplement_4.s273. [DOI] [PubMed] [Google Scholar]
- 41.O'Hanlon DE, Moench TR, Cone RA. In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infect Dis. 2011;11:200. doi: 10.1186/1471-2334-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Hanlon DE, Lanier BR, Moench TR, Cone RA. Cervicovaginal fluid and semen block the microbicidal activity of hydrogen peroxide produced by vaginal lactobacilli. BMC Infect Dis. 2010;10:120. doi: 10.1186/1471-2334-10-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.St John E, Mares D, Spear GT. Bacterial vaginosis and host immunity. Curr HIV/AIDS Rep. 2007;4(1):22–8. doi: 10.1007/s11904-007-0004-y. [DOI] [PubMed] [Google Scholar]
- 44.Cauci S, Culhane JF. Modulation of vaginal immune response among pregnant women with bacterial vaginosis by Trichomonas vaginalis, Chlamydia trachomatis, Neisseria gonorrhoeae, and yeast. Am J Obstet Gynecol. 2007;196(2):133, e1–7. doi: 10.1016/j.ajog.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 45.Cauci S, Driussi S, Guaschino S, Isola M, Quadrifoglio F. Correlation of local interleukin-1beta levels with specific IgA response against Gardnerella vaginalis cytolysin in women with bacterial vaginosis. Am J Reprod Immunol. 2002;47(5):257–64. doi: 10.1034/j.1600-0897.2002.01096.x. [DOI] [PubMed] [Google Scholar]
- 46.Cauci S, Guaschino S, De Aloysio D, et al. Interrelationships of interleukin-8 with interleukin-1beta and neutrophils in vaginal fluid of healthy and bacterial vaginosis positive women. Mol Hum Reprod. 2003;9(1):53–8. doi: 10.1093/molehr/gag003. [DOI] [PubMed] [Google Scholar]
- 47.Wennerholm UB, Holm B, Mattsby-Baltzer I, et al. Interleukin-1alpha, interleukin-6 and interleukin-8 in cervico/vaginal secretion for screening of preterm birth in twin gestation. Acta Obstet Gynecol Scand. 1998;77(5):508–14. [PubMed] [Google Scholar]
- 48.Imseis HM, Greig PC, Livengood CH, 3rd, Shunior E, Durda P, Erikson M. Characterization of the inflammatory cytokines in the vagina during pregnancy and labor and with bacterial vaginosis. J Soc Gynecol Investig. 1997;4(2):90–4. [PubMed] [Google Scholar]
- 49.Hedges SR, Barrientes F, Desmond RA, Schwebke JR. Local and systemic cytokine levels in relation to changes in vaginal flora. J Infect Dis. 2006;193(4):556–62. doi: 10.1086/499824. [DOI] [PubMed] [Google Scholar]
- 50.Mares D, Simoes JA, Novak RM, Spear GT. TLR2-mediated cell stimulation in bacterial vaginosis. J Reprod Immunol. 2008;77:91. doi: 10.1016/j.jri.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ballweber L, Robinson B, Kreger A, et al. Vaginal Langerhans cells non-productively transporting HIV-1 mediate infection of T cells. J Virol. 2011 Oct 5; doi: 10.1128/JVI.05615-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Al-Harthi L, Spear GT, Hashemi FB, Landay A, Sha BE, Roebuck KA. A human immunodeficiency virus (HIV)-inducing factor from the female genital tract activates HIV-1 gene expression through the kappaB enhancer. J Infect Dis. 1998;178(5):1343–51. doi: 10.1086/314444. [DOI] [PubMed] [Google Scholar]
- 53.Spear GT, al-Harthi L, Sha B, et al. A potent activator of HIV-1 replication is present in the genital tract of a subset of HIV-1-infected and uninfected women. Aids. 1997;11(11):1319–26. doi: 10.1097/00002030-199711000-00005. [DOI] [PubMed] [Google Scholar]
- 54.Li Q, Estes JD, Schlievert PM, Duan L, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458(7241):1034–8. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mirmonsef P, Gilbert D, Zariffard MR, et al. The effects of commensal bacteria on innate immune responses in the female genital tract. Am J Reprod Immunol. 2011;65(3):190–5. doi: 10.1111/j.1600-0897.2010.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roy CC, Kien CL, Bouthillier L, Levy E. Short-chain fatty acids: ready for prime time? Nutr Clin Pract. 2006;21(4):351–66. doi: 10.1177/0115426506021004351. [DOI] [PubMed] [Google Scholar]
- 57.Kuchiiwa T, Nio-Kobayashi J, Takahashi-Iwanaga H, Yajima T, Iwanaga T. Cellular expression of monocarboxylate transporters in the female reproductive organ of mice: implications for the genital lactate shuttle. Histochem Cell Biol. 2011;135(4):351–60. doi: 10.1007/s00418-011-0794-2. [DOI] [PubMed] [Google Scholar]
- 58.Pinheiro C, Longatto-Filho A, Ferreira L, et al. Increasing expression of monocarboxylate transporters 1 and 4 along progression to invasive cervical carcinoma. Int J Gynecol Pathol. 2008;27(4):568–74. doi: 10.1097/PGP.0b013e31817b5b40. [DOI] [PubMed] [Google Scholar]
- 59.Cuff MA, Lambert DW, Shirazi-Beechey SP. Substrate-induced regulation of the human colonic monocarboxylate transporter, MCT1. J Physiol. 2002;539(Pt 2):361–71. doi: 10.1113/jphysiol.2001.014241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morris ME, Felmlee MA. Overview of the proton-coupled MCT (SLC16A) family of transporters: characterization, function and role in the transport of the drug of abuse gamma-hydroxybutyric acid. AAPS J. 2008;10(2):311–21. doi: 10.1208/s12248-008-9035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thibault R, Blachier F, Darcy-Vrillon B, de Coppet P, Bourreille A, Segain JP. Butyrate utilization by the colonic mucosa in inflammatory bowel diseases: a transport deficiency. Inflamm Bowel Dis. 2010;16(4):684–95. doi: 10.1002/ibd.21108. [DOI] [PubMed] [Google Scholar]
- 62.Thibault R, De Coppet P, Daly K, et al. Down-regulation of the monocarboxylate transporter 1 is involved in butyrate deficiency during intestinal inflammation. Gastroenterology. 2007;133(6):1916–27. doi: 10.1053/j.gastro.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 63.Le Poul E, Loison C, Struyf S, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278(28):25481–9. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 64.Brown AJ, Goldsworthy SM, Barnes AA, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278(13):11312–9. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 65.Maslowski KM, Vieira AT, Ng A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461(7268):1282–6. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mirmonsef P, Zariffard MR, Gilbert D, Makinde H, Landay AL, Spear GT. Short-Chain Fatty Acids Induce Pro-Inflammatory Cytokine Production Alone and in Combination with Toll-Like Receptor Ligands. Am J Reprod Immunol. 2011 Nov 8; doi: 10.1111/j.1600-0897.2011.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Segain JP, Raingeard de la Bletiere D, et al. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn's disease. Gut. 2000;47(3):397–403. doi: 10.1136/gut.47.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luhrs H, Gerke T, Muller JG, et al. Butyrate inhibits NF-kappaB activation in lamina propria macrophages of patients with ulcerative colitis. Scand J Gastroenterol. 2002;37(4):458–66. doi: 10.1080/003655202317316105. [DOI] [PubMed] [Google Scholar]
- 69.Luhrs H, Kudlich T, Neumann M, et al. Butyrate-enhanced TNFalpha-induced apoptosis is associated with inhibition of NF-kappaB. Anticancer Res. 2002;22(3):1561–8. [PubMed] [Google Scholar]
- 70.Kantor B, Ma H, Webster-Cyriaque J, Monahan PE, Kafri T. Epigenetic activation of unintegrated HIV-1 genomes by gut-associated short chain fatty acids and its implications for HIV infection. Proc Natl Acad Sci U S A. 2009;106(44):18786–91. doi: 10.1073/pnas.0905859106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Quivy V, Adam E, Collette Y, et al. Synergistic activation of human immunodeficiency virus type 1 promoter activity by NF-kappaB and inhibitors of deacetylases: potential perspectives for the development of therapeutic strategies. J Virol. 2002;76(21):11091–103. doi: 10.1128/JVI.76.21.11091-11103.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sanderson IR. Short chain fatty acid regulation of signaling genes expressed by the intestinal epithelium. J Nutr. 2004;134(9):2450S–4S. doi: 10.1093/jn/134.9.2450S. [DOI] [PubMed] [Google Scholar]
- 73.Pilatte Y, Bignon J, Lambre CR. Sialic acids as important molecules in the regulation of the immune system: pathophysiological implications of sialidases in immunity. Glycobiology. 1993;3(3):201–18. doi: 10.1093/glycob/3.3.201. [DOI] [PubMed] [Google Scholar]
- 74.Wiggins R, Hicks SJ, Soothill PW, Millar MR, Corfield AP. Mucinases and sialidases: their role in the pathogenesis of sexually transmitted infections in the female genital tract. Sex Transm Infect. 2001;77(6):402–8. doi: 10.1136/sti.77.6.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cauci S, Culhane JF, Di Santolo M, McCollum K. Among pregnant women with bacterial vaginosis, the hydrolytic enzymes sialidase and prolidase are positively associated with interleukin-1beta. Am J Obstet Gynecol. 2008;198(1):132, e1–7. doi: 10.1016/j.ajog.2007.05.035. [DOI] [PubMed] [Google Scholar]
- 76.McClelland RS. Trichomonas vaginalis infection: can we afford to do nothing? J Infect Dis. 2008;197(4):487–9. doi: 10.1086/526498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Allsworth JE, Ratner JA, Peipert JF. Trichomoniasis and other sexually transmitted infections: results from the 2001-2004 National Health and Nutrition Examination Surveys. Sex Transm Dis. 2009;36(12):738–44. doi: 10.1097/OLQ.0b013e3181b38a4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fichorova RN. Impact of T. vaginalis infection on innate immune responses and reproductive outcome. J Reprod Immunol. 2009;83(1-2):185–9. doi: 10.1016/j.jri.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schwebke JR, Burgess D. Trichomoniasis. Clin Microbiol Rev. 2004;17(4):794–803. doi: 10.1128/CMR.17.4.794-803.2004. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Laga M, Alary M, Nzila N, et al. Condom promotion, sexually transmitted diseases treatment, and declining incidence of HIV-1 infection in female Zairian sex workers. Lancet. 1994;344(8917):246–8. doi: 10.1016/s0140-6736(94)93005-8. [DOI] [PubMed] [Google Scholar]
- 81.McClelland RS, Sangare L, Hassan WM, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis. 2007;195(5):698–702. doi: 10.1086/511278. [DOI] [PubMed] [Google Scholar]
- 82.Van Der Pol B, Kwok C, Pierre-Louis B, et al. Trichomonas vaginalis infection and human immunodeficiency virus acquisition in African women. J Infect Dis. 2008;197(4):548–54. doi: 10.1086/526496. [DOI] [PubMed] [Google Scholar]
- 83.Mavedzenge SN, Pol BV, Cheng H, et al. Epidemiological synergy of Trichomonas vaginalis and HIV in Zimbabwean and South African women. Sex Transm Dis. 2010;37(7):460–6. doi: 10.1097/OLQ.0b013e3181cfcc4b. [DOI] [PubMed] [Google Scholar]
- 84.Kissinger P, Amedee A, Clark RA, et al. Trichomonas vaginalis treatment reduces vaginal HIV-1 shedding. Sex Transm Dis. 2009;36(1):11–6. doi: 10.1097/OLQ.0b013e318186decf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang CC, McClelland RS, Reilly M, et al. The effect of treatment of vaginal infections on shedding of human immunodeficiency virus type 1. J Infect Dis. 2001;183(7):1017–22. doi: 10.1086/319287. [DOI] [PubMed] [Google Scholar]
- 86.Tanton C, Weiss HA, Le Goff J, et al. Correlates of HIV-1 genital shedding in Tanzanian women. PLoS ONE. 2011;6(3):e17480. doi: 10.1371/journal.pone.0017480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lehker MW, Alderete JF. Biology of trichomonosis. Curr Opin Infect Dis. 2000;13(1):37–45. doi: 10.1097/00001432-200002000-00007. [DOI] [PubMed] [Google Scholar]
- 88.Bastida-Corcuera FD, Okumura CY, Colocoussi A, Johnson PJ. Trichomonas vaginalis lipophosphoglycan mutants have reduced adherence and cytotoxicity to human ectocervical cells. Eukaryot Cell. 2005;4(11):1951–8. doi: 10.1128/EC.4.11.1951-1958.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Okumura CY, Baum LG, Johnson PJ. Galectin-1 on cervical epithelial cells is a receptor for the sexually transmitted human parasite Trichomonas vaginalis. Cell Microbiol. 2008;10(10):2078–90. doi: 10.1111/j.1462-5822.2008.01190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ryan CM, Mehlert A, Richardson JM, Ferguson MA, Johnson PJ. Chemical structure of Trichomonas vaginalis surface lipoglycan: a role for short galactose ({beta}1-4/3) N-acetylglucosamine repeats in host cell interaction. J Biol Chem. 2011 Sep 7; doi: 10.1074/jbc.M111.280578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Noel CJ, Diaz N, Sicheritz-Ponten T, et al. Trichomonas vaginalis vast BspA-like gene family: evidence for functional diversity from structural organisation and transcriptomics. BMC Genomics. 2010;11:99. doi: 10.1186/1471-2164-11-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yadav M, Dubey ML, Gupta I, Bhatti G, Malla N. Cysteine proteinase 30 in clinical isolates of T. vaginalis from symptomatic and asymptomatic infected women. Exp Parasitol. 2007;116(4):399–406. doi: 10.1016/j.exppara.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 93.Sommer U, Costello CE, Hayes GR, et al. Identification of Trichomonas vaginalis cysteine proteases that induce apoptosis in human vaginal epithelial cells. J Biol Chem. 2005;280(25):23853–60. doi: 10.1074/jbc.M501752200. [DOI] [PubMed] [Google Scholar]
- 94.Guenthner PC, Secor WE, Dezzutti CS. Trichomonas vaginalis-induced epithelial monolayer disruption and human immunodeficiency virus type 1 (HIV-1) replication: implications for the sexual transmission of HIV-1. Infect Immun. 2005;73(7):4155–60. doi: 10.1128/IAI.73.7.4155-4160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lehker MW, Sweeney D. Trichomonad invasion of the mucous layer requires adhesins, mucinases, and motility. Sex Transm Infect. 1999;75(4):231–8. doi: 10.1136/sti.75.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Levine WC, Pope V, Bhoomkar A, et al. Increase in endocervical CD4 lymphocytes among women with nonulcerative sexually transmitted diseases. J Infect Dis. 1998;177(1):167–74. doi: 10.1086/513820. [DOI] [PubMed] [Google Scholar]
- 97.Kiviat NB, Paavonen JA, Brockway J, et al. Cytologic manifestations of cervical and vaginal infections. I. Epithelial and inflammatory cellular changes. Jama. 1985;253(7):989–96. [PubMed] [Google Scholar]
- 98.Mitchell C, Hitti J, Paul K, et al. Cervicovaginal shedding of HIV type 1 is related to genital tract inflammation independent of changes in vaginal microbiota. AIDS Res Hum Retroviruses. 2011;27(1):35–9. doi: 10.1089/aid.2010.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zariffard MR, Harwani S, Novak RM, Graham PJ, Ji X, Spear GT. Trichomonas vaginalis infection activates cells through toll-like receptor 4. Clin Immunol. 2004;111(1):103–7. doi: 10.1016/j.clim.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 100.Chang JH, Kim SK, Choi IH, Lee SK, Morio T, Chang EJ. Apoptosis of macrophages induced by Trichomonas vaginalis through the phosphorylation of p38 mitogen-activated protein kinase that locates at downstream of mitochondria-dependent caspase activation. Int J Biochem Cell Biol. 2006;38(4):638–47. doi: 10.1016/j.biocel.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 101.Chang JH, Ryang YS, Morio T, Lee SK, Chang EJ. Trichomonas vaginalis inhibits proinflammatory cytokine production in macrophages by suppressing NF-kappaB activation. Mol Cells. 2004;18(2):177–85. [PubMed] [Google Scholar]
- 102.Sutton M, Sternberg M, Koumans EH, McQuillan G, Berman S, Markowitz L. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001-2004. Clin Infect Dis. 2007;45(10):1319–26. doi: 10.1086/522532. [DOI] [PubMed] [Google Scholar]
- 103.Baeten JM, Hassan WM, Chohan V, et al. Prospective study of correlates of vaginal Lactobacillus colonisation among high-risk HIV-1 seronegative women. Sex Transm Infect. 2009;85(5):348–53. doi: 10.1136/sti.2008.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Torok MR, Miller WC, Hobbs MM, et al. The association between Trichomonas vaginalis infection and level of vaginal lactobacilli, in nonpregnant women. J Infect Dis. 2007;196(7):1102–7. doi: 10.1086/521307. [DOI] [PubMed] [Google Scholar]
- 105.Moodley P, Connolly C, Sturm AW. Interrelationships among human immunodeficiency virus type 1 infection, bacterial vaginosis, trichomoniasis, and the presence of yeasts. J Infect Dis. 2002;185(1):69–73. doi: 10.1086/338027. [DOI] [PubMed] [Google Scholar]
- 106.Spear GT, Kendrick SR, Chen HY, et al. Multiplex immunoassay of lower genital tract mucosal fluid from women attending an urban STD clinic shows broadly increased IL1ss and lactoferrin. PLoS One. 2011;6(5):e19560. doi: 10.1371/journal.pone.0019560. [DOI] [PMC free article] [PubMed] [Google Scholar]