Abstract
Ammoxidation of technical lignins under mild conditions is a suitable approach to artificial humic substances. However, carbohydrates as common minor constituents of technical lignins have been demonstrated to be a potential source of N-heterocyclic ecotoxic compounds. Ethyl acetate extracts of ammoxidation mixtures of the monosaccharides glucose and xylose exhibited considerable growth inhibiting activity in the OECD 201 test, with 4-methyl-1H-imidazole, 4-(hydroxymethyl)-1H-imidazole, and 3-hydroxypyridine being the most active compounds. The amount of N-heterocyclic compounds formed at moderate ammoxidation conditions (70 °C, 0.2 MPa O2, 3 h) was significantly lower for the polysaccharides cellulose and xylan (16–30 μg/g of educt) compared to glucose (15.4 mg). Ammoxidation at higher temperature is not recommendable for carbohydrate-rich materials as much higher amounts of N-heterocyclic compounds were formed from both monosaccharides (100 °C: 122.4–160.5 mg/g of educt) and polysaccharides (140 °C: 5.52–16.03 mg/g of educt).
Keywords: ammoxidation, Maillard reaction, lignin, N-modified lignin, artificial humic substances
Introduction
Ammoxidation (or “ammonoxidation”), that is the simultaneous treatment of a given organic substrate with ammonia and oxygen, has been established1 and advanced2,3 as an appropriate method to convert technical, virtually nitrogen-free lignins into nitrogenous soil-improving materials. In particular lignins ammoxidized under comparatively “mild” reaction conditions (T ≤ 100 °C; pO2 ≤ 0.2 MPa; 5% aqueous ammonia) have been demonstrated to share similarities with natural humic substances. They act as soil amendments with slowly nitrogen releasing, water storing, ion exchanging, and texture improving properties, and are hence beneficial for plant growth. Positive effects of ammoxidized lignins on the growth of agricultural crops or trees have been reported, such as in forest plant breeding, ornamental horticulture, or rehabilitation of degraded soils.2,4−6 However, there are still some uncertainties with regard to the application of ammoxidized lignocellulosic materials in soil improvement and crop cultivation. Besides insufficient knowledge about different types of nitrogen bonding and the resulting fertilizing properties,7 the major concern is about possible formation of ecotoxic, carbohydrate-derived nitrogenous compounds under ammoxidation conditions.8
The reaction of mono-, oligo-, and polysaccharides under aqueous alkaline conditions in general, and of sugars with ammonia in Maillard-type reactions in particular, have been comprehensively studied in the past decades. Base-catalyzed degradation of cellulose and monosaccharides was reviewed by Knill and Kennedy,9 the reaction of carbohydrates with ammonia were summarized by Kort,10 while Fay and Bernard11 gave a more recent overview of the Maillard reaction. To the best of our knowledge, no study investigated the types of nitrogenous products obtained by a joint treatment of carbohydrates with aqueous ammonia and oxygen, even though a considerable number of papers on technical aspects of biomass ammoxidation3 and ammoxidative pretreatments (ammonia recycle pretreatment, ARP)8 have been published.3,12
This paper investigates the reaction behavior of cellulose, xylan, d-glucose and d-xylose under ammoxidative conditions at different temperatures (70 °C, 100 °C, 140 °C), oxygen pressures (0.2 MPa, 1.0 MPa), but constant initial ammonia concentration (5%, w/v) and pH of about 11.9. In addition to the weight of the product fractions (solid phase, aqueous phase, ethyl acetate extracts), the composition of the different phases was studied by GC/MS, in part after derivatization. Authentic samples of the main identified N-heterocyclic reaction products were synthesized. These pure compounds were used in ecotoxicity studies with regard to growth inhibition of the fresh-water algae Pseudokirchneriella subcapitata (OECD ecotoxicity test 201), and as analytical standards in GCMS. As phytotoxicity correlated mainly with the content of monosaccharides, gel permeation chromatography was additionally used to study the degradation of polysaccharides under ammoxidation conditions.
Materials and Methods
Beech xylan (97.4% xylose, 0.8% glucose, 0.2% mannose, 0.2% rhamnose, 0.2% cellobiose, 1.1% 4-O-Me-glucuronic acid; MW 10.210 kg/mol) was provided by Lenzing AG (Lenzing, Austria). All other chemicals, including cellulose (acid-washed and milled cotton linters for column chromatography), were purchased from Sigma-Aldrich Handels GmbH (Vienna, Austria) in the highest purity available, and were used, unless otherwise noted, without further purification. Ethyl acetate (EtOAc) was dried over anhydrous calcium chloride, distilled once with acetic anhydride and sulfuric acid, and subsequently over potassium carbonate in order to remove water, ethanol and acetic acid, respectively. Pyridine and ethyl acetate for derivatization were stored over molecular sieves, and filtered through a 0.45 μm syringe filter prior to use.
Ammoxidation
was accomplished in a 100 mL 4566 C Series laboratory-scale pressure vessel (Parr Instruments, Frankfurt, Germany) equipped with a glass liner and a septum valve, and heated with an oil bath. Monosaccharides: 10 mL of 10% aqueous ammonia were placed inside the reactor. After flushing three times with oxygen, the reactor was closed and heated under continuous stirring to the respective temperature (70 °C, 100 or 140 °C). Then, a solution of 1.0 g of the respective monosaccharide in 10 mL of water was added through a septum by a syringe, initiating the reaction (t = 0). The reactor was pressurized with 0.2 and 1 MPa of O2, respectively, and left in connection with the gas supply via a back-pressure valve in order to guarantee constant pressure throughout the reaction. The final pressures including the respective vapor pressure of the aqueous solution of ammonia (5%) were for the 0.2 MPa pressure stage 0.27 MPa (70 °C), 0.38 MPa (100 °C), 0.68 MPa (140 °C), and for the 1.0 MPa pressure stage 1.20 MPa (70 °C), 1.39 MPa (100 °C) and 1.80 MPa (140 °C). Polysaccharides: Deviating from the above-described procedure, a suspension of the respective polysaccharide in 10 mL of water was first pretempered in the reactor before the reaction was initiated by addition of 10 mL of 10% aqueous ammonia.
Workup Procedures
After stirring the reaction mixtures at the respective temperature and pressure for 3 h, the reaction vessel was cooled and depressurized. Workup procedure prior to optimization: The reaction mixture was extracted three times with 20 mL ethyl acetate. The combined extracts were dried over Na2SO4, and evaporated under reduced pressure to dryness. Optimized workup procedure: The reaction mixture was evaporated at 3.5 kPa (35 °C) to remove ammonia, solids were separated by centrifugation, washed with water, and freeze-dried for SEC and elemental analysis. The pH value was adjusted to 8.5 using 2% aqueous ammonia in order to ensure complete deprotonation of imidazole and pyrazine functionalities. Higher concentrations of ammonia should be avoided in order to prevent acetamide formation from ethyl acetate during extraction. After filling up to 20 mL, the aqueous phase was extracted four times with 100 mL of water-saturated ethyl acetate. The combined organic phases were evaporated to approximately 1/10 of their volume under reduced pressure, whereupon the water was removed by azeotropic distillation. The residue was redissolved in 10 mL of dry EtOAc and evaporated at 35 °C (9 kPa) affording a yellowish oil. Aliquots of the extract were used for GC/MS analyses, and for phytotoxicity testing. The aqueous phase was freeze-dried and weighed.
Silylation
An aliquot containing approximately 1 mg of dry matter was lyophilized overnight at −25 °C and 10–20 Pa. Then, 200 μL of dry pyridine, containing 1.5 g/L of the catalyst 4-(N,N-dimethylamino)pyridine (DMAP), and 10 μL of a 10 g/L solution of the internal standard phenyl α-glucopyranoside in pyridine were added. The mixture was vortexed for a minimum of 30 s, before 100 μL of the silylation mixture (BSTFA containing 10% trimethylchlorosilane) was added. Silylation was performed at 70 °C for 2 h. After cooling to room temperature, 900 μL of dry ethyl acetate were added, the mixture was vortexed and analyzed by GC/MS within 24 h. For reference spectra of silylated compounds, a 200 μg aliquot of the respective substance was subjected to the above procedure.
Synthesis of Authentic Samples
(5-Methyl-pyrazin-2-yl)-methanol
Mild oxidation of 2,5-dimethylpyrazine (2.16 g, 20 mmol) with N-chlorosuccinimide (3.19 g, 23.9 mmol) and dibenzoylperoxide (123.6 mg, 0.68 mmol) in 50 mL of CCl4 as described elsewhere.13 Yield after purification by column chromatography: 936 mg (38%) and 143 mg (5.1%) of the byproduct 2,5-bis(hydroxymethyl)pyrazine. The product was stored in a dark place at 4 °C.
1H NMR (CDCl3): δ 2.59 (s, 3H, CH3), 3.16 (s, 1H, OH), 4.18 (s, 2H, CH2OH), 8.43 (s, 1H, pyrazine-H6), 8.53 (s, 1H, pyrazine-H3). 13C NMR (CDCl3): δ 21.1 (CH3), 62.6 (CH2OH), 141.6 (C3), 143.1 (C6), 152.5 (C2), 151.6 (C5).
2-(Arabino-tetrahydroxybutyl)-5-(erythro-2,3,4-trihydroxybutyl)-pyrazine (Deoxyfructosazine)
Synthesis by portionwise addition of d-glucosamine hydrochloride (2.16 g, 10 mmol) to a solution of 3.05 g (25 mmol) of phenylboronic acid in aqueous sodium hydroxide as described elsewhere.14 Yield: 1.31 g (86%).
1H NMR (DMSO-d6): δ 2.71 (1H, dd, CH2, J = 13.8 Hz, 9.3 Hz), 3.04 (1H, dd, CH2, J = 2.8 Hz, 13.8 Hz), 3.36 – 3.45 (m, 3H), 3.53 – 3.63 (m, 3H), 3.72 – 3.77 (m, 1H), 4.45 and 4.68 (7H, br, OH), 4.92 (d, 1H, J = 3.4, tetrahydroxybutyl-H1), 8.38 (d, 1H, J = 1.2), 8.61 (d, 1H, J = 5.2). 13C NMR (DMSO-d6): δ 18.97 (CH2), 56.42, 63.62, 63.98, 71.68, 71.74, 74.24, 75.38 (7 C, CHOH), 142.70, 143.67 (2 C, CHAr), 153.62, 156.151 (2 C, CAr).
4-(Arabino-tetrahydroxybutyl)-imidazole
Synthesis by condensation of d-fructose (5.40 g, 30 mmol), formamidine acetate (5.03 g, 36 mmol) and liquid ammonia (20 mL) at 75 °C using a Parr 4566 C pressure reactor.15 Yield: 2.04 g (36%).
1H NMR (CD3OD): δ 3.60–3.66 (1H, m, CH2OH), 3.68 – 3.75 (2H, dd, tetrahydroxybutyl-H2/H3), 3.77–3.82 (m, 1H, CH2OH), 4.97 (1H, tetrahydroxybutyl-H1, d, J = 1.7 Hz), 7.04 (1H, s, imidazole-H5), 7.61 (1H, d, imidazole-H2, J = 1.0 Hz). 13C NMR (DMSO-d6): δ 64.8 (tetrahydroxybutyl-C4), 68.0 (tetrahydroxybutyl-C1), 73.1 and 75.67 (tetrahydroxybutyl-C2/3), 136.0 (imidazole-C2).
Instrumental Analysis
NMR spectra were recorded on a Bruker Avance II 400 (resonance frequencies 400.13 MHz for 1H and 100.63 MHz for 13C) equipped with a 5 mm observe broadband probe head (BBFO). Standard Bruker pulse programs were used.
GC-MS was performed on a GC 6890N/MSD 5975B instrument equipped with a 30 m × 0.25 mm i.d., 25 μm, capillary HP-5 ms column (Agilent, Germany). Helium total flow was 27.5 mL/min at 46.9 kPa; the column flow was 0.9 mL/min. Temperature program: 50 °C (2 min), 5 °C/min to 280 °C (20 min). 0.2 μL aliquots of the dissolved samples were injected at 230 °C inlet temperature in splitless mode, or with a 1:2 split, depending on the concentration. Ionization was performed in EI mode at 70 eV. Data were acquired and processed with the Agilent Chemstation E.02.02.1431 software package. Unless otherwise noted, tentative peak assignment was accomplished by comparison with the NIST MS library, version 2.0f.
Size exclusion chromatography (SEC): Dionex DG-2410 online degasser; Kontron 420 pump with pulse damper; HP 1100 auto sampler; Gynkotek STH 585 column oven; Shodex RI-71 refractive index and a Wyatt Dawn DSP, λ 488 nm laser multiple-angle light scattering detector. Data evaluation: ASTRA and GRAMS/32 software. Separation was accomplished using four 300 mm × 7.5 mm i.d., 20 μm PL gel mixed A LS columns (Agilent, Germany), an injection volume of 100 μL, eluent flow of 1.00 mL/min and run time of 45 min. Mobile phase: N,N-dimethylacetamide/lithium chloride (0.9% v/w), filtered through a 0.02 μm filter.
Activation of cellulose: 20 mg of dry cellulose was subsequently soaked in a large excess of deionized water, ethanol, and N,N-dimethylacetamide and left in the latter under shaking overnight. After filtration, 2 mL of N,N-dimethylacetamide/LiCl 9% (v/w) was added and the sample was left for dissolution on a vertical shaker overnight. Prior to measurement, 0.3 mL of the respective samples were diluted with 0.9 mL of N,N-dimethylacetamide and filtered. Carbonyl and carboxyl group contents were determined after labeling with the fluorescence markers carbazole-9-carboxylic acid [2-(2-amino-oxyethoxy)ethoxy]amide (CCOA; carbonyl groups)16 and 2-diazomethyl fluorene (FDAM; carboxyl groups), respectively.17
Elemental analyses were performed at the Microanalytical Lab of the University of Vienna.
Ecotoxicity Screening
Dilution series (dilution factors 1, 2, 4, 8, 10, 16, 32, 64, 100, 256, 1000, 1024, and 10 000) of all studied compounds were prepared from respective stock solutions containing 10 mg of sample in 20 mL of water, adjusted to pH 7.0 ± 0.3 with 1 M NaOH. Two mL of this solution was transferred to well plates, inoculated with the exponentially growing test organisms Pseudokirchneriella subcapitata (OECD test 201, approximately 20.000 cells per well) and cultivated for 72 h under following conditions: Illuminated shaker, 6.500–7.000 lx, day/night = 16h/8h, 22 °C. Potassium dichromate was used as a positive reference. All measurements (all concentration levels of samples and reference, blank) were performed in triplicate. Photometric measurements at 485 nm were performed at 0, 24, 48, and 72 h of incubation time.
Quantitation was based on the areas of the growth curves that can be calculated according to the following equations where Mn is the extinction at the time of the nth measuring tn, and A the integral of the growth curve. Inhibition was calculated from the integrals of the growth curves of both the blank (AB) and that of the sample (AS).
Results and Discussion
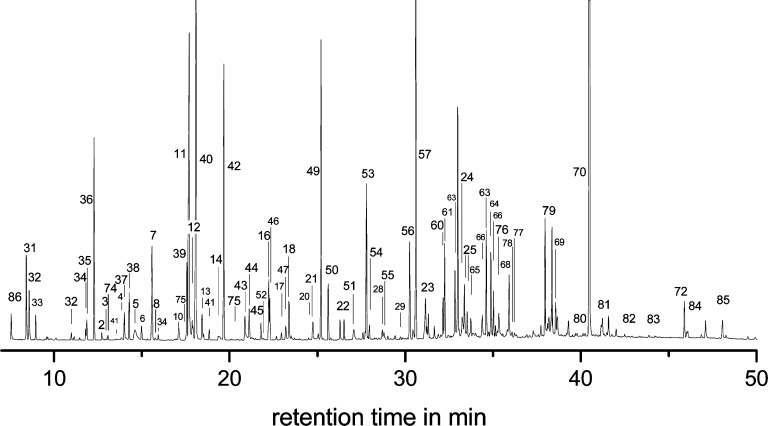
Ammoxidation of both monosaccharides (d-glucose, d-xylan) and polysaccharides (cellulose, xylan) at 70–140 °C (0.2–1.0 MPa O2) afforded a broad spectrum of lower-molecular compounds with distinctly higher total amounts in the case of the monosaccharides as compared to polysaccharides, especially at lower ammoxidation temperature.18 GC/MS analysis of the crude products revealed the presence of a large variety of nitrogenous compounds as shown for d-glucose (100 °C, 0.2 MPa O2, 3 h) in Figure 1. In addition to aminosugars, glycosylamines, amides, urea, and ammonium salts of carbaminic, oxalic, aldonic and deoxyaldonic acids, some unexpected compounds, such as α-hydroxyamides and α-amino acids, were detected (Table 1).18
Figure 1.
Chromatograms of the crude reaction mixture obtained by ammoxidation of d-glucose at 100 °C (0.2 MPa O2, 3 h) after freeze-drying and per-trimethylsilylation.
Table 1. Summary of Nitrogenous Compounds Present in Ammoxidized d-Glucose, d-Xylose, Cellulose, and Xylana.
| ammonia salts of carboxylic acids | glycolic acid (36), glyceric acid (42), erythronic acid (49), threonic acid (50), ribonic acid (56), arabinonic acid (57), mannonic acid (66), gluconic acid (66), lactic acid (35), 3-hydroxypropanoic acid (38), 3,4-dihydroxybutyric acid (46), 2-deoxypentonic acid (53), 3-deoxyhexonic acid (60) |
| amino acids | glycine (41), aminomalonic acid (47), alanine (74), serine (75) |
| amino sugars | glucosamine (62), fructosamine (65), di(glucopyranosyl)amine (73), aminohexopyranoside and aminoglycosides of unknown constitution (69), |
| amides | lactamide (6), glycolamide (7), urea (10), formamide (32), oxamic acid (39), acetamide (86) |
| 1H-imidazoles | 1H-imidazole (2), 4-methyl-1H-imidazole (5), 4-hydroxymethyl-1H-imidazole (43), 4-(1,2-dihydroxyethyl)-1H-imidazole (55), 4-(d-arabino-tetrahydroxybutyl)-1H-imidazole (68), 2-acetyl-4-(tetrahydroxybutyl)-1H-imidazole (77) |
| pyridines | 3-hydroxypyridine (4), 2-hydroxymethylpyridin-5-ol (20) |
| pyrazines | 2-pyrazinol (1), 2-hydroxy-5-methyl-pyrazine (3), 2-pyrazinyl-methanol (7), 2-hydroxy-3-methyl-pyrazine (8), 2-hydroxymethyl-6-methyl-pyrazine (12), 2-(dihydroxyethyl)-pyrazine (18), 2-(dihydroxyethyl)-5-methyl-pyrazine (21), 2,5- and 2,6-(dihydroxymethyl)-pyrazine (22), 2-(dihydroxy-ethyl)-5-(hydroxymethyl)-pyrazine (23), 2-(tetrahydroxybutyl)-pyrazine (24), 2-(tetrahydroxybutyl)-5-methyl-pyrazine (25), 2-(trihydroxypropyl)-pyrazine (28), 2-(trihydroxybutyl)-5-methyl-pyrazine (29), 2-hydroxy-5(and 6)-(tetrahydroxybutyl)-pyrazine and 2-hydroxy-6-(tetrahydroxybutyl)-pyrazine (78), 2,5- and 2,6-deoxy-fructosazine (84), 2,5- and 2,6-fructosazine (85), and 7 further pyrazine derivatives |
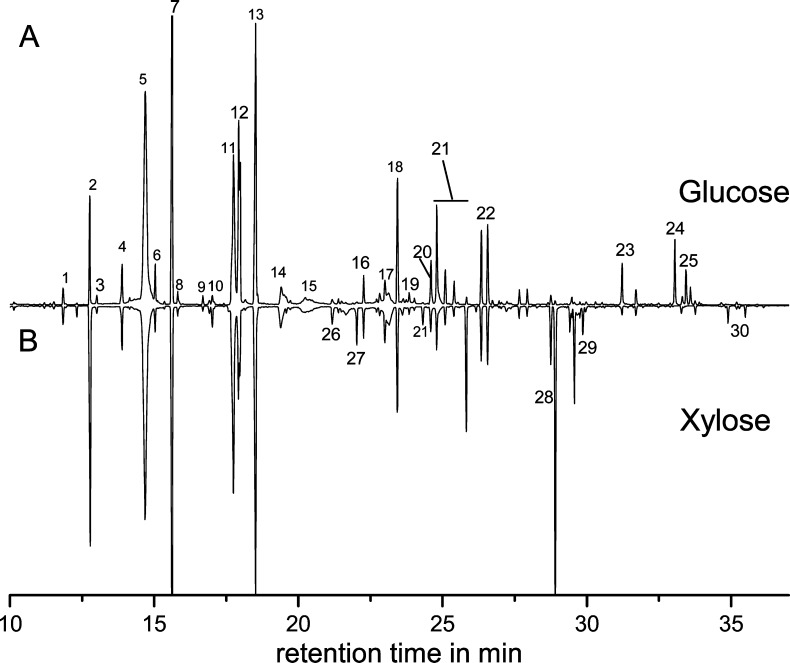
Distinctly higher yields of compounds extractable with ethyl acetate (EtOAc) as obtained after ammoxidation confirmed the formation of less polar compounds from both the monosaccharides glucose and xylose and the polysaccharides cellulose and xylan (Table 2). GC/MS analysis revealed that N-heterocyclic compounds of low H2O/EtOAc partition coefficients (log P ≈ 0) were the main constituents of the organic phases (Figure 2). Therefore, the extraction protocol was optimized toward virtually quantitative (≥97%) extraction of the key compound 4-methyl-1H-imidazole. Following this approach a significantly higher yield of extractives was isolated from the reaction mixtures, in particular for the 100 and 140 °C variants (Table 2). However, independent of the extraction method used, the amount of extractives was consistently 1 order of magnitude higher for the monosaccharides than for the polysaccharides ammoxidized under comparable conditions. The two monosaccharides afforded nearly the same amount of extractives, with xylose giving slightly higher yields. The amount of extractives from xylan was generally higher than that obtained from cellulose.
Table 2. Amounts of Ammoxidation Products (mg Product/100 mg of Educt) in the EtOAc Extracts (Optimized Protocol), Aqueous Phases, and Water-Insoluble Fraction.
| EtOAc extract | water soluble | water insoluble | ammoxidation conditionsa | EtOAc extract | water soluble | water insoluble |
|---|---|---|---|---|---|---|
| Glucose | Cellulose | |||||
| 0 | 104 | 0 | RT, ambient pressure | 0.1 | 2 | 93.7 |
| 1.8 | 67.5 | 0 | 70 °C | 0.4 | 2.1 | 89.6 |
| 14.0 | 77.2 | 0.6 | 100 °C | 0.9 | 2.1 | 91.9 |
| 13.0 | 65.6 | 6.8 | 140 °C | 1.2 | 9.8 | 89.9 |
| Xylose | Xylan | |||||
| 0 | 71.0 | 0 | RT, ambient pressure | 0.1 | 39.0 | 59.1 |
| 3.4 | 91.1 | 0 | 70 °C | 0 | 77.4 | 11.7 |
| n.d. | 82.5 | 0 | 100 °C | 0.2 | 26.3 | 68.5 |
| 17.7 | 71.9 | 2.3 | 140 °C | 3.9 | 43.5 | 41.4 |
Aqueous ammonia (5 w%), 0.2 MPa oxygen partial pressure, 3 h of reaction time.
Figure 2.
Chromatograms of per-trimethylsilylated ethyl acetate extracts of the reaction mixtures obtained after ammoxidation (100 °C, 0.2 MPa O2, 3 h) of d-glucose (A) and d-xylose (B). For peak assignment see Table 1.
The fraction of moderately polar to nonpolar compounds that was extractable with EtOAc increased with the ammoxidation temperature for all studied substrates, and ranged about 14–17.7% for the monosaccharides and 1.2–3.9% for the polysaccharides at 140 °C, relative to the mass of the respective starting material. The amount of dark-colored, water-insoluble products increased correspondingly with temperature, which is indicative of polymerization reactions as shown previously.19,20
Cellulose was increasingly degraded upon ammoxidation beyond 100 °C, which is evident from the amounts of water-soluble products that increased 4-fold when raising the reaction temperature from 100 to 140 °C. For xylan, which was partly soluble in aqueous ammonia (39% relative to the amount of educt) due to its glucuronic acid side groups, the weight of the water-soluble product fraction increased when raising the ammoxidation temperature from room temperature to 70 °C (77.4 mg/100 mg xylan). The water solubility was lower at 100 °C (26.3 mg/100 mg xylan) which is assumed to be due to a loss of glucuronic acid groups. At 140 °C, the fraction of water-soluble products increased up to 43.5%, and the optical appearance changed from white fibrous flakes (70 °C) to brown syrup, suggesting that decomposition of the xylan was so pronounced that water-soluble products of low molecular weight formed.
Compared to the influence of temperature during ammoxidation on the weight of the different fractions, the effect of oxygen pressure was rather small. At least for xylose and xylan (140 °C), a higher oxygen pressure favors the formation of water-soluble products (likely by generation of carboxyl groups) at the expense of water-insoluble ones, whereas the amount of compounds extractable with ethyl acetate remained at the same level.
Size Exclusion Chromatography
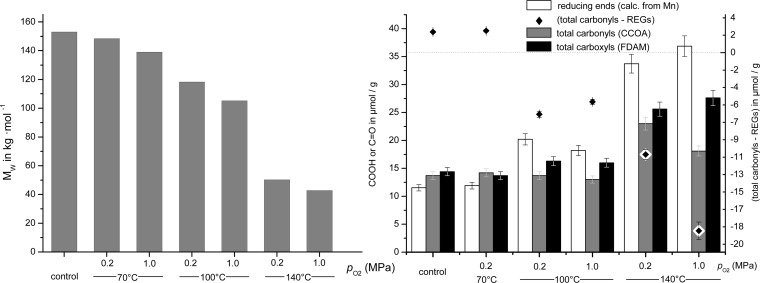
The degradation of polysaccharides under ammoxidation conditions was studied for cellulose. Size exclusion chromatography–multiangle laser light scattering (SEC-MALLS) in combination with fluorescence labeling of carbonyl and carboxyl groups provides additional information on the extent and nature of alkaline degradation in presence of ammonia and oxygen. The total amount of carbonyls and carboxyls was measured according to the CCOA16 and FDAM17 labeling approaches, respectively, and the theoretical number of reducing end groups (REGs) was calculated from the number-average molecular weight (Mn) obtained through SEC-MALLS.
SEC-MALLS analysis of the studied cellulose (cotton linters, M̅W 153 kg/mol) prior to and after ammoxidation confirmed that the increasing amounts of EtOAc-soluble and water-soluble compounds formed at higher temperature correlated with the obtained loss in molar mass of cellulose (Figure 3). However, at moderate ammoxidation temperature of 70 °C, the chemical integrity of cotton linters is little compromised (M̅W = 139 kg/mol). The amount of carbonyls and the difference between total carbonyls and REGs (equates to the content of along-chain carbonyls) remains unaffected, indicating only a very minor extent of oxidative damage along the chain as well as little participation in reactions with ammonia. A significant decrease of M̅W was observed with increasing temperature (100 °C) and oxygen pressure, as expected. At 140 °C, cellulose was severely degraded (M̅W = 43 kg/mol). However, the number of carbonyls increased only slightly. At this temperature, alkaline peeling/stopping commence and fast alkali-induced chain scission (beta-elimination) play a major role. Along-chain carbonyls are immediately consumed by the latter processes, so that their content can be assumed to be nearly zero. Thus, the amount of carbonyls measured is approximately equal to the amount of REGs available for labeling. This amount is smaller than the theoretical amount derived from Mn, as many reducing ends are converted by peeling/stopping-type processes and thus cannot be labeled. In addition, carbonyls and carboxyls of cellulose engage in reactions with ammonia as demonstrated by increasing nitrogen content.
Figure 3.
Mw (left) and carbonyl/carboxyl groups (right) of cotton linter cellulose after ammoxidation at different temperature and oxygen pressure.
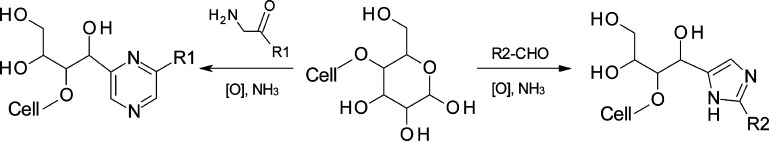
Thereby, fluorescent aromatic structures are formed which is indicated by a weak fluorescence signal of the unlabeled cellulose in SEC-MALLS. The formation of numerous poly(hydroxyalkyl) pyrazine or -imidazole derivatives from monomeric sugars suggests that similar structures can be formed at the reducing ends of cellulose or by reaction of other carbonyl groups along the cellulose chains (Figure 4).
Figure 4.
Proposed formation of N-heterocyclic moieties covalently attached to the reducing ends of cellulose: pyrazines from α-amino carbonyl compounds (left) and 1H-imidazoles from aldehydes (right).
Derivatization and GC/MS Analysis of the EtOAc Extracts
The ethyl acetate extracts were analyzed by GC/MS after per-trimethylsilylation, which is considered to be one of the most versatile derivatization methods.21−23 However, 1H-imidazoles, which turned out to be major ammoxidation products, are somewhat resistant to the initially employed silylation reagent N,O-bis(trimethylsilyl) trifluoroacetamide (BSTFA). In an attempt to optimize the yield of silylated reaction products, a test mixture consisting of 4-methyl-1H-imidazole, glycolic acid, glycine, urea, d-glucose, d-gluconic acid, glucosamine-hydrochloride, and 4-(d-arabino tetrahydroxybutyl)-1H-imidazole was silylated with a mixture of trimethylchlorosilane (TMS-Cl) and/or catalytic amounts of N,N-dimethyl-4-aminopyridine (DMAP) in BSTFA. While urea, carboxy- and hydroxy-functionalities were confirmed to give comparable yields with or without catalysts, the use of TMS-Cl alone increased significantly the yields of per-silylated glucosamine and glycine, and DMAP was especially effective in increasing the yields of 1H-imidazoles with free NH functionalities. A mixture of BSTFA containing 10% of TMCS and 1.5 g/L of DMAP performed best. Higher concentrations of any of the reagents did not further increase yields, but rather promoted side reactions, such as dehydration of silylated urea to N,N′-bis(trimethylsilyl) carbodiimide.
Ethyl acetate is considered to be a solvent that is well-suited for extracting compounds of different polarity from complex mixtures as its polarity index (PI 4.3) is almost exactly between those of water (PI 9.0) and the nonpolar alkanes (PI 0.1).24 The extracts obtained from the ammoxidation mixtures contained a large variety of products, which is shown in the chromatograms of the ethyl acetate extracts of ammoxidized (100 °C, 0.2 MPa O2, 3 h) d-glucose and d-xylose (Figure 2). The comparison of the gas chromatograms revealed many similarities in the product pattern of the EtOAc extracts of ammoxidized d-glucose and d-xylose, even though some of the constituents are present in significantly different amounts. Derivatives of 1H-imidazole, pyrazine, and pyridine that carry short side-chains such as methyl, hydroxymethyl, hydroxyl, or 1,2-dihydroxyethyl groups constitute the major fraction of extractives, with 4-methyl-1H-imidazole (5) and pyrazine-2-methanol (7) being the dominating compounds. Apart from N-heterocyclic compounds, a variety of rather hydrophilic compounds, such as glycol amide, urea and sugars, were also detected, albeit in smaller amounts. Pyrrole, furan and oxazole derivatives, which are known to be formed in Maillard-type reactions,25−28 were not detected. As such compounds are sensitive to oxidative polymerization reactions29,30 their incorporation into the polymeric melanoidin fraction under oxidative conditions is assumed.
Major differences in the peak pattern were found only for those constituents in which carbon skeleton and stereoconfiguration of the parent sugar molecule are partially preserved, such as the tetrahydroxybutyl- (24, 25) and trihydroxybutyl pyrazines (29), which each were only found in the glucose and xylose extracts, respectively, or the different dihydroxyethyl-5-methyl-pyrazine isomers (21).
The amounts of major N-heterocyclic compounds present in the ethyl acetate extracts, which are the most critical constituents with respect to ecotoxicity differ considerably for mono- and polysaccharides (Table 3). Whereas the total amounts of N-heterocyclic compounds reached 5 mg/g for cellulose and 16 mg/g for xylan, they were considerably higher for the monosaccharides (≥100 mg/g of educt).
Table 3. Amounts of Major N-Heterocyclic Compounds (mg/g of Educt) in the Ethyl Acetate Extracts of the Crude Products Obtained at Different Ammoxidation Temperature (0.2 MPa O2, 3 h).
| 70 °C | 100 °C | 140 °C | N-heterocyclic compound | 70 °C | 100 °C | 140 °C |
|---|---|---|---|---|---|---|
| Glucose | Cellulose | |||||
| 0 | 0 | 0 | 2-methyl-1H-imidazole | 0 | 0 | 0.16 |
| 1.2 | 11.9 | 0 | 1H-imidazole | 0.011 | 0.013 | 4.60 |
| 0.5 | 7.5 | 9.7 | 3-hydroxypyridine | 0.011 | 0.004 | 0.10 |
| 7.6 | 76.7 | 35.3 | 4-methyl-1H-imidazole | 0 | 0 | 0.59 |
| 6.1 | 67.4 | 65.0 | pyrazinesa | 0.008 | 0.006 | 0.07 |
| Xylose | Xylan | |||||
| not determined | 0 | 0 | 2-methyl-1H-imidazole | 0 | 0 | 0.33 |
| 33.2 | 1.9 | 1H-imidazole | 0 | 0.150 | 12.10 | |
| 7.5 | 13.1 | 3-hydroxypyridine | 0.004 | 0.132 | 0.95 | |
| 63.6 | 28.5 | 4-methyl-1H-imidazole | 0 | 0 | 1.81 | |
| 18.1 | 24.8 | pyrazinesa | 0.012 | 0.046 | 0.82 | |
Pyrazines: Sum of all found pyrazine derivatives.
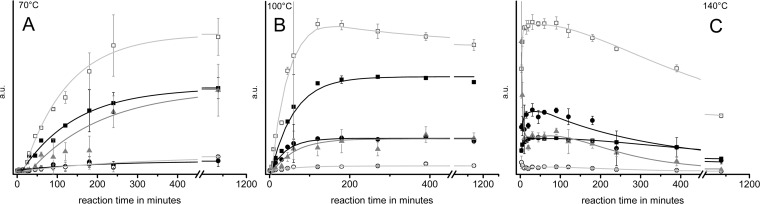
Ammoxidation temperature has a great impact on the amount of N-heterocyclic compounds formed. While for the studied polysaccharides the fraction of extractable nitrogenous products increased with temperature, there was a maximum of yield at 100 °C reaction temperature for the monosaccharides (d-glucose: 158 mg/g of educt, d-xylose: 122 mg/g of educt). Interestingly, the total amount of N-heterocyclic compounds decreased again for the monosaccharides when the temperature was increased from 100 to 140 °C, and additional compounds were formed. Furthermore, larger differences were found between the yields of individual N-heterocyclic compounds at higher temperature (100, 140 °C). 4-Methyl-1H-imidazole contributed the main share to the product spectrum from monosaccarides at all studied temperature levels. However, from the polysaccharides it was formed at high (140 °C) temperature only. 1H-Imidazole was formed in higher yields from xylose and xylan as compared to glucose and cellulose at all temperatures. The yields of 1H-imidazoles are highest at 100 °C. Below 100 °C, pyrazine derivatives were generally formed in minute amounts only. At higher temperatures, comparatively large amounts of pyrazines are formed from glucose, whereas xylose afforded only about one-third of the amount of glucose, and polysaccharides produced them only in traces.
Nitrogenous Constituents of the Crude Reaction Mixture
Besides the afore-discussed nitrogenous constituents of the ethyl acetate extracts, a fraction of more hydrophilic nitrogenous compounds were detected in the crude reaction mixtures. These are N-heterocyclic pyrazine- and 1H-imidazole derivatives carrying hydrophilic side chains, such as deoxyfructosazine and 4-(arabino-tetrahydroxybutyl)-1H-imidazole, aminosugars, such as glucose amine, fructose amine and di(glucopyranosyl)-amine. Surprisingly, some hydroxyamides (glycol- and lactamide) and amino acids (glycine, alanine, serine and 2-amino malonic acid) were also found. For glucose ammoxidized at 100 °C (0.2 MPa O2, 3 h), more than 60 water-soluble nitrogenous compounds were unambiguously confirmed to be present in the crude reaction mixture.18
Formation of N-Heterocyclic Compounds under Ammoxidative Conditions: Mechanistic and Kinetic Considerations
1H-Imidazoles
1H-Imidazoles are well-known products of the reaction of sugars with ammonia.10,26,31,32 However, no mass spectra of trimethylsilylated substituted 1H-imidazoles could be found in the literature. 4(5)-Methyl-, 4(5)-(hydroxymethyl)-, and 4(5)-(tetrahydroxybutyl)-1H-imidazole were therefore identified by comparison with authentic, trimethylsilylated samples. Further 1H-imidazole derivatives known to be formed under these conditions33−35 were tentatively assigned by the fragmentation pattern of their trimethylsilyl derivatives (Table 4). Per-trimethylsilylated polyhydroxyalkyl-1H-midazoles can be easily identified by their abundant M+ ion along with the characteristic m/z 241 fragment ion which is formed by scission of the alkyl chain in beta position to the imidazole ring.
Table 4. Relative Amount of 1H-Imidazole and Its Most Abundant Derivatives in the Crude Products Obtained from Ammoxidation of d-Glucose at 70 °C and 100 °C (0.2 MPa O2, 3 h)a.
| relative
percentage (%)a |
|||
|---|---|---|---|
| Rt (min) | compound | 70 °C | 100 °C |
| 12.32 | 1H-imidazole | 1.58 | 4.51 |
| 14.21 | 4-methyl-1H-imidazole | 0.00 | 43.07 |
| 20.47 | 4-(hydroxymethyl)-1H-imidazole | 67.42 | 46.45 |
| 28.38 | 4-(dihydroxyethyl)-1H-imidazole | 1.58 | 5.64 |
| 34.93 | 4-(2,3,4-trihydroxybutyl)-1H-imidazole | 6.09 | 44.87 |
| 35.41 | 4-(arabino-tetrahydroxybutyl)-1H-imidazole | 834.99 | 74.41 |
| 35.80 | 2-acetyl-4-(tetrahydroxybutyl)-1H-imidazole | 0.45 | 7.22 |
Values were calculated as ratio of the peak areas of the analytes relative to the internal standard phenylglucoside.
N-Silylated, 4-substituted 1H-imidazoles give rather broad peaks with the chosen weakly polar phase, which is useful to identify them within the chromatogram, but increases the detection limit. However, there is evidence from the mass spectra that the special peak shape is not only due to the particular retention behavior on the stationary phase but also due to the existence and virtual coelution of isomer pairs formed by N-trimethylsilylation of 4-substituted 1H-imidazole derivatives at either the N-1 or the N-3 position. Table 4 shows 4-(arabino-tetrahydroxybutyl)-1H-imidazole to be by far the most abundant imidazole derivative formed from d-glucose at 70 °C ammoxidation temperature, followed by 4-(hydroxymethyl)-1H-imidazole. At 100 °C, the total yield of 1H-imidazole derivatives decreased to less than one-third, and the spectrum of products changed considerably. While the amount of tetrahydroxybutyl-1H-imidazole dropped to less than 10%, the content of other 1H-imidazole derivatives such as 4-methyl-1H-imidazole, 4-(2,3,4-trihydroxybutyl)-1H-imidazole, or 2-acetyl-4-(tetrahydroxybutyl)-1H-imidazole increased significantly.
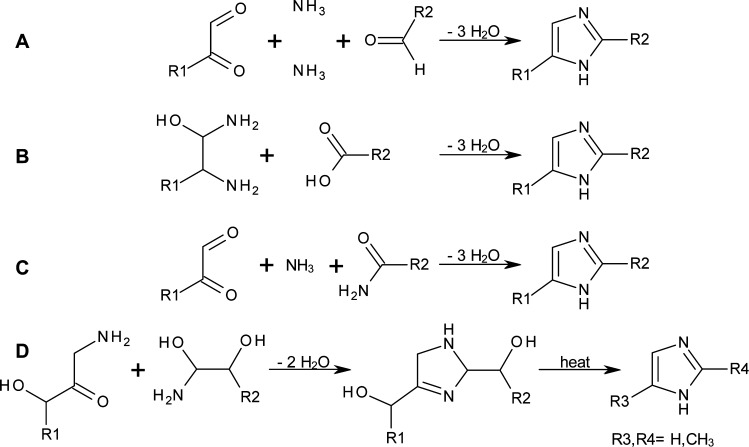
The obtained results provide strong evidence that the formation of 1H-imidazoles involves condensation of an α-dicarbonyl compound, two molecules of ammonia and a highly reactive aldehyde such as formaldehyde or methyl glyoxal (pathway A, Figure 5; Radziszewski reaction).36 Other mechanisms proposed such as condensation of α-amino hemiaminals with carboxylic acids (pathway B),26 reaction of α-dicarbonyl compounds with ammonia and an amide (pathway C)31 or condensation of a glycosyl amine and an amino sugar to a dihydroimidazole with two polyhydroxyalkyl side chains, followed by heat-induced scission of the side-chains (pathway D)10 are not likely either due to the alkaline pH which renders carboxylic acids less reactive, and/or the absence of 2-substituted 1H-imidazoles (R2 substituent in Figure 5) in the product mixtures.
Figure 5.
Main reaction pathways for the formation of 1H-imidazole derivatives from monosaccharides and ammonia (A,36B,26C,31D10). Pathway A is proposed to be the one prevailing under ammoxidation conditions.
Pyrazines
The TMS derivatives of poly(hydroxyalkyl) pyrazines afford readily discernible M+ and characteristic fragment ions, most importantly those produced by cleavage of the C–C bond in α–β position to the pyrazine ring.37 By thoroughly screening the chromatograms for these ions, many combinations of polyhydroxyalkyl and 1-deoxy-polyhydroxyalkyl side chains in the position 2 and 5 (or 2 and 6) of the pyrazine ring were identified. Two peaks with very similar mass spectra were found for all disubstituted pyrazines and assigned to the respective 2,5- and 2,6-disubstituted isomers. For fructosazine and 2-(tetrahydroxybutyl)-pyrazine, additional isomers were found which were assigned to diastereomers with a different configuration of the side chain.
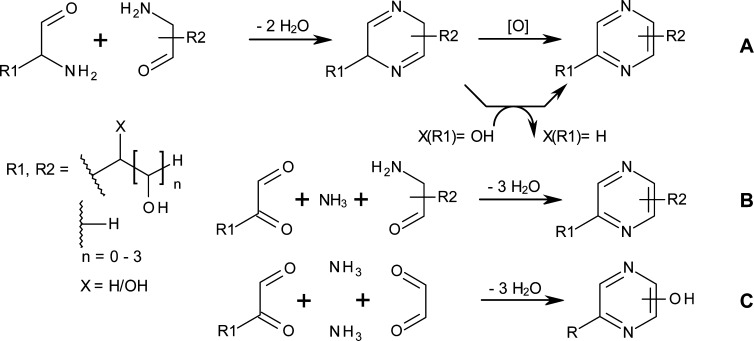
Pyrazines are formed by condensation of two molecules of α-amino carbonyl compounds,10 e.g. amino sugars, initially to a dihydropyrazine and subsequent aromatization to pyrazines. This aromatization step proceeds either through oxidation (pathway A, Figure 6), or elimination of a hydroxy group from the side-chain (pathway B, Figure 6).
Figure 6.
Main reaction pathways for the formation of pyrazine derivatives from two α-aminocarbonyl compounds (pathway A), condensation of amino sugars with α-dicarbonyl derivatives and ammonia (B), or formation of hydroxy-pyrazines by condensation of two α-dicarbonyl compounds and ammonia (C).
The greatest share of the obtained pyrazine derivatives contained a methyl or hydroxymethyl substituent (i.e., C1 substituent in Figure 6), which, according to the above mechanisms, should originate from a C3 fragment. Therefore, we conclude that the main route of glucose degradation comprises its conversion to fructose or 1-amino-1-deoxy-fructose, followed by retro-aldol scission into two C3 fragments. The minor importance of other scissions is reflected by the frequency of the occurrence of pyrazine substituent chain lengths which decreased in the order C1 > C0 > C2 > C4 > C3 for all studied temperatures.
Formation of 2-(tetrahydroxybutyl)-pyrazine derivatives carrying a (poly)hydroxyalkyl substituent of different chain length (n = 0–4) at 5 or 6 position of the heterocyclic ring occurred already at 70 °C ammoxidation temperature, however at a very slow rate (Figure 7). Much higher amounts were obtained at 100 °C. The rate of formation was proportional to the amount of sugars and amino sugars present in the reaction mixture. Prolonged reaction times, and in particular further elevation of the temperature (140 °C), promoted follow-up reactions and hence decreased the concentrations of the 2,5(6)-bis(polyhydroxyalkyl) pyrazines. The formation of 3-methyl-2,5-bis(polyhydroxyalkyl) pyrazines as found previously38 could not be confirmed to occur during ammoxidation. Besides 2,5- and 2,6- polyhydroxyalkyl pyrazines, pyrazin-2-ols were also found in the crude reaction products. For their formation see Figure 6, pathway C.
Figure 7.
Ammoxidation of d-glucose at 70 °C, 100 °C, and 140 °C: Kinetics of the formation of 2-(tetrahydroxybutyl)-pyrazine derivatives with different chain lengths of the substituents in 5(6)-position (isobar 0.2 MPa O2). Derivatives with same chain length are grouped together. Legend: solid black squares, C0; solid black circles, C1; solid gray triangles, C2; open white circles, C3; open white squares, C4.
To the best of our knowledge, this is the first time that this compound class is actually identified in reaction mixtures of sugars and ammonia, although their presence has been hypothesized.39 2-Hydroxypyrazine itself was identified by comparison with an authentic sample, while peak 78 (Figure 1) was tentatively assigned to 2-hydroxy-5(6)-tetrahydroxybutyl pyrazine, based on the mass fragmentation pattern. MS data suggested the presence of other (polyhydroxyalkyl)pyrazinols, however, the respective peaks were too small or too overlapped for unambiguous assignments.
Pyridinols
Pyridinols have long been known as reaction products of glucose and ammonia.40,41 They constitute only a very small fraction of the crude products, but their formation is highly undesirable due to their considerable ecotoxicity, as observed in the conducted OECD 201 screening tests. 3-Hydroxypyridine is the most abundant pyridinol being present in the ammoxidation products of all studied mono- and polysaccharides, with its yield strongly increasing with reaction temperature (Table 3). Furfural is usually considered to be precursor of pyridinols.40,42,43 However, the observed formation of the latter under alkaline conditions and at low temperature (70 °C) points toward a different pathway of formation.
Phytotoxicity Testing
Unicellular algae, such as the fresh-water alga Pseudokirchneriella subcapitata (OECD 201 test) respond with growth inhibition to a large variety of nonspecific toxic substances and are thus widely used as reliable bioindicators for a first phytotoxicity (ecotoxicity) screening of water-soluble chemicals or environmental samples.
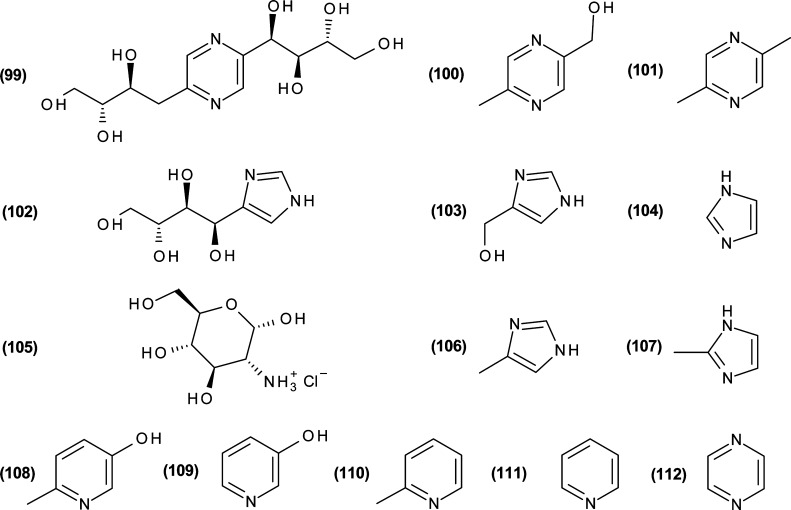
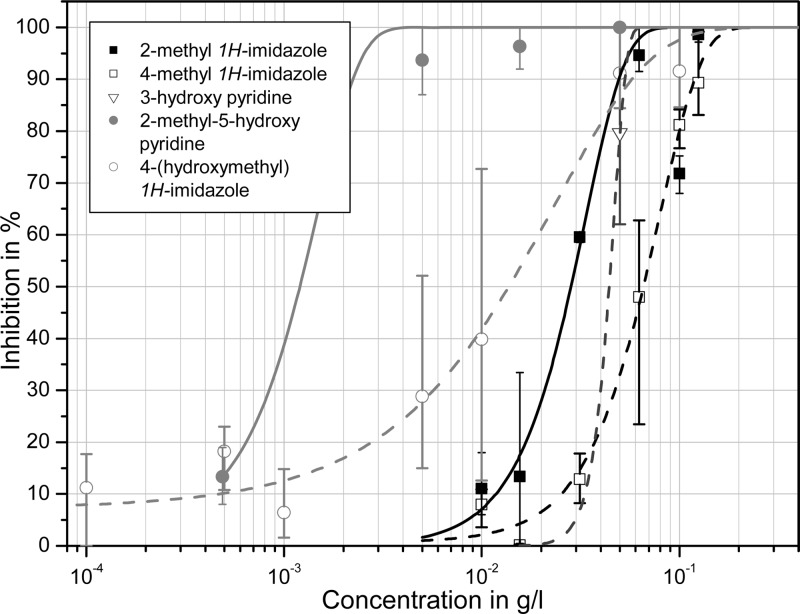
Besides the crude reaction mixtures and the ethyl acetate extracts, phytotoxicity screening was focused on a set of target substances that was compiled based on the analytical results of this study and literature data (Figure 8). Low-molecular, moderately lipophilic N-heterocyclic compounds were in the main focus of the study as many of them are known to exhibit considerable bioactivity.44−47 Hence, two representative hydroxalkyl substituted derivatives were chosen as representatives for each of the two main classes of N-heterocyclic compounds formed by ammoxidation of carbohydrates, that is, 1H-imidazoles and pyrazines: that with the shortest possible side chain (1H-imidazol-4-yl-methanol (103) and 5-methylpyrazin-2-yl-methanol (100), respectively) and that with the longest one (4-(d-arabino-tetrahydroxybutyl)-1H-imidazole (102) and deoxy-fructosazine (103), respectively). The set was complemented by 1H-imidazole (104), 2-methyl-1H-imidazole (107), 4-methyl-1H-imidazole (108), pyrazine (112), 2,5-dimethylpyrazine (101), pyridine (111), and its derivatives 3-pyridinol (109), 6-methyl-3-pyridinol (108), and α-picoline (110). Glucosamine (105) as one of the very first reaction products of glucose with ammonia was also included, as it is known to have phytotoxic effects on corn and barley.48 Sugars, ammonia salts of carboxylic acids (e.g., aldonic acids, deoxy-aldonic acids), and amino acids on the other hand were not considered in this study, as many of them are widespread in nature and usually are not considered to be phytotoxic. The OECD tests revealed that none of the tested pyrazine derivatives had a relevant growth-inhibiting effect toward the unicellular microalgae Pseudokirchneriella subcapitata as all EC50 values were beyond 1 g/L. The toxicity of 1H-imidazoles was confirmed to be much higher as for the pyrazines with 4-(hydroxymethyl)-1H-imidazole (EC50 18 mg/L, Table 5), 2-methyl-1H-imidazole (EC50 28 mg/L), and 4-methyl-1H-imidazole (EC50 66 mg/L) being about 1 order of magnitude more toxic than nonsubstituted 1H-imidazole (EC50 500 mg/L). Among the pyridine derivatives, the nonsubstituted N-heteroaromatic compound showed the highest algae growth-inhibiting response (EC50 0.9 mg/L) followed by 3-hydroxy-6-methyl-pyridine (EC50 1.2 mg/L) and 3-hydroxypyridine (EC50 44 mg/L).
Figure 8.
Target substances for the conducted ecotoxicity tests according to OECD 201.
Table 5. EC50 Values of Selected N-Heterocyclic Compounds As Obtained from the Phytotoxicity Screening According to OECD 201.
| N-heterocyclic compound | EC50 value (mg/L) | ethyl acetate extract of ammoxidized samples | EC50 value (mg/L) |
|---|---|---|---|
| pyridine | 0.9 | d-xylose (100 °C) | 45 |
| 3-hydroxy-6-methyl-pyridine | 1.2 | d-glucose (100 °C) | 62 |
| 4-(hydroxymethyl)-1H-imidazole | 18 | d-glucose (140 °C) | 160 |
| 2-methyl-1H-imidazole | 28 | ||
| 3-hydroxy-pyridine | 44 | ||
| 4-methyl-1H-imidazole | 66 | ||
| 1H-imidazole | 500 |
2,5-Dimethylpyrazine, 4-tetrahydroxybutyl-1H-imidazole, 2-hydroxymethyl-5-methylpyrazine, glucosamine hydrochloride, deoxy-fructosazine, pyrazine, and α-picoline were found to have EC values higher than 1 g/L and were therefore considered not to be relevant from the ecotoxicological point of view. The gentle slope of the 4-(hydroxymethyl)-1H-imidazole inhibition curve may be a hint for several modes of action for this compound (Figure 9).
Figure 9.
Dose–response relationships for different N-heterocyclic compounds as obtained from freshwater algae test according to OECD 201.
All phytotoxicologically active compounds share a medium lipophilicity and basic functionalities with medium basicity of pKB = 6.5–9.0. In principle, this enables them to cross biological membranes easily, and to accumulate in more acidic cell compartments, such as chloroplasts. The less basic (pyrazine) and the more hydrophilic (4-tetrahydroxybutyl-1H-imidazole) do not show any toxic response.
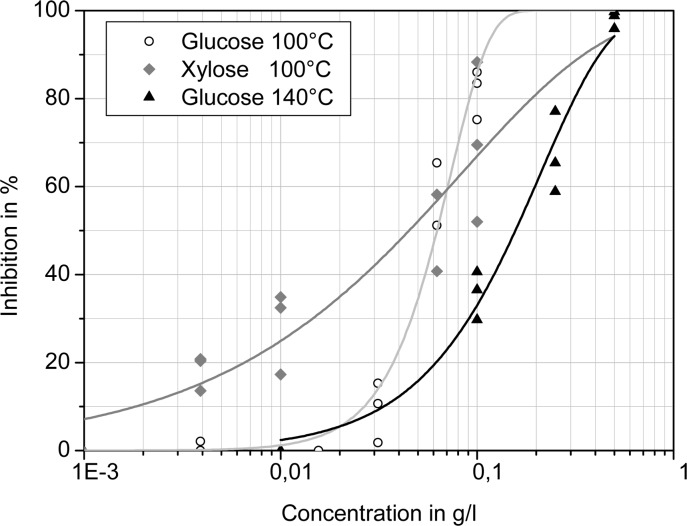
The ethyl acetate extracts of reaction mixtures obtained from ammoxidation of glucose and xylose at 100 and 140 °C, respectively, were shown to have EC50 values similar to that of their main nitrogenous constituent, 4-methyl-1H-imidazole (Table 5). The xylose extract seems to be slightly more bioactive than the glucose extract. The shallow slope of the sigmoidal dose/response curve, which is especially pronounced for the xylose sample, is likely caused by the multitude of different components of the extract (Figure 10). The extract of the glucose ammoxidation at 140 °C is significantly less active than the 100 °C extract, which coincides with the lower percentage of the more toxic imidazoles and higher percentage of less toxic pyrazines formed at higher reaction temperatures. For all ammoxidation batches conducted with the polysaccharides as starting materials, and for all crude products obtained from the monosaccharide at 70 °C reaction temperature, the amount of organically extractable compounds was too low for meaningful phytotoxicity screening.
Figure 10.
Dose–response relationships (OECD 201 test) for selected ethyl acetate extracts of the crude reaction products obtained after ammoxidation of d-glucose and d-xylose.
In contrast to the organic phases, the crude ammoxidation mixtures were demonstrated to exhibit low phytotoxicity, which approximately corresponds to their content of 4-methyl-1H-imidazole. The crude reaction mixtures of both cellulose and xylan, as well as those of glucose and xylose subjected to ammoxidation at 70 °C did not show any inhibition.
The phytotoxicity tests of the ammoxidized samples and their extracts demonstrated that acute phytotoxicity must only be expected from lignocellulosic materials with a high monosaccharide content ammoxidized at temperatures >70 °C. By appropriately choosing reaction temperature and/or a pretreatment procedure capable of largely reducing the monosaccharide content, lignocellulosic biomass can be converted into nitrogen-rich (soil improving) materials without formation of phytotoxic substances.
Supporting Information Available
Synthesis of the authentic samples (5-methyl-pyrazin-2-yl)methanol, 2-(arabino-tetrahydroxybutyl)-5-(erythro-2,3,4-trihydroxybutyl)-pyrazine, and 4-(arabino-tetrahydroxybutyl)-imidazole. EI-MS (70 eV) fragmentation pattern of per-silylated synthesized and commercial N-heterocyclic compounds. Peak numbers, retention times, and peak assignment for all nitrogenous compounds detected in the ethyl acetate extracts and crude ammoxidation products of d-glucose and d-xylose (Table S118). Nitrogen contents of the water-soluble and water-insoluble phases obtained by ammoxidation (3 h) of cellulose, xylan, d-glucose, and d-xylose (Table S2). Ammoxidation (0.2 MPa O2, 3 h) of d-glucose: temperature-dependent formation of 2-(1,2,3,4-tetrahydroxybutyl)-pyrazine derivatives carrying a (poly)hydroxyalkyl substituent of different chain length (n = 0–4) at 5 or 6 position of the heterocyclic ring (Table S3). Growth inhibition of Pseudokirchneriella subcapitata at 0.5 g/L sample concentration for ammoxidized (0.2 MPa O2, 3 h) d-glucose and d-xylose (Table S4). pH of ammoxidation mixtures after 3 h of reaction time (5% NH3, 0.2 MPa O2, 50 g/L substrate; Table S5). EI-MS (70 eV) spectrum and suggested structure of peak 78 (penta(TMS)-2-hydroxy-5(6)-tetrahydroxybutyl pyrazine; Figure S1). This material is available free of charge via the Internet at http://pubs.acs.org.
The financial support by the Christian Doppler Research Society through the CD-laboratory for “Advanced Cellulose Chemistry and Analytics”, the Austrian Research Fund FWF (I154-N19), and the Austrian Research Promotion Agency FFG (834297 ENLIGMA) is gratefully acknowledged.
The authors declare no competing financial interest.
Supplementary Material
References
- Franz A.; Palm A.. Verfahren zur Herstellung organischer Düngemittel. Deutsches Patent DE561487, 1930.
- Flaig W.; Beutelspacher H.; Rietz E. In Chemical Composition and Physical Properties of Humic Substances; Springer, 1975; pp 1–211. [Google Scholar]
- Fischer K.; Liebner F.; Schiene R.; Katzur H. J. N-modified lignin - highly valuable humus substitute and long-lasting fertilizer. Freiberg. Forschungsh. A 2002, A866, 79–88. [Google Scholar]
- Ramirez F.; Gonzalez V.; Crespo M.; Meier D.; Faix O.; Zuniga V. Ammoxidized kraft lignin as a slow-release fertilizer tested on Sorghum vulgare. Bioresour. Technol. 1997, 61, 43–46. [Google Scholar]
- Meier D.; Zuniga-Partida V.; Ramirez-Cano F.; Hahn N.-C.; Faix O. Conversion of technical lignins into slow-release nitrogenous fertilizers by ammoxidation in liquid phase. Bioresour. Technol. 1994, 49, 121–8. [Google Scholar]
- Flaig W. Slow releasing nitrogen fertilizer from the waste product, lignin sulfonates. Chem. Ind. (London) 1973, 553–4. [Google Scholar]
- Schulten H. R.; Schnitzer M. The chemistry of soil organic nitrogen: A review. Biol. Fertil. Soils 1997, 26, 1–15. [Google Scholar]
- Fischer K.; Schiene R., Nitrogenous fertilizers from lignins. In Chemical Modification, Properties and Usage of Lignin; Hu T., Ed.; Kluwer Academics/Plenum Publishers: New York, 2002; pp 167–198. [Google Scholar]
- Knill C. J.; Kennedy J. F. Degradation of cellulose under alkaline conditions. Carbohydr. Polym. 2002, 51, 281–300. [Google Scholar]
- Kort M. J. Reactions of free sugars with aqueous ammonia. Adv. Carbohydr. Chem. 1970, 25, 311–349. [Google Scholar]
- Fay L. B.; Brevard H. Contribution of mass spectrometry to the study of the Maillard reaction in food. Mass Spectrom. Rev. 2005, 24, 487–507. [DOI] [PubMed] [Google Scholar]
- Yang B.; Wyman C. E. Pretreatment: The key to unlocking low-cost cellulosic ethanol. Biofuels, Bioprod. Biorefin. 2008, 2, 26–40. [Google Scholar]
- Iovel I.; Goldberg Y.; Shymanska M. An improved synthesis of 5-methylpyrazine-2-carboxylic acid. Org. Prep. Proc. Int. 1991, 23, 188–190. [Google Scholar]
- Rohovec J.; Kotek J.; Peters J. A.; Maschmeyer T. A clean conversion of d-glucosamine hydrochloride to a pyrazine in the presence of phenylboronate or borate. Eur. J. Org. Chem. 2001, 3899–3901. [Google Scholar]
- Streith J.; Boiron A.; Frankowski A.; Le N. D.; Rudyk H.; Tschamber T. On the way to glycoprocessing inhibitors: A general one-pot synthesis of imidazolosugars. Synthesis 1995, 944–6. [Google Scholar]
- Potthast A.; Röhrling J.; Rosenau T.; Borgards A.; Sixta H.; Kosma P. A novel method for the determination of carbonyl groups in cellulosics by fluorescence labeling. 3. Monitoring oxidative processes. Biomacromolecules 2003, 4, 743–749. [DOI] [PubMed] [Google Scholar]
- Bohrn R.; Potthast A.; Schiehser S.; Rosenau T.; Sixta H.; Kosma P. The FDAM Method: Determination of carboxyl profiles in cellulosic materials by combining group-selective fluorescence labeling with GPC. Biomacromolecules 2006, 7, 1743–1750. [DOI] [PubMed] [Google Scholar]
- Klinger K. M.; Liebner F.; Hosoya T.; Potthast A.; Rosenau T.. Formation of non-heterocyclic nitrogenous compounds upon ammoxidation of monosaccharides. J. Agric. Food Chem. 2013, in press [DOI] [PMC free article] [PubMed]
- Rizzi G. P. chemical structure of colored Maillard reaction products. Food Rev. Int. 1997, 13, 1–28. [Google Scholar]
- Dross A.; Baltes W. The fractionation of caramel ingredients according to their molecular weights. Z. Lebensm.-Unters. Forsch. 1989, 188, 540–4. [Google Scholar]
- Ruiz-Matute A. I.; Hernández-Hernández O.; Rodríguez-Sánchez S.; Sanz M. L.; Martínez-Castro I. Derivatization of carbohydrates for GC and GC–MS analyses. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 2011, 879, 1226–1240. [DOI] [PubMed] [Google Scholar]
- Kataoka H. Derivatization reactions for the determination of amines by gas chromatography and their applications in environmental analysis. J. Chromatogr., A 1996, 733, 19–34. [DOI] [PubMed] [Google Scholar]
- Blau K. H., John, Handbook of Derivatives for Chromatography; John Wiley & Sons Ltd: New York, 1993. [Google Scholar]
- Barwick V. J. Strategies for solvent selection—A literature review. TrAC, Trends Anal. Chem. 1997, 16, 293–309. [Google Scholar]
- Olsson K.; Pernemalm P. A.; Theander O. Formation of aromatic compounds from carbohydrates. VII. Reaction of d-glucose and glycine in slightly acidic, aqueous solution. Acta Chem. Scand., Ser. B 1978, B32, 249–56. [Google Scholar]
- Shibamoto T.; Bernhard R. A. Formation of heterocyclic compounds from the reaction of l-rhamnose with ammonia. J. Agric. Food Chem. 1978, 26, 183–7. [Google Scholar]
- Tressl R.; Kersten E.; Rewicki D. Formation of pyrroles, 2-pyrrolidones, and pyridones by heating of 4-aminobutyric acid and reducing sugars. J. Agric. Food Chem. 1993, 41, 2125–2130. [Google Scholar]
- Yaylayan V. A.; Keyhani A. Elucidation of the mechanism of pyrrole formation during thermal degradation of 13C-labeled l-serines. Food Chem. 2001, 74, 1–9. [Google Scholar]
- Zamora R.; Alaiz M.; Hidalgo F. J. Contribution of pyrrole formation and polymerization to the nonenzymatic browning produced by amino-carbonyl reactions. J. Agric. Food Chem. 2000, 48, 3152–3158. [DOI] [PubMed] [Google Scholar]
- Eiserich J. P.; Macku C.; Shibamoto T. Volatile antioxidants formed from an l-cysteine/d-glucose Maillard model system. J. Agric. Food Chem. 1992, 40, 1982–8. [Google Scholar]
- Moon J.-K.; Shibamoto T. Formation of carcinogenic 4(5)-methylimidazole in maillard reaction systems. J. Agric. Food Chem. 2011, 59, 615–618. [DOI] [PubMed] [Google Scholar]
- Fernandes J. O.; Ferreira M. A. Gas chromatographic-mass spectrometric determination of 4-(5) methylimidazole in ammonia caramel colour using ion-pair extraction and derivatization with isobutylchloroformate. J. Chromatogr., A 1997, 786, 299–308. [Google Scholar]
- Fujii S.; Tsuchida H.; Komoto M. Reaction products of d-glucose and ammonia. X. Isolation and identification of 4(5)-(dl-glycero-2,3-dihydroxypropyl)imidazole. Agric. Biol. Chem. 1966, 30, 73–7. [Google Scholar]
- Klejdus B.; Moravcová J.; Lojková L.; Vacek J.; Kubáň V. Solid-phase extraction of 4(5)-methylimidazole (4Mel) and 2-acetyl-4(5)-(1,2,3,4-tetrahydroxybutyl)-imidazole (THI) from foods and beverages with subsequent liquid chromatographic-electrospray mass spectrometric quantification. J. Sep. Sci. 2006, 29, 378–384. [DOI] [PubMed] [Google Scholar]
- Kroeplien U. R., J.; Van der Greef; Long J.; Robert C. Jr.; Goldstein J. H. 2-Acetyl-4(5)-(1,2,3,4-tetrahydroxybutyl)imidazole: Detection in commercial caramel color III and preparation by a model browning reaction. J. Org. Chem. 1985, 50, 1131–1133. [Google Scholar]
- Yaylayan V. A.; Haffenden L. J. W. Mechanism of imidazole and oxazole formation in [13C-2]-labelled glycine and alanine model systems. Food Chem. 2003, 81, 403–409. [Google Scholar]
- Tsuchida H.; Kitamura K.; Komoto M.; Akomori N. Gas-liquid chromatography and mass spectrometry of trimethylsilyl ethers and butaneboronate-trimethyl-silyl derivatives of polyhydroxyalkylpyrazines. Carbohydr. Res. 1978, 67, 549–563. [Google Scholar]
- Tsuchida H.; Komoto M.; Kato H.; Fujimaki M. Formation of deoxy fructosazine and its 6 isomer on the browning reaction between glucose and ammonia in weak acidic medium. Agric. Biol. Chem. 1973, 37, 2571–2578. [Google Scholar]
- Shibamoto T.; Bernhard R. A. Investigation of pyrazine formation pathways in sugar-ammonia model systems. J. Agric. Food Chem. 1977, 25, 609–614. [Google Scholar]
- Aso K. Formation of β-hydroxypyridine derivatives from hexoses and ammonium salts. I. Bull. Agric. Chem. Soc. Jpn. 1939, 15, 107–8. [Google Scholar]
- Aso K. Formation of β-hydroxypyridine derivatives from hexose and ammonium salts. II. 2-Hydroxymethyl-5-hydroxypyridine. Nippon Nogei Kagaku Kaishi 1940, 16, 249–52. [Google Scholar]
- Müller C.; Diehl V.; Lichtenthaler F. W. Building blocks from sugars. Part 23. Hydrophilic 3-pyridinols from fructose and isomaltulose. Tetrahedron 1998, 54, 10703–10712. [Google Scholar]
- Tsuchida H.; Komoto M.; Kato H.; Fujimaki M. Melanoidin produced from the reaction of glucose and ammonia. III. Isolation and identification of two α-hydroxypyridine derivatives from the nondialyzable melanoidin hydrolyzate. Agric. Biol. Chem. 1973, 37, 403–9. [Google Scholar]
- Panel on food additives and nutrient sources added to food (ANS), scientific opinion on the re-evaluation of caramel colours (E 150 a,b,c,d) as food additives. EFSA J. 2011, 9, 103. [Google Scholar]
- Doležal M.; Kráĺová K. n., Synthesis and evaluation of pyrazine derivatives with herbicidal activity. In Herbicides, Theory and Applications; Soloneski S., Larramendy M. L., Eds.; InTech, 2011. [Google Scholar]
- Chan P. C.; Hills G. D.; Kissling G. E.; Nyska A. Toxicity and carcinogenicity studies of 4-methylimidazole in F344/N rats and B6C3F1 mice. Arch. Toxicol. 2008, 82, 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleeker E. A.; Wiegman S.; de Voogt P.; Kraak M.; Leslie H. A.; de Haas E.; Admiraal W. Toxicity of azaarenes. Rev. Environ. Contam. Toxicol. 2002, 173, 39–83. [PubMed] [Google Scholar]
- Roberts R. M.; Heishman A.; Wicklin C. Growth inhibition and metabolite pool levels in plant tissues fed d-glucosamine and d-galactose. Plant Physiol. 1971, 48, 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.