Abstract
Background
Recent results from the ACOSOG Z0011 trial question the use of intraoperative frozen section (FS) during sentinel lymph node (SLN) biopsy and the role of axillary dissection (ALND) for SLN-positive breast cancer patients. Here we present a 10-year trend analysis of SLN-FS and ALND in our practice.
Methods
We reviewed our prospective SLN database over 10 years (1997–2006, 7509 SLN procedures) for time trends and variation between surgeons in the use of SLN-FS and ALND in patients with cN0 invasive breast cancer.
Results
Use of SLN-FS decreased from 100% to 62% (P < 0.0001) and varied widely by surgeon (66% to 95%). There were no statistically significant trends in the performance of ALND for patients with SLN metastases detected by FS (n = 1370, 99–99%) or routine hematoxylin and eosin (H&E) (n = 333; 69–77%), but only for those detected by serial section H&E with or without immunohistochemistry (n = 438; 73–48%; P = 0.0054) or immunohistochemistry only (n = 294; 48–28%; P < 0.0001). These trends coincided with an increase in the proportion of completion versus immediate ALND (30–40%; P = 0.0710).
Conclusions
Over 10 years, we have observed a diminishing rate of SLN-FS and, for patients with low-volume SLN metastases, fewer ALND, trends that suggest a more nuanced approach to axillary management. If the Z0011 selection criteria had been applied to our cohort, 66% of SLN-FS (4159 of 6327) and 48% of ALND (939 of 1953) would have been avoided, sparing 13% of all patients the morbidity of ALND.
Sentinel lymph node (SLN) biopsy is well established as standard care for patients with clinically node-negative invasive breast cancer. Sixty-nine observational studies and 5 randomized trials have established that SLN biopsy is feasible, accurate, safe, and (compared to axillary lymph node dissection [ALND]) less morbid.1–6 Because ALND is usually recommended for patients with positive SLN, intraoperative assessment of SLN (by frozen section [FS], imprint cytology, or cytologic smear) allows an immediate ALND, avoiding reoperation. Accordingly, our initial practice of SLN biopsy included intraoperative FS followed by immediate ALND if the FS was positive, or reoperative ALND if the SLN was positive by permanent sections.7
Over the past decade, this practice has changed for several reasons. First, we have observed that FS is reasonably sensitive (identifying about 60% of positive SLN), but the yield is low (only 20% of all FS procedures are positive), and even lower for certain low-risk patients.8 Second, we have found no performance advantage for other methods (imprint cytology or cytologic smear) compared to FS.9 Third, we have found that FS-positive patients who had immediate ALND were not consistently spared a reoperation: more than half required further surgery for positive margins after attempted breast-conserving surgery.10 Fourth, we have developed a multivariate nomogram to identify SLN-positive patients at low risk of having additional axillary node metastases.11 As a result of these changes, our rate of ALND has declined for patients with SLN negative on FS but positive by permanent pathology, and we have observed very low rates of axillary local recurrence in SLN-positive patients who did not have ALND.12, 13
Recently, both a large retrospective study from the National Cancer Database and the prospective randomized ACOSOG Z0011 trial (of ALND vs. no further surgery for SLN-positive patients) have reported no differences in the rate of local or distant relapse between patients who had ALND and those who did not.14–16 The ACOSOG trial included patients with hematoxylin and eosin (H&E)-detected macrometastases, suggesting that further modifications to the current policy of FS and immediate or completion axillary dissection may be appropriate. In light of these observations, we examined our trends in the use of SLN-FS and ALND over a 10-year period to ask whether these practices are becoming obsolete.
METHODS
Between May 1997 and December 2006, we performed 10,195 consecutive SLN biopsy procedures on clinically node-negative patients with invasive breast cancer at Memorial Sloan-Kettering Cancer Center (MSKCC), all of whom were entered prospectively in the Breast Service SLN database. We retrospectively reviewed their records under a waiver of authorization from our institutional review board.
We excluded 2607 procedures, including those with a planned backup ALND, immediate ALND performed for gross nodal disease, prior ipsilateral SLN biopsy or ALND, failed mapping, neoadjuvant chemotherapy, nonaxillary SLN, inflammatory cancer, or breast cancer in men. In addition, we excluded 79 procedures performed early in our experience by 3 low-volume surgeons who had all retired by 1999. Our study group comprised the remaining 7302 patients, in whom 7509 SLN biopsy procedures were performed by 11 surgeons. We report results on a per-SLN procedure, not per-patient, basis. The years 1997 and 1998 were pooled as a result of the small number of procedures in 1997, the first year of the study (n = 168, 2.2% of all procedures).
We have previously reported in detail our methods for SLN biopsy and intraoperative FS.8, 9, 17 In brief, we used a combined dye-isotope mapping technique and removed all blue, “hot,” or palpably suspicious nodes. Intraoperative FS was done at the discretion of the surgeon. In general, a portion of the SLN (or, if small, the entire node) was frozen, examined by a single H&E-stained section, and categorized as “frozen section.” The remaining frozen tissue (i.e., “frozen section control”) was thawed, processed routinely, examined as a single permanent H&E-stained section, and categorized as “routine H&E.” The remainder of the node was examined by taking 2 adjacent sections from each of 2 levels 50 μm apart, with 1 section at each level stained with H&E and categorized as “H&E serial sections.” The remaining section at each level was stained by immunohistochemistry (IHC) for cytokeratin and categorized as “IHC only.” Non-SLN (including those in ALND specimens) were examined by routine single-section H&E. ALND was defined on the basis of surgeon intent, not number of nodes removed.
We used the MSKCC nomogram to estimate the risk of additional non-SLN metastases in SLN-positive patients who did not have ALND.11 Local recurrence was defined as any ipsilateral breast or chest wall recurrence. Regional recurrence was defined as recurrence in the axillary, supraclavicular, or internal mammary nodes. All events were validated independently by 2 members of the study team. Median follow-up was 59 months (range, 6–150 months).
We used Cochran-Armitage tests to assess trends over time, Wilcoxon rank sum tests for differences in continuous variables, and Fisher’s exact test for differences in categorical variables. We used generalized estimating equations to examine time trends adjusted for surgeon variability. Adjusted results were similar and are therefore not reported. All statistical analyses were performed by SAS software, version 9.2 (SAS Institute, Cary, NC).
RESULTS
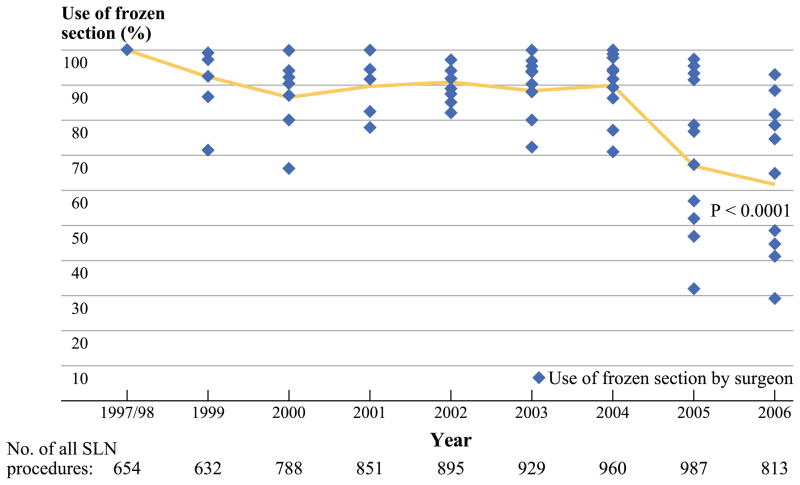
The final study population consisted of 7509 SLN biopsy procedures for clinically node-negative invasive breast cancer. FS was done in 84% overall, but varied widely by surgeon, ranging from 66 to 95%. Use of FS decreased from 100% in 1997–1998 to 62% in 2006 (Fig. 1, P < 0.0001). The decrease in the use of FS over time, however, varied widely among the surgeons across the service. For example, although one surgeon decreased his use of FS only from 100 to 89% during the study period, another surgeon decreased her use from 100 to 29% during the same time. Patients in the FS group (compared to the no-FS patients, Table 1) were younger, were more likely to undergo mastectomy, were more likely to have larger tumors (more often of invasive ductal type), and had higher grade disease, lymphovascular invasion (LVI), and positive SLN (35% vs. 18%, P < 0.001 for all).
FIG. 1.
Use of SLN FS by year and surgeon
TABLE 1.
Clinicopathologic characteristics for 7509 SLN procedures in 7302 patients, by use of FS
| Characteristic | SLN/no FS (n = 1182), n (%) | SLN/FS (n = 6327), n (%) | P valuea |
|---|---|---|---|
| Median age, year (range) | 59 (12–90) | 56 (19–91) | < 0.001 |
| Operation | < 0.001 | ||
| Breast conservation | 857 (72.6) | 4227 (66.9) | |
| Mastectomy | 323 (27.4) | 2093 (33.1) | |
| Missing | 2 | 7 | |
| Median tumor size, cm (range) | 0.7 (0.1–5.0) | 1.3 (0.1–17.0) | < 0.001 |
| Tumor size | < 0.001 | ||
| T1 (≤2 cm) | 1084 (93.1) | 4893 (78.9) | |
| T2 (2–5 cm) | 80 (6.9) | 1250 (20.2) | |
| T3 (> 5 cm) | 0 (0.0) | 59 (1.0) | |
| Tx (unknown) | 18 | 125 | |
| Histologic grade | < 0.001 | ||
| I | 123 (13.8) | 391 (7.3) | |
| II | 281 (31.5) | 1575 (29.6) | |
| III | 489 (54.8) | 3362 (63.1) | |
| Unknown | 289 | 999 | |
| Nuclear grade | < 0.001 | ||
| I | 100 (11.9) | 329 (6.4) | |
| II | 497 (59.2) | 2832 (55.0) | |
| III | 243 (28.9) | 1986 (38.6) | |
| Unknown | 342 | 1180 | |
| Positive SLN | 216 (18.3) | 2219 (35.1) | < 0.001 |
| LVI | |||
| Present | 161 (14.3) | 1554 (24.9) | < 0.001 |
| Missing | 60 | 84 | |
| Multicentric/multifocal tumor | |||
| Present | 280 (23.7) | 1502 (23.7) | 1.000 |
| Missing | 1 | 2 | |
| ER positive | |||
| Present | 852 (81.5) | 4584 (80.2) | 0.330 |
| Missing | 137 | 611 | |
| PR positive | |||
| Present | 621 (59.8) | 3395 (59.7) | 1.000 |
| Missing | 143 | 643 | |
| Tumor type | < 0.001b | ||
| Invasive ductal | 907 (76.8) | 5323 (84.2) | |
| Invasive lobular | 96 (8.1) | 684 (10.8) | |
| Microinvasive DCIS | 148 (12.5) | 96 (1.5) | |
| Other invasive carcinomas | 11 (0.9) | 94 (1.5) | |
| Invasive carcinoma with ductal and lobular features | 19 (1.6) | 128 (2.0) | |
| Missing | 1 | 2 | |
| Median year of surgery (range) | 2005 (1997–2006) | 2002 (1997–2006) | < 0.001 |
SLN sentinel lymph node, FS frozen section, LVI lymphovascular invasion, ER estrogen receptor, PR progesterone receptor, DCIS ductal carcinoma in situ
All P values are based on Fisher’s exact test (categorical covariates) or Wilcoxon rank sum test (continuous covariates), except where otherwise indicated
This P value is based on Pearson’s chi-square test
ALND was performed in 1953 of all 2435 positive SLN biopsy procedures (80%). The frequency of ALND across all SLN-positive patients decreased modestly, from 84% in 1997–1998 to 77% in 2006 (P < 0.0001), and the proportion of completion versus immediate ALND increased from 30 to 40% (P = 0.07). As for FS, ALND patients were younger, were more likely to undergo mastectomy, and were more likely to have tumors of invasive ductal type, larger size, higher grade, greater multifocality/multicentricity, and more LVI (P < 0.001 for all except multifocality/multicentricity; P = 0.002).
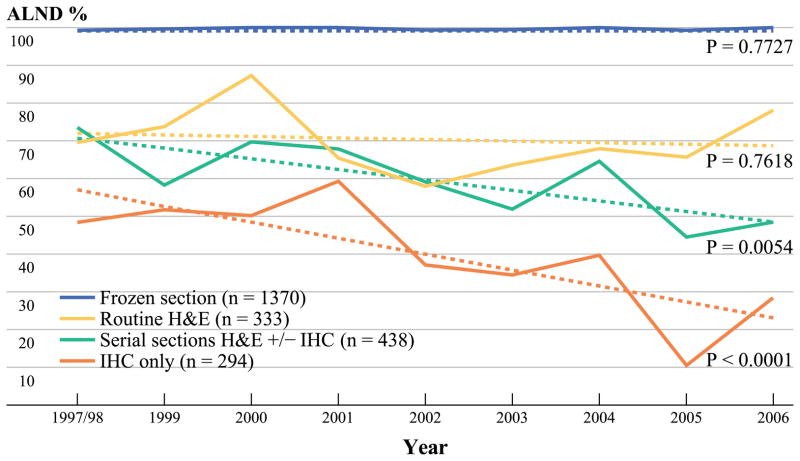
There was no significant downward trend over time (Fig. 2) in the performance of ALND for SLN metastases detected by FS (99–99%, P = 0.77) or routine H&E (69–77%; P = 0.76). In contrast, for 438 positive SLN procedures with metastases detected by serial section H&E, the rate of ALND decreased from 73 to 48% (P = 0.0054), and for 294 metastases detected only by IHC from 48 to 28% (P < 0.0001).
FIG. 2.
Proportion of all positive SLN having ALND by method of detection
When SLN metastases were detected by routine H&E (and FS was either negative or not done), 69% (231 of 333) had ALND. This rate varied widely by surgeon, from 62 to 78%, and performance of ALND was associated with younger age, more LVI, and higher MSKCC nomogram scores (Table 2). Among the ALND patients, 35% had simultaneous breast surgery for margin clearance.
TABLE 2.
Clinicopathologic characteristics for 333 SLN procedures positive by routine H&E, by performance of ALND
| Characteristic | SLN+/no ALND (n = 102), n (%) | SLN+/ALND (n = 231), n (%) | P value |
|---|---|---|---|
| Median age, year (range) | 60 (33–88) | 55 (26–83) | < 0.001 |
| Operation | 0.120 | ||
| Breast conservation | 64 (62.7) | 123 (53.2) | |
| Mastectomy | 38 (37.3) | 108 (46.8) | |
| Completion ALND | |||
| Alone | NA | 151 (65.4) | |
| Simultaneous margin clearance | NA | 80 (34.6) | |
| Median tumor size, cm (range) | 1.4 (0.1–5.0) | 1.5 (0.1–6.0) | 0.356 |
| Tumor size | 0.568 | ||
| T1 (≤2 cm) | 78 (78.8) | 169 (73.8) | |
| T2 (2–5 cm) | 21 (21.2) | 58 (25.3) | |
| T3 (> 5 cm) | 0 (0.0) | 2 (0.9) | |
| Tx (unknown) | 3 | 2 | |
| Histologic grade | 0.546 | ||
| I | 4 (4.7) | 5 (2.5) | |
| II | 28 (32.9) | 64 (31.5) | |
| III | 53 (62.4) | 134 (66.0) | |
| Unknown | 17 | 28 | |
| Nuclear grade | 0.505 | ||
| I | 2 (2.5) | 7 (3.6) | |
| II | 49 (60.5) | 101 (52.6) | |
| III | 30 (37.0) | 84 (43.8) | |
| Unknown | 21 | 39 | |
| LVI present | 25 (24.5) | 98 (42.4) | 0.002 |
| Multicentric/multifocal tumor | 25 (24.5) | 65 (28.1) | 0.593 |
| ER positive | |||
| Present | 82 (82.0) | 190 (82.6) | 0.876 |
| Missing | 2 | 1 | |
| PR positive | |||
| Present | 64 (67.4) | 136 (63.0) | 0.521 |
| Missing | 7 | 15 | |
| Tumor type | 0.447 | ||
| Invasive ductal | 86 (84.3) | 204 (88.3) | |
| Invasive lobular | 11 (10.8) | 19 (8.2) | |
| Microinvasive DCIS | 2 (2.0) | 1 (0.4) | |
| Other invasive carcinomas | 0 (0.0) | 2 (0.9) | |
| Invasive carcinoma with ductal and lobular features | 3 (2.9) | 5 (2.2) | |
| Median year of surgery (range) | 2003 (1997–2006) | 2003 (1997–2006) | 0.904 |
| Median nomogram score, % (range) | |||
| Present | 15 (3–92) | 21 (3–80) | < 0.001 |
| Missing | 7 | 7 | |
SLN sentinel lymph node, H&E hematoxylin and eosin, ALND axillary lymph node dissection, LVI lymphovascular invasion, ER estrogen receptor, PR progesterone receptor, DCIS ductal carcinoma in situ
For patients with SLN metastases detected by routine H&E, the total number of SLN removed was significantly higher in the group without completion ALND (Table 3, P < 0.001), although the number of positive SLN did not differ between groups (P = 0.18). Regarding the number of nodes (SLN plus non-SLN) removed, 58% of patients without ALND had 4 to 9, and 20% had 10 or more. As expected, 92% of patients having ALND had ≥10 axillary nodes removed, but the proportion with >3 positive nodes was small (10%).
TABLE 3.
Number of SLN excised and SLN status in 333 SLN procedures that were positive by routine H&E, by performance of ALND
| SLN | SLN-positive by routine H&E/no ALND (n = 102), n (%) | SLN-positive by on routine H&E/ALND (n = 231), n (%) | P value |
|---|---|---|---|
| SLN excised | < 0.001 | ||
| 1 | 9 (8.8) | 46 (19.9) | |
| 2 | 22 (21.6) | 57 (24.7) | |
| 3 | 16 (15.7) | 45 (19.5) | |
| > 3 | 55 (53.9) | 83 (35.9) | |
| Positive SLN | 0.183 | ||
| 1 | 88 (86.3) | 184 (79.7) | |
| 2 | 9 (8.8) | 38 (16.5) | |
| 3 | 3 (2.9) | 4 (1.7) | |
| > 3 | 2 (2.0) | 5 (2.2) | |
| Negative SLN | < 0.001 | ||
| 0 | 12 (11.8) | 58 (25.1) | |
| 1 | 23 (22.5) | 59 (25.5) | |
| 2 | 16 (15.7) | 43 (18.6) | |
| 3 | 17 (16.7) | 32 (13.9) | |
| > 3 | 34 (33.3) | 39 (16.9) |
SLN sentinel lymph node, H&E hematoxylin and eosin, ALND axillary lymph node dissection
For 102 patients with SLN metastases detected by routine H&E and who did not have ALND, follow-up of ≥6 months was complete for 93% (95 of 102), among whom median follow-up was 59 months (range, 6–150). Fourteen patients (15%) experienced recurrence. As a first event, the crude rate of local recurrence was 2.1%, the crude rate of regional recurrence was 3.2%, and the crude rate of distant recurrence was 9.5% Among the 3 patients with regional node recurrence (2 axillary and 1 supraclavicular) there was no clear pattern of clinicopathologic features (Table 4). One of 60 patients with macrometastases treated by breast conservation (including radiotherapy) had an axillary recurrence, as did 1 of the 35 patients treated by mastectomy. The other nodal recurrence was a supraclavicular recurrence after a mastectomy.
TABLE 4.
Characteristics of three SLN-positive patients by routine H&E who did not have ALND and who developed regional recurrence
| Pattern of regional recurrence | Age (year) | No. of LN+/no. of LN excised | Tumor type | Tumor size (cm) | LVI | ER/PR | Operation | Local RT | Regional RT | Adjuvant systemic therapy | DFI (month) | Nomogram score (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Axilla | 35 | 1/6 | IDC | 1.2 | No | −/unknown | BCT | Breast | No | CT | 11 | 9 |
| Axilla | 43 | 1/9 | IDC | 0.1 | Yes | +/− | Mastectomy | Chest wall | SC | CT, HT | 31 | 7 |
| Supraclavicular | 61 | 1/2 | Invasive carcinoma with ductal and lobular features | 0.35 | No | −/− | Mastectomy | No | No | CT | 76 | 26 |
SLN sentinel lymph node, H&E hematoxylin and eosin, LN lymph node, LVI lymphovascular invasion, ER estrogen receptor, PR progesterone receptor, RT radiotherapy, DFI disease-free interval, IDC invasive ductal carcinoma, BCT breast-conservation therapy, SC supraclavicular, CT chemotherapy, HT hormone therapy
DISCUSSION
During the 10 years under study, we have shown declining rates of intraoperative FS and, for patients in whom FS was either negative or not done, a declining rate of completion ALND in SLN-positive patients (Figs. 1, 2). These trends clearly reflect a selection bias (Tables 1, 2) in which FS and/or completion ALND were more likely to be done in patients at increased risk of local or distant relapse on the basis of age, tumor characteristics (size, grade, and LVI), and nodal status (SLN and non-SLN), and are not at all surprising for a number of reasons.
We have previously studied in detail the performance of SLN FS in our own practice. In a side-by-side comparison of FS with imprint cytology and cytologic smear in the same SLN, we have observed comparable sensitivity overall (60%), high sensitivity for detecting macrometastases (>90%), and low sensitivity for detecting micrometastases (20%).9 We have further shown that the yield of FS (the proportion of all FS that are positive) is relatively low, about 20%, and that in selected patients, it is even lower; for women 60 years of age or older with T1a,b tumors, only 3–8% of FS were positive.8 Finally, we have found that although a positive FS allows an immediate ALND, it does not avoid a reoperation for positive margins of excision; although about 20% of SLN FS were positive overall, our rate of reoperation for positive margins was sufficiently high that a positive FS avoided reoperation for only 7% of patients.10 These observations have all contributed to a decline over time in our overall rate of intraoperative FS, but have not generated a consensus, as is clear from the wide variation we observe between surgeons in the use of FS (66–95%). During the study period, we tried to identify subsets of patients for whom the yield of FS would be higher, but there were no departmental decisions to decrease the performance of FS.
Regarding ALND, we observed no decline in the performance of ALND for patients with SLN positive by FS or routine H&E; a modest decline across all SLN-positive patients (84–77%); and a substantial decline among patients with SLN positive by serial section H&E or IHC only. These trends were associated with a small increase in the proportion of completion (vs. immediate) ALND from 30 to 40%, which did not reach statistical significance (P = 0.07).
These trends have been supported by additional findings from our experience. First among these is the MSKCC nomogram for the prediction of non-SLN status in SLN-positive patients; this well-calibrated multivariate model has been prospectively validated at our own institution and at multiple centers worldwide.11 We have previously observed a declining rate of ALND in our SLN-positive patients over the years after our adoption of the MSKCC nomogram.12 More importantly, we have observed very low rates of axillary local recurrence among our SLN-positive patients whether ALND was done or not. Among our SLN-positive patients who did have versus those who did not have ALND, the MSKCC nomogram predicted residual axillary metastases in 37 and 10%, respectively, but we observed axillary local recurrence at 2 years follow-up in 0.37 and 1.90% (P = 0.004), respectively.10 Making the same comparison in 171 patients whose SLN were positive by IHC only, the MSKCC nomogram predicted residual axillary disease in 8.2% of patients who had ALND and 4.1% of those who did not, but we observed no axillary recurrences in either group at 6 years follow-up.13 We wish to emphasize that our observations do not support the concept of a simple cutoff for performing either FS or ALND on the basis of patient age, tumor characteristics, or nomogram score. In our practice, these decisions have been individualized and reflect a combination of surgeon and patient preference in addition to the clinicopathologic variables noted above. It seems likely, however, that among our patients with H&E-detected SLN metastases, the removal of more total and negative SLN may have influenced the decision to omit ALND.
These trends and findings are not limited to our own institution. The rate of axillary local recurrence in SLN-negative (48 reports) and selected SLN-positive patients (6 reports), all treated without ALND, is comparable: 0.3 and 0.5%, respectively.18, 19 In a report on 403,167 patients with stage I–III breast cancer from the National Cancer Database (1998–2005), 23% of patients with SLN macrometastases and 55% of patients with SLN micrometastases did not have ALND.14 The authors observed a striking trend over time toward fewer ALND in patients with SLN micrometastases, and no advantage for ALND in either local recurrence or relative survival at 5 years. Axillary local recurrence with vs. without ALND in patients with SLN macrometastases was 1.1% and 1%, and with SLN micrometastases was 0.2 and 0.4%, respectively.
We have observed that the decline over time in the rate of ALND for SLN-positive patients was limited to those with metastases detected by serial section H&E or IHC only. Can the use of ALND be reduced further? The most definitive answer to date comes from the ACOSOG Z0011 trial, in which patients with SLN positive by H&E, all having breast conservation with whole breast radiotherapy, were randomized to ALND versus no further surgery (excluding patients with ≥3 positive SLN).15, 16 Although additional positive nodes were found in 27% of the ALND specimens, the rate of regional node recurrence at 6.3 years follow-up was no different between the study arms (0.5% with ALND vs. 0.9% without ALND), and there were no differences in overall or disease-free survival. These data suggest that a policy of “no ALND” is reasonable and safe for virtually all clinically node-negative patients who are having breast conservation (with whole breast radiotherapy) and have 1–2 positive SLN, regardless of the method of detection. If the Z0011 criteria had been applied in our cohort, 66% (4159 of 6327) of all FS and 48% (939 of 1953) of all ALND would have been eliminated, sparing 13% of our total patient population the morbidity of ALND.
Looking ahead, the ACOSOG Z0011 results support marked changes in practice, and we have already incorporated the following into our treatment algorithm for patients with cN0 breast cancers: first, the elimination of intraoperative FS in the setting of breast conservation; and second, the elimination of completion ALND for breast conservation patients with 1–2 positive SLN regardless of metastasis size. The Z0011 results immediately raise questions for further study. Whole breast radiotherapy treats a large portion of the axilla and may in part account for the low rates of axillary local recurrence (0.9% at 6 years) observed in the no-ALND arm of Z0011. Should this raise concern about the growing use of partial breast radiotherapy? Can a policy of “no ALND” be extended to mastectomy patients with positive SLN? With ≥3 positive SLN? With clinically positive nodes? Will the results of the EORTC AMAROS trial (a randomization of SLN-positive patients to ALND vs. axillary radiotherapy) establish the equivalence of ALND and axillary radiotherapy, further reducing the need for ALND?20 Because the time period under study ended in 2006, we believe that the trends we report are the results of our own experience and not the early unreported results of Z0011. The trends that we have already observed in our own practice toward fewer SLN-FS and fewer ALND for SLN-positive patients clearly suggest that both surgeons and patients are comfortable with a more nuanced approach to axillary management, and that our rate of ALND will continue to decline.
Acknowledgments
W.P.W. was partially supported by grants from the Hippocrate Foundation and “Stiftung für Molekularbiologische Tumorforschung.”
Footnotes
Presented in part at the Society of Surgical Oncology’s 64th Annual Cancer Symposium, March 2–5, 2011, San Antonio, TX.
DISCLOSURE The authors have no potential conflicts of interest to report.
References
- 1.Kim T, Giuliano AE, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma. Cancer. 2006;106:4–16. doi: 10.1002/cncr.21568. [DOI] [PubMed] [Google Scholar]
- 2.Veronesi U, Viale G, Paganelli G, et al. Sentinel lymph node biopsy in breast cancer: ten-year results of a randomized controlled study. Ann Surg. 2010;251:595–600. doi: 10.1097/SLA.0b013e3181c0e92a. [DOI] [PubMed] [Google Scholar]
- 3.Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8:881–8. doi: 10.1016/S1470-2045(07)70278-4. [DOI] [PubMed] [Google Scholar]
- 4.Mansel RE, Fallowfield L, Kissin M, et al. Randomized multi-center trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006;98:599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- 5.Zavagno G, De Salvo GL, Scalco G, et al. A Randomized clinical trial on sentinel lymph node biopsy versus axillary lymph node dissection in breast cancer: results of the Sentinella/GIVOM trial. Ann Surg. 2008;247:207–13. doi: 10.1097/SLA.0b013e31812e6a73. [DOI] [PubMed] [Google Scholar]
- 6.Gill G. Sentinel-lymph-node-based management or routine axillary clearance? One-year outcomes of sentinel node biopsy versus axillary clearance (SNAC): a randomized controlled surgical trial. Ann Surg Oncol. 2009;16:266–75. doi: 10.1245/s10434-008-0229-z. [DOI] [PubMed] [Google Scholar]
- 7.O’Hea BJ, Hill ADK, El-Shirbiny A, et al. Sentinel lymph node biopsy in breast cancer: initial experience at Memorial Sloan-Kettering Cancer Center. J Am Coll Surg. 1998;186:423–7. doi: 10.1016/s1072-7515(98)00060-x. [DOI] [PubMed] [Google Scholar]
- 8.Chan SW, Lavigne KA, Port ER, et al. Does the benefit of sentinel node frozen section vary between patients with invasive duct, invasive lobular, and favorable histologic subtypes of breast cancer? Ann Surg. 2008;247:143–9. doi: 10.1097/SLA.0b013e3181581f41. [DOI] [PubMed] [Google Scholar]
- 9.Brogi E, Torres-Matundan E, Tan LK, et al. The results of frozen section, touch preparation, and cytological smear are comparable for intraoperative examination of sentinel lymph nodes: a study in 133 breast cancer patients. Ann Surg Oncol. 2005;12:173–80. doi: 10.1245/ASO.2005.03.067. [DOI] [PubMed] [Google Scholar]
- 10.McLaughlin SA, Ochoa-Frongia LM, Patil SM, et al. Influence of frozen-section analysis of sentinel lymph node and lumpectomy margin status on reoperation rates in patients undergoing breast-conservation therapy. J Am Coll Surg. 2008;206:76–82. doi: 10.1016/j.jamcollsurg.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 11.Van Zee KJ, Manasseh DM, Bevilacqua JL, et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol. 2003;10:1140–51. doi: 10.1245/aso.2003.03.015. [DOI] [PubMed] [Google Scholar]
- 12.Park J, Fey JV, Naik AM, et al. A declining rate of completion axillary dissection in sentinel lymph node-positive breast cancer patients is associated with the use of a multivariate nomogram. Ann Surg. 2007;245:462–8. doi: 10.1097/01.sla.0000250439.86020.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pugliese MS, Karam AK, Hsu M, et al. Predictors of completion axillary lymph node dissection in patients with immunohistochemical metastases to the sentinel lymph node in breast cancer. Ann Surg Oncol. 2010;17:1063–8. doi: 10.1245/s10434-009-0834-5. [DOI] [PubMed] [Google Scholar]
- 14.Bilimoria KY, Bentrem DJ, Hansen NM, et al. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. J Clin Oncol. 2009;27:2946–53. doi: 10.1200/JCO.2008.19.5750. [DOI] [PubMed] [Google Scholar]
- 15.Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252:426–33. doi: 10.1097/SLA.0b013e3181f08f32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs. no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569–75. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cody HS, Borgen PI. State-of-the-art approaches to sentinel node biopsy for breast cancer: study design, patient selection, technique, and quality control at Memorial Sloan-Kettering Cancer Center. Surg Oncol. 1999;8:85–91. doi: 10.1016/s0960-7404(99)00029-8. [DOI] [PubMed] [Google Scholar]
- 18.van der Ploeg IM, Nieweg OE, van Rijk MC, et al. Axillary recurrence after a tumour-negative sentinel node biopsy in breast cancer patients: a systematic review and meta-analysis of the literature. Eur J Surg Oncol. 2008;34:1277–84. doi: 10.1016/j.ejso.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 19.Rutgers EJ. Sentinel node biopsy: interpretation and management of patients with immunohistochemistry-positive sentinel nodes and those with micrometastases. J Clin Oncol. 2008;26:698–702. doi: 10.1200/JCO.2007.14.4667. [DOI] [PubMed] [Google Scholar]
- 20.Straver ME, Meijnen P, van TG, et al. Role of axillary clearance after a tumor-positive sentinel node in the administration of adjuvant therapy in early breast cancer. J Clin Oncol. 2010;28:731–7. doi: 10.1200/JCO.2008.21.7554. [DOI] [PMC free article] [PubMed] [Google Scholar]