Abstract
Objective
Meniscal regeneration was previously shown to be enhanced by injection of mesenchymal stem/stromal cells (MSCs) but the mode of action of the MSCs was not established. The aim of this study was to define how injection of MSCs enhances meniscal regeneration.
Design
A hemi-meniscectomy model in rats was used. Rat-MSCs (rMSCs) or human-MSCs (hMSCs) were injected into the right knee joint after the surgery, and PBS was injected into the left. The groups were compared macroscopically and histologically at 2, 4, and 8 w. The changes in transcription in both human and rat genes were assayed by species-specific microarrays and real-time RT-PCRs.
Results
Although the number of hMSCs decreased with time, hMSCs enhanced meniscal regeneration in a manner similar to rMSCs. hMSCs injection increased expression of rat type II collagen (rat-Col II), and inhibited osteoarthritis progression. The small fraction of hMSCs were activated to express high levels of a series of genes including Indian hedgehog (Ihh), parathyroid hormone-like hormone (PTHLH), and bone morphogenetic protein 2 (BMP2). The presence of hMSCs triggered the subsequent expression of rat-Col II. An antagonist of hedgehog signaling inhibited the expression of rat-Col II and an agonist increased expression of rat-Col II in the absence of hMSCs.
Conclusions
Despite rapid reduction in cell numbers, intra-articular injected hMSCs were activated to express Ihh, PTHLH, and BMP2 and contributed to meniscal regeneration. The hedgehog signaling was essential in enhancing the expression of rat-Col II, but several other factors provided by the hMSCs probably contributed to the repair.
Keywords: MSCs, Meniscus, Indian hedgehog, Parathyroid hormone-like hormone, Bone morphogenetic protein 2
Introduction
Injuries to the fibrocartilage of the knee meniscus illustrate the limited ability of human cartilage to repair itself 1. For symptomatic injuries to the meniscus, a meniscectomy is often performed; a procedure which rapidly leads to osteoarthritis 2. Indications for surgical meniscal repair are limited 3, and the results are not always satisfactory. One potential strategy to enhance meniscal regeneration is to use mesenchymal stem/progenitor cells that can differentiate into cartilage and other skeletal cells 4, 5. A number of reports demonstrated that healing of the surgically injured meniscus was enhanced by injection of the adult stem/progenitor cells from bone marrow referred to as mesenchymal stem or stromal cells (MSCs) 6–8. The observations were initially interpreted on the assumption that the MSCs repaired the tissue by engrafting and differentiating into chondrocytes. However, the number of cells that engrafted was apparently too low to account for the extensive repair frequently observed 9. Similar observations were made with the use of MSCs in other models for human diseases in which repair of tissues was observed without significant long term engraftment of the cells 9, 10. Therefore, the recent focus has been the repair of tissues by MSCs through paracrine factors the cells synthesize in culture 9, 11 and the additional therapeutic factors they are activated to express as a result of cross-talk with injured cells and tissues 10.
In the present study, we hypothesized that intra-articular injection of hMSCs would enhance meniscal regeneration in rats after hemi-meniscectomy. We then capitalized on the species differences to simultaneously track changes in the hMSCs and the endogenous rat cells as the tissue was repaired in order to define how intra-articular injection of MSCs enhances meniscal regeneration.
Materials and methods
Animals
The rat studies were approved by the Institutional Animal Care and Use Committee of Texas A&M Health Science Center. Twelve-week-old male Lewis rats (LEW/Crl; Charles River Laboratories; Wilmington, MA) were used in all experiments with n=5 in each group for quantification of area and histology, and n=4 in each group for RT-PCRs.
Cell preparations
Passage 3 cells were used for all assays. Frozen passage 1 human bone marrow MSCs (hMSCs) were obtained from the Center for the Preparation and Distribution of Adult Stem Cells (http://medicine.tamhsc.edu/irm/msc-distribution.html), and were expanded as described previously 12 Rat MSCs (rMSCs) were isolated from 12-week-old Lewis rats and expanded as described previously 13. Frozen primary human dermal fibroblasts (HDFs); were obtained from Sciencell, (Carlsbad, CA).
To label the cells for some experiments, CM-DiI (Invitrogen; Carlsbad, CA) was added at 5 μL/1×106 cells/ml in PBS. The cells were incubated for 20 min at 37°C in 5% humidified CO2, centrifuged at 480 × g for 5 minutes, and washed twice with PBS.
Meniscectomy and cell injection
Under isoflurane anesthesia a hemi-meniscectomy was performed on both knees as described previously 7. Immediately after surgery, 2 × 106 CM-DiI labeled-hMSCs or rMSCs in 50 μL PBS were injected intra-articularly into the right knee joint. The left knee joint received 50 μL PBS as the control. The rats were allowed to walk freely in the cage.
For experiments with cyclopamine, which is an inhibitor of hedgehog signaling, 2 × 106 hMSCs were injected postoperatively on day 0, and cyclopamine (50 μL of 20 μM in 0.1% DMSO/PBS; Sigma-Aldrich) was injected intra-articularly on postoperative days 0, 1, and 3. Controls were injected with vehicle alone at the same time points. For experiments with Smoothened agonist (SAG), we injected SAG solution (50 μL of 100 nM or 500 nM in PBS; Santa Cruz) intra-articularly on postoperative day 0. We injected cyclopamine, SAG, or vehicle alone (control) bilaterally into knees of each rat (n=4 knees in each group).
Quantification of meniscus area
The whole medial meniscus (n=5 each time point) was collected at 2, 4, and 8 weeks after injection. The menisci were photographed and the area measured with Scion image software.
Histology
After photography for area measurements, menisci were fixed in 4% paraformaldehyde and embedded in paraffin. Five μm horizontal sections were cut and stained with Toluidine blue or subjected to immunohistochemistry.
To assess osteoarthritic changes, proximal tibias were dissected at 2, 4, and 8 weeks after injection and fixed in 4% paraformaldehyde. They were then decalcified (Decalcifying Solution; Richard-Allan Scientific; Kalamazoo, MI) at 4°C for 14 days and embedded in paraffin. The sections stained with Safranin-O and fast green to visualize the cartilage. Two examiners blinded to treatments scored the sections using the Osteoarthritis Research Society International (OARSI) osteoarthritic cartilage histopathology grading system 14. The scoring system ranges from 0 to 24, with 0 representing normal and 24 representing the most severe osteoarthritis.
Immunohistochemistry
Rat type II collagen was visualized using DAB staining of a mouse monoclonal antibody (clone II-4C11) from MP Biomedicals. For immunofluorescence, primary antibodies to Ihh (ab52919), BMP2 (ab6285), PTHLH (ab55631), and PTH-R1 (ab75150) were obtained from Abcam. Species-specific AlexFluor-488 conjugated secondary antibodies were from Invitrogen.
Real-Time RT-PCR analysis for selected mRNAs
RNA was isolated from regenerated menisci using RNA Bee (Tel-Test; Friendswood, TX) according to manufacturer’s instructions and cleaned by RNeasy Mini Kit (QIAGEN; Valencia, CA). Total RNA was used in one-step real-time RT-PCR (ABI 7900 Sequence Detector, Applied Biosystems; Foster City, CA) using QuantiTect Probe RT-PCR Kit (QIAGEN). Reactions were incubated at 50°C for 30 min, 95°C for 15 min, then 40 cycles at 95°C for 15 s followed by 60°C for 1 min.
For normalization of gene expression, human-specific GAPDH primers and probe (TaqMan Gene Expression Assays ID, Hs99999905_m1), rat-specific GAPDH primers and probe (TaqMan Gene Expression Assays ID, Rn01775763_g1), or 18S rRNA primers and probe (TaqMan Gene Expression Assays, ID Hs03003631_g1) were used as internal controls (all from Applied Biosystems; Supplemental Table 3). All primers were tested for species-specificity; the values for critical threshold were indistinguishable from background with RNA from the alternative species.
Statistical analysis
For comparison of two groups, the Mann-Whitney U test was used. For multiple comparisons of three groups or more than three groups, one-way ANOVA followed by Bonferroni’s post hoc test was used. P values less than 0.05 were considered to be statistically significant. Data were analyzed using StatView software.
Additional methods
Detailed methods for cell culture, surgical procedures, immunohistochemistry and microarrays are described in the Supplemental Methods.
Results
Intra-articular injection of hMSCs promoted meniscal regeneration and inhibited development of osteoarthritis
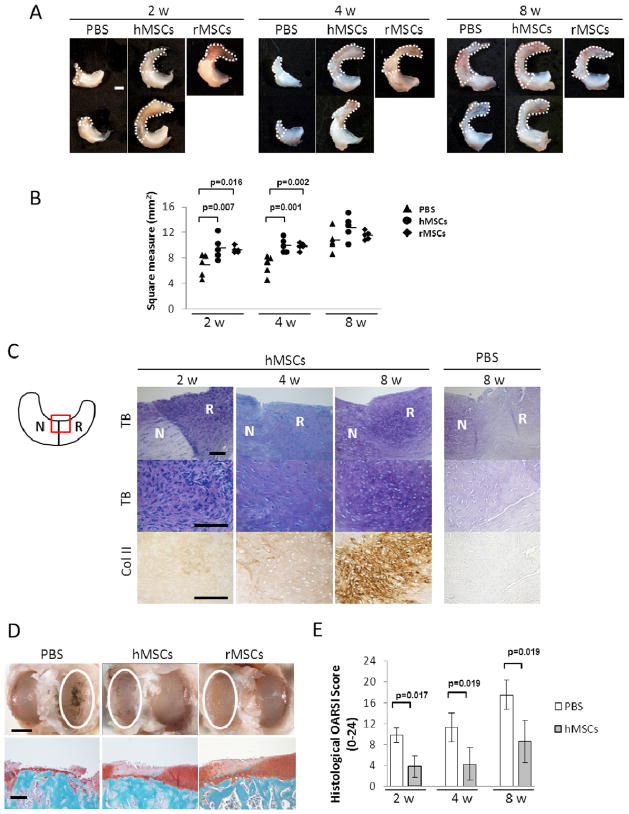
To determine whether intra-articular injection of hMSCs would enhance regeneration of rat meniscus, we performed hemi-meniscectomy 7 in both knees of wild-type Lewis rats. We then closed the knee joint and immediately injected CM-DiI labeled hMSCs (2 × 106 cells in 50 μL PBS) into the right knee joint and an equal volume of PBS into the left knee. For positive controls for the xenotransplantation, we repeated the experiments with the same number of rat MSCs (rMSCs). At 2 and 4 weeks, injection of the hMSCs enhanced regeneration as indicated by the morphology of the meniscus (Fig. 1A) and the size of regenerated area (Fig. 1B). At 8 weeks the beneficial effects of the hMSCs were apparent in histological sections stained with Toluidine blue for metachromasia or labeled with antibodies to type II collagen (Fig. 1C). At 8 weeks the absolute area of regeneration was not significantly different between PBS and hMSC menisci because of the innate ability of rat meniscus to regenerate itself (Fig. 1B). Of special note was that there was no significant difference between the ability of xenotransplantation of hMSCs and syngeneic transplantation of rMSCs to enhance regeneration (Fig. 1B).
Figure 1.
Intra-articular injection of hMSC promoted regeneration of rat meniscus and inhibited development of osteoarthritis after the meniscectomy. (A) Representative macroscopic findings of the meniscus 2, 4, and 8 weeks after the injection of PBS (left), hMSCs (middle), or rMSCs (right). The white dotted line indicates the regenerated tissue. Scale bar, 1 mm. (B) Area of the total meniscus injected with PBS, hMSCs, or rMSCs at 2, 4, and 8 weeks. The horizontal lines are mean values; n = 5 for each group (one-way ANOVA followed by Bonferroni post-tests. P values less than 0.017 were considered to be statistically significant.) (C) Representative sections of the meniscus stained with Toluidine Blue (top and middle), and immunostained for type II collagen (bottom) after PBS or hMSCs injection. The staining in the PBS-treated sample was less with toluidine blue and the anti-body for type II collagen. The schema of the meniscus on the left is shown for orientation. Symbols: N, native meniscus; R, regenerated meniscus; TB, Toluidine Blue; Col II, type II collagen. Scale bar, 100 μm. (D) Representative gross photographs (top) and sections (bottom) of the joint surface of the tibia at 8 weeks. The cartilage was stained with India ink to identify fibrillation and erosion. The white circle indicates the medial tibial plateau. The tibia was sectioned coronally and stained with safranin-O and fast green to identify cartilage (red). Scale bars, 2 mm (top) or 200 μm (bottom). (E) Quantification of histological analysis using the OARSI cartilage osteoarthritis histopathology grading system. Values are mean with lower and upper limit of 95 % CI; n = 5 for each group (Mann-Whitney U test). Symbols: OARSI, Osteoarthritis Research Society International.
We next evaluated articular cartilage damage in adjacent femoral and tibial articular surfaces. Both articular surfaces had osteoarthritic change but they were more severe on the medial tibial plateau than on the medial femoral condyle (data not shown). Therefore, we quantified the osteoarthritic change in the tibial plateau. At 8 weeks after the menisectomy, severe degenerative changes were observed in the medial tibial plateau in PBS knees, but hMSCs inhibited the osteoarthritis progression after meniscectomy (Fig. 1D and E).
Detection of the Injected hMSCs
CM-DiI+hMSCs were readily detected in histological sections of the regenerated meniscus but it was apparent that they decreased rapidly in number with time (Supplemental Fig. 1). The labeled cells seen at 2 and 4 weeks had the morphology of fibroblasts but the small number of CM-Dil-labeled cells seen at 8 weeks had the more spherical morphology of chondrocytes (Supplemental Fig. 1). We confirmed the persistence of the hMSCs by labeling the sections with human-specific nuclear antibody (Supplemental Fig. 2). For a quantitative measure of the engrafted human cells 15, 16, we prepared standard curves by adding pre-determined numbers of hMSCs to the surgically injured menisci just before homogenization, extracted RNA, and assayed it with a quantitative RT-PCR assay specific for human GAPDH (Supplemental Fig. 3A–C). Approximately 2% of hMSCs injected into the synovial space were recovered in the regenerating tissue at day 1, and 1% of hMSCs were recovered at day 3. No hMSCs were detected at day 7 with the assay with the RT-PCR for human GAPDH (Supplemental Fig. 3B). The standard curve (Supplemental Fig. 3A) suggested that the assay was sensitive to detection 200 to 1,000 hMSCs. However, hMSCs were still detected by immunohistochemistry at 8 weeks (below). The discrepancy is probably explained by the standard curve over-estimating the sensitivity of the assay, since it was generated by simply adding hMSCs to the tissue before extraction of the RNA; efficient extraction of RNA from a cartilaginous tissue such as the meniscus that is rich in proteoglycans usually requires digestion of the matrix and isolation of the cells 17. Therefore the real-time RT-PCR assay reflected the rapid decrease in hMSCs in the first few days after administration but probably under-estimated the cells remaining in the tissue at later times.
The injected hMSCs were activated to express Ihh, PTHLH, and BMP2
The use of a xenotransplantation made it possible to follow simultaneously changes in both the donor hMSCs and the endogenous rat cells in the regenerating meniscus. A total of 2 x106 hMSCs were injected into the hemi-meniscectomized knee joint of rat, and RNA was extracted from the meniscus 3 days after the injection, a time at which assays for human GAPDH mRNA indicated there were adequate amounts of human mRNA for analyses. As we observed previously15, 18, an initial impression of the potential cross-talk between the hMSCs and host rodent cells can be obtained by assaying the same RNA on species-specific microarrays.
Therefore, the same samples of RNA were assayed on human microarrays and on rat microarrays, and the data were filtered for cross-hybridization (see Supplemental Methods). The data (Supplemental Table 1) indicated that the human transcriptome was changed with up-regulation of 93 human transcripts. Also, the data suggested that a series of human genes were down-regulated, but the number could not be accurately defined because signals for many human genes were eliminated from the analysis by filtering for cross-hybridization in the samples which contained a low ratio of human RNA to rat RNA.
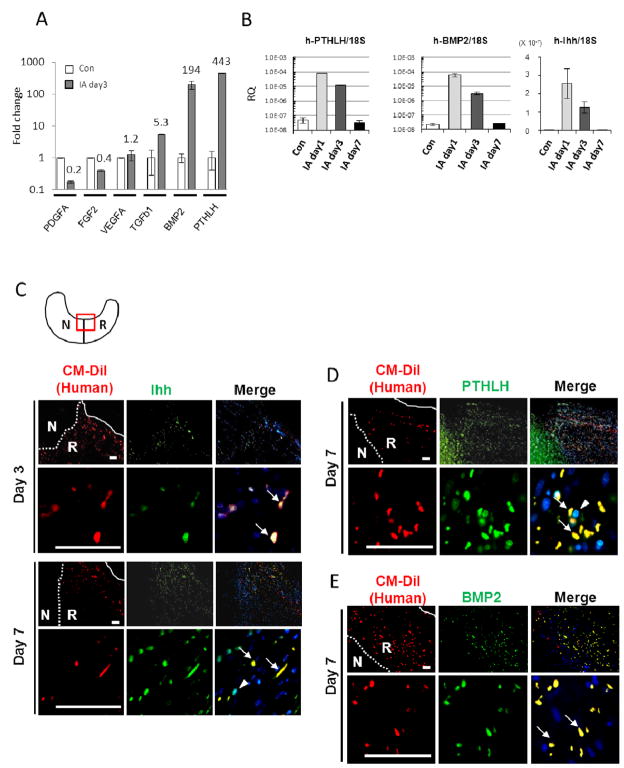
The 40 most highly up-regulated human transcripts (Supplemental Table 1) were subjectively examined for candidate genes of interest, and human-specific real-time RT-PCR assays were used to confirm the microarray data (Fig. 2A). Because administration of hMSCs enhanced regeneration of the meniscus, we elected to focus on the genes related to growth factors, such as PDGF, VEGF, FGF, TGF-b, BMP2 and PTHLH. As observed previously 19, the RT-PCR assays indicated that the increases in expression of some of the genes was larger than the values obtained from the microarrays. On day 3 the increase in PTHLH was 443-fold and the increase in BMP2 was 194-fold. The microarray data did not reveal any changes in Indian hedgehog (Ihh), but Ihh was observed to up-regulate BMP2 and PTHLH during the endochondral ossification 20, 21. Therefore we assayed for Ihh expression. Surprisingly, human Ihh was also increased 22-fold at day 3 compared to control hMSCs. Expression of human Ihh not detected on day 7, apparently because only a small number of hMSCs remained in the tissue (Supplemental Fig. 3B).
Figure 2.
Intra-articular injected hMSCs were activated to express Ihh, PTHLH, and BMP2. (A) Real-time RT-PCR for human-specific mRNA in regenerated meniscus 3 days after injection. Values are mean with lower and upper limit of 95 % CI of fold increase over Con, normalized by ΔΔCt for human-specific GAPDH; n = 4. Symbols: Con, 20,000 hMSCs added to injured meniscus from PBS injected knee before RNA extraction; IA day3, regenerated meniscus 3 days after intra-articular injection of hMSCs. (B) Time sequence of human gene expression by real-time RT-PCR for human PTHLH, BMP2, and Ihh in regenerated meniscus. Values are mean with lower and upper limit of 95 % CI of the relative quantities (RQ) normalized to 18S rRNA reflecting total levels of both human and rat transcripts. (C-E) Immunohistochemistry of anti-human/rat Ihh (C), PTHLH (D), or BMP2 (E) 3 or 7 days after hMSC injection. Primary antibodies for Ihh and PTHLH reacting with both human and rat were used. Low magnification (top) and higher magnification (bottom) of regenerated meniscus. Right column: merged images (CM-DiI, red; Ihh/PTHLH/BMP2, green; DAPI, blue). The white dotted line indicates border between native and regenerated meniscus, and the white solid line indicates outer edge of regenerated meniscus. CM-DiI labeled hMSCs (red) expressed Ihh, PTHLH, and BMP2 (green) (arrows in merge). Note: CM-DiI negative rat-derived cells surrounding hMSCs also expressed Ihh and PTHLH (arrowhead in merge) at day 7. The schema of the meniscus on the top left (C) is shown for orientation. Symbols: N, native meniscus; R, regenerated meniscus. Scale bars, 50 μm.
We next examined the time sequence of changes in the human transcriptome (Fig. 2B). The gene expression of human PTHLH increased 516-fold as early as day 1. The level per human cell (normalized by ΔCt for specific for human GAPDH) increased further between day 3 and day 7 (data not shown), but because the RNAs we have isolated included both human and rat RNAs and the number of human cells was rapidly decreasing, the total level of transcript were normalized again by 18S rRNA, which contains sequences that are 100% conserved among all the eukaryotes. The total level of transcript of PTHLH (normalized by ΔCt for eukaryotic 18S rRNA) was lower on day 7 (Fig. 2B). BMP2 also increased 663-fold at day 1, and further increased at day 7 if expressed on a per human cell basis but not as total tissue content (data not shown).
Immunohistochemistry confirmed that the injected hMSCs that adhered to the injured site expressed Ihh, PTHLH, and BMP2 (Fig. 2C–E).
hMSCs up-regulated expression of chondrogenic genes in rat cells
The data from the rat microarrays indicated that there was up-regulation of expression of 91 rat genes and down regulation of 42 rat genes by a factor of 2-fold or more. The most highly up-regulated 40 rat genes are shown in Supplemental Table 2.
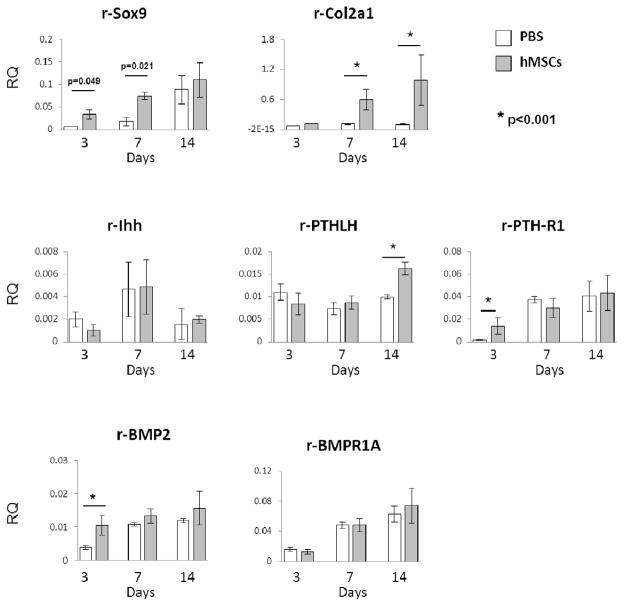
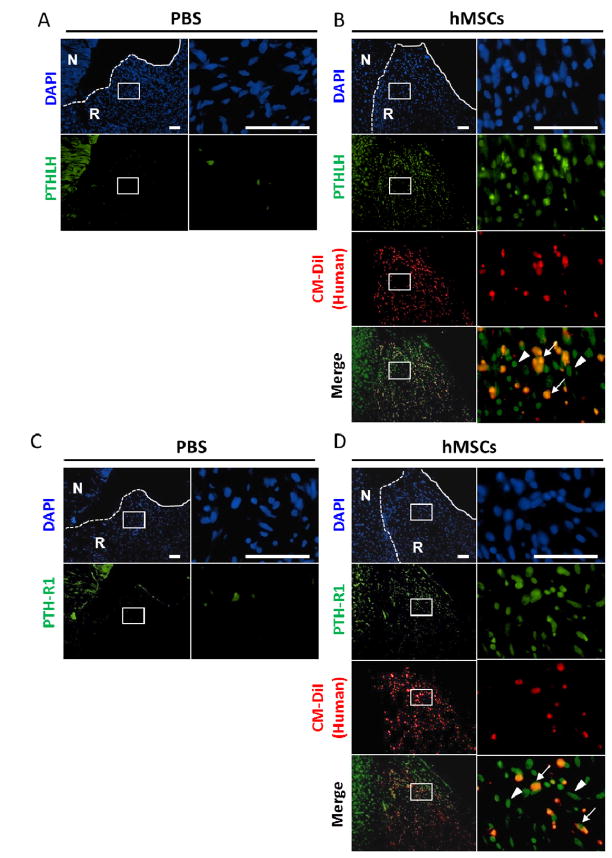
Assays by rat specific real-time RT-PCR were carried out for transcripts of genes expressed during chondrogenesis. Injection of the hMSCs increased the levels of Sox9 mRNA at day 3 and day 7 (Fig. 3). As expected from the assays by immunohistochemistry (see Fig. 1C), injection of the hMSCs increased expression of the rat Col2a1 gene at day 7 and 14. The increases in several other rat genes were more variable. There was no consistent effect of the hMSCs on expression of rat Ihh, or the rat BMP receptor BMPR1A. However, hMSC injection increased the expression of rat receptor for PTHLH (PTH-R1) and BMP2 on day 3, and PTHLH on day 14. The increased expression of PTHLH and PTH-R1 were evaluated by immunohistochemistry (Fig. 4). Interestingly, not only human (CM-DiI positive) cells but also rat (CM-DiI negative) cells surrounding hMSCs expressed PTHLH and PTH-R1 at day 7.
Figure 3.
hMSCs up-regulated expression of chondrogenic genes in rat cells. Real-time RT-PCR assay for rat-specific mRNA (Sox9, Col2a1, Ihh, PTHLH, PTH-R1, BMP2, and BMPR1A) in the regenerated meniscus 3, 7 and 14 days after PBS or hMSC injection. Values are mean with lower and upper limit of 95 % CI of the relative quantities (RQ) normalized to rat-specific GAPDH; n = 4 for each group. *p< 0.001 (Mann-Whitney U test).
Figure 4.
A large number of PTHLH/PTH-R1 positive cells were found in the regenerated tissue after injection of hMSCs. (A–D) Immunohistochemistry of anti-human/rat PTHLH (A, B) or PTH-R1 (C, D) 7 days after the injection of PBS (A, C) or hMSCs (B, D). Low magnification images are shown in the left and higher magnification of the regenerated tissue are shown in the right. Staining of nuclei (DAPI, blue), CM-DiI (red), and PTHLH or PTH-R1 (green) are shown. The white dotted line indicates the border between the native meniscus and regenerated tissue, and the white solid line indicates the outer edge of the regenerated tissue. A large number of PTHLH or PTH-R1 positive cells (green) were observed in the regenerated meniscus after the injection of hMSCs (B, D). In contrast, few PTHLH or PTH-R1 positive cells were present in the PBS injection group (B, D). Note that not only CM-DiI positive human cells (arrows in merge) but also CM-DiI-negative rat cells (arrowheads) expressed PTHLH or PTH-R1.
The role of human Ihh
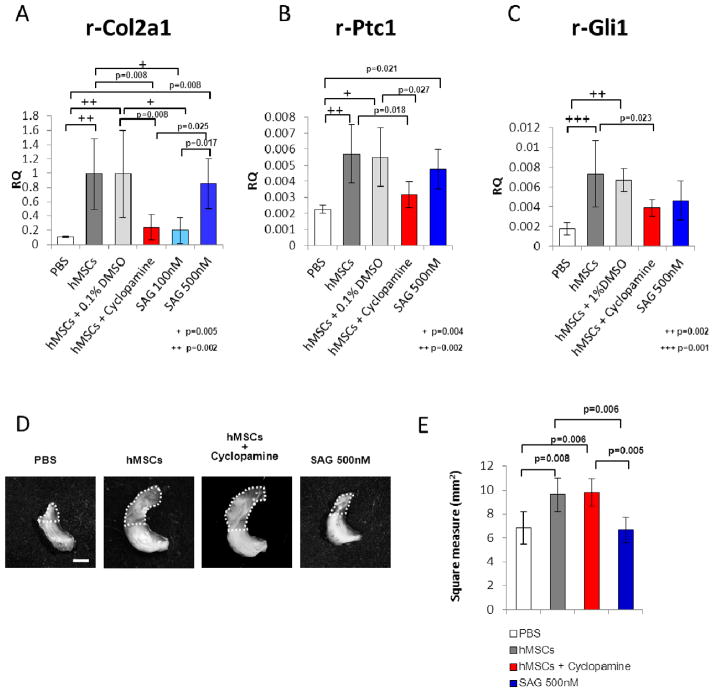
We elected to test the hypothesis that activation of hMSCs to express Ihh was an essential step in enhancing the regeneration of the rat meniscus. To test the hypothesis, we used cyclopamine, a steroid alkaloid that inhibits Ihh signaling by serving as an antagonist to the downstream target Smoothened. We elected to inject cyclopamine on day 0, 1, and 3 because it had a half-life of only 4 hours after ip or po administration 22 and a similar dosage schedule was used previously in experiments on repair of a cranial defect 23. In the presence of cyclopamine, hMSCs did not enhance expression of rat Col2a1(Fig. 5A). Therefore the results were consistent with the hypothesis that expression of Ihh was essential. As expected, administration of cyclopamine also decreased expression of Patched (Ptc1) and Gli1, downstream targets of hedgehog signaling (Fig. 5B and C). However, cyclopamine did not inhibit morphological regeneration of the meniscus as fibrous tissue (Fig. 5D and E). As a further test of the hypothesis we used SAG, a Smoothened agonist of hedgehog signaling. We elected to inject SAG on day 0 because expression of human Ihh reached a peak on day 1 and then declined (Fig. 2B).
Figure 5.
Effects of an inhibitor and an angonist of hedgehog signaling. (A) Real-time RT-PCR assay for rat-specific Col2a1 mRNA. RNA was recovered from the regenerated meniscus 2 weeks after the various treatments, including injection of PBS, hMSCs, hMSCs plus 0.1% DMSO (as a control of cyclopamine), hMSCs plus cyclopamine (50 μL of a 20 μM in 0.1% DMSO), 100 nM or 500 nM Smoothened agonist (SAG), or fibroblasts. Values are mean with lower and upper limit of 95 % CI of the relative quantities (RQ) normalized to rat-specific GAPDH; n = 4 for each group. + p=0.005, ++ p= 0.002 (one-way ANOVA followed by Bonferroni post-tests. P values less than 0.0033 were considered to be statistically significant.) (B, C) Real-time RT-PCR assay for rat-specific Ptc1 (B) and Gli1 (C), downstream targets of hedgehog signaling. RNA was recovered from the regenerated meniscus 3 days after the various treatments. Values are mean with lower and upper limit of 95 % CI of the relative quantities (RQ) normalized to rat-specific GAPDH; n = 4 for each group. + p=0.004, ++ p= 0.002 in B, ++ p=0.002, +++ p=0.001 in C (one-way ANOVA followed by Bonferroni post-tests. P values less than 0.005 were considered to be statistically significant.) (D) Representative macroscopic photographs of the meniscus 2 weeks after the various treatments. Scale bar: 1 mm. (E) Area of the total meniscus 2 weeks after the various treatments. Values are mean with lower and upper limit of 95 % CI; n = 4 for each group (one-way ANOVA followed by Bonferroni post-tests. P values less than 0.0083 were considered to be statistically significant.)
Injection of SAG after the meniscal surgery enhanced expression of rat Ptc1, Gli1, and Col2a1 gene in the absence of hMSCs (Fig. 5A–C). However, injection of SAG did not increase the area of repair (Fig. 5D and E). The results suggested that expression of Ihh by the hMSCs was essential for enhancing the rat Col II expression but probably not in itself sufficient for the enhanced regeneration observed with administration of hMSCs in the model (Supplemental Fig. 4).
Discussion
As reported previously 6–8, injection of MSCs enhanced the regeneration of surgically injured meniscus. Of special interest was the observation here that xenotransplantation of human MSCs were as effective as syngeneic rat MSCs in repairing the rat meniscus 7. The effectiveness of the hMSCs in the immunocompetent rats is consistent with previous reports that MSCs were immune privileged 24–26 and that hMSCs had about the same half life after injection into the hippocampus of wild-type mice as after injection into the hippocampus of immunodeficient mice 18. The hMSCs promoted both the synthesis of rat type II collagen in the regenerating meniscus and apparently the resilience of the tissue since they reduced the tibial osteoarthritis that developed in control knees. The hMSCs employed here may have been particularly effective because they were prepared with a standardized protocol that generates cultures enriched for early progenitor cells 12, 27, 28.
We previously evaluated the fate of rMSCs by in vivo imaging and histology in the same rat hemi-meniscectomy model 7. In vivo imaging analysis demonstrated that more than 1,000 of intra-articular injected rMSCs were detected in the knee joints for 28 days. The number of intra-articular injected LacZ+ rat cells were also decreased with time, but small number of LacZ+ cells were observed in the regenerated meniscus up to 12 weeks. In the present study, we did not directly compare the number of injected cells in rMSCs vs hMCSs; however apparently the rat cells survived somewhat longer than human cells 7.
The use of xenotransplantation of hMSCs made it possible to use species-specific assays obtain an initial impression of cross-talk between the hMSCs and the host cells in the meniscus15, 18 Real-Time RT-PCR assays of mRNA for human-specific GAPDH demonstrated that only a small number of the hMSCs injected into the synovial space engrafted to the regenerating edge of the meniscus. The cells disappeared rapidly but immunohistochemistry of the tissue demonstrated that a small number were detectable at 8 weeks. Microarray data demonstrated that shortly after the hMSCs had engrafted, there were marked changes in their transcriptomes with up-regulation of 93 human genes. Therefore, exposure of the hMSCs to the in vivo microenvironment had a major effect on the human cells. Real time RT-PCR assays to confirm some of the microarray data demonstrated several hundred fold increases in the levels of transcripts for the cartilage characteristic genes PTHLH and BMP2. These increases were accompanied by increases in the expression of human Ihh.
The time course for changes in the transcriptomes of the human cells and the rat cells in the regenerating edge of the meniscus suggested that the activation of the human cells to express Ihh, PTHLH and BMP2 promoted the subsequent expression of the Col2a1 gene by the rat cells (Fig. 6). The results indicated that the expression of Ihh was critical since an inhibitor of hedgehog signaling negated the effects of hMSCs in promoting expression of the rat Col2a1 gene. The inhibitor was not species-specific and therefore inhibited expression of both human and rat Ihh. Unfortunately, several experiments to use siRNAs to knock down the Ihh transcripts in the hMSCs under the conditions used previously 15 were unsuccessful: the transcripts were knocked down with the negative controls with the scrambled siRNA controls to the same extent as with the specific siRNAs. However, the conclusion that expression of Ihh by the hMSCs played a critical role was supported by the observation that an agonist of hedgehog signaling prompted expression of the rat Col2a1 gene in the absence of hMSCs.
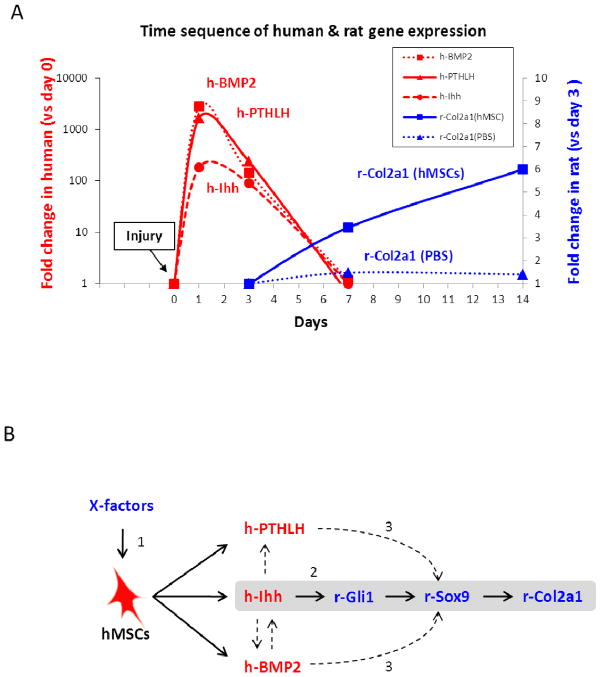
Figure 6.
Possible mechanism of rat meniscal regeneration by intra-articular injection of hMSCs. (A) Summary graph of time sequence of human and rat gene expression changes by species-specific real time RT-PCR. Intra-articular injected hMSCs were activated to express human Ihh, PTHLH, and BMP2 (red) as early as day 1. Values for human genes are expressed as fold increase over values for controls (Con) of cultured hMSCs, normalized by ΔΔCt for 18S to reflect total levels of both human and rat transcripts. Rat Col2a1 gene expression in the regenerated meniscus was increased after the injection of hMSCs (blue). Values for rat genes are expressed as fold increase over values for PBS day 3, normalized by ΔΔCt for rat GAPDH. Values are means from RT-PCR data presented in Fig. 2B and Fig. 3. Symbols: Con, 20,000 hMSCs added to injured meniscus from PBS injected knee before RNA extraction. (B) Summary diagram of the possible mechanism for rat meniscal regeneration by hMSCs. (1) Intra-articular injected hMSCs were activated to express Ihh, PTHLH, and BMP2 when they were exposed to the in vivo microenvironment. (2) Human Ihh expressed by the hMSCs enhanced expression of rat Col2a1 gene probably through the downstream hedgehog target of rat Gli1 and then through Sox9. hMSCs did not enhance expression of rat Gli1 and Col2a1 in the presence of cyclopamine. In contrast, injection of Smoothened agonist enhanced expression of rat Gli1 and Col2a1. (3) PTHLH and BMP2 may also have enhanced expression of rat Col2a1 through rat Sox9. Symbols: X-factors, unknown factors which activate hMSCs to express Ihh, PTHLH, and BMP2.
The agonist of hedgehog signaling did not however fully duplicate the effects of the hMSCs because it did not enhance restoration of the morphology of the meniscus. Therefore the hMSCs made additional contributions to the regeneration process that probably involved the expression of human PTHLH, BMP2, and perhaps other factors.
The increased expression of the Col2a1 produced by the agonist of hedgehog signaling raises the possibility that such agonists might provide a therapy for improving cartilage repair. However, the effects of hedgehog signaling are highly dependent on context 29. Lin et al. 30 demonstrated that hedgehog signaling was increased in human osteoarthritic samples and in mice with surgically induced osteoarthritis. Also, transgenic mice over-expressing hedgehog were more predisposed to osteoarthritis than wild-type mice. In addition, they observed that pharmacological and genetic inhibition of hedgehog signaling decreased the development of osteoarthritis. Experiments within cultured chondrocytes were consistent with these observations. The reasons for the dramatic differences from the observations made here are not apparent. The data however suggest that the relatively brief increase in hedgehog signaling we observed after a single administration of hMSCs immediately after hemi-menisectomy enhanced a process of orderly chondrogenesis much as occurs during normal development of the skeleton. In contrast, the more chronic increases in hedgehog signaling that Lin et al.26 examined in the setting of the extensive tissue destruction seen in osteoarthritis apparently drives a disordered hypertrophy of chondrocytes that exacerbates the pathological changes. One consequence of these observations is that agonists of hedgehog signaling may improve meniscal regeneration and chondrogenesis after acute injuries, but chronic administration of the same agents may increase the severity of osteoarthritis 30.
The results are consistent with previous observations with MSCs in that increases in expression of Ihh and PTHLH were seen during chondrogenic differentiation of MSCs in vitro 31, 32. The results are also consistent with previous observations on the roles of Ihh, PTHLH and BMP2 in chondrogenesis. Ihh is one of the master regulator of both chondrocyte and osteoblast differentiation during endochondral bone formation. Ihh is expressed in mesenchymal cells at the early stage of pre-chondrocytes and the late stage of pre-hypertrophic chondrocytes 33. Ihh stimulates chondrocyte proliferation directly and, through stimulation of PTHLH synthesis, determines the distance from the end of the bone at which chondrocytes stop proliferating and undergo hypertrophic differentiation 34. The results are also consistent with previous observations that Gli, a downstream target of hedgehog signaling, can up-regulate Sox9 by binding to its promoter 35, 36. During endochondral ossification, PTHLH is synthesized by chondrocytes and perichondrial cells at the ends of the developing bones. It stimulates the proliferation of chondrocytes and suppresses their terminal differentiation 31, 37, 38. PTHLH up-regulates Sox9 transcription 39, which has been shown to promote the differentiation of mesenchymal cells into chondroblasts 40. Thus, PTHLH may have acted upon the endogenous rat mesenchymal cells and induced their differentiation through the Sox9 pathway. In addition, PTHLH up-regulates the expression of Bcl2, an apoptosis inhibitor 41, which also may have a role in regulating rat matrix production 42. BMP signaling has been shown to play an important role in the development of bone and cartilage. BMP2 has been demonstrated to be a potent stimulator of chondrocyte metabolism and differentiation in vitro 43–45 and in vivo 46, 47. For example, BMP2 stimulated DNA synthesis of bovine meniscal cells in vitro in a dose-dependent manner 48. In addition, Ihh is a direct target gene of BMP2 49, and Ihh promotes BMP expression. In the injured meniscus, BMP2 produced by hMSCs may have stimulated endogenous rat mesenchymal cells to proliferate and differentiate. It may also have activated Ihh signaling in human and rat cells.
In effect, the observations presented here demonstrated that the human MSCs that engrafted in the knee joint of the hemi-meniscectomized rat were activated by the microenvironment of the injured meniscus to express extremely high levels of three genes that play critical roles in the development of normal cartilage: Ihh, PTHLH and BMP2. The human cells rapidly decreased in number but their presence triggered the subsequent expression of type II collagen by the rat meniscal cells. The expression of human Ihh was essential for up-regulating rat genes for type II collagen, Patched, and Gli1, an observation that raised the possibility that agonist of Ihh signaling may be useful in treating acute injuries to the meniscus and perhaps articular cartilage. However, for clinical application, interspecies differences have to be considered. The inherent healing capacity of the human meniscus has been shown to be lacking in the inner third and is very limited in the middle third 50. We used a rat model, and rat meniscus has a greater spontaneous healing potential 7. To demonstrate the effectiveness of agonist of Ihh signaling for meniscus regeneration, further experimental studies in larger animal models are needed. In addition, our data lack details about biomechanical properties and the contents of collagen and proteoglycans in the regenerated meniscus. Also, it is uncertain whether the regenerated menisci will prevent secondary osteoarthritic change in the long term. Therefore, further studies should be carried out.
Supplementary Material
Acknowledgments
Role of the funding source
Not applicable.
We thank Laura Quinlivan for assistance with animal experiments and Dina Gaupp, Center for Gene Therapy, Tulane University Health Sciences Center, for assistance with histological sectioning. This study was supported in part by National Institutes of Health/National Center for Research Resources Grant P40 RR 17447.
Footnotes
Contributions
Study design: MH, IS, TM and DP. Study conduct: DP. Data collection: MH, HC, and JY. Data analysis: MH, HC, and JY. Data interpretation: MH, HC, RL, JY, IS, and DP. Drafting manuscript: MH, HC, and DP. Revising manuscript content: RR and DP. Approving final version of manuscript: MH, HC, RL, RR, JY, TM, IS, and DP. DP takes responsibility for the integrity of the data analysis.
Conflict of interest
DJP is a co-founder of Temple Therapeutics LLC. The other authors state that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen FH, Rousche KT, Tuan RS. Technology Insight: adult stem cells in cartilage regeneration and tissue engineering. Nat Clin Pract Rheumatol. 2006;2:373–382. doi: 10.1038/ncprheum0216. [DOI] [PubMed] [Google Scholar]
- 2.Wieland HA, Michaelis M, Kirschbaum BJ, Rudolphi KA. Osteoarthritis - an untreatable disease? Nat Rev Drug Discov. 2005;4:331–344. doi: 10.1038/nrd1693. [DOI] [PubMed] [Google Scholar]
- 3.Sgaglione NA, Steadman JR, Shaffer B, Miller MD, Fu FH. Current concepts in meniscus surgery: resection to replacement. Arthroscopy. 2003;19 (Suppl 1):161–188. doi: 10.1016/j.arthro.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 4.Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient’s bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007;211:27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- 5.Prockop DJ, Olson SD. Clinical trials with adult stem/progenitor cells for tissue repair: let’s not overlook some essential precautions. Blood. 2007;109:3147–3151. doi: 10.1182/blood-2006-03-013433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdel-Hamid M, Hussein MR, Ahmad AF, Elgezawi EM. Enhancement of the repair of meniscal wounds in the red-white zone (middle third) by the injection of bone marrow cells in canine animal model. Int J Exp Pathol. 2005;86:117–123. doi: 10.1111/j.0959-9673.2005.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horie M, Sekiya I, Muneta T, Ichinose S, Matsumoto K, Saito H, et al. Intra-articular Injected synovial stem cells differentiate into meniscal cells directly and promote meniscal regeneration without mobilization to distant organs in rat massive meniscal defect. Stem Cells. 2009;27:878–887. doi: 10.1634/stemcells.2008-0616. [DOI] [PubMed] [Google Scholar]
- 8.Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48:3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 9.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 10.Prockop DJ, Kota DJ, Bazhanov N, Reger RL. Evolving paradigms for repair of tissues by adult stem/progenitor cells (MSCs) J Cell Mol Med. 2010;14:2190–2199. doi: 10.1111/j.1582-4934.2010.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skalnikova H, Motlik J, Gadher SJ, Kovarova H. Mapping of the secretome of primary isolates of mammalian cells, stem cells and derived cell lines. Proteomics. 2011;11:691–708. doi: 10.1002/pmic.201000402. [DOI] [PubMed] [Google Scholar]
- 12.Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, Prockop DJ. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20:530–541. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- 13.Javazon EH, Colter DC, Schwarz EJ, Prockop DJ. Rat marrow stromal cells are more sensitive to plating density and expand more rapidly from single-cell-derived colonies than human marrow stromal cells. Stem Cells. 2001;19:219–225. doi: 10.1634/stemcells.19-3-219. [DOI] [PubMed] [Google Scholar]
- 14.Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14:13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishida Y, Sugahara-Kobayashi M, Takahashi Y, Nagata T, Ishikawa K, Asai S. Screening for control genes in mouse hippocampus after transient forebrain ischemia using high-density oligonucleotide array. J Pharmacol Sci. 2006;101:52–57. doi: 10.1254/jphs.fp0050881. [DOI] [PubMed] [Google Scholar]
- 17.Baldwin CT, Reginato AM, Smith C, Jimenez SA, Prockop DJ. Structure of cDNA clones coding for human type II procollagen. The alpha 1(II) chain is more similar to the alpha 1(I) chain than two other alpha chains of fibrillar collagens. Biochem J. 1989;262:521–528. doi: 10.1042/bj2620521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohtaki H, Ylostalo JH, Foraker JE, Robinson AP, Reger RL, Shioda S, et al. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci U S A. 2008;105:14638–14643. doi: 10.1073/pnas.0803670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ylostalo J, Bazhanov N, Prockop DJ. Reversible commitment to differentiation by human multipotent stromal cells in single-cell-derived colonies. Exp Hematol. 2008;36:1390–1402. doi: 10.1016/j.exphem.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minina E, Kreschel C, Naski MC, Ornitz DM, Vortkamp A. Interaction of FGF, Ihh/Pthlh, and BMP signaling integrates chondrocyte proliferation and hypertrophic differentiation. Dev Cell. 2002;3:439–449. doi: 10.1016/s1534-5807(02)00261-7. [DOI] [PubMed] [Google Scholar]
- 21.Minina E, Wenzel HM, Kreschel C, Karp S, Gaffield W, McMahon AP, et al. BMP and Ihh/PTHrP signaling interact to coordinate chondrocyte proliferation and differentiation. Development. 2001;128:4523–4534. doi: 10.1242/dev.128.22.4523. [DOI] [PubMed] [Google Scholar]
- 22.Lipinski RJ, Hutson PR, Hannam PW, Nydza RJ, Washington IM, Moore RW, et al. Dose- and route-dependent teratogenicity, toxicity, and pharmacokinetic profiles of the hedgehog signaling antagonist cyclopamine in the mouse. Toxicol Sci. 2008;104:189–197. doi: 10.1093/toxsci/kfn076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levi B, James AW, Nelson ER, Li S, Peng M, Commons GW, et al. Human adipose-derived stromal cells stimulate autogenous skeletal repair via paracrine Hedgehog signaling with calvarial osteoblasts. Stem Cells Dev. 20:243–257. doi: 10.1089/scd.2010.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fibbe WE, Nauta AJ, Roelofs H. Modulation of immune responses by mesenchymal stem cells. Ann N Y Acad Sci. 2007;1106:272–278. doi: 10.1196/annals.1392.025. [DOI] [PubMed] [Google Scholar]
- 25.Le Blanc K. Mesenchymal stromal cells: Tissue repair and immune modulation. Cytotherapy. 2006;8:559–561. doi: 10.1080/14653240601045399. [DOI] [PubMed] [Google Scholar]
- 26.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 27.Larson BL, Ylostalo J, Prockop DJ. Human multipotent stromal cells undergo sharp transition from division to development in culture. Stem Cells. 2008;26:193–201. doi: 10.1634/stemcells.2007-0524. [DOI] [PubMed] [Google Scholar]
- 28.Lee RH, Hsu SC, Munoz J, Jung JS, Lee NR, Pochampally R, et al. A subset of human rapidly self-renewing marrow stromal cells preferentially engraft in mice. Blood. 2006;107:2153–2161. doi: 10.1182/blood-2005-07-2701. [DOI] [PubMed] [Google Scholar]
- 29.Katoh Y, Katoh M. Hedgehog target genes: mechanisms of carcinogenesis induced by aberrant hedgehog signaling activation. Curr Mol Med. 2009;9:873–886. doi: 10.2174/156652409789105570. [DOI] [PubMed] [Google Scholar]
- 30.Lin AC, Seeto BL, Bartoszko JM, Khoury MA, Whetstone H, Ho L, et al. Modulating hedgehog signaling can attenuate the severity of osteoarthritis. Nat Med. 2009;15:1421–1425. doi: 10.1038/nm.2055. [DOI] [PubMed] [Google Scholar]
- 31.Fischer J, Dickhut A, Rickert M, Richter W. Human articular chondrocytes secrete parathyroid hormone-related protein and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis. Arthritis Rheum. 2010;62:2696–2706. doi: 10.1002/art.27565. [DOI] [PubMed] [Google Scholar]
- 32.Sekiya I, Vuoristo JT, Larson BL, Prockop DJ. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci U S A. 2002;99:4397–4402. doi: 10.1073/pnas.052716199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science. 1996;273:613–622. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- 34.Deschaseaux F, Sensebe L, Heymann D. Mechanisms of bone repair and regeneration. Trends Mol Med. 2009;15:417–429. doi: 10.1016/j.molmed.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Bien-Willner GA, Stankiewicz P, Lupski JR. SOX9cre1, a cis-acting regulatory element located 1. 1 Mb upstream of SOX9, mediates its enhancement through the SHH pathway. Hum Mol Genet. 2007;16:1143–1156. doi: 10.1093/hmg/ddm061. [DOI] [PubMed] [Google Scholar]
- 36.Park J, Zhang JJ, Moro A, Kushida M, Wegner M, Kim PC. Regulation of Sox9 by Sonic Hedgehog (Shh) is essential for patterning and formation of tracheal cartilage. Dev Dyn. 2010;239:514–526. doi: 10.1002/dvdy.22192. [DOI] [PubMed] [Google Scholar]
- 37.Kim YJ, Kim HJ, Im GI. PTHrP promotes chondrogenesis and suppresses hypertrophy from both bone marrow-derived and adipose tissue-derived MSCs. Biochem Biophys Res Commun. 2008;373:104–108. doi: 10.1016/j.bbrc.2008.05.183. [DOI] [PubMed] [Google Scholar]
- 38.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 39.Huang W, Chung UI, Kronenberg HM, de Crombrugghe B. The chondrogenic transcription factor Sox9 is a target of signaling by the parathyroid hormone-related peptide in the growth plate of endochondral bones. Proc Natl Acad Sci U S A. 2001;98:160–165. doi: 10.1073/pnas.011393998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rabie AB, Hagg U. Factors regulating mandibular condylar growth. Am J Orthod Dentofacial Orthop. 2002;122:401–409. doi: 10.1067/mod.2002.125713. [DOI] [PubMed] [Google Scholar]
- 41.Amling M, Neff L, Tanaka S, Inoue D, Kuida K, Weir E, et al. Bcl-2 lies downstream of parathyroid hormone-related peptide in a signaling pathway that regulates chondrocyte maturation during skeletal development. J Cell Biol. 1997;136:205–213. doi: 10.1083/jcb.136.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng L, Balakir R, Precht P, Horton WE., Jr Bcl-2 regulates chondrocyte morphology and aggrecan gene expression independent of caspase activation and full apoptosis. J Cell Biochem. 1999;74:576–586. [PubMed] [Google Scholar]
- 43.Aikawa T, Shirasuna K, Iwamoto M, Watatani K, Nakamura T, Okura M, et al. Establishment of bone morphogenetic protein 2 responsive chondrogenic cell line. J Bone Miner Res. 1996;11:544–553. doi: 10.1002/jbmr.5650110416. [DOI] [PubMed] [Google Scholar]
- 44.Duprez DM, Coltey M, Amthor H, Brickell PM, Tickle C. Bone morphogenetic protein-2 (BMP-2) inhibits muscle development and promotes cartilage formation in chick limb bud cultures. Dev Biol. 1996;174:448–452. doi: 10.1006/dbio.1996.0087. [DOI] [PubMed] [Google Scholar]
- 45.Sekiya I, Larson BL, Vuoristo JT, Reger RL, Prockop DJ. Comparison of effect of BMP-2, -4, and -6 on in vitro cartilage formation of human adult stem cells from bone marrow stroma. Cell Tissue Res. 2005;320:269–276. doi: 10.1007/s00441-004-1075-3. [DOI] [PubMed] [Google Scholar]
- 46.Glansbeek HL, van Beuningen HM, Vitters EL, Morris EA, van der Kraan PM, van den Berg WB. Bone morphogenetic protein 2 stimulates articular cartilage proteoglycan synthesis in vivo but does not counteract interleukin-1alpha effects on proteoglycan synthesis and content. Arthritis Rheum. 1997;40:1020–1028. doi: 10.1002/art.1780400605. [DOI] [PubMed] [Google Scholar]
- 47.van Beuningen HM, Glansbeek HL, van der Kraan PM, van den Berg WB. Differential effects of local application of BMP-2 or TGF-beta 1 on both articular cartilage composition and osteophyte formation. Osteoarthritis Cartilage. 1998;6:306–317. doi: 10.1053/joca.1998.0129. [DOI] [PubMed] [Google Scholar]
- 48.Bhargava MM, Attia ET, Murrell GA, Dolan MM, Warren RF, Hannafin JA. The effect of cytokines on the proliferation and migration of bovine meniscal cells. Am J Sports Med. 1999;27:636–643. doi: 10.1177/03635465990270051601. [DOI] [PubMed] [Google Scholar]
- 49.Seki K, Hata A. Indian hedgehog gene is a target of the bone morphogenetic protein signaling pathway. J Biol Chem. 2004;279:18544–18549. doi: 10.1074/jbc.M311592200. [DOI] [PubMed] [Google Scholar]
- 50.Cannon WD, Jr, Vittori JM. The incidence of healing in arthroscopic meniscal repairs in anterior cruciate ligament-reconstructed knees versus stable knees. Am J Sports Med. 1992;20:176–181. doi: 10.1177/036354659202000214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.