Summary
Because of the number of factors affecting the nutritional and metabolic status in patients with advanced chronic kidney disease or who are on maintenance dialysis, the prevention and treatment of protein-energy wasting (PEW) of chronic kidney disease should involve a comprehensive combination of maneuvers to diminish protein and energy depletion, in addition to therapies that will avoid further losses. The available evidence suggests that nutritional supplementation, administered orally or parenterally, is effective in the treatment of maintenance dialysis patients with PEW in whom oral dietary intake from regular meals cannot maintain adequate nutritional status. Increased oral nutrient intake during dialysis and at home is the ideal choice for this intervention. In clinical practice, the advantages of intradialytic oral nutritional supplements include proven efficacy and compliance. Therefore, at a minimum, oral nutritional supplementation given intradialytically should be attempted in maintenance dialysis patients with PEW, accompanied by individualized dietary advice for appropriate intake at home. In ones who cannot tolerate oral feeding, other forms of nutritional supplementation including intradialytic parenteral nutritional are a reasonable strategy. Although not proven conclusively, nutritional interventions in the form of supplementation may lead to considerable improvements in mortality, hospitalization, and treatment costs.
Keywords: Wasting, cachexia, ESRD, malnutrition, IDPN
Among the many risk factors that affect outcome of end-stage renal disease (ESRD) patients, especially those on maintenance dialysis, a state of metabolic and nutritional derangements, more aptly called protein-energy wasting (PEW) of chronic kidney disease (CKD), plays a major role.1,2 Multiple studies now indicate that PEW is associated closely with major adverse clinical outcomes and results in increased rates of hospitalization and death in ESRD patients. Given the significance of the problem, as well as the complexity of the pathophysiologic basis of PEW of CKD, it is evident that the prevention and treatment options of this comorbid condition are both critical and complex.
Because of the large number of factors affecting nutritional and metabolic status in patients with advanced CKD or who are on maintenance dialysis, prevention and treatment of PEW should involve a comprehensive combination of maneuvers to diminish protein and energy depletion, in addition to therapies that will avoid further losses (Table 1).3 In addition to a number of strategies discussed in detail in accompanying articles within this issue, there is a strong rationale for nutritional supplementation interventions among patients with ESRD. In the subsequent section, the rationale and efficacy of nutritional support for the chronically wasted CKD patient is discussed.
Table 1.
Suggested Table to Monitor Nutritional Status and Guide Therapy in Kidney Failure
| Simple (Monthly) Assessment |
Findings | Possible Interventions |
|---|---|---|
| BW Serum albumin |
Continuous decline or <85% IBW <4.0 g/dL |
Suspect uremic malnutrition and perform more detailed nutritional assessment |
| Serum creatinine | Relatively low predialysis values |
No intervention needed at this point |
| Detailed Assessment | Possible Interventions (simple) | |
| Serum prealbumin | <30 mg/dL, and/or | Dietary counseling: DPI ≥ 1.2 g/kg/d, energy intake 30–35 kcal/d |
| Serum transferrin | <200 mg/dL, and/or | |
| IGF-1 | <200 ng/mL, and/or | CHD and peritoneal dialysis |
| LBM and/or fat mass | Unexpected decrease | Increase dialysis dose to Kt/V > 1.4 |
| SGA | Worsening | Use biocompatible membranes |
| Upper gastrointestinal motility enhancer |
||
| CKD | ||
| Consider timely initiation of CDT | ||
|
Repeat Detailed Assessment (2–3 months from previous) |
Possible Interventions (moderate to complex) |
|
| Serum prealbumin | <30 mg/dL, and/or | Nutritional supplements: |
| Serum transferrin | <200 mg/dL, and/or | Oral, enteric tube feeding, IDPN (requires Medicare approval) |
| IGF-I | <200 ng/mL, and/or | |
| Serum creatinine | Relatively low predialysis values, and/or |
Anabolic factors (experimental): rhGH, rhIGF-I |
| LBM and/or fat mass | ||
| C-reactive protein< | unexpected decrease >10 mg/L |
Appetite stimulants (experimental) |
| Anti-inflammatory (experimental) |
Adapted with permission from Pupim et al.38
Abbreviations: BW, body weight; IBW, ideal body weight; IGF-1, insulin-like growth factor-1; LBM, lean body mass; SGA, subjective global assessment; DPI, dietary protein intake; CDT, chronic dialysis treatment.
WHY PROVIDE NUTRITIONAL SUPPLEMENTATION TO PATIENTS WITH ADVANCED CKD?
The observation that CKD patients decrease their protein and energy intake as they lose their kidney function4 has led the nephrology community to believe that uremia per se is a net protein catabolic state, primarily owing to the decreased nutrient intake.5 However, in an intriguing editorial, Lim and Kopple6 suggested that CKD per se, even when advanced, does not engender net protein breakdown. They based this conclusion on published nitrogen balance studies as well as whole-body amino acid turnover kinetics,7,8 which show that there is a concomitant decrease in both protein synthesis and degradation in patients with advanced CKD, resulting in a net nitrogen balance not different from matched healthy controls, albeit at a significantly low protein turnover rate. This is a physiologically expected adaptation because the rate of protein turnover is related directly to the production of certain end-products known as uremic toxins that accumulate in advanced CKD.
These observations indicate that advanced CKD leads to a syndrome of metabolic and nutritional abnormalities that result in a low protein turnover state, but not necessarily with significant impact on net whole-body protein balance. A decreased dietary protein and energy intake, regardless of the cause (ie, anorexia of advanced CKD or prescription of low dietary protein intake) can be compensated with an adjustment in the protein degradation with no significant impact on net balance. Therefore, clinically stable advanced CKD patients are able to preserve their protein stores throughout the progression of kidney disease, even in the setting of decreased dietary protein intake. However, the net gain or loss of cell and tissue protein in human beings ultimately is determined by a balance between 2 opposite processes, protein synthesis and degradation. At times of accelerated protein degradation owing to increased metabolic needs, such as acute illnesses or stress conditions, it is likely that these patients cannot initiate the appropriate compensatory mechanisms, such as increased protein synthesis. The lack of response can be owing to either inadequate dietary intake or a defect in incorporation of the available nutrients inherent to uremia or concurrent illnesses. Ikizler et al9 examined this issue in 18 maintenance hemodialysis (MHD) patients who were admitted to a regular ward for various reasons. Their results showed that hospitalized MHD patients had inadequate protein and energy intake, and this was evidenced by dietary protein and energy intake levels of 66% and 50% of suggested values, respectively. Nitrogen balance was negative in 12 of 18 patients by an average of −2.11 ± 2.77 g of nitrogen/day (range, −9.91 to +3.89 g of nitrogen/d). In a subsequent study, Steiber et al10 examined dietary protein and calorie intake in 42 MHD patients. In this sample, the mean protein and calorie intakes were 10 kcal/kg and 0.4 g protein/kg. During the first 48 hours of hospitalization, only 14% of the patients met their estimated kcal needs and only 7% met their protein needs. Therefore, it is obvious that some form of nutritional supplementation is necessary in hospitalized acutely catabolic maintenance dialysis patients to avoid the development of PEW.1
Dialysis Therapy as a Catabolic Stimulus
A number of studies have shown that although the recommended dietary protein intake level of 0.6 to 0.8 g/kg/d is safe for stable CKD patients not yet on maintenance dialysis, recommended levels of protein and energy intake are relatively higher for ESRD patients (1.2 g/kg/d and 35 kcal/kg/d, respectively).11,12 As noted in the aforementioned discussion, this is primarily owing to the increased metabolic needs of ESRD patients with ongoing catabolic processes such as hospitalization. An additional cause of increased metabolic and nutritional stress is the dialytic therapy. Until recently, data from previous studies have been unclear as to the effects of the hemodialysis procedure on protein and energy homeostasis. Borah et al13 studied nitrogen balance on dialysis and nondialysis days during high (1.4 g/kg/d) and low (0.5 g/kg/d) protein intake. They found that patients were in negative nitrogen balance on all days on the low-protein diet. However, patients were in negative nitrogen balance on dialysis days (but not on nondialysis days) even with high protein intake. In addition, the rate of nitrogen generation was higher on dialysis days, particularly in the hours immediately after dialysis, suggesting that dialysis is a catabolic event. Several recent studies unequivocally showed that the catabolic effects of hemodialysis, especially on the protein homeostasis, are profound, affecting both whole-body and skeletal muscle protein homeostasis. All of these careful metabolic studies consistently showed a decrease in protein synthesis at the whole-body level and one specific study showed an additional increase in whole-body protein breakdown.14 In addition, 2 separate studies showed a significant increase in net skeletal muscle protein breakdown; in one study, these undesirable effects persisted for at least 2 hours after the completion of hemodialysis.15,16
Can the Protein Catabolic Effects of Hemodialysis Procedure Be Prevented or Treated?
The tendency towards PEW instigated by decreased protein and energy intake in this population, coupled with increased protein catabolism and energy expenditure during the dialysis procedure, all potentially could be ameliorated by increasing nutrient intake through supplementation, especially during dialysis. Nutritional supplementation in the ESRD population, as in others, can be delivered in oral or parenteral form.
Oral Nutritional Supplementation
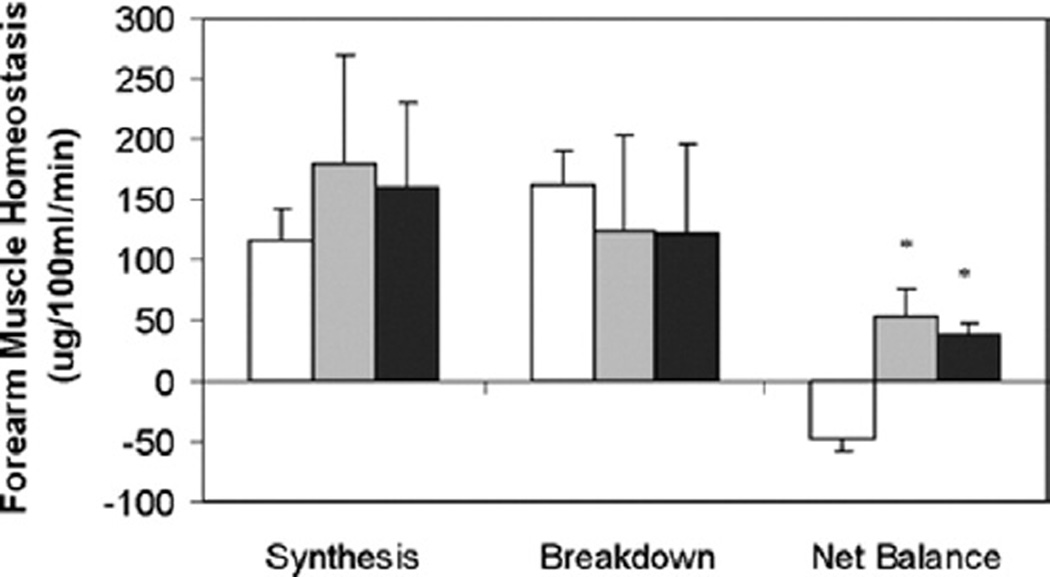
The effectiveness of oral nutritional supplementation has not been clear-cut in ESRD patients. Until recently, the results have been mixed, and most available studies are hampered by design and power issues. Nevertheless, several more recent reports have provided intriguing data on the effectiveness of oral nutritional supplementation in ESRD patients, especially when provided intradialytically. In a detailed metabolic study, Veeneman et al17 reported the effects of feeding during hemodialysis on whole-body protein balance using stable isotope tracer methodology. The feeding was in the form of yogurt, cream, and protein-enriched milk powder, given as 6 equal portions during the hemodialysis procedure as well as on a nondialysis day. Their results showed that consumption of a protein- and energy-enriched meal during hemodialysis resulted in a positive protein balance to the same extent as on a nondialysis day. In a more recent study, Pupim et al18 examined the efficacy of intradialytic oral nutritional supplementation in comparison with no supplementation or intradialytic parenteral nutrition (IDPN) supplementation in 8 MHD patients with signs of PEW. Both IDPN supplementation and intradialytic oral nutritional supplementation resulted in highly positive whole-body net balance, as compared with neutral balance in the control session when no supplementation was provided. Similarly, skeletal muscle protein homeostasis during hemodialysis also improved with both IDPN and intradialytic oral nutrition as compared with the unsupplemented group (Fig. 1). Although the anabolic effects of parenteral supplementation dissipated in the postdialytic period, oral supplementation led to sustained anabolic effects.
Figure 1.
Forearm muscle protein homeostasis dynamic components during HD, comparing control (□), IDPN ( ), and PO (■) in 8 CHD patients with deranged nutritional status. Skeletal muscle protein homeostasis during HD improved with both IDPN and PO versus control (P = .005 and .009 for IDPN versus control and PO versus control, respectively). Oral supplementation resulted in persistent anabolic benefits in the post-HD phase for muscle protein metabolism, when anabolic benefits of IDPN dissipated (data not shown in figure). Units are ug/100 mL/min. *P < .05 versus control. Adapted with permission from American Society of Nephrology.18
), and PO (■) in 8 CHD patients with deranged nutritional status. Skeletal muscle protein homeostasis during HD improved with both IDPN and PO versus control (P = .005 and .009 for IDPN versus control and PO versus control, respectively). Oral supplementation resulted in persistent anabolic benefits in the post-HD phase for muscle protein metabolism, when anabolic benefits of IDPN dissipated (data not shown in figure). Units are ug/100 mL/min. *P < .05 versus control. Adapted with permission from American Society of Nephrology.18
The studies by Veeneman et al17 and Pupim et al18 indicated that oral feeding of MHD patients results in acute improvements in protein balance. However, these studies were not designed to establish whether the apparent short-term benefits of oral nutritional supplementation will translate into long-term improvements in the overall nutritional status of the MHD patient with PEW. Several studies have provided stimulating data regarding beneficial effects of prolonged oral nutritional supplementation in maintenance dialysis patients. Caglar et al19 reported that intradialytic oral nutritional supplementation improved several nutritional parameters (including serum albumin and serum prealbumin concentrations as well as subjective global assessment) in a large group of MHD patients with PEW. A significant aspect of this study was that nutritional supplementation was given during HD, which not only improved compliance to the treatment but also provided supplements at a time when catabolism was at its highest level in these patients.19 By using a different approach, Eustace et al20 reported that oral amino acid supplements, administered 3 times a day over 3 months, significantly improved serum albumin concentration in MHD patients in a prospective, randomized, placebocontrolled pilot study. Of note, subjects in the very low serum albumin strata (<3.5 g/dL) improved more than those in the low albumin strata (3.5–3.8 g/dL, P < .01). Improvements also were seen in grip strength and SF-12 mental health score. These effects were more pronounced in the CHD patients than in peritoneal dialysis patients. More recently, Kalantar-Zadeh et al21 reported in a controlled-design study that in hypoalbuminemic MHD patients, a short-term (4 weeks) in-center intradialytic oral nutritional intervention was associated with a significant increase in serum albumin levels. The supplementation consisted of one can of Nepro (Abbott Nutrition, Columbus, OH) and one can of Oxepa (Abbott Nutrition, Columbus, OH) administered during HD and was also found to be practical, convenient, and well tolerated.
Daily (nondialytic) Oral Nutritional Supplementation
Although provision of nutrients during hemodialysis is an attractive approach, primarily because of the magnitude of the catabolic processes during dialysis, intradialytic oral nutrition by itself may be inadequate to achieve optimal dietary intake in certain subgroups of maintenance dialysis patients. For these patients, additional forms of supplementation such as enteral (including oral protein, amino acid tablets and energy supplementation, nasogastric tubes, and percutaneous endoscopic gastroscopy or jejunostomy tubes) can be considered.22 In a recent meta-analysis, Stratton et al23 performed a systematic review aimed at determining the potential benefits of enteral multinutrient support (oral or tube) in MHD patients. The outcome measures sought were clinical (quality of life, complications, and mortality), biochemical (serum albumin and electrolyte levels), and nutritional (dietary intake and anthropometry). The analysis included 18 studies (5 randomized controlled trials, 13 nonrandomized controlled trials) and suggested that enteral nutritional support increased total (energy and protein) intake and increased serum albumin concentration on average by 0.23 g/dL, with no adverse effects on electrolyte status (serum phosphate and potassium). The investigators also emphasized that the improvement in nutritional markers may well translate into improvement in clinical outcome, especially in patients with overt PEW.
Although provocative, the aforementioned studies can only be considered as preliminary. Despite a plethora of epidemiologic data and a number of rather suboptimal-designed interventional studies, it is important to recognize that causation cannot be inferred and that these findings warrant larger randomized clinical trials. The results of a recent, much larger magnitude, and better-designed study (French Intradialytic Nutrition Evaluation [FINE]) are now available to provide us such information, albeit with certain limitations (see below).24
IDPN
Although the gastrointestinal route is always preferred as the primary choice for nutritional supplementation, parenteral provision of nutrients, especially during the HD procedure (IDPN), has been shown to be a safe and convenient approach for individuals who cannot tolerate oral or enteral administration of nutrients. Several studies, although not all, have shown evidence for nutritional improvements with the use of IDPN in MHD patients with overt PEW. Many of these studies focusing on IDPN involved a limited sample size (which did not allow appropriate stratification of patients) over a short period of time, which hindered the ability of these trials to properly address the objective. Hence, the observed inconsistency of the results between these studies.25,26 The high cost of IDPN therapy and the regulatory concerns remain the greatest barriers to performing adequately powered clinical trials.27 As a result, there have been regulatory and financial concerns in advocating for the utilization of this potentially beneficial treatment.
To further explore its efficacy, we used stable isotope infusion techniques to directly measure specific components of protein and energy metabolism in MHD patients after administration of IDPN.28 We performed a randomized cross-over study in which all the patients were studied with and without IDPN (IDPN and control protocols). The results showed that IDPN promoted a 96% increase in whole-body protein synthesis and a 50% decrease in wholebody proteolysis as compared with the control protocol. In addition, IDPN provided significantly higher forearm muscle protein synthesis compared with the control (260%). Although there were no differences in forearm muscle proteolysis between protocols, the net result was a change from negative (muscle loss) to positive (muscle accretion) balance during IDPN administration. The clinical relevance of this gain can be appreciated when one calculates that during the 3.5 hours when IDPN was being infused during hemodialysis, approximately 51.5 g of whole-body protein were anabolized compared with an essentially catabolic process in the absence of IDPN. If the body’s fat-free mass is 73% water, the observed changes account for an uptake of an additional 191 g of fat-free mass gain as a result of the IDPN treatment. In a subsequent study, our laboratory also reported the effects of IDPN administration on the albumin fractional synthetic rate in 7 CHD patients using stable isotope methodology.29 The results of this study indicated that IDPN increases the hepatic synthesis of albumin as a part of an improvement in the whole-body protein homeostasis.
These preliminary observations provide support to the limited number of long-term clinical studies reporting benefits to IDPN administration in ESRD patients. Cano et al,30 in a randomized controlled study, reported improvements in multiple nutritional parameters with IDPN in a group of 26 malnourished chronic hemodialysis patients. In a retrospective analysis of more than 1,500 chronic hemodialysis patients treated with IDPN, Chertow et al25 reported a decreased risk of death with the use of IDPN, particularly in patients with serum albumin concentrations less than 3.5 g/dL and serum creatinine concentrations less t han 8 mg/dL; these patients showed substantial improvements in the nutritional parameters after use of IDPN. Over a 9-month period, Mortelmans et al26 prospectively evaluated 26 chronic hemodialysis malnourished patients who failed to improve with diet counseling. They reported significant increases in body weight, fat mass, and triceps skinfold with IDPN.
Similar studies using amino acids in dialysate (AAD) as a nutritional intervention in malnourished peritoneal dialysis patients also have provided conflicting results. It is worth mentioning that patients on peritoneal dialysis are prone to muscle wasting through different mechanisms, and therefore the observations regarding the causes and treatment strategies for PEW in MHD patients cannot be readily extrapolated to PD patients. Although detailed metabolic studies examining the role of amino acid and protein losses on protein turnover have not been performed, 2 metabolic studies have indicated beneficial effects of amino acid supplementation through dialysate. On the other hand, a long-term clinical trial did not show a conclusive nutritional improvement through such a strategy in PD patients.31–33 Jones et al32 reported benefits with 1 or 2 exchanges per day of AAD with increases in serum transferrin and total protein concentrations as well as a tendency for plasma amino acid profiles to become more normal. Of interest, there were significant improvements in serum albumin and prealbumin concentrations in malnourished peritoneal dialysis patients, particularly in those who had serum albumin concentrations in the lowest tertile.32 It also should be noted that an increase in serum urea concentrations associated with exacerbation of uremic symptoms, as well as metabolic acidosis, remains a potential complication of AAD.34
The FINE Study
Overall, the earlier-mentioned data suggest that IDPN and AAD may be useful in the treatment of MHD patients with PEW and offers an alternative method of nutritional intervention in patients in whom oral or enteral intake cannot be maintained. As is the case for oral nutritional supplementation, these data can be considered only as preliminary, and there is a need for large-scale, well-designed, nutritional intervention studies of IDPN in chronic dialysis patients with overt PEW. The results of a recent study may shed some light on this controversy, albeit with inherent limitations. Cano et al24 reported the results of the largest and arguably best executed nutritional intervention study in MHD patients with PEW. Despite the negative tone of the article’s title, the study actually provided encouraging data on nutritional supplementation efforts in these individuals. In this large randomized clinical trial, the investigators of the FINE study randomly assigned 186 MHD patients with PEW to receive 1 year of IDPN and oral nutritional supplementation or oral supplementation alone.24 After stratification by center, patients were randomized to receive IDPN (for 1 year) and standard oral supplements providing 500 kcal/d and 25 g/d protein, or oral supplements alone. The nutritional supplement goal was to bring patients’ intakes up to the recommended amounts of 30 to 35 kcal/ kg/d and 1.2 g/kg/d, respectively. The primary outcome, 2-year mortality, was similar in the 2 groups (39% in the control group and 43% in the IDPN group), suggesting that oral nutritional supplementation is equally effective as IDPN when oral intake is possible. Increases in serum prealbumin were associated with decreases in 2-year mortality and hospitalization rates, providing prospective evidence of a link between response to nutritional therapy and improved outcomes.
Despite the negative primary outcome of the study, there were several important observations of the FINE study that provide much cause for optimism First, as the investigators point out, the route of administration of nutritional supplementation (ie, oral or combined oral–parenteral) does not have any significant effect on survival in MHD patients with PEW, assuming that equal and adequate amounts of protein and calories are provided. Similarly, the route of administration does not influence the improvements in most nutritional markers that are observed after supplementation. These findings are not unexpected; several reports have shown that intradialytic oral and parenteral nutritional supplementation improve whole-body and skeletal muscle protein homeostasis to a comparable extent in the short term (Fig. 1).18
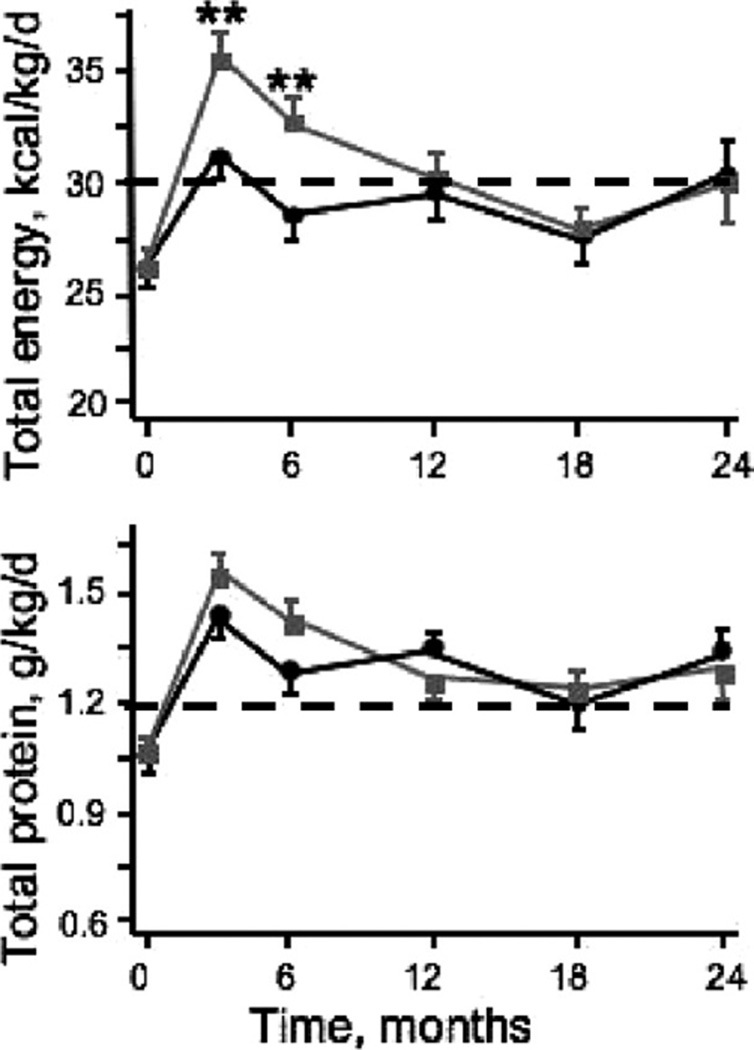
Second, despite the lack of an appropriate control group, the results of the FINE study imply that nutritional supplementation does indeed improve nutritional markers in CHD patients with PEW if the targets for dietary protein and energy intake recommended by the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (>1.2 g/kg/d and >30 kcal/kg/d, respectively) are achieved (Fig. 2). It is of note that the improvement in serum albumin level reported by Cano et al24 (~2 g/L) is highly consistent with that described in the majority of other published studies reporting the effectiveness of nutritional interventions.23 These data also confirm the appropriateness of the Kidney Disease Outcomes Quality Initiative dietary protein and calorie intake guidelines.35
Figure 2.
Changes in total energy and protein intakes during the 2-year follow-up evaluation in control (black line) and IDPN (gray line) groups (means ± SEM) in the FINE study participants. Although there were between-group differences in energy intake at months 3 and 6 (P < .01), both groups achieved the minimum K/DOQI recommended thresholds for protein and energy intake in maintenance hemodialysis patients (dotted lines). In both groups, nutritional support induced comparable increases in serum albumin levels at months 3, 6, 12, and 18 (P < .01) and in serum prealbumin levels at months 3 to 24 (P < .02). Adapted with permission from American Society of Nephrology.24
Third, the results imply that nutritional interventions in general improve survival in MHD patients. This conclusion, however, should be applied with caution because the study did not include a no-intervention arm, and the nutritional improvements may just reflect a regression-to-the-mean phenomenon. Although this was a critical limitation of the study, the investigators appropriately noted that it would have been unethical to withhold nutritional therapy. One can, however, compare the overall 2-year mortality rate in the study (42%) with the published mortality rate obtained from European registry data, adjusted for at least one of the FINE study inclusion criteria (a serum albumin level <35 g/L; 49%). This comparison indicates an approximately 15% improvement in overall mortality, a survival benefit that, if it is a treatment effect, is unmatched by any other proposed therapy for high-risk CHD patients to date. Finally, the results indicate that simple nutritional markers, such as serum prealbumin level, can be used as surrogate markers not only of nutritional status but also possibly of hospitalization and survival.
CHANGING PARADIGMS IN NUTRITIONAL AND METABOLIC MANAGEMENT OF CKD PATIENTS ON MAINTENANCE DIALYSIS THERAPY
There is now indisputable evidence to indicate that the dietary protein and energy requirements of ESRD patients are much higher than the general population based on clearcut scientific rationale. Although advanced kidney disease per se may not induce a highly protein and energy catabolic state, it is conducive to the development of PEW owing to associated metabolic derangements and comorbid conditions. Recurrent hospitalization episodes are an obvious potentially preventable cause. Certain conditions associated with advanced kidney disease (ie, chronic inflammation, insulin resistance, and insulin deficiency) can be the additional culprits of the progressive muscle wasting observed in these patients. The hemodialysis procedure is an additional apparent protein catabolic procedure, a potential justification for intradialytic nutritional supplementation.
An equally important issue to consider in maintenance dialysis patients is the relevance of overweight and obesity. Despite the potential adverse consequences of obesity in earlier stages of kidney disease, there is now a plethora of epidemiologic studies indicating that higher body mass index, regardless of its etiology (ie, increased adiposity and/or lean body mass), is associated with significantly better survival in ESRD patients.36 Although the exact mechanism(s) underlying this association have not been elucidated, it points to a potentially beneficial effect of increasing the protein and energy intakes to levels higher than those required to maintain a neutral nitrogen balance alone, if weight gain is one potential outcome of this intervention.
Finally, it also is important to assess the impact of nutritional supplements not only in terms of changes in nutritional parameters, but to extrapolate these observations to potential improvements in hospitalization, mortality, and cost effectiveness. In a recent study, Lacson et al37 showed that an increase in serum albumin concentration in the order of 2 g/L, the average improvement reported in most if not all nutritional intervention studies, in 50% of the US dialysis population was associated with projections of approximately 1,400 lives saved, approximately 6,000 hospitalizations averted, and approximately $36 million in Medicare cost savings resulting from a reduction of approximately 20,000 hospital days over 1 year (Table 2).7
Table 2.
Projections of Possible Impact of a Systematic Intervention That Improves Albumin Level by 0.2 g/dL in 25% to 75% of Patients With a Baseline Serum Albumin Level of 3.5 g/dL or Less From the FMCNA Dialysis Population
| FMCNA Population, Possible Impact of Intervention |
Percentage of Patients With Albumin Level ≤3.5 g/dL Improved by 0.2 g/dL |
||
|---|---|---|---|
| 25% | 50% | 75% | |
| Number of hospitalization potential events avoided (%) |
823 (0.80) | 1,646 (1.59) | 2,468 (2.59) |
| Potential hospital days avoided (%) | 2,624 (0.67) | 5,247 (1.35) | 7,871 (2.02) |
| Potential percentage decline in crude death rate | −0.24 | −0.48 | −0.72 |
| Potential lives saved | 189 | 369 | 553 |
Abbreviations: FMCNA, Fresenius Medical Care, North America.
Reprinted with permission from Lacson et al.37
SUMMARY
In summary, the available evidence suggests that nutritional supplementation, administered orally or parenteraly, is effective in the treatment of maintenance dialysis patients with PEW in whom oral dietary intake from regular meals cannot maintain adequate nutritional stores. Clearly, increased oral nutrient intake during dialysis and at home is ideal. In clinical practice, the advantages of intradialytic oral nutritional supplements include proven efficacy and compliance. Therefore, at a minimum, oral nutritional supplementation given intradialytically should be attempted in maintenance dialysis patients with PEW, accompanied by individualized dietary advice for appropriate intake at home. In persons who cannot tolerate oral feeding, using other forms of nutritional intake, including IDPN, is a reasonable strategy. Although not proven conclusively, nutritional interventions in the form of supplementation that increase serum albumin by 0.2 g/dL or greater may lead to considerable improvements in mortality, hospitalization, and treatment costs.
Acknowledgments
This work is supported in part by National Institutes of Health grants R01-DK45604, R01-HL070938, K24-DK62849, and UL1 RR024975.
REFERENCES
- 1.Ikizler TA, Hakim RM. Nutrition in end-stage renal disease. Kidney Int. 1996;50:343–357. doi: 10.1038/ki.1996.323. [DOI] [PubMed] [Google Scholar]
- 2.Kopple JD. Effect of nutrition on morbidity and mortality in maintenance dialysis patients. Am J Kidney Dis. 1994;24:1002–1009. doi: 10.1016/s0272-6386(12)81075-4. [DOI] [PubMed] [Google Scholar]
- 3.Johansen KL, Painter PL, Sakkas GK, Gordon P, et al. Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: a randomized, controlled trial. J Am Soc Nephrol. 2006;17:2307–2314. doi: 10.1681/ASN.2006010034. [DOI] [PubMed] [Google Scholar]
- 4.Ikizler TA, Greene J, Wingard RL, Parker RA, et al. Spontaneous dietary protein intake during progression of chronic renal failure. J Am Soc Nephrol. 1995;6:1386–1391. doi: 10.1681/ASN.V651386. [DOI] [PubMed] [Google Scholar]
- 5.Anderstam B, Mamoun AH, Sodersten P, Bergstrom J. Middle-sized molecule fractions isolated from uremic ultrafiltrate and normal urine inhibit ingestive behavior in the rat. J Am Soc Nephrol. 1996;7:2453–2460. doi: 10.1681/ASN.V7112453. [DOI] [PubMed] [Google Scholar]
- 6.Lim VS, Kopple JD. Protein metabolism in patients with chronic renal failure: role of uremia and dialysis. Kidney Int. 2000;58:1–10. doi: 10.1046/j.1523-1755.2000.00135.x. [DOI] [PubMed] [Google Scholar]
- 7.Reaich D, Channon SM, Scrimgeour CM, Daley SE, et al. Correction of acidosis in humans with CRF decreases protein degradation and amino acid oxidation. Am J Physiol. 1993;265:E230–E235. doi: 10.1152/ajpendo.1993.265.2.E230. [DOI] [PubMed] [Google Scholar]
- 8.Lim VS, Yarasheski KE, Flanigan MJ. The effect of uraemia, acidosis, and dialysis treatment on protein metabolism: a longitudinal leucine kinetic study. Nephrol Dial Transplant. 1998;13:1723–1730. doi: 10.1093/ndt/13.7.1723. [DOI] [PubMed] [Google Scholar]
- 9.Ikizler TA, Greene JH, Wingard RL, Hakim RM. Nitrogen balance in hospitalized chronic hemodialysis patients. Kidney Int Suppl. 1996;50:S53–S56. [PubMed] [Google Scholar]
- 10.Steiber AL, Weatherspoon LJ, Handu D. Clinical and dietary indicators associated with uremic status in hospitalized dialysis patients. J Ren Nutr. 2002;12:49–54. doi: 10.1053/jren.2002.28353. [DOI] [PubMed] [Google Scholar]
- 11.K/DOQI, National Kidney Foundation. Clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis. 2000;35:S1–S140. doi: 10.1053/ajkd.2000.v35.aajkd03517. [DOI] [PubMed] [Google Scholar]
- 12.Kopple J, Massry S. Nutritional management of renal disease. Baltimore, MD: Williams & Wilkins; 1997. p. 929. [Google Scholar]
- 13.Borah M, Schoenfeld P, Gotch F, Sargent J, et al. Nitrogen balance during intermittent dialysis therapy of uremia. Kidney Int. 1978;14:491–500. doi: 10.1038/ki.1978.154. [DOI] [PubMed] [Google Scholar]
- 14.Lim VS, Ikizler TA, Raj DS, Flanigan MJ. Does hemodialysis increase protein breakdown? Dissociation between whole-body amino acid turnover and regional muscle kinetics. J Am Soc Nephrol. 2005;16:862–868. doi: 10.1681/ASN.2004080624. [DOI] [PubMed] [Google Scholar]
- 15.Ikizler TA, Pupim RB, Brouillette JR, Levenhagen DK, et al. Hemodialysis stimulates muscle and whole-body protein loss and alters substrate oxidation. Am J Physiol Endocrinol Metab. 2002;282:E107–E116. doi: 10.1152/ajpendo.2002.282.1.E107. [DOI] [PubMed] [Google Scholar]
- 16.Raj DS, Zager P, Shah VO, Dominic EA, et al. Protein turnover and amino acid transport kinetics in endstage renal disease. Am J Physiol Endocrinol Metab. 2004;286:E136–E143. doi: 10.1152/ajpendo.00352.2003. [DOI] [PubMed] [Google Scholar]
- 17.Veeneman JM, Kingma HA, Boer TS, Stellaard F, et al. Protein intake during hemodialysis maintains a positive whole body protein balance in chronic hemodialysis patients. Am J Physiol Endocrinol Metab. 2003;284:E954–E965. doi: 10.1152/ajpendo.00264.2002. [DOI] [PubMed] [Google Scholar]
- 18.Pupim LB, Majchrzak KM, Flakoll PJ, Ikizler TA. Intradialytic oral nutrition improves protein homeostasis in chronic hemodialysis patients with deranged nutritional status. J Am Soc Nephrol. 2006;17:3149–3157. doi: 10.1681/ASN.2006040413. [DOI] [PubMed] [Google Scholar]
- 19.Caglar K, Fedje L, Dimmitt R, Hakim RM, et al. Therapeutic effects of oral nutritional supplementation during hemodialysis. Kidney Int. 2002;62:1054–1059. doi: 10.1046/j.1523-1755.2002.00530.x. [DOI] [PubMed] [Google Scholar]
- 20.Eustace JA, Coresh J, Kutchey C, Te PL, et al. Randomized double-blind trial of oral essential amino acids for dialysis-associated hypoalbuminemia. Kidney Int. 2000;57:2527–2538. doi: 10.1046/j.1523-1755.2000.00112.x. [DOI] [PubMed] [Google Scholar]
- 21.Kalantar-Zadeh K, Braglia A, Chow J, Kwon O, et al. An anti-inflammatory and antioxidant nutritional supplement for hypoalbuminemic hemodialysis patients: a pilot/feasibility study. J Ren Nutr. 2005;15:318–331. doi: 10.1016/j.jrn.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Cockram DB, Hensley MK, Rodriguez M, Agarwal G, et al. Safety and tolerance of medical nutritional products as sole sources of nutrition in people on hemodialysis. J Ren Nutr. 1998;8:25–33. doi: 10.1016/s1051-2276(98)90034-6. [DOI] [PubMed] [Google Scholar]
- 23.Stratton RJ, Bircher G, Fouque D, Stenvinkel P, et al. Multinutrient oral supplements and tube feeding in maintenance dialysis: a systematic review and meta-analysis. Am J Kidney Dis. 2005;46:387–405. doi: 10.1053/j.ajkd.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 24.Cano NJ, Fouque D, Roth H, Aparicio M, et al. Intradialytic parenteral nutrition does not improve survival in malnourished hemodialysis patients: a 2-year multicenter, prospective, randomized study. J Am Soc Nephrol. 2007;18:2583–2591. doi: 10.1681/ASN.2007020184. [DOI] [PubMed] [Google Scholar]
- 25.Chertow GM, Ling J, Lew NL, Lazarus JM, et al. The association of intradialytic parenteral nutrition with survival in hemodialysis patients. Am J Kidney Dis. 1994;24:912–920. doi: 10.1016/s0272-6386(12)81060-2. [DOI] [PubMed] [Google Scholar]
- 26.Mortelmans AK, Duym P, Vanderbroucke J, De-Smet R, et al. Intradialytic parenteral nutrition in malnourished hemodialysis patients: a prospective long-term study. JPEN J Parenter Enteral Nutr. 1999;23:90–95. doi: 10.1177/014860719902300290. [DOI] [PubMed] [Google Scholar]
- 27.McCann LM, Foulks CJ. Nutritional recommendations for patients undergoing continous peritoneal dialysis. Semin Dial. 1992;5:136–141. [Google Scholar]
- 28.Pupim LB, Flakoll PJ, Brouillette JR, Levenhagen DK, et al. Intradialytic parenteral nutrition improves protein and energy homeostasis in chronic hemodialysis patients. J Clin Invest. 2002;110:483–492. doi: 10.1172/JCI15449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pupim L, Flakoll PJ, Ikizler TA. Nutritional supplementation acutely increases albumin fractional synthetic rate in chronic hemodialysis patients. J Am Soc Nephrol. 2004;15:1920–1926. doi: 10.1097/01.asn.0000128969.86268.c0. [DOI] [PubMed] [Google Scholar]
- 30.Cano N, Labastie-Coeyrehourq J, Lacombe P, Stroumza P, et al. Perdialytic parenteral nutrition with lipids and amino acids in malnourished hemodialysis patients. Am J Clin Nutr. 1990;52:726–730. doi: 10.1093/ajcn/52.4.726. [DOI] [PubMed] [Google Scholar]
- 31.Garibotto G, Sofia A, Canepa A, Saffioti S, et al. Acute effects of peritoneal dialysis with dialysates containing dextrose or dextrose and amino acids on muscle protein turnover in patients with chronic renal failure. J Am Soc Nephrol. 2001;12:557–567. doi: 10.1681/ASN.V123557. [DOI] [PubMed] [Google Scholar]
- 32.Jones M, Hagen T, Boyle CA, Vonesh E, et al. Treatment of malnutrition with 1.1% amino acid peritoneal dialysis solution: results of a multicenter outpatient study. Am J Kidney Dis. 1998;32:761–769. doi: 10.1016/s0272-6386(98)70131-3. [DOI] [PubMed] [Google Scholar]
- 33.Tjiong HL, van den Berg JW, Wattimena JL, Rietveld T, et al. Dialysate as food: combined amino acid and glucose dialysate improves protein anabolism in renal failure patients on automated peritoneal dialysis. J Am Soc Nephrol. 2005;16:1486–1493. doi: 10.1681/ASN.2004050402. [DOI] [PubMed] [Google Scholar]
- 34.Kopple JD, Bernard D, Messana J, Swartz R, et al. Treatment of malnourished CAPD patients with an amino acid based dialysate. Kidney Int. 1995;47:1148–1157. doi: 10.1038/ki.1995.164. [DOI] [PubMed] [Google Scholar]
- 35.Kopple JD. National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis. 2001;37:S66–S70. doi: 10.1053/ajkd.2001.20748. [DOI] [PubMed] [Google Scholar]
- 36.Kalantar-Zadeh K, Kuwae N, Wu DY, Shantouf RS, et al. Associations of body fat and its changes over time with quality of life and prospective mortality in hemodialysis patients. Am J Clin Nutr. 2006;83:202–210. doi: 10.1093/ajcn/83.2.202. [DOI] [PubMed] [Google Scholar]
- 37.Lacson E, Jr, Ikizler TA, Lazarus JM, Teng M, et al. Potential impact of nutritional intervention on ESRD hospitalization, death and treatment costs. J Renal Nutr. 2007;17:363–371. doi: 10.1053/j.jrn.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Pupim LB, Cuppari L, Ikizler TA. Nutrition and metabolism in kidney disease. Semin Nephrol. 2006;26:134–157. doi: 10.1016/j.semnephrol.2005.09.010. [DOI] [PubMed] [Google Scholar]