Abstract
Wnt signaling stabilizes β-catenin through the LRP6 receptor signaling complex, which antagonizes the β-catenin destruction complex. The Axin scaffold and associated glycogen synthase kinase-3 (GSK3) have central roles in both assemblies, but the transduction mechanism from the receptor to the destruction complex is contentious. We report that Wnt signaling is governed by phosphorylation regulation of Axin scaffolding function. Phosphorylation by GSK3 kept Axin activated (“open”) for β-catenin interaction and poised for engagement of LRP6. Formation of the Wnt-induced LRP6-Axin signaling complex promoted Axin dephosphorylation by protein phosphatase-1, and inactivated (“closed”) Axin through an intra-molecular interaction. Inactivation of Axin diminished its association with β-catenin and LRP6, thereby inhibiting β-catenin phosphorylation and enabling activated LRP6 to selectively recruit active Axin for inactivation reiteratively. Our findings reveal mechanisms for scaffold regulation and morphogen signaling.
Signaling by secreted Wnt morphogens governs developmental, homeostatic, and pathological processes by regulating β-catenin stability, and represents a critical target for cancer and disease therapeutics (1, 2). Without Wnt stimulation, cytosolic β-catenin concentrations are kept low because a “destruction complex” assembled by the Axin scaffold binds to β-catenin, Adenomatosis polyposis coli (APC), casein kinase-1α (CK1α), and glycogen synthase kinase-3 (GSK3), and promotes phosphorylation of β-catenin by CK1α and GSK3 thus ensuring β-catenin ubiquitination and degradation (1–3). Upon Wnt stimulation, a receptor complex on the cell surface is formed between Frizzled (Fz) and LDL receptor-related protein 6 (LRP6), resulting in phosphorylation and activation of LRP6 and its recruitment of Axin (4–7). Assembly of the Fz-LRP6 complex and associated Dishevelled (Dvl) and the Axin destruction complex, referred to collectively as the “LRP6 signaling complex (signalosome)”, inhibits phosphorylation of β-catenin thereby causing its stabilization (6–10). The mechanism by which LRP6 activation leads to β-catenin stabilization remains enigmatic (1, 2, 11).
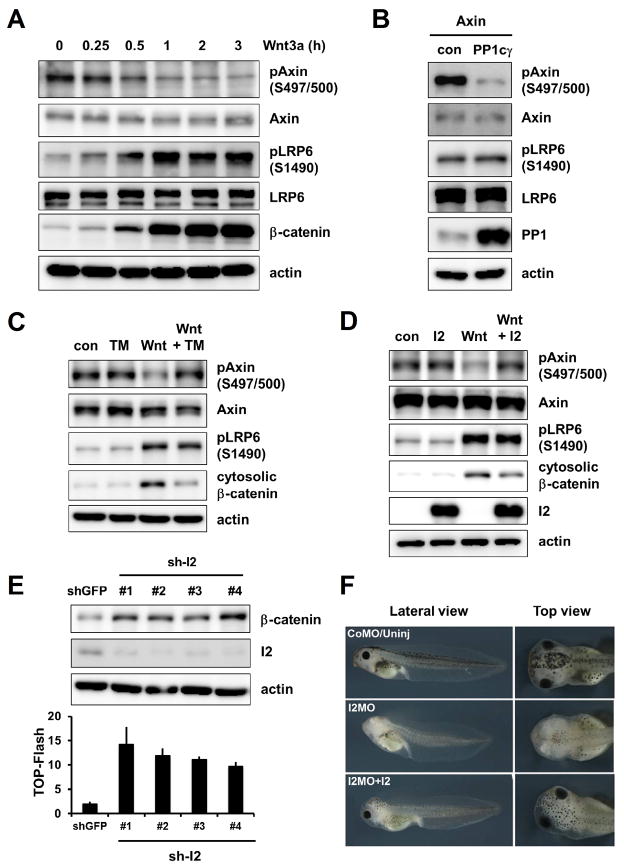
Axin is a phospho-protein and central to assemblies of both destruction (12–15) and signaling complexes (4–10), and becomes dephosphorylated upon Wnt stimulation (16, 17). We generated an antibody, Ab-pS497/500 (fig. S1A to S1C), for Axin phosphorylated at serines 497 and 500, which are GSK3 phosphorylation sites in vitro (18). Axin phosphorylation at S497/S500 was decreased within 15–30 min of Wnt3a treatment of mouse L fibroblasts (Fig. 1A), embryonic fibroblasts (fig. S1D), and human embryonic kidney (HEK) 293T cells (Fig. 1C and 1D). Wnt-induced dephosphorylation of Axin likely reflects the counterbalance between GSK3 and a protein phosphatase (PP) such as PP1, whose catalytic subunit, PP1c, was identified in an RNAi screen in Drosophila cells as a requirement for Wnt/β-catenin signaling (19). Through a functional cDNA overexpression screen in HEK293T cells, we identified PP1cγ, one of the three PP1c genes in the human genome (20), as an activator of the Wnt/β-catenin signaling reporter TOP-Flash (fig. S2A). PP1cγ overexpression decreased phosphorylation of Axin but not of LRP6 (Fig. 1B); a pharmacological PP1 inhibitor, Tautomycin (TM), prevented Wnt-induced dephosphorylation of Axin without affecting LRP6 phosphorylation (Fig. 1C and fig. S3).
Fig. 1.
Wnt-induced Axin dephosphorylation by PP1 and effects of I2 on Wnt signaling and Xenopus anteriorization. (A) Wnt3a-induced Axin dephosphorylation, LRP6 phosphorylation and β-catenin stabilization in L cells. Protein detections were performed by immunoblotting throughout the paper unless specified otherwise. actin: a loading control. (B) Effect of PP1cγ overexpression on phosphorylation of Axin but not LRP6 in HEK293T cells. (C and D) Effects of TM (C) or I2 overexpression (D) on Wnt3a-induced Axin dephosphorylation and β-catenin stabilization in HEK293T cells. (E) Effects of I2 depletion with shRNAs (sh-I2) on β-catenin stabilization (top) and TOP-Flash (bottom) in HEK293T cells. shGFP: an shRNA against GFP. Error bars represent SD of triplicates. (F) Effect of I2 depletion with an I2MO in embryos and its rescue by human I2 mRNA. CoMo/Uninj: control MO-injected/uninjected.
PP1 has pleiotropic roles and its specificity is conferred by hundreds of PP1c-binding proteins (20). Inhibitor-2 (I2, or PPP1R2) is a specific inhibitor of PP1c (20). Overexpression of I2 countered Wnt3a-induced Axin dephosphorylation (without affecting LRP6 phosphorylation) and β-catenin stabilization (Fig. 1D and fig. S2B), inhibited Wnt3a- or PP1cγ-activated TOP-Flash (fig. S2C and S2D), and antagonized β-catenin stabilization by an activated LRP6 (fig. S2E). Depletion of the endogenous I2 with shRNAs resulted in accumulation of β-catenin and increased TOP-Flash (Fig. 1E and fig. S2F). A morpholino antisense-oligonucleotide (MO) that targets Xenopus I2 mRNA and blocked I2 protein synthesis caused deficiency in Xenopus head development and reduced anterior marker expression, which were restored by human I2 mRNA injection or knockdown of β-catenin (Fig. 1F and fig. S4). Thus I2 antagonizes Wnt/β-catenin signaling and participates in vertebrate anteriorization, which requires Wnt pathway inhibition (21).
Recent models of Wnt signaling (22, 23) have overlooked Axin phosphorylation, and one argued that Wnt/LRP6 signaling maintains an intact Axin destruction complex without inhibiting β-catenin phosphorylation (22). We reevaluated this critical issue. We found that in multiple Wnt-responsive cells, the rate of β-catenin phosphorylation was suppressed under Wnt stimulation (fig. S5) as reported (3, 24), and this correlated with Axin dephosphorylation.
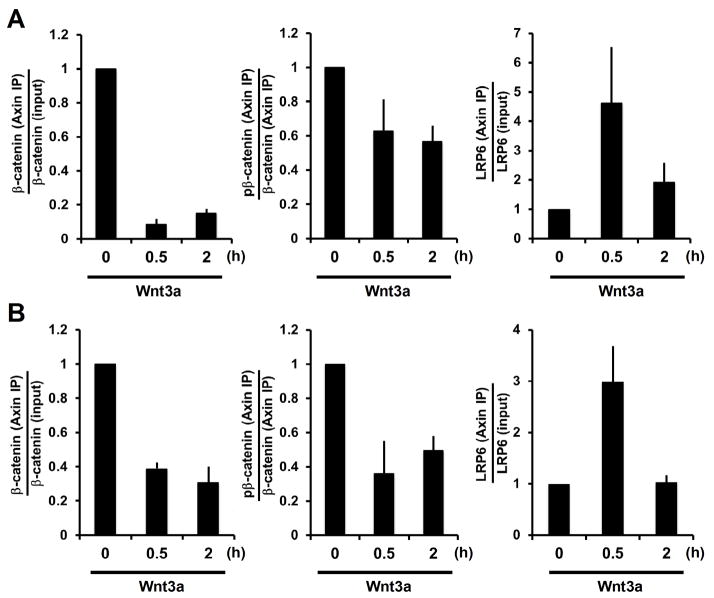
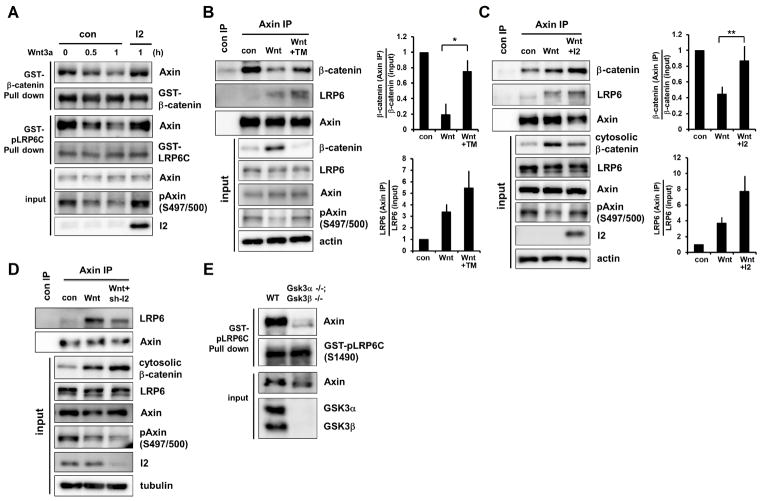
Association of Axin and β-catenin is the cornerstone binary interaction within the destruction complex. Co-immunoprecipitation (co-IP) of endogenous proteins showed that the amount of β-catenin associated with Axin appeared to be unchanged or decrease after 0.5 hours of Wnt treatment but to increase after 2 hours (fig. S6A and S6B). However normalization to the amount of cytosolic β-catenin made it clear that Wnt stimulation weakened the interaction between β-catenin and Axin (Fig. 2A and 2B, left panels). We estimated that the dissociation constant (Kd) of Axin-β-catenin association was increased by Wnt3a (fig. S6C), signifying a weaker interaction. Indeed Axin from extracts of Wnt3a-treated cells exhibited reduced capability to associate with β-catenin in an in vitro binding assay (Fig. 3A) (17). Such diminished Axin-β-catenin association observed in vitro and in vivo correlated with, and appeared to be attributable to Axin dephosphorylation by PP1, because it could be partially restored by I2 overexpression or TM treatment (Fig. 3A to 3C). For β-catenin co-immunoprecipitated with Axin from Wnt3a-treated cells, the amount of phosphorylation relative to that of β-catenin was reduced (Fig. 2A and 2B, middle panels; fig. S6A and S6B), implying a diminished rate of β-catenin phosphorylation in the Axin complex in Wnt-treated cells. Association of Axin with GSK3 remained constant regardless of Wnt treatment (fig. S6A and S6B) (22, 25). Thus Wnt signaling inhibits Axin-β-catenin association and β-catenin phosphorylation, consistent with earlier findings (3, 17), kinetic modeling (24), and the prevailing model (1, 11, 14, 15) (see Fig. S6C).
Fig. 2.
Wnt regulation of Axin-β-catenin and Axin-LRP6 association, and of β-catenin phosphorylation in the Axin complex. (A and B) Quantifications of Wnt3a effects on ratios of Axin-associated β-catenin versus input β-catenin (left), Axin-associated phospho-β-catenin versus Axin-associated β-catenin (middle), and Axin-associated LRP6 versus total LRP6 (right) in L (A) and HEK293T (B) cells. Error bars represent SEM of triplicates.
Fig. 3.
Effects of Wnt-regulated Axin phosphorylation on its binding to β-catenin and LRP6. (A) HEK293T cells overexpressing I2 (or control) were treated with Wnt3a. Lysates were incubated with GST-β-catenin or GST-phospho-LRP6C in vitro. Bound or input Axin, and phospho-Axin were examined. (B and C) L cells treated with TM (B) and HEK293T cells overexpressing I2 (or control) (C) were stimulated with Wnt3a for 0.5 hours. Lysates were immunoprecipitated with an Axin antibody, and Axin-associated β-catenin and LRP6, and input proteins were examined. Quantifications show Axin-associated β-catenin versus cytosolic β-catenin (top), and Axin-associated LRP6 versus total LRP6 (bottom). Note that TM or I2 reduced Wnt3a-induced β-catenin levels (Input). Error bars represent SEM of triplicates. *P<0.05 and **P<0.01 with student-Newman-Keuls test. (D) HEK293T cells expressing an I2 (or control) shRNA were treated with Wnt3a for 0.5 hours. Lysates were immunoprecipitated with an Axin antibody, and Axin-associated LRP6 and input lysates were examined. (E) In vitro binding to GST-phospho-LRP6C by Axin from lysates of WT or Gsk3α−/−;Gsk3β−/− mouse ES cells. Bound or input Axin was examined.
Wnt-induced phosphorylation of LRP6 recruits Axin to assemble the signaling complex (5–7). Intriguingly, co-IP of endogenous proteins showed that Wnt-induced association of LRP6 and Axin was prominent at 0.5 hours but diminished after 2 hours (Fig. 2A and 2B, right panels; and fig. S6A and S6B), despite of persistence of phospho-LRP6 (fig. S5). The diminished binding between phospho-LRP6 and Axin appeared to result from Axin dephosphorylation by PP1, because the binding was enhanced by I2 overexpression or TM treatment (Fig. 3B and 3C) and reduced by I2 depletion (Fig. 3D). Axin from extracts of Wnt3a-treated cells showed reduced capability to associate with phospho-LRP6 in an in vitro binding assay (fig. S7A), and this reduction appeared to result from Axin dephosphorylation by PP1 because it was prevented by I2 overexpression (Fig. 3A). Complementarily, Axin from extracts of cells treated with a pharmacological GSK3 inhibitor, BIO or SB216763, or of Gsk3α−/−;Gsk3β−/− cells (26), had minimal association with phospho-LRP6 (Fig. 3E and fig. S7B). Thus phosphorylation of Axin by GSK3 enhanced, whereas dephosphorylation of Axin by PP1 diminished, Axin’s ability to associate with phospho-LRP6 (and β-catenin), implying that activated LRP6 selectively recruits the phosphorylated form of Axin that is active in β-catenin association and degradation.
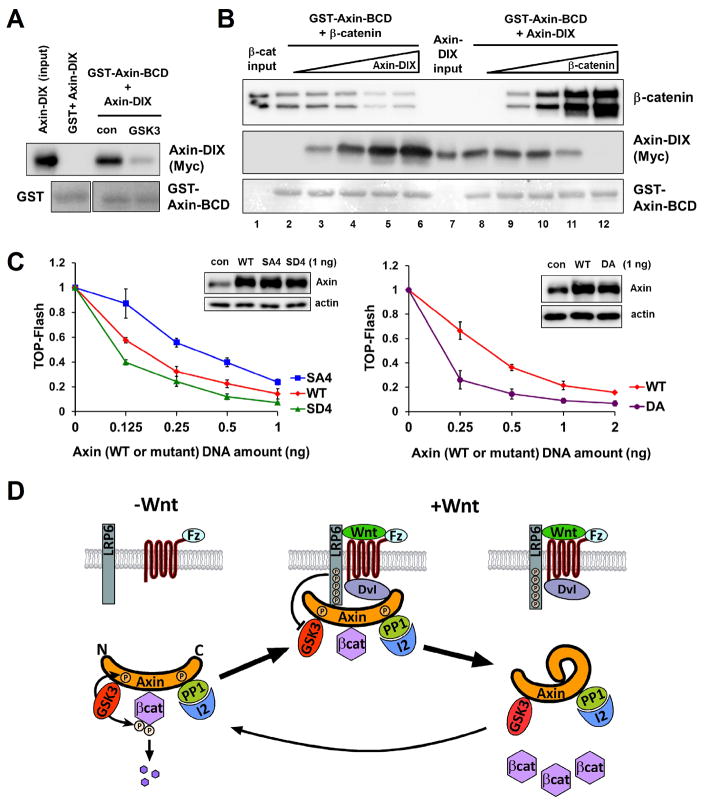
Above results imply that Axin may undergo a phosphorylation-dependent conformational change. Axin is an intrinsically disordered protein with individual partner-binding domains (fig. S8A) (14, 15). Axin’s β-catenin binding domain (Axin-BCD) associated, in vitro and when overexpressed in cells, with Axin DIX domain (Axin-DIX) but not homologous Dvl2 DIX domain (Dvl-DIX) (Fig. 4A, fig. S8B and S9), reflecting a specific and direct interaction. Phosphorylation of Axin-BCD by GSK3 in vitro inhibited BCD-DIX binding (Fig. 4A and fig. S8C). Therefore there may be an intra-molecular BCD-DIX interaction that is prevented upon Axin phosphorylation by GSK3, providing an explanation for how phosphorylation of Axin enhances its association with β-catenin and phospho-LRP6. Indeed Axin intra- and inter-molecular interactions appeared to be mutually exclusive because β-catenin and Axin-DIX competed for binding to Axin-BCD (Fig. 4B and fig. S10A). A positively charged histidine-rich region of BCD (fig. S9, S10B, and S11) and a negatively charged loop of DIX (fig. S12A and S13) participated in BCD-DIX interaction, which appeared to be disrupted by negative charges generated in BCD through phosphorylation by GSK3 (figs. S10C, S10D, and S11). Axin(SD4), which contains phosphomimetic aspartic acid substitutions of 4 serines (including S497 and S500) in BCD (fig. S11), and Axin(DA), which contains alanine substitutions of acidic residues in DIX (fig. S13), were each expected to have a weaker intra-molecular interaction (fig. S10D and S12A) and were indeed more effective in inhibiting Wnt/β-catenin signaling than the WT Axin (Fig. 4C). Axin(SA4), which contains alanine substitutions of the 4 serines in BCD (fig. S11) and was predicted to have a stronger intra-molecular interaction (fig. S10C), was less effective in inhibiting Wnt/β-catenin signaling (Fig. 4C). These results support a model that Axin “autoinhibits” through the BCD-DIX intra-molecular interaction. Live cell FRET (fluorescence resonance energy transfer) imaging corroborated this model by demonstrating a Wnt3a-induced proximity of Axin’s carboxyl DIX to its amino terminus (fig. S14 to S17), likely through Axin dephosphorylation.
Fig. 4.
A phosphorylation-regulated Axin intra-molecular interaction and a Wnt signaling model. (A) Association of Axin-DIX with GST-Axin-BCD and its inhibition by GSK3 phosphorylation of Axin-BCD. (B) Competition of β-catenin association with GST-Axin-BCD by Axin-DIX (lanes 1–6) and vice versa (lanes 7–12). Purified recombinant proteins were used in these in vitro assays. Axin-DIX or β-catenin was detected by immunoblotting and GST or GST-Axin-BCD by Ponceau staining (A and B). The lower band of β-catenin (B) was a proteolytic fragment. (C) Comparisons of Axin(SD4), Axin(SA4), and Axin(DA) with Axin in antagonizing Wnt-induced TOP-Flash in HEK293T cells. X-axes represent DNA doses transfected. Note larger TOP-Flash differences at lower overexpression doses. Insets show levels of overexpressed Axin at the 1ng dose and that of endogenous Axin (con). (D) An “Axin inactivation” model for Wnt stabilization of β-catenin. APC and CK1α were omitted for clarity. See text and fig. S18.
We propose a Wnt signaling model (Fig. 4D) that unifies findings on the two Axin complexes mediating LRP6 signaling and β-catenin destruction. Without Wnt, Axin is associated with and phosphorylated by GSK3 and is in an activated (“open”) conformation for β-catenin binding and phosphorylation and poised for engagement of LRP6 (Fig. 4D). With Wnt, LRP6 undergoes Fz/Dvl-dependent phosphorylation and recruits the active Axin destruction complex to form the signaling complex, in which GSK3 bound to Axin is inhibited by phospho-LRP6 (27–29), leading to initial inhibition of β-catenin phosphorylation and tipping the balance towards Axin dephosphorylation by PP1. Dephosphorylated Axin adopts an inactivated (“closed”) conformation through intra-molecular autoinhibition, and becomes incompetent for association with β-catenin or phospho-LRP6, leading to disassembly of destruction and signaling complexes (Fig. 4D). Phospho-LRP6 is thus freed for another round of recruitment of phosphorylated-activated Axin for inactivation while ignoring dephosphorylated-inactivated Axin, and the steps likely reiterate to keep β-catenin phosphorylation suppressed. Wnt-induced biphasic assembly and disassembly of the LRP6 signaling complex appear to enable phospho-LRP6 to inactivate Axin in a “catalytic” manner, underlying Wnt stabilization of β-catenin in broad component stoichiometries. GSK3 acts as an “assembler” of destruction (16–18) and signaling complexes (4, 6, 8–10) through phosphorylation of Axin and LRP6, whereas PP1 dephosphorylates Axin (19) to disassemble both complexes while leaving phospho-LRP6 unperturbed for continuous signaling. Our model further explains the β-catenin stabilization kinetics. Elevating levels of β-catenin, by competing against Axin intra-molecular autoinhibition, could promote reassembly of the Axin-GSK3-β-catenin complex and counter its disassembly by Wnt (Fig. 4D), thereby plateauing when equilibrium is achieved. This implies a safeguard mechanism by which rising concentrations of β-catenin could trigger its own degradation to avoid excessive accumulation. Axin represents a scaffold with an on/off switch controlled through a ligand- and phosphorylation-dependent intra-molecular interaction, which additionally could serve as a feedback sensor of target (β-catenin) concentrations. Similar scaffold functions could occur in other pathways as highlighted by yeast Ste5 (30).
Supplementary Material
Acknowledgments
We thank Y. Wu for the BCD2 construct, X. Yu for fig. S11 and S13, B. Doble and J. Woodgett for Gsk3-null mES cells, A. Klein, M. Kirschner, T, Schwarz, J-h. Wang, and X. H. lab members for comments, M. Lin, B. Yu, M. Wan, X. Cao, and W. Wei for help. S.E.K. and H. H were supported partially by an NRF (2009-357-C00096, Korea) and CIHR (Canada) postdoctoral fellowship, respectively. J.G.A. was funded partially by CNPq (Brazil) as a visiting scientist to X.H. lab. L. P. acknowledges support by NIH (R00EB008737 and R01EB015481). X.H. acknowledges support by NIH (RO1 GM074241) and Boston Children’s Hospital Intellectual and Developmental Disabilities Research Center (P30 HD-18655).
Footnotes
References and Notes
- 1.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Liu C, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 4.Mao J, et al. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell. 2001;7:801. doi: 10.1016/s1097-2765(01)00224-6. [DOI] [PubMed] [Google Scholar]
- 5.Tamai K, et al. A mechanism for Wnt coreceptor activation. Mol Cell. 2004;13:149. doi: 10.1016/s1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- 6.Zeng X, et al. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson G, et al. Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature. 2005;438:867. doi: 10.1038/nature04170. [DOI] [PubMed] [Google Scholar]
- 8.Bilic J, et al. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- 9.Zeng X, et al. Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development. 2008;135:367. doi: 10.1242/dev.013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarz-Romond T, Metcalfe C, Bienz M. Dynamic recruitment of axin by Dishevelled protein assemblies. J Cell Sci. 2007;120:2402. doi: 10.1242/jcs.002956. [DOI] [PubMed] [Google Scholar]
- 11.Metcalfe C, Bienz M. Inhibition of GSK3 by Wnt signalling--two contrasting models. J Cell Sci. 2011;124:3537. doi: 10.1242/jcs.091991. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda S, et al. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. Embo J. 1998;17:1371. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Behrens J, et al. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- 14.Kimelman D, Xu W. beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene. 2006;25:7482. doi: 10.1038/sj.onc.1210055. [DOI] [PubMed] [Google Scholar]
- 15.Stamos JL, Weis WL. The b-catenin destruction complex. Cold Spring Harbor perspectives in biology. 2013;5 doi: 10.1101/cshperspect.a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamamoto H, et al. Phosphorylation of axin, a Wnt signal negative regulator, by glycogen synthase kinase-3beta regulates its stability. J Biol Chem. 1999;274:10681. doi: 10.1074/jbc.274.16.10681. [DOI] [PubMed] [Google Scholar]
- 17.Willert K, Shibamoto S, Nusse R. Wnt-induced dephosphorylation of axin releases beta-catenin from the axin complex. Genes Dev. 1999;13:1768. doi: 10.1101/gad.13.14.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jho E, Lomvardas S, Costantini F. A GSK3beta phosphorylation site in axin modulates interaction with beta-catenin and Tcf-mediated gene expression. Biochem Biophys Res Commun. 1999;266:28. doi: 10.1006/bbrc.1999.1760. [DOI] [PubMed] [Google Scholar]
- 19.Luo W, et al. Protein phosphatase 1 regulates assembly and function of the beta-catenin degradation complex. Embo J. 2007;26:1511. doi: 10.1038/sj.emboj.7601607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Virshup DM, Shenolikar S. From promiscuity to precision: protein phosphatases get a makeover. Mol Cell. 2009;33:537. doi: 10.1016/j.molcel.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Niehrs C. Regionally specific induction by the Spemann-Mangold organizer. Nat Rev Genet. 2004;5:425. doi: 10.1038/nrg1347. [DOI] [PubMed] [Google Scholar]
- 22.Li VSW, et al. Wnt Signaling through Inhibition of beta-Catenin Degradation in an Intact Axin1 Complex. Cell. 2012;149:1245. doi: 10.1016/j.cell.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Taelman VF, et al. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell. 2010;143:1136. doi: 10.1016/j.cell.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernandez AR, Klein AM, Kirschner MW. Kinetic responses of beta-catenin specify the sites of Wnt control. Science. 2012;338:1337. doi: 10.1126/science.1228734. [DOI] [PubMed] [Google Scholar]
- 25.Valvezan AJ, Zhang F, Diehl JA, Klein PS. Adenomatous polyposis coli (APC) regulates multiple signaling pathways by enhancing glycogen synthase kinase-3 (GSK-3) activity. J Biol Chem. 2012;287:3823. doi: 10.1074/jbc.M111.323337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doble BW, Patel S, Wood GA, Kockeritz LK, Woodgett JR. Functional redundancy of GSK-3alpha and GSK-3beta in Wnt/beta-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev Cell. 2007;12:957. doi: 10.1016/j.devcel.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu GG, Huang H, Garcia Abreu J, He X. Inhibition of GSK3 phosphorylation of beta-catenin via phosphorylated PPPSPXS motifs of Wnt coreceptor LRP6. PLoS One. 2009;4:e4926. doi: 10.1371/journal.pone.0004926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cselenyi CS, et al. LRP6 transduces a canonical Wnt signal independently of Axin degradation by inhibiting GSK3’s phosphorylation of beta-catenin. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:8032. doi: 10.1073/pnas.0803025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piao S, et al. Direct inhibition of GSK3beta by the phosphorylated cytoplasmic domain of LRP6 in Wnt/beta-catenin signaling. PLoS One. 2008;3:e4046. doi: 10.1371/journal.pone.0004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zalatan JG, Coyle SM, Rajan S, Sidhu SS, Lim WA. Conformational control of the Ste5 scaffold protein insulates against MAP kinase misactivation. Science. 2012;337:1218. doi: 10.1126/science.1220683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.