Abstract
Objective
African Americans experience preterm birth at nearly twice the rate of Whites. Chronic stress associated with minority status is implicated in this disparity. Inflammation is a key biological pathway by which stress may affect birth outcomes. This study examined effects of race and pregnancy on stress-induced inflammatory responses.
Methods
Thirty-nine women in the 2nd trimester of pregnancy (19 African American; 20 White) and 39 demographically similar nonpregnant women completed an acute stressor (Trier Social Stress Test). Psychosocial characteristics, health behaviors, and affective responses were assessed. Serum interleukin(IL)-6 was measured via high sensitivity ELISA at baseline, 45 minutes, and 120 minutes post-stressor.
Results
IL-6 responses at 120 minutes post-stressor were 46% higher in African Americans versus Whites (95%CI:8%-81%; t(72)=3.51, p=.001). This effect was present in pregnancy and nonpregnancy. IL-6 responses at 120 minutes post-stressor tended to be lower (15%) in pregnant versus nonpregnant women (95%CI:-5%-32%; p=0.14). Racial differences in inflammatory responses were not accounted for by demographics, psychological characteristics, health behaviors, or differences in salivary cortisol across the study session. Pregnant Whites showed lower negative affective responses than nonpregnant women of either race (ps≤.007).
Conclusion
This study provides novel evidence that stress-induced inflammatory responses are more robust among African American women versus Whites during pregnancy and nonpregnancy. The ultimate impact of stress on health is a function of stressor exposure and physiological responses. Individual differences in stress-induced inflammatory responses represent a clear target for continued research efforts in racial disparities in health during pregnancy and nonpregnancy.
Keywords: women, pregnancy, pregnant, race, racial disparities, inflammation, inflammatory response, interleukin-6, IL-6, proinflammatory cytokines, preterm birth, stress, trier social stress test; acute stressor, affective responses, cortisol
Introduction
Preterm birth affects 12-13% of births in the US and is a leading cause of infant mortality (1, 2). The estimated societal economic burden is at least $26.2 billion per year, or $51,600 per preterm infant (1). In the US, the preterm birth rate is approximately 18% among African American women and 10.5-11.5% among non-Hispanic White, Asian, and Hispanic women (3). Although numerous explanations have been forwarded, demographic characteristics and health behaviors do not adequately account for this racial disparity. Associations between race and preterm birth, as well as low birth weight, and infant mortality remain after accounting for indicators of socioeconomic status including educational attainment, income, and occupational status (4-8).
Because traditional explanations have failed to adequately explain the racial disparity in preterm birth, theories have increasingly focused on understanding the health implications of chronic stress associated with minority status (1, 9-13). Supporting the conceptualization of minority status as a chronic stressor, perceived racial discrimination has repeatedly been linked to increased risk of preterm delivery and low birth weight (12, 14-18). These relationships remain after accounting for traditional behavioral risk factors, suggesting a role for more direct physiological links between stress and preterm birth.
Inflammation is a key biological pathway by which psychological stress may affect birth outcomes. Available data suggest that healthy pregnancy elicits mild elevations in both pro- and antiinflammatory serum cytokine levels and that exaggerated increases in circulating inflammatory markers are predictive of greater risk of spontaneous preterm delivery (19-24). Moreover, successful pregnancy in humans has been associated with attenuated proinflammatory cytokine production in response to in vitro immune challenges, with the most marked changes in the 3rd trimester (25-29). This adaptation may be critical in preventing rejection of the fetus by the maternal immune system and protecting the fetus from excessive maternal inflammatory responses to infectious agents (30, 31). Failure to demonstrate attenuation of inflammatory responses has been reported among women who subsequently experience miscarriage or deliver small for gestational age babies (27) as well as in nonpregnant women with a history of recurrent spontaneous miscarriage versus women with a history of successful pregnancy (32). Thus, factors influencing appropriate adaptation of inflammatory responses may affect risk of adverse pregnancy outcomes.
Psychosocial factors including perceived stress, stressful life events, depressive symptoms, and trauma have been associated with higher circulating inflammatory markers including interleukin(IL)-6, tumor necrosis factor(TNF)-α, and IL-1RA (33-38) as well as exaggerated inflammatory responses to in vivo and in vitro immune challenges in pregnant women (39, 40). Linking such effects to birth outcomes, in a study of 173 women followed across pregnancy, an association between prenatal stress and gestational age at birth was mediated by levels of circulating inflammatory markers (41).
Notably, we know of no studies examining inflammatory responses to acute psychological stress in pregnancy, or the extent to which psychosocial factors modify this response. Data in nonpregnant adults show that interleukin(IL)-6 responses to acute stressors are delayed and extended as compared to responses of the sympathetic nervous system and hypothalamic-pituitary-adrenal (HPA) axis. Increases in IL-6 from baseline have been observed beginning at 30-45 minutes post-stressor with continuing increases at final follow-up timepoints of up to two hours post-stressor (42, 43). Thus, exposure to elevated inflammatory markers may be considerable in duration among individuals who experience frequent stressors in daily life.
Prior studies show that African American race and greater exposure to racial discrimination predict greater cardiovascular reactivity to a variety of acute stressors (44-48). In addition, stressors of a racially provocative nature elicit stronger cardiovascular responses among African Americans than do stressors that are racially neutral (49). Thus, race is a factor which may affect not only frequency of stressor exposure (particularly racial discrimination), but also the magnitude of physiological response to stressors. The extent to which race modifies inflammatory responses to acute stress is not known. However, other sociodemographic factors indicative of chronic stress including low socioeconomic status and clinical depression have been associated with exaggerated stress-induced IL-6 responses (43, 50, 51).
In sum, given the growing literature linking both stressor exposure and inflammation to racial disparities in adverse pregnancy outcomes, examination of effects of race and pregnancy on stress-induced inflammatory responses is warranted. The first goal of this study was to examine the effect of race on stress-induced inflammatory responses. It was hypothesized that during pregnancy and nonpregnancy African American women would exhibit more robust stress-induced IL-6 responses than their White counterparts. The second goal of this study was to examine the effect of pregnancy on stress-induced inflammatory responses. It was hypothesized that pregnant women would show attenuation of IL-6 responses as compared to nonpregnant women and that this attenuation would be more notable among Whites versus African Americans. Finally, in exploratory analyses, we examined associations between cortisol and inflammatory responses to determine whether pregnancy or race-related differences in cortisol may mediate the hypothesized differences in IL-6 response.
Methods
Participants
Participants included 40 pregnant women (20 African American; 20 White) who were assessed during the second trimester of pregnancy (21-24 weeks gestation) and 40 nonpregnant control participants matched for age, race, parity, and income. Study visits were conducted between August 2009 and November 2011. The study was approved by the Ohio State University Biomedical Sciences Institutional Review board (IRB). Women were recruited from the Ohio State University Wexner Medical Center General Prenatal Clinic, which serves a racially diverse group of primarily socioeconomically disadvantaged women. In addition, women were recruited from the general community of Columbus, Ohio. Blood sampling was unsuccessful for two women (one nonpregnant African American, one pregnant African American). Thus, the final sample included 78 women (39 pregnant and 39 nonpregnant).
In terms of the trimester of assessment, we focused on the 2nd trimester rather than the 1st trimester because a primary goal was to examine differences in stress reactivity due to pregnancy status; more significant adaptations in cardiovascular, neuroendocrine and immune function are evidenced by the 2nd trimester than in the 1st trimester. We chose to focus on the 2nd rather than 3rd trimester for two reasons. First, increasing evidence suggests that stressors which occur earlier in pregnancy are more likely to have detrimental effects (e.g., 52). However, research to date has focused almost exclusively on stress reactivity during the third trimester (53). Second, assessment in the 2nd trimester avoids systematic exclusion of women who may go on to deliver preterm during the 3rd trimester.
Women were ineligible if they reported current tobacco use or chronic health problems which affect immune, endocrine, or cardiovascular function including cancer, diabetes, chronic hypertension, gestational hypertension, preeclampsia, or anemia at the time of screening. In addition, women were excluded if they were taking anti-depressants, anti-anxiety medications, or mood stabilizers. If a woman reported antibiotic use, she was scheduled at least two weeks following usage.
Women were excluded if they reported consuming more than 300mg of caffeine per day. Women reporting use of any recreational drugs (e.g., marijuana, cocaine, methamphetamines) in the previous 6 months were excluded. Women were excluded if they were obese, defined as a pre-pregnancy (if pregnant) or current (if nonpregnant) body mass index (BMI) ≥ 30 (kg/m2). Because the racial disparity in preterm birth most clearly affects US-born African American women, women who were not US-born were ineligible.
Women were not eligible as nonpregnant control participants if they had given birth within the past 6 months or were currently breastfeeding. Among the pregnant participants, women were excluded if they had multifetal gestation or known fetal anomaly. Because previous pregnancy has been associated with more significant physiological changes in subsequent pregnancy [208], pregnant participants with at least one previous live birth were targeted. Pregnancy timing in terms of maternal age varies considerably with sociodemographic factors such as income and marital status. Thus, to provide ideal demographic matching, nonpregnant women with a prior live birth were also targeted. In the final sample, 76 of 78 (97.4%) women had a prior live birth (38 pregnant and 38 nonpregnant). Two nulliparous women were included due to mistaken endorsement of a prior live birth by one nonpregnant woman at the time of screening who was subsequently matched with a pregnant nulliparous woman.
Study Visit Overview
All study sessions were conducted in the afternoon, beginning at 12:00 pm. Upon arrival to the Ohio State University Clinical Research Center, participants provided informed consent and were given a standardized lunch to ensure a euglycemic state. Following lunch, a catheter was inserted into an antecubital vein to allow for serial blood sampling and baseline questionnaires were completed assessing mood. Following a 20 minute acclimation/rest period, baseline blood samples were obtained. Next, the Trier Social Stress Test (TSST) was initiated. Serum samples were collected at 45 minutes and 120 minutes following the conclusion of the TSST for assessment of circulating IL-6. Across the course of the 4.5 hour study protocol, a total of 122 mL of blood was drawn. Saliva samples were collected by salivette at 25 minutes prior to stressor initiation and at 0, 15, 30, 45, 60, and 90 minutes post-stressor.
Laboratory Stressor: The Trier Social Stress Test (TSST)
This commonly used and well-validated laboratory stressor reliably evokes physiological reactivity as well as increases in self-reported stress (54-57). The TSST is a 20-minute task which requires participants to make a speech and perform mental arithmetic in front of an “audience” of 2-3 evaluators. For this study, the audience was composed of two female evaluators, one African American and one white. The participant was told to imagine that she had applied for a job and been invited to an interview by the selection committee. She was informed that she would be given 10 minutes to prepare a speech about why she would be best for the job and 5 minutes to talk with the committee, followed by a second experimental task. Following the speech, the participant completed a 5 minute mental arithmetic task involving serial subtraction. The difficulty of the subtraction task was adjusted each minute on the basis of the participant’s performance during the previous minute to improve the equivalence of the stress task across participants as described elsewhere (42, 58). To enhance the evaluative aspect of the task, the speech and math tasks were videotaped and participants were informed that these would be used for later “behavioral analysis”.
Psychosocial Measures
Questionnaires were used to assess various psychological constructs as well as subjective responses to the stressor in order to determine the similarity between groups on these factors. All questionnaires, with the exception of the baseline Positive and Negative Affect Scale (PANAS), were completed post-stressor. The Experiences of Discrimination Scale was administered after the final blood draw to ensure that racial differences in recall of potentially stressful events did not differentially affect inflammatory responses.
The Positive and Negative Affect Scale (PANAS) was administered three times to measure transient affective responses to the stressor: at the conclusion of the acclimation/rest period, immediately upon conclusion of the stressor, and at 120 minutes post-stressor. With excellent norms and strong reliability and validity, this is an excellent self-report measure of transient affective states (59).
Depressive symptoms were assessed with the Center for Epidemiological Studies Depression Scale (CES-D). This measure is brief and shows good reliability and validity (60, 61). Further, CES-D scores during pregnancy are associated with negative outcomes including restricted fetal growth (62), spontaneous preterm birth (63), and impaired neuromotor performance among neonates (64). A cut-off of ≥ 16 is commonly used to indicate clinically meaningful depressive symptoms.
The state subscale of the State-Trait Anxiety Inventory (STAI) includes 20 items which measure state anxiety (65). This measure shows good internal consistency and test-retest reliability (66).
The 14-item version of the Perceived Stress Scale (PSS), also widely used and well-validated, was used to measure the subjective experiences of stress and coping with stress during the past month (67). The PSS measures a construct that is independent of depressive symptomatology (67). Demonstrating predictive validity in pregnant populations, the PSS has been associated with maternal neuroendocrine function (68, 69) and risk of bacterial vaginosis (70).
The Cook-Medley Hostility Scale is a 50-item set of true-false items which sum to yield a hostility score (71). A subset of 13 items measure cynical hostility. The scale has good internal consistency and test-retest reliability (72). Hostility tends to be positively correlated with perceived racism (73, 74) and has been associated with stronger cardiovascular reactions to stress (75, 76).
Social support was measured using the Multidimensional Scale of Perceived Social Support (MSPSS). This 12-item measure assesses support from family, friends, and a significant other. It has been validated for use among pregnant women (77, 78).
The short form of the Childhood Trauma Questionnaire (CTQ-SF) is a 28-item self-report measure of childhood or adolescent abuse and neglect (79, 80). This scale shows measurement invariance across diverse samples and good criterion-related validity among adolescents in relation to corroborative data (79). Standard cut-offs on the CTQ were used to classify women as having experienced or not experienced childhood abuse in the form of emotional abuse, physical abuse, and/or sexual abuse (81).
Sleep quality was assessed with the Pittsburgh Sleep Quality Index (PSQI). This scale has good diagnostic sensitivity and specificity in distinguishing good and poor sleepers (82). A score > 5 is indicative of clinically disturbed sleep.
The Experiences of Discrimination (EOD) scale is a 9-item measure assessing the occurrence and frequency of discrimination due to race/ethnicity. Specifically, participants indicate whether they have experienced discrimination over their lifetime (Yes or No) in the following settings: 1) at school, 2) getting hired or getting a job, 3) at work, 4) getting housing, 5) getting medical care, 6) getting service in a store or restaurant, 7) getting credit, bank loans or a mortgage, 8) on the street or in a public setting, 9) from the police or in the courts. For items endorsed, participants rate the frequency of this occurrence: once, 2-3 times, or 4+ times. This scale has high test-retest reliability and predictive validity for health outcomes in Black adults (83-85). Moreover, validation studies indicate that scores are not related to social desirability (83).
Health Behaviors
As described, women reporting tobacco use at the time of screening were excluded from participation. Exercise was operationalized as the frequency of engaging in vigorous physical activity long enough to build up a sweat with a range of “less than once per month” to “more than once per week”. Prenatal vitamin use was defined as never, some days (1-3 days per week), most days (4-6 days per week) and every day (7 days per week).
Interleukin-6
Serum was prepared from clotted blood samples from all participants at each of the three assessment time points (baseline, 45 minutes post-stressor, and 120 minutes post-stressor). Serum levels of IL-6 were measured using ultra-sensitive kits from Meso Scale Discovery. All samples were batched and assayed on kits from the same lot. The lower limit of detection was 0.61 pg/ml. All samples were above the limit of detection. Inter- and intra-assay coefficients of variation were 10.65% and 10.66%, respectively.
Salivary Cortisol
Saliva was collected via salivette at seven timepoints: 25 minutes prior to the stressor (baseline), immediately upon completion of the stressor, and at 15, 30, 45, 60, and 90 minutes post-stressor. Determinations are made using the Cortisol Coat-A-Count RIA kit (Siemens Medical Solutions Diagnostics, 5700 West 9th Street, Los Angeles, Ca. 90045). Intra-assay coefficient of variation was 4.3% and inter-assay coefficient of variation was 5.2%. The sensitivity of the assay was 0.025 μg/dl. Assays were counted and calculated on the Packard Cobra II Gamma Counter (PerkinElmer, 710 Bridgeport Avenue, Shelton, Ct. 06484).
Statistical Analyses
Summary statistics were reported as mean and standard deviation for continuous variables and frequency and percentage for categorical variables. Participants were grouped into four categories based on race and pregnancy status. Demographic variables, health behaviors, and psychosocial assessments were compared using analysis of variance for continuous outcomes, chi-squared or Fisher’s exact tests for categorical outcomes, and the nonparametric Jonckheere-Terpstra (JT) test for ordered categorical outcomes. Differences by pregnancy and race overall and also between each pair of race/pregnancy subgroups were tested.
Linear mixed models were used to test for differences in stress responses across race and pregnancy. Outcome variables were IL-6, salivary cortisol, and PANAS positive and negative affect. The distributions of IL-6 and salivary cortisol measures were right-skewed; thus, IL-6 and salivary cortisol measures were log-transformed to better satisfy the normality assumptions of the mixed model analyses. For each model, the independent fixed effects were the fully saturated interactions and main effects for race, pregnancy, and time. For IL-6 and PANAS positive/negative, models were fit to the change scores at the two post-stressor time points, controlling for the baseline value of the outcome variable by including it as a covariate. With the inclusion of the baseline covariate, this model yields equivalent p-values whether post-scores or change scores are modeled; the change score outcomes are preferred as they yield estimates directly indicating change from baseline. As a sensitivity analysis, we also evaluated IL-6 with the baseline level included as a dependent variable along with the two post-stressor time points. For salivary cortisol, since there were six follow-up time points instead of two, the baseline value was included as a dependent variable at time = 0 and subsequent time points were tested against the baseline parameter estimate. A random subject effect was also included in each model, accounting for the correlation across time within a subject. Parameter contrasts were set up in SAS® PROC MIXED to test race and pregnancy effects at each time point. The contrasts produce a one degree of freedom test with a t-statistic. Marginal distributions of race and pregnancy were used as the coefficients for interaction terms including race or pregnancy, as appropriate.
To assess the association of cortisol with change in IL-6, the area under the curve (AUC) for salivary cortisol from 25 minutes pre-stressor to 90 minutes post-stressor was calculated for each participant using the trapezoidal rule. Correlations between cortisol area under the curve and IL-6 change from baseline at 45 and 120 minutes were calculated.
The study was powered based on enrolling 40 pregnant and 40 nonpregnant women (50% African American and 50% White), which would yield greater than 90% power to detect the expected effect sizes of 1 standard deviation in terms of Cohen’s d for pregnancy and race effects on IL-6 responses.
Results
Demographic Characteristics
Women did not significantly differ by race or pregnancy status in age, education, income, nulliparity, or BMI (based on pre-pregnancy weight for pregnant women; Table 1). Nonpregnant women were less likely to be married than pregnant women (X2(1) = 8.67, p = .003). This effect was driven by nonpregnant African Americans who were less likely to be married than pregnant White (X2(1) = 11.3, p = .001) or pregnant African American women (X2(1) = 5.7, p = .017).
Table 1.
Demographic Characteristics
| Nonpregnant White (n=20) |
Nonpregnant African American (n=19) |
Pregnant White (n=20) |
Pregnant African American (n=19) |
|
|---|---|---|---|---|
| Age [Mean (SD)]1 | 24.85 (3.10) | 23.16 (4.20) | 23.90 (3.21) | 23.68 (3.56) |
| Marital Status [n (%)]*2 | ||||
| Married | 5 (25%) | 1 (5%) | 11 (55%) | 7 (37%) |
| In a relationship | 11 (55%) | 7 (37%) | 8 (40%) | 12 (63%) |
| Single | 4 (20%) | 11 (58%) | 1 (5%) | 0 (0%) |
| Education [n (%)]3 | ||||
| High school graduate or less | 6 (30%) | 9 (47%) | 9 (45%) | 5 (25%) |
| Some college | 6 (30%) | 7 (37%) | 7 (35%) | 9 (47%) |
| College degree (2 or 4 yr) | 8 (40%) | 2 (11%) | 4 (20%) | 4 (21%) |
| Income [n (%)]3 | ||||
| <$15,000 | 7 (35%) | 8 (42%) | 8 (40%) | 9 (47%) |
| $15,000-29,999 | 7 (35%) | 6 (32%) | 6 (30%) | 6 (35%) |
| ≥ $30,000 | 6 (30%) | 5 (26%) | 6 (30%) | 4 (21%) |
| Nulliparous# [n(%)]2 | 0 (0%) | 1 (5%) | 0 (0%) | 1 (5%) |
| BMI†[Mean (SD)]1 | 24.13 (3.88) | 23.57 (3.48) | 24.32(2.72) | 23.57 (3.70) |
Analysis of variance, 2-tailed
Chi-square test
Jonckheere-Terpstratest, 2-tailed
nonpregnant African American women were significantly less likely to be married than pregnant White (X2(1) = 11.3, p = .001) or pregnant AfricanAmerican women (X2(1) = 5.7, p = .017).
women with prior pregnancies were targeted for recruitment; see methods
based on pre-pregnancy weight for pregnant women; obese women (BMI ≥ 30) were excluded from participation
Inflammatory Responses to Acute Stress
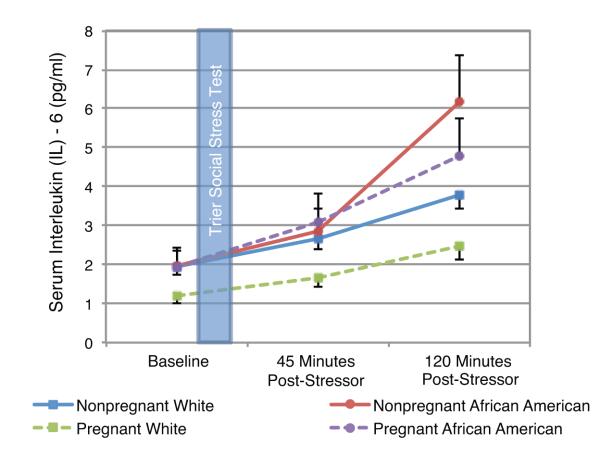
Baseline IL-6 levels were significantly lower in pregnant White versus nonpregnant White women (t(74) = 2.54, p = .013). Linear mixed model results demonstrated that, controlling for baseline IL-6, African American women exhibited significantly greater IL-6 increases at 120 minutes post-stressor as compared to Whites (t(72) = 3.51, p = .001). Controlling for baseline, IL-6 levels at 120 minutes post-stressor were 46% higher among African American women (95% CI: 18% to 81%; Fig 1).
Fig 1. Inflammatory Responses to the Trier Social Stress Test in Pregnant and Nonpregnant Women.
Controlling for baseline, IL-6 levels at 120 minutes post-stressor were 46% higher in African Americans versus Whites (95% CI: 18% to 81%; t(72) = 3.51, p = .001). This effect of race was significant during pregnancy and nonpregnancy. Note: Data are pictured in raw values. Analyses were conducted using log-transformed values.
Model contrasts demonstrated that nonpregnant African Americans showed significantly greater inflammatory responses at 120 minutes post-stressor than either pregnant (t(72) = 3.50, p = .001) or nonpregnant (t(72) = 2.26, p = .027) Whites. Pregnant African American women had significantly greater inflammatory responses than pregnant Whites (t(72) = 2.67, p = .009). Thus, effects of race were observed in pregnancy as well as nonpregnancy. In a sensitivity analysis modeling IL-6 at baseline, 45 minutes, and 120 minutes, the race by time interaction was significant (F(2,146) = 5.15, p=.007). In terms of pregnancy effects, controlling for baseline, IL-6 levels at 120 minutes post-stressor tended to be lower (15%) in pregnant versus nonpregnant women (95% CI: -5% to 32%; p = .14).
Salivary Cortisol
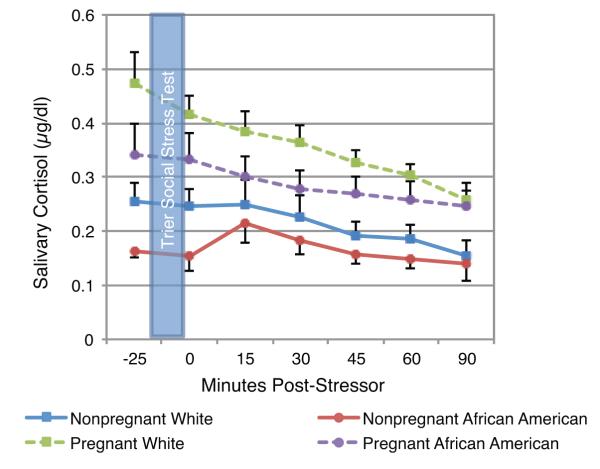
At baseline, cortisol was significantly higher in pregnant women than in nonpregnant women (t(72) = 15.30, p < .001; Fig 2). A non-significant trend was seen for lower baseline cortisol among African Americans versus Whites (t(72) = 2.65, p = .11). Across all participants combined, salivary cortisol was significantly lower than baseline immediately post-stressor (t(72) = 2.03, p = .046) and at 30, 45, 60, and 90 minutes post-stressor (30 minutes: t(72) = 3.08, p = .003; 45 minutes: t(72) = 4.82, p < .001; 60 minutes: (t(72) = 5.99, p < .001; 90 minutes: t(72) = 9.70, p < .001). There were no significant increases from baseline in cortisol in any of the groups. There were no significant differences in estimated slopes for linear change in salivary cortisol from 25 minutes pre-stressor to 90 minutes post-stressor with the exception of a flatter cortisol slope among nonpregnant African-Americans compared to pregnant (t(73) = 2.26, p = .027) and nonpregnant (t(73) = 2.05, p = .044) Whites.
Fig 2. Cortisol Responses to the Trier Social Stress Test in Pregnant and Nonpregnant Women.
At baseline, cortisol was significantly higher in pregnant women than in non-pregnant women (t(72) = 15.30, p < .001). A non-significant trend was seen for lower baseline cortisol among African Americans versus Whites (t(72) = 2.65, p = .11). The AUC of cortisol across the study session was significantly higher among pregnant versus non-pregnant women (t(75) = 4.45, p <.001) and marginally lower among African Americans versus Whites (t(75) = 1.77, p = .08). Note: Data are pictured in raw values. Analyses were conducted using log-transformed values.
The AUC of cortisol across the study session was marginally higher among Whites versus African Americans (t(75) = 1.77, p = .080) and significantly higher among pregnant versus nonpregnant women (t(75) = 4.45, p <.001). In the overall sample, total salivary cortisol (AUC) was not associated with the change from baseline in IL-6 at 45 (r = 0.14, p =.22) or 120 (r = −0.03, p = .81) minutes post-stressor. Moreover, after controlling for cortisol AUC, race remained significantly associated with change in IL-6 at 120 minutes post-stressor (t(71) = 3.95, p< .001).
Subjective Responses to Acute Stress
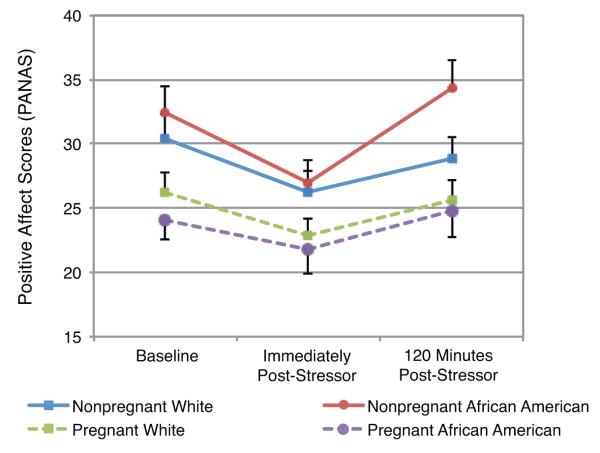
At baseline (prior to stressor onset), pregnant women reported significantly less positive affect as measured by the PANAS as compared to nonpregnant women (t(74) = 12.6, p < .001). Controlling for baseline, positive affect did not differ based on race or pregnancy status immediately post-stressor (race: t(74)=0.10, p = .92; pregnancy: t(74) = 0.22, p = .82) or at 120 minutes post-stressor (race: t(74) = 1.86, p = .067; pregnancy: t(74) = 1.33, p = .19; Fig 3).
Fig 3. Changes in Positive Affect in Response to the Trier Social Stress Test.
Pregnant women reported significantly less positive affect at baseline as compared to non-pregnant women (F(1,74) = 12.6, p < .001). Controlling for baseline positive affect, positive affect immediately post-stressor or 120 minutes post-stressor did not differ based on race or pregnancy status (ps ≥ .06).
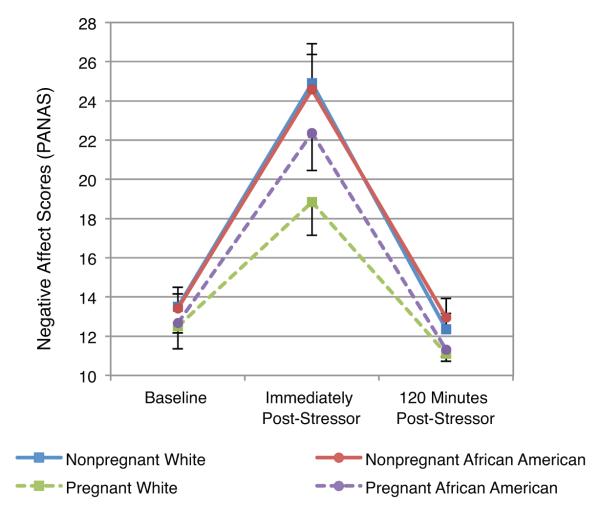
Women did not differ in negative affect at baseline on the basis of either race or pregnancy status (race: t(74) = 0.06, p = .95; pregnancy: t(74) = 0.98, p = .33). Immediately post-stressor, pregnant women had a significantly lower increase in negative affect than nonpregnant women (t(74) = 7.65, p = .007; Fig 4). This effect was driven by pregnant Whites who had lower increases in negative affect than both nonpregnant African Americans (t(74) = 2.76, p = .007) and nonpregnant Whites (t(74) = 2.94, p = .004), while pregnant African Americans did not differ significantly from either nonpregnant African Americans (t(74) = 1.11, p=.27) or nonpregnant Whites (t(74) = 0.95, p=.35). The race by pregnancy interaction was not significant immediately post-stressor (t(74) = 1.38, p=0.17). Women did not differ in negative affect at 120 minutes post-stressor.
Fig 4. Changes in Negative Affect in Response to the Trier Social Stress Test.
Women did not differ in negative affect at baseline based on either race or pregnancy status. Immediately post-stressor, pregnant women had a significantly lower increase in negative affect than nonpregnant women (t(74) = 7.65, p = .007). This effect was driven by pregnant Whites who had lower increases in negative affect than either nonpregnant African Americans or nonpregnant Whites (ps ≤ .01).
Health Behaviors
Health behaviors are presented in Table 2. Participation in vigorous activity was significantly greater among Whites versus African Americans (JT, Z = 2.35, p = .019) and in nonpregnant versus pregnant women (JT, Z = 3.20, p = .001). These differences were driven by nonpregnant White women, who reported taking part in vigorous activity significantly more frequently than each of the other 3 groups (ps < .003). Observed differences in inflammatory responses did not change after controlling for physical activity. Groups did not differ in smoking status or prenatal vitamin use (between pregnant groups).
Table 2.
Health Behaviors
| Nonpregnant White (n=20) |
Nonpregnant African American (n=19) |
Pregnant White (n=20) |
Pregnant African American (n=19) |
|
|---|---|---|---|---|
| Smoking Status 1 | ||||
| Current | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Past | 8 (40%) | 4 (21%) | 9 (45%) | 4 (21%) |
| Never | 12 (60%) | 15 (79%) | 11 (55%) | 15 (79%) |
| Prenatal Vitamin Use 2 | n/a | n/a | ||
| Never | 8 (40%) | 5 (26%) | ||
| Some days (1-3/week) | 4 (20%) | 2 (11%) | ||
| Most days (4-6/week) | 5 (25%) | 2 (11%) | ||
| Every day (7 days/week) | 3 (15%) | 10 (53%) | ||
| Exercise # 2 | ||||
| Less than once per month | 0 (0%) | 3 (16%) | 8 (40%) | 9 (47%) |
| Once per month | 1 (5%) | 2 (11%) | 0 (0%) | 2 (11%) |
| 2-3 times per month | 2 (10%) | 7 (37%) | 5 (25%) | 3 (16%) |
| Once per week | 7 (35%) | 4 (21%) | 3 (15%) | 2 (11%) |
| More than once per week | 10 (50%) | 3 (16%) | 4 (20%) | 3 (16%) |
Chi-square test
Jonckheere-Terpstrates (JT)t, 2-tailed
Participation in vigorous activity was significantly greater among Whites versus AfricanAmericans (JT, p = .019) and in nonpregnant versus pregnant women (JT, p = .001). These differences were driven by nonpregnant White women, who reported taking part in vigorous activity significantly more frequently than each of the other 3 groups (ps < .003).
Psychosocial Factors
In terms of psychosocial factors, 26.9% of women overall scored at or above a clinical cut-off of 16 on the CES-D indicating significant depressive symptoms. The mean state anxiety score (STAI) was 35.12 which is the 56%ile for women in this age range (86). The mean perceived stress score (PSS) was 17.92 (SD = 6.52), which is also slightly higher than a mean of 16.14 (SD=7.56) among women in a population-based sample of women (87). The overall mean total score on the social support measure (MSPSS) was 5.59 (SD = 1.06) which is slightly less than that reported in a prior study of pregnant women (Mean = 6.01; SD = 0.90) (77). In the overall sample, 56.4% were classified as poor sleepers on the basis of a score of > 5 on the PSQI. In addition, 52.5% met criteria for childhood abuse on the basis of scores on the CTQ.
Analyses were conducted to psychosocial factors based on race and pregnancy status assessed (Table 3). Women did not differ by race or pregnancy status in the proportion of women with CES-D > 16 or the proportion reporting childhood abuse (all X2 (1) < 1.89, ps > .17), or in state anxiety as measured by the STAI (both t(74) > 0.53, ps > .60) . Perceived support in total (t(74) = 2.26, p = .026) and from a significant other (t(74) = 2.67, p = .009) was greater in pregnant versus nonpregnant women. African American women reported greater racial discrimination both in terms of the number of situations (JT, Z = 2.70, p = .007) and total frequency (JT, Z = 2.52, p = .012). Among African American women greater racial discrimination was not significantly related to scores on the CES-D, STAI, or MSPSS (ps ≥ .39). The correlation between the PSS and EOD scores in terms of number of situations (r = .26, p = .11) and total frequency (r = .32, p = .056) approached statistical significance. Observed racial differences in inflammatory responses did not change when controlling for social support or racial discrimination in separate models.
Table 3.
Psychological Functioning
| Nonpregnant White (n=20) |
Nonpregnant African American (n=19) |
Pregnant White (n=20) |
Pregnant African American (n=19) |
|
|---|---|---|---|---|
| Depressive Symptoms 1 | ||||
| CES-D ≥ 16; n (%) | 6 (30%) | 7 (36.8%) | 3 (15.0%) | 5 (26.3%) |
| Anxiety 2 | ||||
| State Anxiety [STAI; Mean (SD)] | 35.25 (13.14) | 36.26 (10.40) | 34.80 (8.82) | 34.17 (9.46) |
| Perceived Stress 2 | ||||
| PSS Score; Mean (SD) | 17.79 (7.6) | 19.72 (6.24) | 17.10 (6.56) | 17.21 (5.66) |
| Impaired Sleep Quality 1 | ||||
| PSQI Score > 5 | 11 (55%) | 13 (68%) | 11 (55%) | 9 (45%) |
| Social Support [Mean (SD)] #2 | ||||
| Total MPSSS | 5.22 (1.02) | 5.44 (1.16) | 5.81 (1.14) | 5.92 (0.83) |
| Family Support | 5.40 (1.64) | 5.43 (1.20) | 5.66 (1.59) | 5.75 (1.59) |
| Friend Support | 4.94 (1.24) | 5.55 (1.27) | 5.52 (1.37) | 5.74 (1.07) |
| Significant Other Support | 5.33 (1.84) | 5.33 (1.98) | 6.25 (1.22) | 6.28 (0.91) |
| Hostility 2 | ||||
| Cook-Medley Score; Mean (SD) | 19.40 (7.44) | 22.05 (7.74) | 20.10 (7.91) | 20.42 (6.69) |
| Childhood Trauma 1 | ||||
| Abused; n (%) | 10 (50%) | 12 (63.2%) | 8 (40%) | 11 (57.9%) |
| Racial Discrimination ^ 3 | ||||
| EOD Score Situations | ||||
| 0 | 12 (60%) | 6 (32%) | 11 (55%) | 7 (37%) |
| 1-2 | 7 (35%) | 7 (37%) | 7 (35%) | 5 (26%) |
| >2 | 1 (5%) | 6 (32%) | 2 (10%) | 7 (37%) |
| EOD Score Frequency | ||||
| 0 | 12 (60%) | 6 (32%) | 11 (55%) | 7 (37%) |
| 1-5 | 6 (30%) | 7 (37%) | 7 (35%) | 4 (21%) |
| 6-10 | 1 (5%) | 2 (11%) | 0 (0%) | 7 (37%) |
| >10 | 1 (5%) | 4 (21%) | 2 (10%) | 1 (5%) |
Chi-square test
Analysis of variance, 2-tailed
Jonckheere-Terpstratest, 2-tailed
Perceived support was significantly greater among pregnant versus nonpregnant women in total (t(74) = 2.26, p = .026) and from a significant other (t(74) = 2.67, p = .009).
Racial discrimination was significantly greater among African Americans versus Whites in terms of both the number of situations (Jonckheere-Terpstra (JT), p = .007) and total frequency of discrimination (JT, p = .012) as reported on the EOD.
Discussion
The first goal of this study was to examine effects of race on inflammatory responses to acute stress. As hypothesized, African American women exhibited greater serum IL-6 responses to the stressor as compared to Whites. Model contrasts demonstrated that this effect of race was significant during both pregnancy and nonpregnancy. This effect was not accounted for by demographic variables or health behaviors.
Notably, African Americans and Whites were similar in all psychosocial variables measured with the exception of greater reported experiences of discrimination among African Americans. The relationship between race and inflammatory responses remained after controlling for perceived racial discrimination, indicating exposure to racism as measured by this scale did not account for observed racial differences. Despite this result, the potential role for racial discrimination in the observed effects cannot be discounted. It is of note that a relatively high percentage of White women in the current study (42.5%) reported experiencing racial discrimination in one or more major life situation. However, the qualitative experience and social implications of racial discrimination among Whites versus African Americans differ (88). Thus, a simple comparison or statistical control for frequency of exposures among Whites versus African Americans may not meaningfully capture the psychosocial impact of such exposures. Moreover, this study did not have adequate statistical power to compare inflammatory responses among African Americans on the basis of the severity of perceived racial discrimination. Within group variability may ultimately be more meaningful with regard to physical health effects of racial discrimination. For example, our prior work shows that among pregnant African Americans, those reporting greater racial discrimination have higher Epstein Barr Virus antibody titers, indicating poorer cellular immune function (89). Finally, it is also possible that a different measure of racial discrimination may provide more predictive value in this context.
These data have implications for understanding biological mechanisms by which racial minority status may confer increased risk of adverse birth outcomes. The majority of studies to date have focused on stressor exposure and/or subjective experiences of stress in predicting preterm birth. However, the ultimate physical impact of stress on health is a function of exposure as well as physiological response. As reviewed, inflammatory processes are implicated in adverse birth outcomes including preterm birth (9). Therefore, more robust and extended inflammatory responses may confer increased risk of adverse birth outcomes, particularly among women who experience more frequent acute stressor exposure. Moreover, prior data show that upon repeated exposures to an acute laboratory stressor, habituation of cortisol and blood pressure reactivity is evidenced, but stress-induced elevations in IL-6 remain similar (42). Therefore, the experience of continued or repeated psychosocial stressors may confer considerable exposure to inflammatory markers.
An exploratory aim of this study was to examine the correspondence between cortisol and stress-induced IL-6 responses. Baseline and cortisol AUC was higher among pregnant versus nonpregnant women, as expected. Compared to Whites, African Americans showed a trend toward lower baseline cortisol (p = .11) and lower cortisol AUC (p = .080). The study protocol was conducted in the early afternoon. Prior data show that, compared to Whites, African Americans have lower cortisol levels in the morning/early afternoon and higher levels in the evening resulting in a flatter diurnal slope (90, 91). Thus, these data are consistent with prior studies. Cortisol AUC was not associated with IL-6 responses in the overall sample, and the noted racial differences in IL-6 responses were not modified by inclusion of cortisol AUC in the model.
Some prior data suggests that activity of the HPA axis is inversely associated with IL-6 responses to acute stress (92), although other studies have found this relationship to be inconsistent (42). It is important to consider that although cortisol has robust anti-inflammatory effects on cytokine-producing cells, extended exposure to elevated levels of glucocorticoids (GC), such as that seen in conditions of repeated or chronic stress, may produce GC insensitivity at the level of both cytokine producing cells and the HPA axis (93, 94). GC insensitivity is marked by a diminished ability of the HPA axis and cytokine producing cells to respond to cortisol, resulting in more sustained HPA axis responses and greater production of inflammatory markers. Thus, in the context of GC resistance, the expected inverse relationship between cortisol and inflammatory markers may be diminished or eliminated. Therefore, without knowledge of GR function or typical cortisol exposure (i.e., measurement of the complete diurnal slope), the lack of association between IL-6 and salivary cortisol responses in the current study is difficult to interpret.
Of note, in this study, no significant cortisol increases were seen in response to the stressor. Due to typical diurnal decline in cortisol, effects of stress on cortisol responses are less apparent without the use of a non-stressor control day, particularly when measurement occurs in the afternoon (95). In the absence of stress-induced cortisol increases, stressor exposure may slow the downward diurnal cortisol slope, but this effect within subjects is not quantifiable without comparison to a non-stress day. In addition, prior studies show that cortisol reactivity to acute stressors is less robust among women than men (96). Thus, these factors may explain the lack of increase in cortisol in response to the stressor.
The current study included 39 pregnant women, among whom only two (5.1%) delivered preterm (one African American; one White). This preterm birth rate is lower than in the US population overall and reflects the exclusion of women with high risk health conditions (e.g., hypertension) and health behaviors (e.g., smokers). Thus, this study was not appropriately powered to detect potential associations between inflammatory responses to acute stress and birth outcomes. This is a clear direction for future studies.
Although the focus of the current study was on pregnancy, the evidenced effect of race on inflammatory responses has implications for health outcomes well beyond pregnancy. In particular, there are substantial racial disparities in cardiovascular diseases (97). Black women as well as men experience greater prevalence, earlier disease onset, and greater cardiovascular mortality compared to White individuals of the same age (98). Inflammation is an established mechanistic factor in cardiovascular disease risk (99, 100). Moreover, it has been demonstrated that stress-induced increases in IL-6 predict ambulatory blood pressure three years later (101). The current data provide novel evidence of racial differences in stress-induced inflammatory responses. Attention is needed regarding the potential role of inflammatory responses to acute stress in racial disparities in cardiovascular disease risk.
In terms of pregnancy status, contrary to prediction, there was no significant difference between pregnant women, who were assessed in the 2nd trimester of pregnancy, and nonpregnant women in terms of inflammatory responses, although there was a trend toward this effect (p=0.14). IL-6 increases in response to the stressor were 15% lower in pregnant versus nonpregnant women (95% CI: −5% to 32%). This effect size translates to a Cohen’s d of .17 which is a small effect. Prior data in humans and animals indicate that inflammatory responses to both in vivo and in vitro biological challenges (e.g., lipopolysaccharide) are attenuated in pregnancy (25-29). It has been postulated that this attenuation of inflammatory responses represents an adaptive mechanism by which the fetus is protected from excessive maternal responding. Following from this argument, adaptation of the inflammatory responses to psychological challenge may be similarly beneficial. Data from a larger cohort would provide greater statistical power to determine if this effect is present.
Prior data has shown that negative affective responses to a speech task are associated with the magnitude of inflammatory response (102). In the current study, there was no main effect of race on negative affective responses. However, pregnant White women reported significantly smaller increases in negative affect than did nonpregnant Whites or nonpregnant Blacks. Prior evidence suggests that stressors are experienced as less stressful during pregnancy versus nonpregnancy. For example, upon exposure to an earthquake, women rated the experience as more stressful in earlier versus later stages of pregnancy and showed increasingly shorter gestation upon earlier exposure (52). It is notable that, in the current sample, pregnant African American women did not show attenuation of negative affective responses compared to nonpregnant African Americans.
The current study used the Trier Social Stress Test (TSST). The TSST is mild stressor designed to be similar to stressors such as public speaking that most people encounter at some time in their daily life. It has previously been used with women during pregnancy and postpartum (e.g., 103, 104). However, the vast majority of previous studies of reactivity to psychological stressors during pregnancy have used tasks such as mirror tracing, Stroop selective attention tasks, and cold pressor tasks (for review see 53, 105). Due to its similarity to naturally occurring stressors, the TSST arguably provides a stronger approximation of reactivity to everyday psychological stressors than these other types of tasks. In addition, evidence suggests pregnant women exhibit significantly higher reactivity to public speaking as compared to other types of acute stressors (106). Therefore, use of the TSST in this study provides both strong external validity and improved power to detect effects of the stressor on physiological parameters of interest relative to other stressors.
This study included assessment of IL-6 at 45 minutes and 120 minutes post-stressor, given that inflammatory responses to laboratory stressors are delayed (107). Although these are appropriate sampling timepoints, longer follow-up would provide valuable data regarding the full duration and magnitude of inflammatory marker exposure in different groups. This should be considered in future studies. In addition, this study included assessment of pregnant women in the second trimester. It has been hypothesized that stress responsivity is progressively attenuated as pregnancy progresses (105). Longitudinal assessment during each trimester of pregnancy and/or cross-sectional studies with demographically matched groups of women during each trimester would provide valuable data regarding the adaptation of inflammatory responses across the course of gestation. Relatedly, in the nonpregnant control group, effects of menstrual cycle phase were not assessed. Prior data indicate that menstrual cycle phase does not appreciably alter heart rate, blood pressure, catecholamine, lipid, or inflammatory cytokine responses to acute stress (108, 109), but does alter cortisol responses (110).
Women with a previous live birth were targeted for recruitment and comprised 76/78 (97.4%) of the final sample. This strengthens the study design because available data suggest that physiological adaptation to pregnancy may be greater during subsequent versus initial pregnancy (111). In addition, recruitment of nonpregnant controls with a prior live birth provided a sample with the best demographic equivalence to pregnant participants in terms of women experiencing motherhood at a given stage of life. Although this study design was chosen based on these empirical considerations, results may differ among women who are nulliparous.
This study included stringent inclusion/exclusion criteria. All women were normal weight, generally healthy, and denied use of recreational drugs or smoking at the time of eligibility screening. However, we did not confirm drug or smoking status via objective measures (e.g., serum cotinine). Self-reported smoking likely underestimates true smoking behavior, particularly among pregnant women (112). These stringent inclusion/exclusion requirements increase homogeneity within groups, increasing statistical power. However, as noted earlier, these criteria also exclude women at greatest risk for adverse birth outcomes. Moving forward, future studies should endeavor to include women with more diverse health behaviors and demographic characteristics to provide power to examine inflammatory responses in relation to birth outcomes and typify the mediating and moderating role of the factors in predicting stress-induced inflammatory responses.
This study was powered to detect effects of race and pregnancy status. Psychological data (e.g., hostility, depressive symptoms, etc.) were collected to determine the comparability of groups in factors which may affect physiological responses to acute stress. However, this sample size did not permit analyses of meditational or moderation effects of these constructs (e.g., if hostility, sleep quality, or other factors differentially affected inflammatory responses by race or pregnancy status). In addition, we did not conduct clinical interviews to determine history of clinical depression. Future research should aim to examine such psychological mediators and moderators.
In sum, this study provides novel data regarding inflammatory responses to acute stress among women on the basis of race and pregnancy status. These data represent a promising direction in delineating pathways by which stress may affect pregnancy outcome and fetal development. Although numerous studies demonstrate a link between various psychosocial stress exposures and pregnancy outcomes, comparatively few have focused on 1) biological mechanisms underlying these links or 2) individual differences in responses to stress. In addition, these results may possibly have relevance beyond pregnancy for racial disparities in diseases with an inflammatory component, particularly cardiovascular diseases. As the ultimate impact of stressor exposure is a function of not only the occurrence of the stressor but also the individual response to the stressor, focus on individual differences in stress-induced inflammatory responses represents a clear target for continued research efforts.
Acknowledgments
We appreciate the contributions of Clinical Research Assistants Colleen Sagrilla, Kelly Marceau, and Rebecca Long to data collection. We would like to thank our study participants. We also thank the staff at the OSU Clinical Research Center and Wexner Medical Center Prenatal Clinic.
Role of the Funding Sources This study was supported by NICHD (HD061644, LMC and HD067670, LMC). The project described was supported by the Ohio State University Clinical Research Center, funded by the National Center for Research Resources, Grant UL1RR025755 and is now at the National Center for Advancing Translational Sciences, Grant 8UL1TR000090-05. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. Lisa Christian had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Abbreviations
- IL-6
Interleukin-6
- ELISA
Enzyme-linked Immunosorbent Assay
- TNF-α
Tumor Necrosis Factor-α
- IL-1RA
Interleukin-1 Receptor Antagonist
- BMI
Body Mass Index
- TSST
Trier Social Stress Test
- PANAS
Positive and Negative Affect Scale
- CES-D
Center for Epidemiological Studies Depression Scale
- STAI
State-Trait Anxiety Inventory
- PSS
Perceived Stress Scale
- CTQ-SF
Childhood Trauma Questionnaire- Short Form
- PSQI
Pittsburgh Sleep Quality Index
- EOD
Experiences of Discrimination Scale
- MSPSS
Multidimensional Scale of Perceived Social Support
- AUC
Area under the curve
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Committee on Understanding Premature Birth and Assuring Healthy Outcomes . In: Preterm birth : causes, consequences, and prevention. Behrman RE, Butler AS, editors. National Academies Press; Washington, D.C.: 2007. [PubMed] [Google Scholar]
- 2.Callaghan WM, MacDorman MF, Rasmussen SA, Qin C, Lackritz EM. The contribution of preterm birth to infant mortality rates in the United States. Pediatrics. 2006;118(4):1566–1573. doi: 10.1542/peds.2006-0860. [DOI] [PubMed] [Google Scholar]
- 3.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, Munson ML. Births: final data for 2005. Natl Vital Stat Rep. 2007;56(6):1–103. [PubMed] [Google Scholar]
- 4.Mcgrady GA, Sung JFC, Rowley DL, Hogue CJR. Preterm Delivery and Low-Birth-Weight among 1st-Born Infants of Black-and-White College Graduates. American Journal of Epidemiology. 1992;136(3):266–276. doi: 10.1093/oxfordjournals.aje.a116492. [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg RL, Cliver SP, Mulvihill FX, Hickey CA, Hoffman HJ, Klerman LV, Johnson MJ. Medical, psychosocial, and behavioral risk factors do not explain the increased risk for low birth weight among black women. American Journal of Obstetrics and Gynecology. 1996;175(5):1317–1324. doi: 10.1016/s0002-9378(96)70048-0. [DOI] [PubMed] [Google Scholar]
- 6.Collins JW, Hawkes EK. Racial differences in post-neonatal mortality in Chicago: what risk factors explain the black infant’s disadvantage? Ethnicity and Health. 1997;2(1-2):117–25. doi: 10.1080/13557858.1997.9961820. [DOI] [PubMed] [Google Scholar]
- 7.Shiono PH, Rauh VA, Park M, Lederman SA, Zuskar D. Ethnic differences in birthweight: the role of lifestyle and other factors. American Journal of Public Health. 1997;87(5):787–93. doi: 10.2105/ajph.87.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoendorf KC, Hogue CJR, Kleinman JC, Rowley D. Mortality among Infants of Black as Compared with White College-Educated Parents. New England Journal of Medicine. 1992;326(23):1522–1526. doi: 10.1056/NEJM199206043262303. [DOI] [PubMed] [Google Scholar]
- 9.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simhan HN, Krohn MA, Roberts JM, Zeevi A, Caritis SN. Interleukin-6 promoter - 174 polymorphism and spontaneous preterm birth. American Journal of Obstetrics and Gynecology. 2003;189(4):915–918. doi: 10.1067/s0002-9378(03)00843-3. [DOI] [PubMed] [Google Scholar]
- 11.Mulherin Engel SA, Erichsen HC, Savitz DA, Thorp J, Chanock SJ, Olshan AF. Risk of spontaneous preterm birth is associated with common proinflammatory cytokine polymorphisms. Epidemiology. 2005;16(4):469–477. doi: 10.1097/01.ede.0000164539.09250.31. [DOI] [PubMed] [Google Scholar]
- 12.Giscombe CL, Lobel M. Explaining Disproportionately High Rates of Adverse Birth Outcomes Among African Americans: The Impact of Stress, Racism, and Related Factors in Pregnancy. Psychological Bulletin. 2005;131(5):662–683. doi: 10.1037/0033-2909.131.5.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green NS, Damus K, Simpson JL, Iams J, Reece EA, Hobel CJ, Merkatz IR, Greene MF, Schwarz RH, M.D.S.A. Committee Research agenda for preterm birth: Recommendations from the March of Dimes. American Journal of Obstetrics and Gynecology. 2005;193(3):626–635. doi: 10.1016/j.ajog.2005.02.106. [DOI] [PubMed] [Google Scholar]
- 14.Collins JW, David RJ, Handler A, Wall S, Andes S. Very low birthweight in African American infants: the role of maternal exposure to interpersonal racial discrimination. American Journal of Public Health. 2004;94(12):2132–2138. doi: 10.2105/ajph.94.12.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dole N, Savitz DA, Siega-Riz AM, Hertz-Picciotto I, McMahon MJ, Buekens P. Psychosocial factors and preterm birth among African American and White women in central North Carolina. American Journal of Public Health. 2004;94(8):1358–1365. doi: 10.2105/ajph.94.8.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mustillo S, Krieger N, Gunderson EP, Sidney S, McCreath H, Kiefe CI. Self-reported experiences of racial discrimination and Black-White differences in preterm and low-birthweight deliveries: the CARDIA Study. American Journal of Public Health. 2004;94(12):2125–31. doi: 10.2105/ajph.94.12.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dole N, Savitz DA, Hertz-Picciotto I, Siega-Riz AM, McMahon MJ, Buekens P. Maternal stress and preterm birth. American Journal of Epidemiology. 2003;157:14–24. doi: 10.1093/aje/kwf176. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg L, Palmer JR, Wise LA, Horton NJ, Corwin MJ. Perceptions of racial discrimination and the risk of preterm birth. Epidemiology. 2002;13(6):646–52. doi: 10.1097/00001648-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Curry AE, Vogel I, Skogstrand K, Drews C, Schendel DE, Flanders WD, Hougaard DM, Thorsen P. Maternal plasma cytokines in early- and mid-gestation of normal human pregnancy and their association with maternal factors. Journal of Reproductive Immunology. 2008;77(2):152–160. doi: 10.1016/j.jri.2007.06.051. [DOI] [PubMed] [Google Scholar]
- 20.Vassiliadis S, Ranella A, Papadimitriou L, Makrygiannakis A, Athanassakis I. Serum levels of pro- and anti-inflammatory cytokines in nonpregnant women, during pregnancy, labour and abortion. Mediators of Inflammation. 1998;7(2):69–72. doi: 10.1080/09629359891199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austgulen R, Lien E, Liabakk NB, Jacobsen G, Arntzen KJ. Increased Levels of Cytokines and Cytokine Activity Modifiers in Normal-Pregnancy. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 1994;57(3):149–155. doi: 10.1016/0028-2243(94)90291-7. [DOI] [PubMed] [Google Scholar]
- 22.Opsjon SL, Wathen NC, Tingulstad S, Wiedswang G, Sundan A, Waage A, Austgulen R. Tumor-Necrosis-Factor, Interleukin-1, and Interleukin-6 in Normal Human-Pregnancy. American Journal of Obstetrics and Gynecology. 1993;169(2):397–404. doi: 10.1016/0002-9378(93)90096-2. [DOI] [PubMed] [Google Scholar]
- 23.Makhseed M, Raghupathy R, Azizieh F, Farhat R, Hassan N, Bandar A. Circulating cytokines and CD30 in normal human pregnancy and recurrent spontaneous abortions. Human Reproduction. 2000;15(9):2011–2017. doi: 10.1093/humrep/15.9.2011. [DOI] [PubMed] [Google Scholar]
- 24.Curry AE, Vogel I, Drews C, Schendel D, Skogstrand K, Flanders WD, Hougaard D, Olsen J, Thorsen P. Mid-pregnancy maternal plasma levels of interleukin 2, 6, and 12, tumor necrosis factor-alpha, interferon-gamma, and granulocyte-macrophage colony-stimulating factor and spontaneous preterm delivery. Acta Obstetricia Et Gynecologica Scandinavica. 2007;86(9):1103–1110. doi: 10.1080/00016340701515423. [DOI] [PubMed] [Google Scholar]
- 25.Denney JM, Nelson EL, Wadhwa PD, Waters TP, Mathew L, Chung EK, Goldenberg RL, Culhane JF. Longitudinal modulation of immune system cytokine profile during pregnancy. Cytokine. 2011;53(2):170–7. doi: 10.1016/j.cyto.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elenkov IJ, Wilder RL, Bakalov VK, Link AA, Dimitrov MA, Fisher S, Crane M, Kanik KS, Chrousos GP. IL-12, TNF-alpha, and hormonal changes during late pregnancy and early postpartum: Implications for autoimmune disease activity during these times. Journal of Clinical Endocrinology and Metabolism. 2001;86(10):4933–4938. doi: 10.1210/jcem.86.10.7905. [DOI] [PubMed] [Google Scholar]
- 27.Marzi M, Vigano A, Trabattoni D, Villa ML, Salvaggio A, Clerici E, Clerici M. Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clinical and Experimental Immunology. 1996;106(1):127–133. doi: 10.1046/j.1365-2249.1996.d01-809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fofie AE, Fewell JE, Moore SL. Pregnancy influences the plasma cytokine response to intraperitoneal administration of bacterial endotoxin in rats. Experimental Physiology. 2004;90(1):95–101. doi: 10.1113/expphysiol.2004.028613. [DOI] [PubMed] [Google Scholar]
- 29.Aguilar-Valles A, Poole S, Mistry Y, Williams S, Luheshi GN. Attenuated fever in rats during late pregnancy is linked to suppressed interieukin-6 production after localized inflammation with turpentine. Journal of Physiology-London. 2007;583(1):391–403. doi: 10.1113/jphysiol.2007.132829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blackburn ST, Loper DL. Maternal, fetal, and neonatal physiology: a clinical perspective. WB Saunders; Philadelphia: 1992. [Google Scholar]
- 31.Stables D. Physiology in Childbearing. Bailliere Tindall; Edinburgh: 1999. [Google Scholar]
- 32.Makhseed M, Raghupathy R, Azizieh F, Al-Azemi MMK, Hassan NA, Bandar A. Mitogen-induced cytokine responses of maternal peripheral blood lymphocytes indicate a differential Th-type bias in normal pregnancy and pregnancy failure. American Journal of Reproductive Immunology. 1999;42(5):273–281. doi: 10.1111/j.1600-0897.1999.tb00101.x. [DOI] [PubMed] [Google Scholar]
- 33.Coussons-Read ME, Okun ML, Schmitt MP, Giese S. Prenatal stress alters cytokine levels in a manner that may endanger human pregnancy. Psychosomatic Medicine. 2005;67(4):625–31. doi: 10.1097/01.psy.0000170331.74960.ad. [DOI] [PubMed] [Google Scholar]
- 34.Christian LM, Franco A, Glaser R, Iams J. Depressive symptoms are associated with elevated serum proinflammatory cytokines among pregnant women. Brain, Behavior, and Immunity. 2009;23(6):750–754. doi: 10.1016/j.bbi.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruiz RJ, Stowe RP, Goluszko E, Clark MC, Tan A. The relationships among acculturation, body mass index, depression, and interleukin 1-receptor antagonist in Hispanic pregnant women. Ethn Dis. 2007;17(2):338–43. [PubMed] [Google Scholar]
- 36.Blackmore ER, Moynihan JA, Rubinow DR, Pressman EK, Gilchrist M, O’Connor TG. Psychiatric symptoms and proinflammatory cytokines in pregnancy. Psychosomatic Medicine. 2011;73(8):656–63. doi: 10.1097/PSY.0b013e31822fc277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cassidy-Bushrow AE, Peters RM, Johnson DA, Templin TN. Association of depressive symptoms with inflammatory biomarkers among pregnant African-American women. Journal of Reproductive Immunology. 2012;94(2):202–9. doi: 10.1016/j.jri.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Paul K, Boutain D, Agnew K, Thomas J, Hitti J. The relationship between racial identity, income, stress and C-reactive protein among parous women: Implications for preterm birth disparity research. Journal of the National Medical Association. 2008;100(5):540–546. doi: 10.1016/s0027-9684(15)31300-6. [DOI] [PubMed] [Google Scholar]
- 39.Christian LM, Franco A, Iams JD, Sheridan J, Glaser R. Depressive symptoms predict exaggerated inflammatory response to in vivo immune challenge during human pregnancy. Brain, Behavior, and Immunity. 2010;24(1):49–53. doi: 10.1016/j.bbi.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coussons-Read ME, Okun ML, Nettles CD. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain, Behavior, and Immunity. 2007;21:343–350. doi: 10.1016/j.bbi.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Coussons-Read ME, Lobel M, Carey JC, Kreither MO, D’Anna K, Argys L, Ross RG, Brandt C, Cole S. The occurrence of preterm delivery is linked to pregnancy-specific distress and elevated inflammatory markers across gestation. Brain Behavior and Immunity. 2012;26(4):650–659. doi: 10.1016/j.bbi.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.von Kanel R, Kudielka BM, Preckel D, Hanebuth D, Fischer JE. Delayed response and lack of habituation in plasma interleukin-6 to acute mental stress in men. Brain Behavior and Immunity. 2006;20(1):40–48. doi: 10.1016/j.bbi.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 43.Brydon L, Edwards S, Mohamed-Ali V, Steptoe A. Socioeconomic status and stress-induced increases in interleukin-6. Brain, Behavior, and Immunity. 2004;18(3):281–290. doi: 10.1016/j.bbi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 44.Anderson NB, Lane JD, Monou H, Williams RB, Houseworth SJ. Racial-Differences in Cardiovascular Reactivity to Mental Arithmetic. International Journal of Psychophysiology. 1988;6(2):161–164. doi: 10.1016/0167-8760(88)90047-5. [DOI] [PubMed] [Google Scholar]
- 45.Lepore SJ, Revenson TA, Weinberger SL, Weston P, Frisina PG, Robertson R, Portillo MM, Jones H, Cross W. Effects of social stressors on cardiovascular reactivity in Black and White women. Annals of Behavioral Medicine. 2006;31(2):120–127. doi: 10.1207/s15324796abm3102_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Light KC, Sherwood A. Race, borderline hypertension, and hemodynamic responses to behavioral stress before and after beta-adrenergic blockade. Health Psychol. 1989;8(5):577–95. doi: 10.1037//0278-6133.8.5.577. [DOI] [PubMed] [Google Scholar]
- 47.Hatch M, Berkowitz G, Janevic T, Sloan R, Lapinski R, James T, Barth WH. Race, cardiovascular reactivity, and preterm delivery among active-duty military women. Epidemiology. 2006;17(2):178–182. doi: 10.1097/01.ede.0000199528.28234.73. [DOI] [PubMed] [Google Scholar]
- 48.Light KC, Obrist PA, Sherwood A, James SA, Strogatz DS. Effects of race and marginally elevated blood pressure on responses to stress. Hypertension. 1987;10(6):555–63. doi: 10.1161/01.hyp.10.6.555. [DOI] [PubMed] [Google Scholar]
- 49.Brondolo E, Rieppi R, Kelly KP, Gerin W. Perceived racism and blood pressure: A review of the literature and conceptual and methodological critique. Annals of Behavioral Medicine. 2003;25(1):55–65. doi: 10.1207/S15324796ABM2501_08. [DOI] [PubMed] [Google Scholar]
- 50.Pace TWW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. American Journal of Psychiatry. 2006;163(9):1630–U1. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 51.Fagundes CP, Glaser R, Hwang BS, Malarkey WB, Kiecolt-Glaser JK. Depressive symptoms enhance stress-induced inflammatory responses. Brain, Behavior, and Immunity. 2012 doi: 10.1016/j.bbi.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glynn LM, Wadhwa PD, Dunkel-Schetter C, Chicz-DeMet A, Sandman CA. When stress happens matters: Effects of earthquake timing on stress responsivity during pregnancy. American Journal of Obstetrics and Gynecology. 2001;184:637–642. doi: 10.1067/mob.2001.111066. [DOI] [PubMed] [Google Scholar]
- 53.de Weerth C, Buitelaar JK. Physiological stress reactivity in human pregnancy - a review. Neuroscience and Biobehavioral Reviews. 2005;29:295–312. doi: 10.1016/j.neubiorev.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 54.Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’-A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 55.Kirschbaum C, Klauer T, Filipp SH, Hellhammer DH. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosomatic Medicine. 1995;57:23–31. doi: 10.1097/00006842-199501000-00004. [DOI] [PubMed] [Google Scholar]
- 56.Kirschbaum C, Bartussek D, Strasburger CJ. Cortisol responses to psychological stress and correlations with personality traits. Personal and Individual Differences. 1992;13:1353–1357. [Google Scholar]
- 57.Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology. 1994;19:313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 58.Schommer NC, Hellhammer DH, Kirschbaum C. Dissociation between reactivity of the hypothalamus-pituitary-adrenal axis and the sympathetic-adrenal-medullary system to repeated psychosocial stress. Psychosomatic Medicine. 2003;65(3):450–460. doi: 10.1097/01.psy.0000035721.12441.17. [DOI] [PubMed] [Google Scholar]
- 59.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54(1063-70) doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 60.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 61.Basco MR, Krebaum SR, Rush AJ. In: Outcome measures of depression, in Measuring patient changes in mood, anxiety, and personality disorders. Strupp HH, Horowitz LM, Lambert MJ, editors. American Psychological Association; Washington D. C.: 1997. pp. 207–245. [Google Scholar]
- 62.Hoffman S, Hatch MC. Depressive symptomatology during pregnancy: evidence for an association with decreased fetal growth in pregnancies of lower social class women. Health Psychology. 2000;19:535–543. [PubMed] [Google Scholar]
- 63.Orr ST, James SA, Blackmore Prince C. Maternal prenatal depressive symptoms and spontaneous preterm births among African-American women in Baltimore, Maryland. American Journal of Epidemiology. 2002;156(9):797–802. doi: 10.1093/aje/kwf131. [DOI] [PubMed] [Google Scholar]
- 64.Lundy BL, Jones NA, Field T, Nearing G, Davalos M, Pietro PA, Schanberg S, Kuhn C. Prenatal depression effects on neonates. Infant Behavior and Development. 1999;22:119–129. [Google Scholar]
- 65.Spielberger CD. State-trait anxiety inventory : a comprehensive bibliography. 2nd ed Consulting Psychologists Press; Palo Alto, CA (577 College Ave., Palo Alto 94306): 1989. p. 115. [Google Scholar]
- 66.Barnes LLB, Harp D, Jung WS. Reliability generalization of scores on the Spielberger state-trait anxiety inventory. Educational and Psychological Measurement. 2002;62(4):603–618. [Google Scholar]
- 67.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- 68.Wadhwa PD, Dunkel-Schetter C, Chicz-DeMet A, Porto M, Sandman CA. Prenatal psychosocial factors and the neuroendocrine axis in human pregnancy. Psychosomatic Medicine. 1996;58(5):432–446. doi: 10.1097/00006842-199609000-00006. [DOI] [PubMed] [Google Scholar]
- 69.Hobel CJ, Dunkel-Schetter C, Roesch SC, Castro LC, Arora CP. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks’ gestation in pregnancies ending in preterm delivery. American Journal of Obstetrics and Gynecology. 1999;180:S257–263. doi: 10.1016/s0002-9378(99)70712-x. [DOI] [PubMed] [Google Scholar]
- 70.Culhane JF, Rauh V, McCollum KF, Hogan VK, Agnew K, Wadhwa PD. Maternal stress is associated with bacterial vaginosis in human pregnancy. Maternal and Child Health Journal. 2001;5(2):127–134. doi: 10.1023/a:1011305300690. [DOI] [PubMed] [Google Scholar]
- 71.Cook WW, D.M.P.h.a.P.-v.s.f.t.M.J.o.A.P. Medley Proposed hostility and Pharisaic-virtue scales for the MMPI. Journal of Applied Psychology. 1954;38:414–418. 38, 414-418. [Google Scholar]
- 72.Barefoot JC, Dodge KA, Peterson BL, Dahlstrom G, Williams RB., Jr. The Cook-Medley Hostility Scale: Item content and ability to predict survival. Psychosomatic Medicine. 1989;51:46–57. doi: 10.1097/00006842-198901000-00005. [DOI] [PubMed] [Google Scholar]
- 73.Brondolo E, Kelly KP, Coakley V, Gordon T, Thompson S, Levy E, Cassells A, Tobin JN, Sweeney M, Contrada RJ. The perceived ethnic discrimination questionnaire: Development and preliminary validation of a community version. Journal of Applied Social Psychology. 2005;35(2):335–365. [Google Scholar]
- 74.Branscombe NR, Schmitt MT, Harvey RD. Perceiving pervasive discrimination among African Americans: Implications for group identification and well-being. Journal of Personality and Social Psychology. 1999;77(1):135–149. [Google Scholar]
- 75.Suls J, Wan CK. The relationship between trait hostility and cardiovascular reactivity: A quantitative review and analysis. Psychophysiology. 1993;30:615–626. doi: 10.1111/j.1469-8986.1993.tb02087.x. [DOI] [PubMed] [Google Scholar]
- 76.Davis MC, Matthews KA, McGrath CE. Hostile attitudes predict elevated vascular resistance during interpersonal stress in men and women. Psychosomatic Medicine. 2000;62(1):17–25. doi: 10.1097/00006842-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 77.Zimet GD, Powell SS, Farley GK, Werkman S, Berkoff KA. Psychometric Characteristics of the Multidimensional Scale of Perceived Social Support. Journal of Personality Assessment. 1990;55(3-4):610–617. doi: 10.1080/00223891.1990.9674095. [DOI] [PubMed] [Google Scholar]
- 78.Zimet GD, Dahlem NW, Zimet SG, Farley GK. The Multidimensional Scale of Perceived Social Support. Journal of Personality Assessment. 1988;52(1):30–41. doi: 10.1080/00223891.1990.9674095. [DOI] [PubMed] [Google Scholar]
- 79.Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect. 2003;27(2):169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 80.Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(3):340–348. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 81.Bernstein DP, Fink L. Childhood Trauma Questionnaire: A retrospective self-report. The Psychological Corporation; San Antonio, Texas: 1998. [Google Scholar]
- 82.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 83.Krieger N, Smith K, Naishadham D, Hartman C, Barbeau EM. Experiences of discrimination: Validity and reliability of a self-report measure for population health research on racism and health. Social Science and Medicine. 2005;61(7):1576–1596. doi: 10.1016/j.socscimed.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 84.Krieger N. Racial and Gender Discrimination - Risk-Factors for High Blood-Pressure. Social Science and Medicine. 1990;30(12):1273–1281. doi: 10.1016/0277-9536(90)90307-e. [DOI] [PubMed] [Google Scholar]
- 85.Krieger N, Sidney S. Racial discrimination and blood pressure: The CARDIA study of young black and white adults. American Journal of Public Health. 1996;86(10):1370–1378. doi: 10.2105/ajph.86.10.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Spielberger CD. State-Trait Anxiety Inventory for Adults: Manual, Instrument, and Scoring Guide. Consulting Psychologists Press, Inc; 1983. [Google Scholar]
- 87.Cohen S, Janicki-Deverts D. Who’s Stressed? Distributions of Psychological Stress in the United States in Probability Samples from 1983, 2006, and 2009. Journal of Applied Social Psychology. 2012;42(6):1320–1334. [Google Scholar]
- 88.Byrd DR. Race/Ethnicity and self-reported levels of discrimination and psychological distress, California, 2005. Prev Chronic Dis. 2012;9:E156. doi: 10.5888/pcd9.120042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Christian LM, Iams JD, Porter K, Glaser R. Epstein-Barr virus reactivation during pregnancy and postpartum: effects of race and racial discrimination. Brain, Behavior, and Immunity. 2012;26(8):1280–7. doi: 10.1016/j.bbi.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosomatic Medicine. 2006;68(1):41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- 91.Skinner ML, Shirtcliff EA, Haggerty KP, Coe CL, Catalano RF. Allostasis model facilitates understanding race differences in the diurnal cortisol rhythm. Development and Psychopathology. 2011;23(4):1167–86. doi: 10.1017/S095457941100054X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kunz-Ebrecht SR, Mohamed-Ali V, Feldman PJ, Kirschbaum C, Steptoe A. Cortisol responses to mild psychological stress are inversely associated with proinflammatory cytokines. Brain, Behavior, and Immunity. 2003;17(5):373–83. doi: 10.1016/s0889-1591(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 93.Spencer RL, Miller AH, Stein M, McEwen BS. Corticosterone regulation of type I and type II adrenal steroid receptors in brain, pituitary, and immune tissue. Brain Research. 1991;549(2):236–46. doi: 10.1016/0006-8993(91)90463-6. [DOI] [PubMed] [Google Scholar]
- 94.Sapolsky RM, McEwen BS. Down-regulation of neural corticosterone receptors by corticosterone and dexamethasone. Brain Research. 1985;339(1):161–165. doi: 10.1016/0006-8993(85)90638-9. [DOI] [PubMed] [Google Scholar]
- 95.Lovallo WR, Farag NH, Vincent AS. Use of a resting control day in measuring the cortisol response to mental stress: Diurnal patterns, time of day, and gender effects. Psychoneuroendocrinology. 2010;35(8):1253–1258. doi: 10.1016/j.psyneuen.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kirschbaum C, Wust S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosomatic Medicine. 1992;54(6):648–57. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- 97.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, Newby LK, Pina IL, Roger VL, Shaw LJ, Zhao D, Beckie TM, Bushnell C, D’Armiento J, Kris-Etherton PM, Fang J, Ganiats TG, Gomes AS, Gracia CR, Haan CK, Jackson EA, Judelson DR, Kelepouris E, Lavie CJ, Moore A, Nussmeier NA, Ofili E, Oparil S, Ouyang P, Pinn VW, Sherif K, Smith SC, Jr., Sopko G, Chandra-Strobos N, Urbina EM, Vaccarino V, Wenger NK. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the American Heart Association. Journal of the American College of Cardiology. 2011;57(12):1404–23. doi: 10.1016/j.jacc.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hansson GK. Mechanisms of disease - Inflammation, atherosclerosis, and coronary artery disease. New England Journal of Medicine. 2005;352(16):1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 100.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 101.Brydon L, Steptoe A. Stress-induced increases in interleukin-6 and fibrinogen predict ambulatory blood pressure at 3-year follow-up. Journal of Hypertension. 2005;23(5):1001–1007. doi: 10.1097/01.hjh.0000166841.57474.d0. [DOI] [PubMed] [Google Scholar]
- 102.Carroll JE, Low CA, Prather AA, Cohen S, Fury JM, Ross DC, Marsland AL. Negative affective responses to a speech task predict changes in interleukin (IL)-6. Brain, Behavior, and Immunity. 2011;25(2):232–8. doi: 10.1016/j.bbi.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nierop A, Bratsikas A, Klinkenberg A, Nater UM, Zimmermann R, Ehlert U. Prolonged salivary cortisol recovery in second-trimester pregnant women and attenuated salivary alpha-amylase responses to psychosocial stress in human pregnancy. Journal of Clinical Endocrinology and Metabolism. 2006;91(4):1329–1335. doi: 10.1210/jc.2005-1816. [DOI] [PubMed] [Google Scholar]
- 104.Redwine LS, Altemus M, Leong YM, Carter CS. Lymphocyte responses to stress in postpartum women: relationship to vagal tone. Psychoneuroendocrinology. 2001;26(3):241–251. doi: 10.1016/s0306-4530(00)00049-4. [DOI] [PubMed] [Google Scholar]
- 105.Christian LM. Physiological reactivity to psychological stress in human pregnancy: Current knowledge and future directions. Progress in Neurobiology. 2012;99:106–116. doi: 10.1016/j.pneurobio.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.De Weerth C, Wied CCGD, Jansen LMC, Buitelaar JK. Cardiovascular and cortisol responses to a psychological stressor during pregnancy. Acta Obstetricia Et Gynecologica Scandinavica. 2007;86(10):1181–1192. doi: 10.1080/00016340701547442. [DOI] [PubMed] [Google Scholar]
- 107.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain, Behavior, and Immunity. 2007;21(7):901–12. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 108.Stoney CM, Owens JF, Matthews KA, Davis MC, Caggiula A. Influences of the normal menstrual cycle on physiologic functioning during behavioral stress. Psychophysiology. 1990;27:125–135. doi: 10.1111/j.1469-8986.1990.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 109.Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM, Price LH. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010;35(13):2617–2623. doi: 10.1038/npp.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine. 1999;61(2):154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 111.Clapp JF, Capeless E. Cardiovascular function before, during, and after the first and subsequent pregnancies. American Journal of Cardiology. 1997;80(11):1469–1473. doi: 10.1016/s0002-9149(97)00738-8. [DOI] [PubMed] [Google Scholar]
- 112.Shipton D, Tappin DM, Vadiveloo T, Crossley JA, Aitken DA, Chalmers J. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study. British Medical Journal. 2009;339 doi: 10.1136/bmj.b4347. [DOI] [PMC free article] [PubMed] [Google Scholar]