Abstract
Laboratory surveillance data from the Animal Health Laboratory, University of Guelph, on the etiological diagnoses of neonatal diarrhea in piglets were analyzed to determine the relative importance and trends of different enteric pathogens in Ontario. A total of 237 cases, including live and dead 1- to 7-day-old piglets, were submitted for diagnosis of gastrointestinal illness between 2001 and 2010. The combined frequencies for cases of gastrointestinal illness involving Escherichia coli, Clostridium perfringens type A, rotavirus, and Clostridium difficile, either as single pathogens or a complex of pathogens, accounted for 56% of the total cases. In a total of 33% of cases of gastrointestinal illness, an etiological agent was not identified. The frequency of cases diagnosed with enterotoxigenic E. coli was decreased from 2007. Cases submitted in 2010 were more likely to be diagnosed with C. perfringens type A compared to cases submitted in 2002 to 2007 (P < 0.05). There was a significant trend for cases submitted in the winter to be diagnosed with C. perfringens type A, enterotoxigenic E. coli, rotavirus, and Cystoisospora suis (formerly Isospora suis) (P < 0.05). Enterotoxigenic E. coli was less likely diagnosed if C. difficile, C. perfringens, or rotavirus were detected (P < 0.05). Younger piglets were more likely to be diagnosed with C. perfringens type A (P < 0.05) and C. difficile (P < 0.05) than older piglets. This study shows that E. coli, C. perfringens type A, rotavirus, and C. difficile are enteric pathogens of concern for Ontario swine farrowing operations and further research is required to understand the reasons for the cases that are not diagnosed.
Résumé
Les données de surveillance provenant du Animal Health Laboratory de l’University of Guelph sur les diagnostics étiologiques des diarrhées néonatales des porcelets ont été analysées afin de déterminer l’importance relative et les tendances des différents agents pathogènes entériques en Ontario. Entre 2001 et 2010, 237 cas de porcelets vivants et morts âgés de 1 à 7 jours ont été soumis pour un diagnostic de maladie gastrointestinale. Les fréquences combinées pour les cas de maladies gastrointestinales impliquant Escherichia coli, Clostridium perfringens type A, rotavirus, et Clostridium difficile, soit comme seul agent pathogène ou un complexe d’agents pathogènes, représentaient 56 % du total des cas. Pour 33 % des cas de maladies gastrointestinales, aucun agent étiologique n’a été identifié. La fréquence des cas avec un diagnostic d’E. coli entérotoxigénique a diminué à compter de 2007. Les cas soumis en 2010 étaient plus susceptibles d’avoir un diagnostic d’infection à C. perfringens type A comparativement aux cas soumis de 2002 à 2007 (P < 0,05). Il y avait une tendance significative pour les cas soumis en hiver d’avoir un diagnostic avec C. perfringens type A, E. coli entérotoxigénique, rotavirus, et Cystoisospora suis (anciennement Isospora suis) (P < 0,05). Les E. coli entérotoxigéniques étaient moins souvent diagnostiquées si C. difficile, C. perfringens, ou du rotavirus étaient détectés (P < 0,05). Les porcelets plus jeunes étaient plus susceptibles d’être diagnostiqués avec du C. perfringens type A (P < 0,05) et C. difficile (P < 0,05) que les porcelets plus vieux. Cette étude démontre que E. coli, C. perfringens type A, rotavirus, et C. difficile sont des agents pathogènes d’intérêt pour les opérations de mise-bas en Ontario et des recherches supplémentaires sont requises pour comprendre les raisons pour lesquelles certains cas ne sont pas diagnostiqués.
(Traduit par Docteur Serge Messier)
Introduction
Neonatal piglet diarrhea is a major cause of pre-weaning mortality, resulting in significant economic loss for swine producers. The relative importance of different diseases contributing to neonatal diarrhea in piglets appears to be changing, possibly because of changes in husbandry and management practices, advances in diagnostic techniques, and/or the emergence of new diseases. Reports in the United States have indicated an increase in cases of neonatal diarrhea in piglets attributed to Clostridium difficile in a survey conducted in 2000 (1), and in Clostridium perfringens type A in diagnostic laboratory submission data from 2004 to 2009 (2). These reports also indicate a relative decrease in cases attributed to enterotoxigenic Escherichia coli (ETEC), transmissible gastroenteritis (TGE) virus, and Clostridium perfringens type C compared to retrospective data from previous decades (1,2).
In recent years, clostridial infections as causes of neonatal diarrhea in piglets have gained prominence. Clostridium perfringens are divided into 5 toxinotypes (2,3), and some studies have found an association between C. perfringens type A strains containing the beta2 toxin gene (cpb2) and neonatal diarrhea in piglets (4,5). The current diagnostic criteria for C. perfringens type A enteritis is not specific and is generally based on a combination of isolation of large numbers of C. perfringens type A from the intestinal contents, exclusion of other causes of diarrhea, and possibly the detection of cpb2 through polymerase chain reaction (PCR) (3). Diarrhea associated with C. difficile can occur in piglets between 1 to 7 d of age, with some reports also including sudden death and scrotal edema (2,3). Diagnosis of C. difficile-associated neonatal diarrhea in piglets is generally confirmed by histopathologic lesions in the colon and, less often, the small intestine, in combination with toxin detection in intestinal content via enzyme-linked immunosorbent assay (ELISA) kits (1,3).
The purpose of this study was to use laboratory diagnostic data to identify the frequency and trends of different pathogens contributing to neonatal diarrhea in piglets from Ontario swine farms between 2001 and 2010.
Materials and methods
Data collection
Data were provided by the Animal Health Laboratory (AHL) at the University of Guelph, and included laboratory submissions from swine farms in Ontario from 2001 to 2010. The data included cases in which > 1 live or dead piglets between 1 to 7 d of age were submitted for a gastrointestinal tract (GIT) illness. A GIT case was defined as a case submitted for one or more of the following reasons: enteritis, diarrhea, or scours. For farms with multiple submissions, each submission was counted as 1 case. The data set for GIT cases submitted from January 2001 to April 2007 was provided in text file, and the data set for GIT cases submitted from May 2007 to December 2010 was provided in a spreadsheet (Microsoft Excel 2007; Microsoft Corporation, Redmond, Washington, USA).
Diagnostic methods
Necropsy and histopathologic examination were done in all cases. The etiological diagnoses of GIT cases were based on pathologists’ interpretation of gross and histopathologic lesions, in combination with results of microbiologic tests. Ancillary microbiologic tests common to all cases with necropsy evidence of gastrointestinal disease included bacterial culture from the small intestine and colon, and rotavirus latex agglutination (RLA) testing. Additional tests done for each case were at the discretion of the individual pathologist assigned to the case, as directed by lesions indicative of specific etiologic agents. A diagnosis of colibacillosis required histologic evidence of colonization of the luminal surface of enterocytes by short rod-shaped bacilli, isolation of E. coli from intestine, and identification of ETEC strains through agglutination serotyping or genotyping through gel-based PCR. Diagnosis of TGE was based on histologic evidence of atrophic enteritis and identification of TGE virus antigen by fluorescent antibody test (FAT) or immunohistochemistry (IHC). A diagnosis of rotavirus enteritis required histologic evidence of atrophic enteritis and identification of rotavirus antigen within enterocytes by FAT, or in feces or intestinal content by latex agglutination test (LAT), which detected rotavirus A. From September 2010 onwards, rotavirus infection was confirmed by multiplex real-time reverse-transcriptase polymerase chain reaction (real time RT-PCR) for rotavirus A and C, or gel-based RT-PCR for rotavirus B (6). Diagnosis of coccidiosis caused by Cystoisospora suis (formerly Isospora suis) was based on histologic identification of Cystoisospora merozoites, schizonts, or oocysts in the cytoplasm of enterocytes, often in combination with lesions of villus atrophy, or identification of coccidial oocysts in feces by sucrose wet mount or fecal flotation. Diagnosis of C. perfringens type A was based on the presence of long bacilli consistent with C. perfringens on the intestinal mucosal surface, in combination with isolation of C. perfringens in large numbers from intestinal content or, in some cases, genotyping of C. perfringens strains through gel-based PCR, which detected the toxinotype (A, B, C, D, E) and cpb2. Confirmation of C. difficile-associated enterocolitis was based on presence of typical histologic lesions of focal suppurative inflammation and necrosis (“volcano” lesions), in combination with the detection of C. difficile toxins (A and B) in intestinal content or feces via ELISA, or isolation of C. difficile (the culture method was available from March 2008 onward) (7). Bacterial culture of the intestinal contents of the piglets was used to identify other bacterial pathogens, including Salmonella enterica serovars and Enterococcus durans. Cryptosporidium parvum was identified based on the presence of typical histological lesions, including the presence of appropriately sized and shaped protozoa adhered to the luminal surface of the enterocytes. Concurrent porcine reproductive and respiratory syndrome virus (PRRSV) infection in some pigs with GIT disease was determined by identification of PRRSV antigen in lung by IHC, or detection of viral nucleic acid in lung using IHC or gel-based PCR (before June 2010) or multiplex real-time RT-PCR (from June 2010 onward) (8).
Data analysis
The 2 data sets were merged in a spreadsheet and imported for statistical analysis (Stata 10 Intercooled for Windows XP; StataCorp LP, College Station, Texas, USA). Multivariable logistic regression models were used to analyze the association between the diagnosis of ETEC, C. perfringens, C. difficile, rotavirus, and Cystoisospora suis, and independent variables; including age of piglet in days, year of submission, season of submission, and diagnosis of other enteric pathogens. The seasons were categorized using the calendar dates for the equinoxes and solstices for each year in Canada. A manual stepwise procedure was used to build the models. Univariable analysis for the association between diagnosis of the enteric pathogen and independent variables was done using single logistic regression, the predictor variables where P < 0.20 were selected for inclusion in multivariable analyses. Pair-wise correlation coefficients were calculated between independent variables, and coefficients with an absolute value greater than 0.8 were considered collinear. If 2 independent variables were significantly correlated, the more informative variable was used in the final model. A variable was identified as a confounder if it changed the coefficient of the main effects by ≥ 20% when the potential confounder variable was removed. If a variable was determined to be a confounder, it was included in the final model regardless of its statistical significance. Interaction was evaluated between the detection of each pathogen. Each interaction term was assessed for statistical significance with the main effects model, and the interaction terms with P < 0.05 were selected for inclusion in multivariable analysis. Interaction terms that were not significant in the final model were removed if removal of the interaction term did not result in a significant change in the likelihood ratio test. The logistic regression models were assessed for goodness-of-fit, which was indicated by P > 0.05 in the Pearson’s chi-squared test.
Results
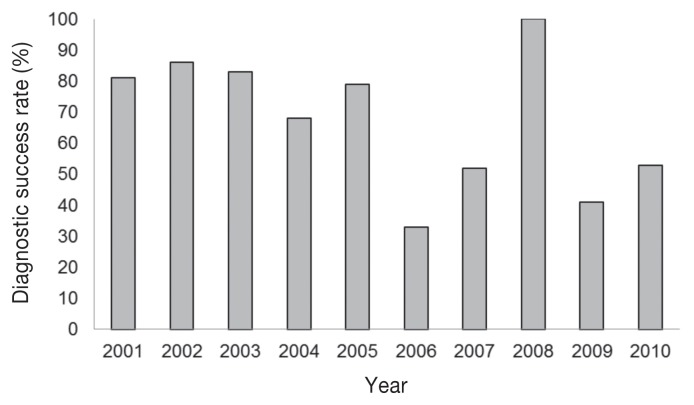
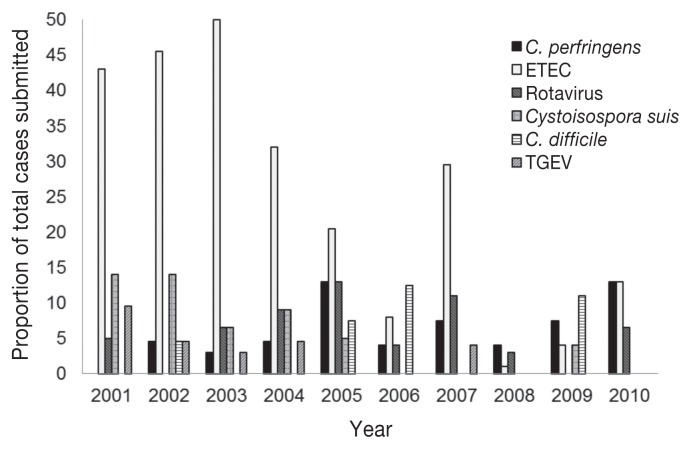
A total of 237 GIT cases involving the submission of live or dead piglets, 1 to 7 d of age were submitted to the AHL, from 2001 to 2010. The number of these GIT cases submitted per year ranged from 10 to 39, with a mean of 23.7 ± 7.97 cases per year. A successful diagnosis was defined as a gastrointestinal case in which the etiological agent was identified. The diagnostic success rate for GIT cases submitted per year is indicated in Figure 1. The annual prevalence for GIT cases diagnosed with single etiological agents is indicated for ETEC, C. perfringens, rotavirus, Cystoisospora suis, C. difficile, and TGE virus (Figure 2).
Figure 1.
Diagnostic success rate for gastrointestinal tract (GIT) cases in 1- to 7-day-old pigs submitted to Animal Health Laboratory (AHL) from 2001 to 2010.
Figure 2.
Annual prevalence for gastrointestinal tract (GIT) cases submitted to Animal Health Laboratory (AHL) diagnosed with a single etiological agent.
There were 79 (33%) GIT cases submitted to the AHL in which an etiological agent was not identified. There were a total of 51 (22%) GIT cases in the fall, 50 cases in the spring (21%), 46 (19%) cases in the summer, and 90 (38%) cases in the winter. The diagnosis of pathogens involved in each GIT case is given in Table I.
Table I.
Pathogens involved in gastrointestinal tract (GIT) disease cases for 1- to 7-dayold pigs submitted to the Animal Health Laboratory, University of Guelph, from 2001 to 2010
| Pathogens involved in GIT case | Number of cases for single etiological diagnosis (% of total GIT cases) | Number of cases for multiple etiological diagnosis (percent of total GIT cases) |
|---|---|---|
| Escherichia coli (n = 73) | 63 (26.6) | 10 (4.2) |
| Clostridium perfringens (n = 28) | 19 (8.0) | 9 (4.3) |
| Rotavirus (n = 28) | 18 (7.6) | 10 (4.2) |
| Clostridium difficile (n = 21) | 10 (4.2) | 11 (4.6) |
| Cystoisospora suis (n = 15) | 13 (5.5) | 2 (0.84) |
| Transmissible gastroenteritis virus (n = 7) | 6 (2.5) | 1 (0.42) |
| Salmonella sp. (n = 6) | 2 (0.84) | 4 (1.7) |
| Porcine reproductive and respiratory syndrome virus (n = 3) | 2 (0.84) | 1 (0.42) |
| Cryptosporidium parvum (n = 2) | 1 (0.42) | 1 (0.42) |
| Enterococcus durans (n = 2) | 1 (0.42) | 1 (0.42) |
Enterotoxigenic E. coli infection was diagnosed as the cause of GIT disease for 63 cases that involved a single etiological agent, and 10 cases that involved multiple etiological agents (31% of total cases; Table I). Enterotoxgenic E. coli was less likely to be recovered from a GIT case if C. difficile, C. perfringens, or rotavirus were detected (P < 0.05; Table II). Enterotoxgenic E. coli was more likely to be diagnosed for GIT cases that occurred in the winter compared to the spring and the summer (P < 0.05; Table II). There was a more likely tendency for a GIT case to be diagnosed with ETEC between years 2001 and 2004 than in 2009 (P < 0.05; Table II).
Table II.
Logistic regression comparing the association of enterotoxigenic Escherichia coli diagnosis and independent variables
| Independent variables | Odds ratio | Standard error | 95% Confidence interval | P-value |
|---|---|---|---|---|
| Clostridium difficile detection | ||||
| No | Referent | |||
| Yes | 0.11 | 0.125 | 0.013–.97 | 0.047 |
| Clostridium perfringens detection | ||||
| No | Referent | |||
| Yes | 0.20 | 0.148 | 0.049–0.85 | 0.029 |
| Rotavirus detection | ||||
| No | Referent | |||
| Yes | 0.29 | 0.170 | 0.95–0.91 | 0.034 |
| Seasona | ||||
| Winter | Referent | |||
| Spring | 0.38 | 0.171 | 0.16–0.92 | 0.032 |
| Summer | 0.37 | 0.177 | 0.15–0.94 | 0.037 |
| Yeara | ||||
| 2009 | Referent | |||
| 2001 | 9.6 | 7.60 | 2.0–45 | 0.004 |
| 2002 | 9.6 | 7.56 | 2.1–45 | 0.004 |
| 2003 | 12 | 8.68 | 2.7–50 | 0.001 |
| 2004 | 5.0 | 3.95 | 1.1–24 | 0.042 |
| 2005 | 4.0 | 3.03 | 0.89–18 | 0.07 |
| 2007 | 4.3 | 3.39 | 0.93–20 | 0.062 |
Parameters for fall, 2006, 2008, and 2010 are not shown (P > 0.1).
Clostridium perfringens was diagnosed as the cause of GIT disease for 19 cases that involved a single etiological agent, and 9 cases that involved multiple etiological agents (12% of total cases; Table II). A total of 155 GIT cases were cultured for C. perfringens and the organism was isolated in 133 (86%) cases. The GIT cases were less likely to be diagnosed with C. perfringens with increasing age of the piglets (P < 0.05) and if ETEC was detected (P = 0.065; Table III). Clostridium perfringens was more likely diagnosed for GIT cases that occurred in the winter compared to the fall and spring (P < 0.05; Table III), and more likely diagnosed for GIT cases that occurred in the summer compared to the fall (OR = 0.13, P = 0.022) and the spring (OR = 0.13, P = 0.029). The odds of a GIT case being diagnosed with C. perfringens was greater in 2010 compared to the years between 2002 and 2007 (P < 0.10). Clostridium perfringens isolates from 40 cases were genotyped for major toxin genes and cpb2. All isolates from the 40 cases belonged to toxinotype A and cpb2 was detected in the C. perfringens isolates of 38 (95%) cases. Of the 40 GIT cases where C. perfringens isolates were genotyped, 17 cases were diagnosed with C. perfringens as the etiological agent (42.5%). Genotyping was not done on the isolates in 11 cases diagnosed with C. perfringens as the etiological agent.
Table III.
Logistic regression comparing the association of Clostridium perfringens diagnosis and independent variables
| Independent variables | Odds ratio | Standard error | 95% Confidence interval | P-value |
|---|---|---|---|---|
| Average age of submitted piglets (d) | 0.69 | 0.106 | 0.51–0.93 | 0.016 |
| Seasona | ||||
| Winter | Referent | |||
| Spring | 0.13 | 0.116 | 0.024–0.73 | 0.021 |
| Fall | 0.13 | 0.108 | 0.028–0.65 | 0.013 |
| ETEC detection | ||||
| No | Referent | |||
| Yes | 0.27 | 0.192 | 0.068–1.1 | 0.065 |
| Yeara | ||||
| 2010 | Referent | |||
| 2002 | 0.074 | 0.0967 | 0.0059–0.94 | 0.045 |
| 2003 | 0.089 | 0.0956 | 0.011–0.73 | 0.025 |
| 2004 | 0.056 | 0.0713 | 0.0046–0.68 | 0.024 |
| 2005 | 0.19 | 0.163 | 0.037–1.0 | 0.052 |
| 2006 | 0.040 | 0.0500 | 0.0033–0.47 | 0.011 |
| 2007 | 0.078 | 0.0840 | 0.0095–0.64 | 0.018 |
| 2009 | 0.17 | 0.159 | 0.029–1.0 | 0.056 |
Parameters for 2001 (no cases of C. perfringens diagnosed), 2008, and summer (P > 0.1) are not shown.
ETEC — enterotoxigenic E. coli.
Rotavirus was diagnosed as the cause of GIT disease for 18 cases that involved a single etiological agent, and 10 cases that involved multiple etiological agents (12% of total cases). Rotavirus was more likely diagnosed for GIT cases that occurred in the fall compared to spring (OR = 0.14, P = 0.012) and the summer (OR = 0.15, P = 0.016).
Clostridium difficile was diagnosed as the cause of GIT disease for 10 cases that involved a single etiological agent and 11 cases that involved multiple etiological agents (9% of total cases). Clostridium difficile was diagnosed less with increasing age of the piglets (OR = 0.68, P = 0.031). Clostridium difficile was diagnosed less if ETEC was detected (OR = 0.085, P = 0.045), but diagnosed more if Salmonella sp. was detected (OR = 20, P = 0.007).
Cystoisospora suis was diagnosed as the cause of GIT disease for 13 cases that involved a single etiological agent and 2 cases that involved multiple etiological agents (6% of total cases). Coccidiosis was diagnosed more in GIT cases that occurred in the summer compared to spring (OR = 0.26, P = 0.03) and winter (OR = 0.046, P = 0.004). Transmissible gastroenteritis virus was diagnosed as the cause of GIT disease for 6 cases that involved a single etiological agent and 1 case that involved multiple etiological agents (3% of total cases). Porcine reproductive and respiratory syndrome was diagnosed as the cause of GIT disease for 2 cases that involved a single etiological agent and 1 case that involved multiple etiological agents (0.01% of total cases).
Discussion
Cases of neonatal diarrhea were most frequent during winter, a well-established finding in countries with harsh winters (9). The major known pathogens that contributed to neonatal diarrhea in piglets from 2001 to 2010 were ETEC, rotavirus, C. perfringens, and C. difficile. The combined GIT cases involving these pathogens contribute to more than half of the GIT cases submitted to the AHL. Interestingly, there was marked annual variation in specific etiological diagnoses although the reason for this annual variation is unclear.
Enterotoxigenic E. coli was a common cause of neonatal diarrhea from 2001 to 2005 in cases involving single etiological agents, but there was a relative decrease in the number of cases diagnosed with this agent from 2006 onward. Enterotoxigenic E. coli was more frequently diagnosed for GIT cases that occurred in the winter compared to the spring and summer. Post-weaning ETEC enteritis has been documented to occur most frequently in the fall and winter (10), and another study reported that cases of neonatal colibacillosis in piglets were least prevalent in the spring compared to other seasons (11).
Rotavirus was an important pathogen contributing to neonatal diarrhea in piglets. The detection of rotavirus may be influenced by the sampling time of infected piglets during the course of disease, with the highest viral load occurring at the acute stage. The AHL has reported that approximately 50% of rotavirus cases were attributed to rotavirus group A (6); it is possible that rotavirus infection caused by other groups (B or C) were underdiagnosed at the AHL, since PCR methods available for detecting these rotavirus groups were only available from 2010 onward. The introduction of RT-PCR was reported by the AHL to increase the diagnosis of rotavirus B and rotavirus C infection (12). The AHL reported that from 2010 to April 2011, 17% of cases were positive by rotavirus latex agglutination, whereas 68% of cases were positive by rotavirus group RT-PCR tests. Of these, 6 cases were positive for rotavirus A, 8 cases were positive for rotavirus B, 1 case was positive for rotavirus A and B, and 1 case was positive for rotavirus A and C (12).
Studies conducted on the frequency of different swine enteric pathogens have also shown that rotavirus is a common pathogen isolated from diarrheic neonatal piglets (1,13,14). Our study reported that rotavirus was more likely to be diagnosed for GIT cases that occurred in the fall compared to the spring and summer, suggesting that rotaviral infection often occurred in the fall season.
Although C. perfringens type C infections in swine herds have been identified in some geographic areas (2), C. perfringens type C was not identified in any Ontario GIT cases submitted to the AHL between 2001 and 2010. Clostridium perfringens type A was the second most frequent etiological diagnosis of GI illness in piglets within the first week of their life in this study. Analysis showed that the likelihood of C. perfringens diagnosis decreased with increasing piglet age. It is thought that C. perfringens associated diarrhea usually occurs within 48 h after birth (2). However, it is possible that diarrhea associated with C. perfringens type A is misdiagnosed, since the pathogenic mechanism is unknown and current diagnostic methods are not specific for C. perfringens-associated enteritis. In fact, C. perfringens was considered as the etiological diagnosis for only 42.5% of cases where genotyping was done on the isolates, without concurrent histologic evidence of bacterial proliferation at the intestinal mucosal surface, positive cpb2 status, or both.
Previous studies have indicated an association with cpb2-positive C. perfringens type A and neonatal diarrhea in piglets (4,5). However, a recent study showed that the beta2 toxin (CPB2) was detected in the intestinal content of both healthy and diarrheic piglets, and that enumeration of cpb2-positive C. perfringens type A did not provide a useful diagnosis for C. perfringens type A enteritis (14). Because cpb2-positive C. perfringens were isolated from diarrheic piglets where an etiological agent was not identified in that study, it was suggested that C. perfringens type A enteritis was often misdiagnosed in cases where no other known pathogens except C. perfringens was identified (14).
Cases of neonatal diarrhea attributed to C. difficile appeared sporadic and approximately half of the total cases associated with C. difficile involved a complex of other pathogens. For example, C. difficile was more likely to be diagnosed for GIT cases when Salmonella spp. was also isolated. Some studies indicate that C. difficile is an emerging pathogen in neonatal diarrhea (1,14). The bacterial culture test for C. difficile became available at the AHL in 2008 (7); the difficulty of culturing this anaerobic organism suggests that cases attributed to C. difficile may be underdiagnosed (3). There is currently no consensus on the best method for the diagnosing C. difficile enteritis in humans, and guidelines for diagnosing C. difficile enteritis in animals are unavailable (15). A recent study reported that the commercial tests available for detection of C. difficile toxins have lower sensitivity and specificity in piglets compared to humans (15). A 2-step algorithm was recommended for the detection of C. difficile in herds of swine, similar to that used in medical diagnostic laboratories. A first step suggested was the use of a test with high negative predictive value such as real-time PCR, followed by confirmation of the positive results with toxigenic culture as a second step (15). However, the study was more focused on the presence or absence of the organism in herds rather than specific diagnosis of C. difficile enteritis in piglets (15). Clostridium difficile toxins can be detected in healthy piglets and, therefore, its presence alone does not confirm the diagnosis. However, C. difficile may be an important pathogen causing subclinical infection in piglets (16). Clearly, further work is required to develop a sensitive diagnostic approach for C. difficile enteritis in piglets.
Cystoisospora suis was diagnosed as a single etiological agent in cases of neonatal diarrhea that occurred mainly from 2001 to 2005. The general lack of diarrheal cases attributed to Cystoisospora suis from 2006 onwards suggests that it is currently not a major pathogen of concern for 1- to 7-day-old piglets. This is in agreement with reports that conclude coccidiosis is associated with a diarrhea that begins after 1 or 2 wk of age (13,17). Coccidiosis was diagnosed more in GIT cases that occurred in the summer compared to the spring and the winter, in agreement with another report that indicated neonatal piglet coccidiosis cases were most frequently identified in the summer (9). Cases neonatal diarrhea attributed to TGE virus were sporadic and absent from 2008 onwards, indicating that it is not a current concern for the Ontario swine industry.
The number of neonatal piglet GIT cases submitted to the AHL generally decreased in the 10-year period, which may reflect the decrease in number of swine operations in Ontario during this time, an actual decrease in prevalence of neonatal diarrhea due to improved farm management practices, or other factors, including reduced submissions because of poor economic returns for Ontario swine producers from from 2007 to 2010. Furthermore, no etiological agent was determined in one third of the GIT cases submitted to the AHL. The lack of diagnosis for some GIT cases is possibly due to inappropriate samples submitted to the diagnostic laboratory; piglets in the acute stage of disease have the highest pathogen load and, therefore, are more likely to yield useful diagnostic information (2). It is also possible that there may be emerging pathogens causing neonatal diarrhea in piglets that are not detected by current diagnostic methods. Prevalence studies on infectious causes of diarrhea in piglets conducted in different countries could not identify etiological agents in 17% to 58% of the pigs examined (1,13,17). Similarly, a recent study conducted in Ontario did not identify the etiological agent in 38% of neonatal diarrheic pigs (14). It is possible that some GIT cases had an etiological agent identified, but were not recorded electronically due to data entry error. Another reason for the lack of diagnosis in some GIT cases is that the producer may choose not to pursue additional laboratory tests for cost reasons. There may be other unknown pathogens involved in undiagnosed GIT cases submitted to the AHL and further investigation is required to determine the current causes of diarrhea in piglets from Ontario swine farms. The results of the study may not represent the actual prevalences of pathogens causing neonatal diarrhea in piglets in the field.
This study identified several current pathogens involved in neonatal diarrhea for Ontario swine farms. Clostridium difficile appears to be an emerging pathogen, and ETEC and rotavirus remain pathogens of concern for neonatal diarrhea in piglets. Further research in the diagnostic method of these pathogens may be useful in improving the diagnostic rate for GIT cases. The data suggested that C. perfringens type A may be an important pathogen for neonatal diarrhea in piglets, but the current lack of specific diagnostic criteria for this pathogen made it difficult to determine the significance of this pathogen.
Acknowledgments
This project was supported by the OMAF and MRA-UG Agreement through the Animal Health Strategic Investment fund (AHSI) managed by the Animal Health Laboratory of the University of Guelph.
References
- 1.Yaeger M, Funk N, Hoffman L. A survey of agents associated with neonatal diarrhea in Iowa swine including Clostridium difficile and porcine reproductive and respiratory syndrome virus. J Vet Diag Invest. 2002;14:281–287. doi: 10.1177/104063870201400402. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz KJ. Clostridium-associated diseases in swine. Proc Annu Meet AASV. 2009:415–421. [Google Scholar]
- 3.Songer JG, Uzal FA. Clostridial enteric infections in pigs. J Vet Diag Invest. 2005;17:528–536. doi: 10.1177/104063870501700602. [DOI] [PubMed] [Google Scholar]
- 4.Garmory HS, Chanter N, French NP, Bueschel D, Songer JG, Titball RW. Occurrence of Clostridium perfringens b2-toxin amongst animals, determined using genotyping and subtyping PCR assays. Epidemiol Infect. 2000;124:61–67. doi: 10.1017/s0950268899003295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bueschel DM, Jost BH, Billington SJ, Trinh HT, Songer JG. Prevalence of cpb2, encoding beta2 toxin, in Clostridium perfringens field isolates: Correlation of genotype with phenotype. Vet Microbiol. 2002;94:121–129. doi: 10.1016/s0378-1135(03)00081-6. [DOI] [PubMed] [Google Scholar]
- 6.Carman S. New swine Rotavirus group A/C real-time RT-PCR and swine Rotavirus group B RT-PCR tests available at the AHL. AHL Newsletter. 2010;14:25. [Google Scholar]
- 7.Slavic D. Clostridium difficile culture and toxin testing. AHL Newsletter. 2008;12:1. [Google Scholar]
- 8.Carman S, McEwen B, Fairles J. Tetracore Next generation NA/EU PRRSV multiplex real-time RTPCR is now available at the AHL. AHL Newsletter. 2010;14:15. [Google Scholar]
- 9.Sanford SE, Josephson GKA. Porcine neonatal coccidiosis. Can Vet J. 1981;22:282–285. [PMC free article] [PubMed] [Google Scholar]
- 10.Amezcua R, Friendship RM, Dewey CE, Gyles C, Fairbrother JM. Presentation of postweaning Escherichia coli diarrhea in southern Ontario, prevalence of hemolytic E. coli serogroups involved, and their antimicrobial resistance patterns. Can J Vet Res. 2002;66:73–78. [PMC free article] [PubMed] [Google Scholar]
- 11.Morin M, Turgeon D, Jolette J, et al. Neonatal diarrhea of pigs in Quebec: Infectious causes of significant outbreaks. Can J Comp Med. 1983;47:11–17. [PMC free article] [PubMed] [Google Scholar]
- 12.Carman S, Hazlett M, McEwen B, Rossov K. New Rotavirus PCR test improves diagnostic rate for porcine diarrhea. AHL Newsletter. 2011;15:13. [Google Scholar]
- 13.Katsuda K, Kohmoto M, Kawashima K, Tsunemitsu H. Frequency of enteropathogen detection in suckling and weanling pigs with diarrhea in Japan. J Vet Diag Invest. 2006;18:350–354. doi: 10.1177/104063870601800405. [DOI] [PubMed] [Google Scholar]
- 14.Farzan A, Kircanki J, DeLay J, et al. An investigation into the association between cpb2-encoding C. perfringens type A and diarrhea in neonatal piglets. Can J Vet Res. 2013;77:45–53. [PMC free article] [PubMed] [Google Scholar]
- 15.Keessen EC, Hopman NEM, van Leengoed LAMG, et al. Evaluation of four different diagnostic tests to detect Clostridium difficile in piglets. J Clin Microbiol. 2011;49:1816–1821. doi: 10.1128/JCM.00242-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yaeger MJ, Kinyon JM, Songer GJ. A prospective, case control study evaluating the association between Clostridium difficile toxins in the colon of neonatal swine and gross and microscopic lesions. J Vet Diag Invest. 2007;19:52. doi: 10.1177/104063870701900108. [DOI] [PubMed] [Google Scholar]
- 17.Wieler LH, Ilieff A, Herbst W, et al. Prevalence of enteropathogens in suckling and weanling suckling pigs with diarrhea in southern Germany. J Vet Med B. 2001;48:151–159. doi: 10.1111/j.1439-0450.2001.00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]