Abstract
The objectives of this study were to determine the analgesic and motor effects of a high-volume intercoccygeal epidural injection of bupivacaine at 2 concentrations in cows. A prospective, randomized, blinded, crossover trial was conducted on 6 adult cows. An indwelling epidural catheter was placed in the first intercoccygeal space and advanced 10 cm cranially. All the cows received 3 treatments with a washout period of 48 h: saline (control), 0.125% bupivacaine (high dose), or 0.0625% bupivacaine (low dose), at a final volume of 0.15 mL per kilogram of body weight, infused manually into the epidural space over a period of 15 min. The anal and tail tone and motor deficits of the pelvic limbs were evaluated in 5 of the cows with use of a numerical rating scale and a visual analogue scale (VAS). Sensory block was assessed in 4 of the cows by the response to needle pricks in different regions with the use of a VAS. Measurements were obtained before and at different time points after injection, up to 360 min. Analysis of variance for repeated measures and post-hoc Tukey’s and Dunnett’s tests were used. Differences were considered significant when the P-value was ≤ 0.05. One cow became recumbent 6 h after injection. Anal and tail tones were significantly decreased and motor deficits of the pelvic limbs were significantly increased after bupivacaine treatment compared with control treatment. The overall mean VASpain scores ± standard deviation were 66 ± 8 after control treatment, 52 ± 5 after low-dose bupivacaine treatment, and 43 ± 5 after high-dose bupivacaine treatment. The pain scores were significantly lower in caudal regions up to the saphenous nerve after high-dose bupivacaine treatment compared with control treatment and significantly lower in the anus, vulva, and tail after low-dose bupivacaine treatment compared with control treatment. Thus, analgesia with moderate motor deficits of the pelvic limbs may be obtained with 0.125% bupivacaine administered epidurally.
Résumé
Les objectifs de la présente étude étaient de déterminer chez la vache les effets analgésiques et moteurs d’une injection épidurale inter-coccygienne d’un volume important de bupivacaïne à deux concentrations. Une étude croisée prospective, randomisée, et à l’aveugle a été réalisée chez 6 vaches adultes. Un cathéter épidural à demeure a été placé dans le premier espace inter-coccygien et avancé cranialement de 10 cm. Toutes les vaches ont reçu 3 traitements avec une période d’évacuation de 48 h : saline (témoin), 0,125 % de bupivacaïne (dose élevée) ou 0,0625 % de bupivacaïne (faible dose), à un volume final de 0,15 mL par kilo de poids corporel, infusé manuellement dans l’espace épidural sur une période de 15 min. Le tonus anal et de la queue ainsi que les déficits moteurs des membres pelviens ont été évalués chez 5 des vaches au moyen d’une échelle numérique de pointage et une échelle analogue visuelle (VAS). Le bloc sensitif a été évalué chez 4 des vaches par la réponse à des piqûres d’aiguille dans différentes régions avec l’utilisation d’une VAS. Les mesures ont été obtenues avant et à différents temps après l’injection, jusqu’à 360 min. Une analyse de variance pour mesures répétées et les tests post-hoc de Tukey et de Dunnett ont été utilisés. Les différences étaient considérées significatives lorsque la valeur de P était ≤ 0,05. Une vache est demeurée couchée 6 h après l’injection. Le tonus anal et de la queue était réduit de manière significative et les déficits moteurs des membres pelviens étaient significativement augmentés après le traitement à la bupivacaïne comparativement au traitement témoin avec la saline. Dans l’ensemble les scores moyens ± l’écart-type de VASdouleur étaient 66 ± 8 après le traitement témoin, 52 ± 5 après le traitement à faible dose de bupivacaïne, et 43 ± 5 après le traitement avec la dose élevée de bupivacaïne. Les scores de douleur étaient significativement plus faibles dans les régions caudales jusqu’au nerf saphène après le traitement avec les doses élevées de bupivacaïne comparativement au traitement témoin et significativement plus faibles au niveau de l’anus, la vulve et la queue après le traitement avec les faibles doses de bupivacaïne comparativement au traitement témoin. Ainsi, une analgésie avec des déficits moteurs modérés des membres pelviens peut être obtenue avec de la bupivacaïne à 0,125 % administrée par voie épidurale.
(Traduit par Docteur Serge Messier)
Introduction
Epidural administration of local anesthetics in the first intercoccygeal space is frequently used to provide anesthesia and analgesia for caudal surgical and obstetric procedures in large animals. The short-acting local anesthetic lidocaine is typically used for this purpose (1,2). However, local anesthetics administered epidurally can affect motor nerve function in addition to sensory nerve fibers, which can lead to serious complications secondary to ataxia, muscle weakness, or paralysis of the pelvic limbs. Volume of administration has been shown to be a major factor influencing cranial distribution of local anesthetics within the epidural space (3); therefore, the general recommendation for intercoccygeal epidural administration of 2% lidocaine in large animals is 1 mL per 100 kg of body weight (4). Unfortunately, this limits the anesthesia to the most caudal dermatomes; specifically, the perineum, tail, anus, vulva, and vagina (4). Administration of higher volumes of lidocaine in the sacrococcygeal or first intercoccygeal space has been used clinically in calves with minimal untoward cardiovascular effects and excellent anesthesia up to the level of the umbilicus (5,6). However, this technique caused paralysis of the pelvic limbs and recumbency, and therefore its use is limited to calves, small ruminants, and pigs.
Other techniques to desensitize the flank of cattle and horses without affecting the motor function of the pelvic limbs have been described; these include paravertebral thoracolumbar anesthesia (4,7) and segmental dorsolumbar epidural anesthesia (8–11). However, these techniques do not provide analgesia to the pelvic limbs.
Bupivacaine is a longer-lasting local anesthetic with potent analgesic action. Concurrent intrathecal administration of opioids and bupivacaine decreased the opioid requirements and the development of opioid tolerance compared with intrathecal administration of opioids alone (12). When administered epidurally at low concentrations (< 0.125%) in humans, bupivacaine produces analgesia while minimally affecting motor function (13–17). Epidural coadministration of methadone, ketamine, and low-concentration bupivacaine provided good analgesia without motor deficits in a cow with complex regional pain syndrome (18). In dogs, the analgesic and motor effects of 3 concentrations of bupivacaine administered epidurally were compared (19): the lowest concentration of bupivacaine studied (0.25%) produced less analgesia but also less motor deficit than the higher concentrations (0.5% and 0.75%). To our knowledge, no controlled studies have been conducted on the analgesic and motor effects of epidural administration of high volumes and low concentrations of bupivacaine in large animals. The possibility of achieving analgesia without motor function deficits of the pelvic limbs would have important clinical implications in the analgesic management of painful conditions associated with the abdomen and/or pelvic limbs in large animals.
The objectives of this study were to evaluate, in adult cows, the analgesic and motor effects of 2 low concentrations of bupivacaine administered epidurally into the first intercoccygeal space at high volume. The hypothesis was that low concentrations would produce analgesia of caudal dermatomes as well as of the pelvic limbs and flanks without interfering with motor function.
Materials and methods
The study was approved by the Animal Care Committee of the University of Guelph, and the animals were maintained in accordance with the Canadian Council on Animal Care guidelines (20). The 7 adult nonlactating Holstein Friesian cows were determined to be healthy by physical examination before entry to the study and daily physical examination throughout the study period. Cows with obvious musculoskeletal disease were excluded. No cows were pregnant, as determined by rectal ultrasonographic examination before entry to the study. During the study period the cows were placed in individual stables, restrained in a head gate, and offered hay and water ad libitum. During the time between treatments they were kept in multiple indoor bovine stables and fed only hay.
One of the cows was used in a pilot study, carried out to determine the volume and concentration of bupivacaine that could be injected epidurally without causing pronounced dysfunction of the pelvic limbs. The other 6 cows were used in a prospective, randomized, blinded, crossover study. All the cows received 3 treatments, administered in a random order with a washout period of at least 48 h. The treatments were as follows: sterile isotonic saline (control), bupivacaine (Marcaine, 0.5%; Hospira, Montreal, Quebec) diluted in sterile saline to a concentration of 0.0625% (low dose), and bupivacaine diluted in sterile saline to a concentration of 0.125% (high dose). All treatments were administered at a dose of 0.15 mL per kilogram of body weight and infused manually over a period of 15 min via an indwelling epidural catheter placed into the first intercoccygeal space.
The epidural catheter (Med-Rx Epidural Anesthesia Kit; Benlan, Oakville, Ontario) was always placed by the same investigator (ER) on the first treatment day with sterile technique. The catheter was inserted into the first intercoccygeal space after the area had been clipped and aseptically prepared with clorhexidine soap, ethanol, and clorhexidine solution. A small skin incision was made with a no. 15 scalpel blade after subcutaneous infiltration of 2 mL of 2% lidocaine. A Tuohy needle (17-gauge, 3.5-in, Huber point) was then inserted through the skin and directed into the epidural space at a 30° angle to the contour of the rump. Correct placement of the tip of the needle into the epidural space was confirmed by the “hanging drop” technique with the use of sterile saline. The catheter (19-gauge) was introduced into the epidural space through the Tuohy needle and advanced cranially for 10 cm, up to approximately the level of the 4th sacral vertebra. Correct catheter placement was assumed if there was no resistance to insertion. The catheter was cut to a length of approximately 20 cm and a filter (0.22 μm) placed at the end of the catheter and sutured to the skin. The catheter was fixed with cyanoacrylate glue and the entry site covered with gauze impregnated with povidone iodine solution and a drape (Opsite; Smith and Nephew Medical Limited, Hull, England). The catheter was left in place for the duration of the study and was flushed every 8 h with 3 mL of sterile heparinized saline (50 IU/mL). It was removed after the 3 treatments had been completed (minimum 7 d, maximum 10 d) and visually inspected for any signs of kinking or contamination.
Cardiorespiratory variables, motor tone, and analgesia were evaluated and recorded at the following time points: before the epidural injection (time −5 min; baseline), upon completion of the injection (time 0 min), and 15, 30, 45, 60, 90, 120, 180, 240, 300, and 360 min after completion of the injection.
Cardiorespiratory variables recorded included heart rate (determined by direct auscultation of the heart for 1 min), respiratory rate (determined by counting chest movements for 1 min), and arterial blood pressure (AP; measured noninvasively by oscillometric technique). The oscillometric monitor (Cardell Veterinary Monitor 9402 BP/SpO2; Sharn Veterinary, Tampa, Florida, USA) was connected to a large-animal cuff (model SV10; Sharn Veterinary), which was placed around the base of the tail. Three consecutive readings of the oscillometric systolic (SAP), diastolic (DAP), and mean (MAP) arterial blood pressures were collected at each time point, and the average value was calculated and used for statistical analysis.
Tail and anal tone as well as motor deficits of the pelvic limbs were scored by a blinded evaluator (LMR) on a numerical rating scale (NRS; Appendix). To evaluate for motor deficits of the pelvic limbs, the investigator pushed the cow on the hindquarters and pulled the tail sideways while the cow remained restrained in a head gate, and this was recorded on video. Additionally, the degree of motor deficit of the pelvic limbs was scored from the video recordings by a blinded investigator (ER) using a visual analogue scale (VAS) for motor tone (VASmotor), which consisted of a 100-mm-long line with no marks, where 0 mm meant absence of ataxia and 100 mm meant extreme ataxia, with the animal falling down.
Appendix.
Numerical rating scale for evaluating motor function
Tail tone when evaluator moves the tail up and down
|
The degree of analgesia was determined by assessing the animal’s response to the application of standard noxious stimuli to skin and muscle, which consisted of pricks with a 25-gauge, 1-in hypodermic needle and a 22-gauge, 1.5-in needle, respectively, applied sequentially at different regions innervated by specific nerves, as shown in Figure 1(21). The pricks were done bilaterally from caudal to cranial, always by the same blinded investigator (LMR), and were recorded on video for later evaluation by a different blinded investigator (ER). Muscle pricks were done only if the animal did not respond to the skin pricks in that region or if the animal’s response was questionable (i.e., slight movement or movement possibly related to another cause). A VAS for avoidance behavior in response to the needle pricks (VASpain) was used: 0 mm meant no response and 100 mm meant an aggressive response (i.e., kicking at the investigator doing the pricks). Needle pricks were done up to at least 60 min after epidural injection in all cows. When a region was clearly positive to the pricks (i.e., the animal showed a clear avoidance response) at all time points up to 60 min after epidural injection or at any time point after 60 min, no more pricks were done in that region, and the last VASpain value obtained was recorded for the subsequent time points. The maximum VASpain value obtained in each region at each time point with either skin or muscle pricks was used for statistical analysis.
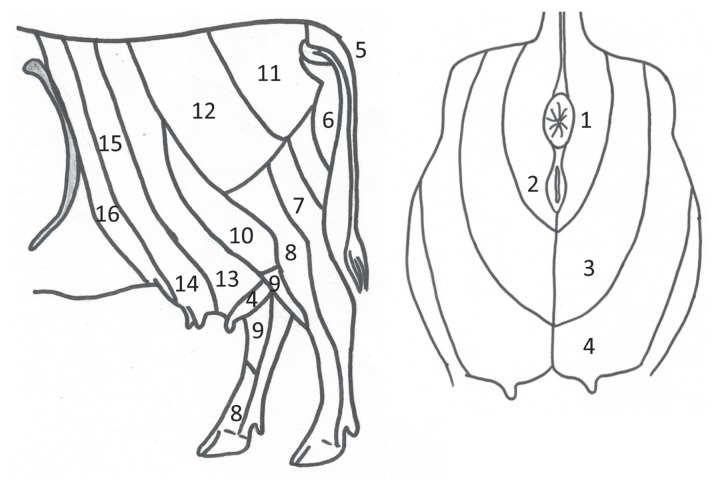
Figure 1.
Regions used for needle-prick stimulation in 4 cows to evaluate analgesia in response to an intercoccygeal epidural injection of saline or bupivacaine [0.0625% (low dose) or 0.125% (high dose)] (0.15 mL/kg in all treatments). Modified from Budras and Habel (21). 1 — anus; 2 — vulva; 3 — perineum; 4 — udder caudal; 5 — tail; 6 — pudendal nerve; 7 — tibial nerve; 8 — common peroneal nerve; 9 — saphenous nerve; 10 — lateral cutaneous femoral nerve; 11 — middle clunial nerve; 12 — cranial clunial nerve; 13 — udder middle; 14 — udder cranial; 15 — second lumbar vertebral nerve; 16 — first lumbar vertebral nerve.
For statistical analysis commercial software (SAS, version 9.1.3; SAS Institute, Cary, North Carolina, USA) was used, and the following were done: a Shapiro–Wilk test to assess normal distribution of the data; log transformation when appropriate; a logit transformation applied to the VAS scores; analysis of variance, general linear model for repeated measures, accounting for random effects (cow and period) and fixed effects (treatment, time, and treatment–time interaction); and post-hoc analysis with Tukey’s and Dunnett’s tests. A P-value ≤ 0.05 was considered significant.
Results
The mean weight of the cows was 658 ± 81 [standard deviation (SD)] kg. The total volume of epidural treatments was 102.8 ± 7.5 mL. One cow did not respond to any of the treatments. This treatment failure was possibly due to misplacement of the epidural catheter. Data for this cow were therefore not used for statistical analysis. Another cow was used only for motor and cardiorespiratory evaluation owing to behavioral limitations. Therefore, 5 cows were used for motor and cardiorespiratory evaluation and 4 cows for evaluation of analgesia.
The cardiorespiratory parameters were not significantly different among treatments or within a treatment over time relative to the baseline values and remained within normal limits (Table I).
Table I.
Cardiorespiratory values for 5 cows before and at different time points (overall value) after an intercoccygeal epidural injection of saline (control), 0.0625% bupivacaine (low dose) or 0.125% bupivacaine (high dose), final volume 0.15 mL/kg in all treatments
| Parameter | Mean ± standard deviation (SD); treatment group | |||
|---|---|---|---|---|
|
| ||||
| 5 min before injection | Average in the 6 h after injection | |||
|
| ||||
| Control | Low dose | High dose | ||
| HR (beats/min) | 57 ± 8 | 55 ± 7 | 54 ± 7 | 55 ± 7 |
| RR (breaths/min) | 31 ± 9 | 32 ± 8 | 33 ± 10 | 35 ± 12 |
| SAP (mmHg) | 119 ± 12 | 122 ± 10 | 126 ± 13 | 127 ± 12 |
| DAP (mmHg) | 56 ± 11 | 58 ± 10 | 63 ± 8 | 58 ± 13 |
| MAP (mmHg) | 77 ± 11 | 78 ± 11 | 83 ± 9 | 80 ± 13 |
HR — heart rate; RR — respiratory rate; SAP — systolic arterial blood pressure; DAP — diastolic arterial blood pressure; MAP — mean arterial blood pressure.
All the cows remained standing during the 6-h study period. One cow became recumbent immediately after being released from the head gate 6 h after epidural injection of the high dose of bupivacaine. The cow remained recumbent for a few minutes and then stood up unassisted without problems.
The NRS scores for anal and tail tone and for motor deficits of the pelvic limbs are summarized in Table II. The effects of treatment, time, and treatment–time interaction were significant for anal and tail tone (P < 0.001). Compared with baseline the anal scores were significantly increased from 0 to 240 min after high-dose bupivacaine treatment and from 0 to 180 min after low-dose treatment, and compared with the control treatment the anal scores were significantly increased from 0 to 300 min after high-dose bupivacaine treatment and from 0 to 180 min after low-dose treatment. Compared with both baseline and control treatment the tail scores were significantly increased from 0 to 360 min after high-dose bupivacaine treatment and from 0 to 300 min after low-dose treatment. There was also a significant effect of treatment (P < 0.001), time (P < 0.001), and treatment–time interaction (P = 0.008) for the NRS scores for motor deficits of the pelvic limbs: the scores were significantly increased from baseline and compared with control treatment from 0 to 360 min after high-dose bupivacaine treatment and at 15 and 45 minutes after low-dose treatment.
Table II.
Scores for anal and tail motor tone and motor deficits of the pelvic limbs for the 5 cows at different time points (overall value) after the injections
| Scoring scale; parameter | Median score (and range) or mean ± SD; treatment group | ||
|---|---|---|---|
|
| |||
| Control | Low dose | High dose | |
| NRS: anal tone | 1 (1–1) | 2 (1–4)a | 2 (1–4)a |
| NRS: tail tone | 1 (1–2) | 2 (1–4)a | 3 (1–4)a,b |
| NRS: motor deficits of pelvic limbs | 1 (1–2) | 1 (1–3) | 2 (1–3)a,b |
| VASmotor | 0.4 ± 1.9 | 11.3 ± 15.5a | 37.2 ± 24.4a,b |
Significantly different from the value for the control treatment (P < 0.001).
Significantly different from the value for the low-dose treatment (P < 0.001).
NRS — numerical rating scale (see Appendix); VASmotor — visual analogue scale for motor tone.
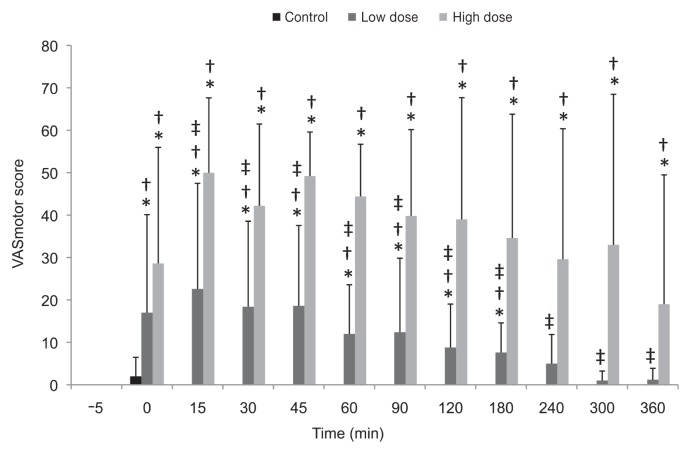
The VASmotor scores are summarized in Table II and Figure 2. The effects of treatment, time, and treatment–time interaction were significant (P < 0.001). The motor deficits of the pelvic limbs were evident immediately after epidural injection of low-dose and high-dose bupivacaine and lasted for 3 and up to 6 h, respectively.
Figure 2.
Mean scores ± standard deviation for motor deficits of the pelvic limbs in 5 cows, determined from video recordings on a visual analogue scale for motor tone 5 min before and at different time points after the control, low-dose, or high-dose injection.
* Significantly different from the pretreatment value. † Significantly different from the control value at the same time point. ‡ Significantly different from the high-dose value at the same time point.
The overall VASpain scores in response to needle pricks were higher for each region on the left side than on the right side, but no statistical comparisons were made between sides. The overall mean scores (± SD) for all regions together over time, excluding baseline, were 66 ± 8, 52 ± 5, and 43 ± 5 for the control, low-dose bupivacaine, and high-dose bupivacaine treatments, respectively. The regions that showed a significant treatment effect were tail (P = 0.007), anus (P < 0.001), vulva (P < 0.001), perineum (P < 0.001), udder caudal (P < 0.001), pudendal nerve (P = 0.026), tibial nerve (P < 0.001), common peroneal nerve (P < 0.001), saphenous nerve (P = 0.008), and udder middle (P < 0.001) on the left side and tail (P < 0.001), anus (P < 0.001), perineum (P < 0.001), and tibial nerve (P = 0.002) on the right side. The differences in scores are summarized in Table III. The regions that showed a significant treatment–time interaction were anus (P = 0.035), vulva (P = 0.047), perineum (P = 0.002), udder caudal (P = 0.047), and middle clunial nerve (P = 0.015) on the left side and anus (P = 0.035) and middle clunial nerve (P < 0.001) on the right side.
Table III.
Scores for avoidance behavior in response to needle pricks in 4 cows at different time points (overall value) after the injections; only regions that showed a significant treatment effect (P < 0.05) for analgesia are included
| Mean score ± SD; treatment group | |||
|---|---|---|---|
|
|
|||
| Region; side | Control | Low dose | High dose |
| Anus | |||
| Left | 66 ± 18 | 31 ± 23 | 11 ± 20a,b |
| Right | 51 ± 10 | 14 ± 19a | 7 ± 16a,b |
| Vulva, left | 66 ± 21 | 35 ± 26a | 9 ± 15a,b |
| Perineum | |||
| Left | 71 ± 15 | 54 ± 21 | 29 ± 28a,b |
| Right | 65 ± 22 | 31 ± 26 | 11 ± 20a,b |
| Udder caudal, left | 56 ± 37 | 43 ± 32 | 28 ± 34a,b |
| Tail | |||
| Left | 79 ± 12 | 35 ± 26a | 16 ± 24a |
| Right | 72 ± 6 | 39 ± 25a | 19 ± 27a,b |
| Pudendal nerve, left | 81 ± 8 | 57 ± 21 | 47 ± 32a |
| Tibial nerve | |||
| Left | 85 ± 11 | 68 ± 22a | 66 ± 27a |
| Right | 70 ± 12 | 66 ± 22 | 55 ± 29a,b |
| Common peroneal nerve, left | 67 ± 11 | 61 ± 24 | 46 ± 31a,b |
| Saphenous nerve, left | 65 ± 11 | 54 ± 18 | 47 ± 31a |
| Udder middle, left | 55 ± 14 | 49 ± 25a | 26 ± 21a,b |
Significantly different from the value for the control treatment (P < 0.001).
Significantly different from the value for the low-dose treatment (P < 0.001).
With high-dose bupivacaine treatment the onset and duration of analgesia were variable among regions: between 0 and 30 min for onset and between 300 and 360 min for duration. The VASpain scores were significantly decreased below baseline from 0 to 300 min in the anus and vulva, at 30 min in the region of the middle clunial nerve, and from 30 to 360 min in the perineum and udder caudal on the left side, as well as from 0 to 300 min in the anus and from 0 to 90 min in the region of the middle clunial nerve on the right side. The scores were significantly lower than with the control treatment from 30 to 360 min in the anus and perineum, from 15 to 360 min in the vulva, at 30 min in the region of the middle clunial nerve, and from 60 to 360 min in the udder caudal on the left side, as well as from 30 to 360 min in the anus and at 60 min in the region of the middle clunial nerve on the right side.
At the time of removal, no catheters had signs of kinking or contamination.
Discussion
This study showed that administration of 0.15 mL/kg of 0.125% bupivacaine 10 cm cranial to the first intercoccygeal space of adult cows produced complete or partial sensory block in caudal regions up to the level of the cutaneous area innervated by the saphenous nerve and caused mild to moderate motor deficits of the tail, anus, and pelvic limbs, with no untoward cardiorespiratory effects. However, administration of 0.0625% bupivacaine did not produce consistent sensory block and caused mild motor deficits of the tail, anus, and pelvic limbs, with no untoward cardiorespiratory effects.
Lumbosacral administration of high volumes of 2% lidocaine (0.18 to 0.24 mL/kg BW) in combination with xylazine has been reported to provide adequate analgesia to permit umbilical surgery in calves (22). Also in calves, sacrococcygeal epidural administration of a high volume (0.4 mL/kg) of 2% lidocaine had no clinically significant cardiovascular effects and provided good conditions for surgery in the abdomen up to the level of the umbilicus (5,6). In buffalo calves the use of epidural 0.75% ropivacaine in the lumbosacral space at doses between 0.05 and 0.1 mL/kg BW induced good analgesia up to the thorax and recumbency (23). In all these studies, paralysis of the pelvic limbs was obtained by injecting high volumes of local anesthetics epidurally.
In the present study, consistent analgesia was observed on the left side up to the level of the saphenous nerve after epidural injection of 0.125% bupivacaine. Previous studies in humans have also found consistent analgesia at this concentration of bupivacaine administered epidurally, with minimal motor dysfunction (13–17). On the other hand, consistent analgesia with epidural administration of 0.0625% bupivacaine was not obtained in this study, in accordance with the study in humans by Brennum et al (16), who observed that epidural administration of 0.075% bupivacaine induced only a slight and nonsignificant attenuation in pain ratings of brief argon laser, mechanical, and electrical stimuli. In human obstetric clinical studies, analgesia for labor was considered insufficient with 0.0625% bupivacaine administered epidurally; supplemental bupivacaine doses were necessary (14,15,24). However, other studies in humans have demonstrated a good analgesic effect of 0.0625% bupivacaine when administered epidurally in combination with fentanyl or sufentanil during labor (25–27). The reasons for the discrepancy among studies using 0.0625% bupivacaine could be multiple, but most likely the concomitant use of opioids is the reason, as it has been shown that the epidural use of opioids combined with local anesthetics results in more intense analgesia than individual administration of the drugs owing to their synergistic antinociceptive interaction at the level of the spinal cord (28).
Response to needle pricks was used in the present study to evaluate analgesia, as it is a common method in research studies in humans and animals (23,29,30) and is frequently used clinically to test whether surgery can be done after a local block in standing large animals. Pinpricks are considered mechanical stimuli of brief duration that activate fast Aδ nociceptors (31), which might not be the same type of stimulus induced by surgery or painful conditions, such as inflammation. Hypoalgesia for such brief stimuli is obtained before hypoalgesia for stimuli of greater spatial and temporal dimensions, which may explain the frequent clinical scenario in which insufficient surgical analgesia is encountered in spite of good pinprick analgesia (16,29). A poor correlation between lack of pinprick sensation and absence of postoperative pain in humans has also been demonstrated (32). In the study by Brennum et al (16) in humans, epidural administration of 0.075% bupivacaine selectively induced hypoalgesia for heat but not for mechanical or electrical stimuli, whereas the 0.125% concentration induced hypoalgesia for heat and mechanical stimuli but not electrical stimuli, and only bupivacaine 0.5% induced total anesthesia, with lack of pain in response to the 3 types of stimuli. It is possible that an analgesic effect of 0.0625% bupivacaine administered epidurally to animals could be demonstrated with a different type of stimulus, such as thermal, or by administering the bupivacaine in combination with opioids. Alternatively, including a greater number of cows in the study might have increased the power to reveal an analgesic effect of 0.0625% bupivacaine administered epidurally.
The scale used to evaluate response to needle pricks (VAS for avoidance behavior) was selected because it is commonly used to evaluate pain in animals (33) and allows evaluation on the basis of an animal’s individual behavior (i.e., the animal can serve as its own control). Moreover, because it is a continuous scale, statistical analysis is facilitated and statistical power increased. Most studies using pinpricks in animals use categorical scales (11,19,30). Nevertheless, there is currently no “gold standard” pain scale, and all scales have limitations, as they are based on subjective interpretation of animal behavior. Some pain scales also use objective parameters such as heart and respiratory rates (34), but these parameters may be affected by many other factors unrelated to pain. After careful evaluation of the video recordings, we decided to use the VAS scale for avoidance response, as some cows had low responses at baseline. Also, with this scale it is possible to evaluate behavioral changes on the basis of the individual, which is not possible with predefined categorical scoring systems. The assignment of a positive or negative response when pinpricks are done is not always straightforward, as some animals might feel pain but not respond owing to fear, or they might have hypoalgesia but not complete sensory block and therefore might still respond to the stimulus although the painful perception is decreased. Also because of learned behavior some animals might respond even when there is no associated pain. Randomization of the treatments should have minimized any possible effect of learned behavior on our results.
Sympathetic B fibers are readily blocked by local anesthetics, inducing cutaneous vasodilation and a rise in skin temperature. Skin temperature has been used in some studies in cattle in an attempt to evaluate success or failure of sensory blockade produced by epidural administration of local anesthetics (11). Skin temperature was not measured in the present study to evaluate sensory blockade as it has been shown to be a nonsensitive indicator of sympathectomy induced by epidurally administered anesthetics in humans (35). Also a skin temperature change was not observed after epidural administration of lidocaine in cows (11), and the authors suggested that the thickness of cattle skin may prevent marked changes in skin temperature. In addition, skin temperature may be influenced by other factors, such as environmental temperature.
The onset of motor deficits of the anus, tail, and pelvic limbs was immediate with both bupivacaine concentrations, but the deficits lasted longer with 0.125% bupivacaine (up to 6 h for tail and pelvic limbs). The onset of analgesia was immediate in the caudal regions and later in the more cranial regions. The duration of analgesia varied among regions, lasting up to 360 min in some of them with the high concentration of bupivacaine. In dogs the duration of motor effects exceeded the duration of sensory block after epidural administration of bupivacaine at concentrations of 0.25%, 0.5%, and 0.75% (19). In humans the duration of motor block was similar to the duration of hypoesthesia for mechanical and electrical stimuli after epidural administration of 0.25% and 0.5% bupivacaine (16). In the present study, measurements were done up to 6 h after injection, and the complete offset of sensory and motor effects could not be determined.
In humans, epidural administration of bupivacaine at concentrations of 0.075% and 0.125% did not induce motor blockade, as assessed by the knee-extension strength test (16), and the blockade was considered mild after administration of 0.125% bupivacaine, as evaluated by the ability of the patient to move the lower extremities (17). However, neither of these studies evaluated the ability of the person to stand or walk without ataxia. In the present study the motor effects were evaluated with the cows standing restrained in a head gate, with sideways movement allowed; however, they were not walked to evaluate the degree of ataxia because in the pilot study these doses of epidural bupivacaine induced mild to moderate motor deficits of the pelvic limbs and therefore the animals could fall if they were walked. In fact, 1 cow fell down after being released from the head gate 6 h after epidural administration of 0.125% bupivacaine. Therefore, this technique should be used with caution in large-animal clinical practice.
With the volume of bupivacaine used in this study, we expected analgesia up to the flank. However, it was obtained only up to the region of the saphenous nerve with 0.125% bupivacaine. The limited craniad epidural spread could be due to the rate of administration (0.01 mL/kg BW per minute), as a slow rate would be expected to generate lower epidural pressures, which could limit the spread (36). However, studies in humans showed that rapid injection of local anesthetics into the epidural space produced greater initial pressures and a faster onset of sensory block than slow injection, but there was no difference in the final extent and duration of the block (37,38). Multiple factors can affect the spread of the epidural block: in humans these factors include age, weight, dose of local anesthetic, addition of opioids, site of injection, and body height (39). In dogs, body position after epidural administration affected the spread of a methylene blue solution (40). Even though total sensory block was not obtained in the present study, hypoalgesia in more cranial regions might have been present and not demonstrated with the methods used.
A washout period of at least 48 h was chosen from epidural pharmacokinetic data in isoflurane-anesthetized sheep: the reported elimination half-life of bupivacaine was 142.5 min (41). The pharmacokinetic parameters of bupivacaine administered epidurally have not been reported in cattle to our knowledge.
In the present study, the left side had lower VASpain scores than the right side in most dermatomes. The side difference could have been due to lateralization of the epidural catheter towards the left side in the studied cows. However, such lateralization could not be confirmed, as no radiographs were obtained and no postmortem examinations conducted. One cow did not respond to any of the treatments, and the treatment failure was probably due to misplacement of the catheter out of the epidural space, but this could not be confirmed.
One limitation of the present study was the small sample size and thus the low power to detect more significant effects of the treatments. The study was initially designed with 6 cows, similar to previous crossover studies in cattle (1,11,30), but unfortunately the sample size was reduced to 5 cows for motor and cardiorespiratory evaluation and 4 cows for evaluation of analgesia during the course of the study. None the less, post-hoc power calculations revealed that the power was 71% and 99% to detect differences in motor tone and analgesia, respectively, between the use of a high dose of bupivacaine and the use of saline.
In conclusion, high-volume intercoccygeal epidural administration of 0.125% bupivacaine provided partial or complete sensory block in most caudal regions and in the pelvic limbs of cows. However, high-volume intercoccygeal epidural administration of 0.0625% bupivacaine did not provide consistent sensory block with the methods used in this study. This technique should be used with caution in large-animal clinical practice as it causes mild to moderate motor deficits of the pelvic limbs, which may cause recumbency if the animal is moved or walked.
Acknowledgments
The Department of Clinical Studies of the Ontario Veterinary College, University of Guelph, Guelph, Ontario, funded the study.
References
- 1.DeRossi R, Zanenga NF, Alves OD, Carneiro RP, Ossuna MR, Jorge TP. Effects of caudal epidural ketamine and/or lidocaine on heifers during reproductive procedures: A preliminary study. Vet J. 2010;185:344–346. doi: 10.1016/j.tvjl.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 2.Mosure WL, Meyer RA, Gudmundson J, Barth AD. Evaluation of possible methods to reduce pain associated with electroejaculation in bulls. Can Vet J. 1998;39:504–506. [PMC free article] [PubMed] [Google Scholar]
- 3.Freire CD, Torres ML, Fantoni DT, Cavalcanti RL, Noel-Morgan J. Bupivacaine 0.25% and methylene blue spread with epidural anesthesia in dog. Vet Anaesth Analg. 2010;37:63–69. doi: 10.1111/j.1467-2995.2009.00493.x. [DOI] [PubMed] [Google Scholar]
- 4.Skarda RT, Tranquilli WJ. Local and regional anesthetic and analgesic techniques: Ruminants and swine. In: Tranquilli WJ, Thurmon JC, Grimm KA, et al., editors. Lumb & Jones Veterinary Anesthesia and Analgesia. 4th ed. Ames, Iowa: Blackwell Publishing; 2007. pp. 643–681. [Google Scholar]
- 5.Meyer H, Starke A, Kehler W, Rehage J. High caudal epidural anaesthesia with local anaesthetics or alpha(2)-agonists in calves. J Vet Med A Physiol Pathol Clin Med. 2007;54:384–389. doi: 10.1111/j.1439-0442.2007.00942.x. [DOI] [PubMed] [Google Scholar]
- 6.Meyer H, Kastner SB, Beyerbach M, Rehage J. Cardiopulmonary effects of dorsal recumbency and high-volume caudal epidural anaesthesia with lidocaine or xylazine in calves. Vet J. 2010;186:316–322. doi: 10.1016/j.tvjl.2009.08.020. Epub 2009 Sep 18. [DOI] [PubMed] [Google Scholar]
- 7.Moon PF, Suter CM. Paravertebral thoracolumbar anaesthesia in 10 horses. Equine Vet J. 1993;25:304–308. doi: 10.1111/j.2042-3306.1993.tb02968.x. [DOI] [PubMed] [Google Scholar]
- 8.Skarda RT, Muir WW. Segmental epidural and subarachnoid analgesia in conscious horses: A comparative study. Am J Vet Res. 1983;44:1870–1876. [PubMed] [Google Scholar]
- 9.Lee I, Yamagishi N, Oboshi K, Ayukawa Y, Sasaki N, Yamada H. Comparison of xylazine, lidocaine and the 2 drugs combined for modified dorsolumbar epidural anaesthesia in cattle. Vet Rec. 2004;155:797–799. [PubMed] [Google Scholar]
- 10.Hiraoka M, Miyagawa T, Kobayashi H, Takahashi T, Kishi H, Lee I. Successful introduction of modified dorsolumbar epidural anesthesia in a bovine referral center. J Vet Sci. 2007;8:181–184. doi: 10.4142/jvs.2007.8.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeRossi R, Bertoni RA, Ruzzon RH, Verde-Selva AB, Frazilio FO. Segmental dorsolumbar epidural analgesia via the caudal approach using multiple port catheters with ketamine or lidocaine or in combination in cattle. Vet Anaesth Analg. 2010;37:451–459. doi: 10.1111/j.1467-2995.2010.00559.x. [DOI] [PubMed] [Google Scholar]
- 12.van Dongen RT, Crul BJ, van Egmond J. Intrathecal coadministration of bupivacaine diminishes morphine dose progression during long-term intrathecal infusion in cancer patients. Clin J Pain. 1999;15:166–172. doi: 10.1097/00002508-199909000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Bleyaert A, Soetens M, Vaes L, Van Steenberge AL, Van der Donck A. Bupivacaine, 0.125 per cent, in obstetric epidural analgesia: Experience in three thousand cases. Anesthesiology. 1979;51:435–438. doi: 10.1097/00000542-197911000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Cohen SE, Yeh JY, Riley ET, Vogel TM. Walking with labor epidural analgesia: The impact of bupivacaine concentration and a lidocaine–epinephrine test dose. Anesthesiology. 2000;92:387–392. doi: 10.1097/00000542-200002000-00019. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez-Pereles MC, Uribarri FJ, Gragera I. 0.0625% bupivacaine compared with 0.125% bupivacaine continuously perfused epidurally during vaginal delivery. Rev Esp Anestesiol Reanim. 1993;40:9–11. [PubMed] [Google Scholar]
- 16.Brennum J, Nielsen PT, Horn A, Arendt-Nielsen L, Secher NH. Quantitative sensory examination of epidural anaesthesia and analgesia in man; dose–response effect of bupivacaine. Pain. 1994;56:315–326. doi: 10.1016/0304-3959(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 17.Owen MD, D’Angelo R, Gerancher JC, et al. 0.125% ropivacaine is similar to 0.125% bupivacaine for labor analgesia using patient-controlled epidural infusion. Anesth Analg. 1998;86:527–531. doi: 10.1097/00000539-199803000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Bergadano A, Moens Y, Schatzmann U. Continuous extradural analgesia in a cow with complex regional pain syndrome. Vet Anaesth Analg. 2006;33:189–192. doi: 10.1111/j.1467-2995.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 19.Gomez de Segura IA, Menafro A, Garcia-Fernandez P, Murillo S, Parodi EM. Analgesic and motor-blocking action of epidurally administered levobupivacaine or bupivacaine in the conscious dog. Vet Anaesth Analg. 2009;36:485–494. doi: 10.1111/j.1467-2995.2009.00469.x. [DOI] [PubMed] [Google Scholar]
- 20.Olfert ED, Cross BM, McWilliam AA, editors. Guide to the Care and Use of Experimental Animals. 2nd ed. Vol. 1. Ottawa, Ontario: Canadian Council on Animal Care; 1993. [Last accessed June 16, 2013]. Available from www.ccac.ca/Documents/Standards/Guidelines/Experimental_Animals_Vol1.pdf. [Google Scholar]
- 21.Budras K-D, Habel RE. Bovine Anatomy. An Illustrated Text. Hannover, Germany: Schlutersche GmbH & Company; 2003. p. 20. [Google Scholar]
- 22.Lewis CA, Constable PD, Huhn JC, Morin DE. Sedation with xylazine and lumbosacral epidural administration of lidocaine and xylazine for umbilical surgery in calves. J Am Vet Med Assoc. 1999;214:89–95. [PubMed] [Google Scholar]
- 23.Amarpal, Kinjavdekar P, Aithal HP, et al. Comparison of two doses of ropivacaine for lumbosacral epidural analgesia in buffalo calves (Bubalus bubalis) Vet Rec. 2007;160:766–769. doi: 10.1136/vr.160.22.766. [DOI] [PubMed] [Google Scholar]
- 24.Li DF, Rees GA, Rosen M. Continuous extradural infusion of 0.0625% or 0.125% bupivacaine for pain relief in primigravid labour. Br J Anaesth. 1985;57:264–270. doi: 10.1093/bja/57.3.264. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez-Guisasola J, Serrano ML, Cobo B, et al. A comparison of 0.0625% bupivacaine with fentanyl and 0.1% ropivacaine with fentanyl for continuous epidural labor analgesia. Anesth Analg. 2001;92:1261–1265. doi: 10.1097/00000539-200105000-00034. [DOI] [PubMed] [Google Scholar]
- 26.Russell R, Reynolds F. Epidural infusions for nulliparous women in labour. A randomised double-blind comparison of fentanyl/bupivacaine and sufentanil/bupivacaine. Anaesthesia. 1993;48:856–861. doi: 10.1111/j.1365-2044.1993.tb07413.x. [DOI] [PubMed] [Google Scholar]
- 27.Russell R, Quinlan J, Reynolds F. Motor block during epidural infusions for nulliparous women in labour: A randomized double-blind study of plain bupivacaine and low dose bupivacaine with fentanyl. Int J Obstet Anesth. 1995;4:82–88. doi: 10.1016/0959-289x(95)82997-o. [DOI] [PubMed] [Google Scholar]
- 28.Kaneko M, Saito Y, Kirihara Y, Collins JG, Kosaka Y. Synergistic antinociceptive interaction after epidural coadministration of morphine and lidocaine in rats. Anesthesiology. 1994;80:137–150. doi: 10.1097/00000542-199401000-00021. [DOI] [PubMed] [Google Scholar]
- 29.Adrendt-Nielsen L, Oberg B, Bjerring P. Quantitative assessment of extradural bupivacaine analgesia. Br J Anaesth. 1990;65:633–638. doi: 10.1093/bja/65.5.633. [DOI] [PubMed] [Google Scholar]
- 30.De Rossi R, Bucker GV, Varela JV. Perineal analgesic actions of epidural clonidine in cattle. Vet Anaesth Analg. 2003;30:64–71. [PubMed] [Google Scholar]
- 31.Mackenzie RA, Burke D, Skuse NF, Lethlean AK. Fibre function and perception during cutaneous nerve block. J Neurol Neurosurg Psychiatry. 1975;38:865–873. doi: 10.1136/jnnp.38.9.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curatolo M, Kaufmann R, Petersen-Felix S, Arendt-Nielsen L, Scaramozzino P, Zbinden AM. Block of pinprick and cold sensation poorly correlate with relief of postoperative pain during epidural analgesia. Clin J Pain. 1999;15:6–12. doi: 10.1097/00002508-199903000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Dobromylskyj P, Flecknell PA, Lascelles BD, Livingston A, Taylor P, Waterman-Pearson A. Pain assessment. In: Flecknell PA, Waterman-Pearson A, editors. Pain Management in Animals. 1st ed. London: Saunders Elsevier; 2000. pp. 53–80. [Google Scholar]
- 34.Firth AM, Haldane SL. Development of a scale to evaluate postoperative pain in dogs. J Am Vet Med Assoc. 1999;214:651–659. [PubMed] [Google Scholar]
- 35.Ginosar Y, Weiniger CF, Meroz Y, et al. Pulse oximeter perfusion index as an early indicator of sympathectomy after epidural anesthesia. Acta Anaesthesiol Scand. 2009;53:1018–1026. doi: 10.1111/j.1399-6576.2009.01968.x. [DOI] [PubMed] [Google Scholar]
- 36.Usubiaga JE, Wikinski JA, Usubiaga LE. Epidural pressure and its relation to spread of anesthetic solutions in epidural space. Anesth Analg. 1967;46:440–446. [PubMed] [Google Scholar]
- 37.Kanai A, Suzuki A, Hoka S. Rapid injection of epidural mepivacaine speeds the onset of nerve blockade. Can J Anaesth. 2005;52:281–284. doi: 10.1007/BF03016064. [DOI] [PubMed] [Google Scholar]
- 38.Cardoso MM, Carvalho JC. Epidural pressures and spread of 2% lidocaine in the epidural space: Influence of volume and speed of injection of the local anesthetic solution. Reg Anesth Pain Med. 1998;23:14–19. doi: 10.1016/s1098-7339(98)90105-5. [DOI] [PubMed] [Google Scholar]
- 39.Curatolo M, Orlando A, Zbinden AM, Scaramozzino P, Venuti FS. A multifactorial analysis of the spread of epidural analgesia. Acta Anaesthesiol Scand. 1994;38:646–652. doi: 10.1111/j.1399-6576.1994.tb03971.x. [DOI] [PubMed] [Google Scholar]
- 40.Gorgi AA, Hofmeister EH, Higginbotham MJ, Kent M. Effect of body position on cranial migration of epidurally injected methylene blue in recumbent dogs. Am J Vet Res. 2006;67:219–221. doi: 10.2460/ajvr.67.2.219. [DOI] [PubMed] [Google Scholar]
- 41.Ratajczak-Enselme M, Estebe JP, Rose FX, et al. Effect of epinephrine on epidural, intrathecal, and plasma pharmacokinetics of ropivacaine and bupivacaine in sheep. Br J Anaesth. 2007;99:881–890. doi: 10.1093/bja/aem291. [DOI] [PubMed] [Google Scholar]