Abstract
A sandwich-dot enzyme-linked immunosorbent assay (dot ELISA) was developed for the detection of canine distemper virus (CDV). In 56 dogs suspected to have CD the rates of detection of CDV antigen in samples of blood lymphocytes and palpebral conjunctiva by dot ELISA and ELISA were, respectively, 91% (49/54) and 81% (44/54) for the lymphocyte samples and 88% (28/32) and 75% (24/32) for the conjunctival samples. The CDV detection limits were 10 ng/50 μL for dot ELISA and 40 ng/50 μL for ELISA. The reliability of dot ELISA relative to electron microscopy was 96% with 22 samples: all 21 samples in which CDV particles were observed by electron microscopy yielded positive results with dot ELISA; the single sample in which particles were not observed yielded false-positive results with dot ELISA. The results indicate that the dot ELISA developed can serve as a reliable rapid diagnostic test in suspected cases of CD and also be useful for epidemiologic surveillance of the disease.
Résumé
Une épreuve immuno-enzymatique sandwich par point (dot ELISA) a été développée afin de détecter le virus du distemper canin (CDV). Chez 56 chiens suspectés d’avoir le CD, les taux de détection d’antigène du CDV dans des échantillons de lymphocytes sanguins et de la conjonctive palpébrale par dot ELISA et ELISA étaient, respectivement, 91 % (49/54) et 81 % (44/54) pour les échantillons de lymphocytes et 88 % (28/32) et 75 % (75/32) pour les échantillons de conjonctive. Les limites de détection de CDV étaient 10 ng/50 μL pour le dot ELISA et 40 ng/50 μL pour l’ELISA. La fiabilité du dot ELISA relativement au microscope électronique était de 96 % avec 22 échantillons : les 21 échantillons à partir desquels des particules de CDV furent observées ont donné des résultats positifs au dot ELISA; le seul échantillon à partir duquel aucune particule ne fut observée a donné un résultat faussement positif au dot ELISA. Les résultats indiquent que l’épreuve dot ELISA développée peut servir en tant que test diagnostique rapide et fiable lors de cas suspectés de CD et peut également être utile pour la surveillance épidémiologique de la maladie.
(Traduit par Docteur Serge Messier)
Introduction
Canine distemper (CD) is a highly contagious disease that affects dogs of all ages. It has high morbidity and mortality rates and occurs worldwide. Canine distemper virus (CDV), a member of the family Paramyxoviridae, genus Morbillivirus(1), causes acute generalized infection or chronic localized and persistent infection in the central nervous system (2). Infected dogs have either the catarrhal form of distemper or epileptiform convulsions in the initial stages of the disease. Since the virus shows strong infectivity and the infection has a high mortality rate, most dog breeders suffer serious economic losses with CDV infection (1,3).
Several serologic assays for evaluating antibody status have been used to confirm clinical CD (4–7). However, only a low antibody response can be detected in the first few weeks after infection (8). Furthermore, many puppies with maternal antibodies and vaccinated dogs may have high titers of neutralizing antibodies (5). Therefore, detection of the neutralizing antibodies is not fully reliable for a diagnosis of CD, and more attention has been paid to detecting CDV antigens. The most reliable method of detecting CDV in infected dogs is virus isolation (9); however, the method is time-consuming and frequently unsuccessful when the infection is not in an acute stage (8). Other laboratory tools, such as staining for inclusion bodies (10) and fluorescent antibody testing (8), also produce a negative result in subacute or chronic cases. An enzyme-linked immunosorbent assay (ELISA) using protein A and monoclonal antibody (11), an immunochromatographic assay (12), and an immunocapture ELISA (13) were developed to detect CDV in cell cultures and clinical specimens. They all have high specificity and sensitivity; however, they require an ELISA reader and have limitations for field studies (13,14). Recently, methods to detect the CDV nucleocapsid protein gene by means of reverse-transcription polymerase chain reaction (PCR) (10,15) and real-time PCR (16) have been developed. These methods can be carried out only in equipped laboratories but are highly sensitive and useful. Rapid and sensitive laboratory and field tests for the diagnosis of CDV infection are essential for CD control.
Sandwich-dot ELISA (dot ELISA) is a sensitive and specific technique for detecting various virus antigens that has wide clinical diagnostic applications (9,17,18). The aim of this study was to establish a rapid and sensitive laboratory and field test for the diagnosis of CDV infection. Our results indicate that the monoclonal-antibody-based dot ELISA has virtues such as reliability, simplicity of performance, and good reproducibility.
Materials and methods
Cells and virus strains
Vero cells were seeded into 75-cm2 cell-culture flasks. After being grown for 24 h in modified Eagle’s medium supplemented with 100 U/mL of penicillin G, 100 mg of streptomycin (GIBCO BRL, Carlsbad, California, USA), and 5% fetal bovine serum (Sigma Chemical Company, St. Louis, Missouri, USA) at 37°C in 5% CO2, monolayer cultures that were 80% to 90% confluent were infected with CDV (19). Noninfected Vero cells were used as controls.
Yongchun Jin (Yanbian University, Jilin, China) kindly provided 20 CDV (YJ-IV) strains, 4 canine parvovirus (CPV) (JIN-C-4) strains, 3 infectious canine hepatitis virus (ICHV) (TS-25) strains, and 3 rabies virus (RV) (RU-34) strains, to be used as indicator viruses in the dot ELISA.
Purification of virus antigen
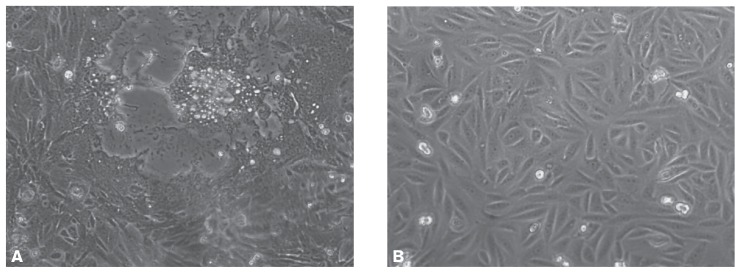
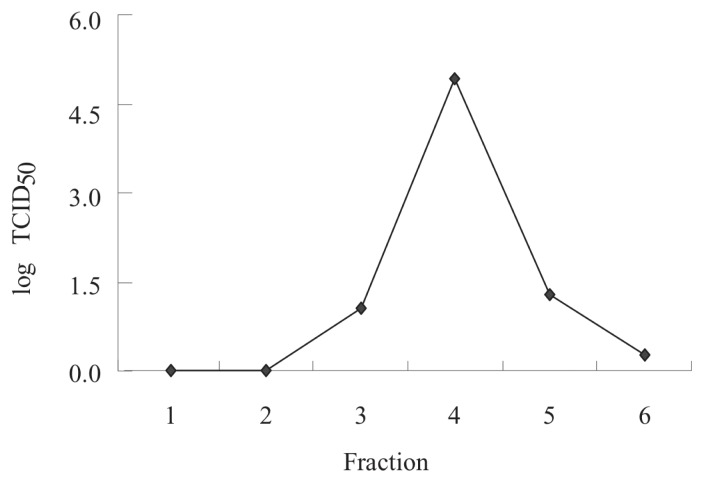
When approximately 75% of the CDV-inoculated Vero cells showed cytopathic effects (CPE) (Figure 1, bottom panel), 72 h after inoculation, the cell-associated viruses were harvested by freezing and thawing the cells 3 times in serum-free medium. The resultant cells and medium were then centrifuged at 650 ×g for 20 min to remove cellular debris. The supernatant was filtered through 0.22-μm filters (Millipore Corporation, Bedford, Massachusetts, USA). The partially purified virus was placed on a discontinuous sucrose gradient of 30%, 45%, and 60% (w/v) and centrifuged for 18 h at 54 000 ×g to separate CDV particles from cellular proteins responsible for cross-reactivity with the virus. Among the 6 major fractions obtained, infectivity in Vero cell culture was greatest for fraction 4, at a 50% tissue culture infective dose (TCID50) of 105.0. This fraction was therefore used as the antigen for specific antibody production and for the dot ELISA.
Figure 1.
Vero cells grown for 24 h and then inoculated (A) with canine distemper virus (CDV) or not inoculated (B). After 72 h about 90% of the CDV-inoculated Vero cells showed cytopathic effects. Images were acquired with a Leica DM IRB microscope (Leica Microsystems, Heerbrugg, Germany) at a magnification of × 200.
Preparation of polyclonal IgG against CDV
In brief, rabbits were given a subcutaneous injection of 500 μg of gradient-purified CDV (fraction 4) Gradient purified CDV antigen of fraction 4 used for preparation of the monoclonal IgG.mixed with an equal volume of complete Freund’s adjuvant (1,3). Two weeks later a booster dose, 500 μg of purified CDV mixed with an equal volume of incomplete Freund’s adjuvant, was injected subcutaneously. Two weeks later 500 μg of purified CDV was injected intraperitoneally. Blood was drawn 10 d after the last injection and 33% ammonium sulfate added to precipitate the protein. The partially purified IgG was dialyzed overnight against 0.02 M phosphate-buffered saline (PBS), pH7.2, and then the IgG was isolated by affinity chromatography with a HiTrap Protein G HP column (GE Healthcare, Fairfield, Connecticut, USA).
Preparation of monoclonal IgG against CDV
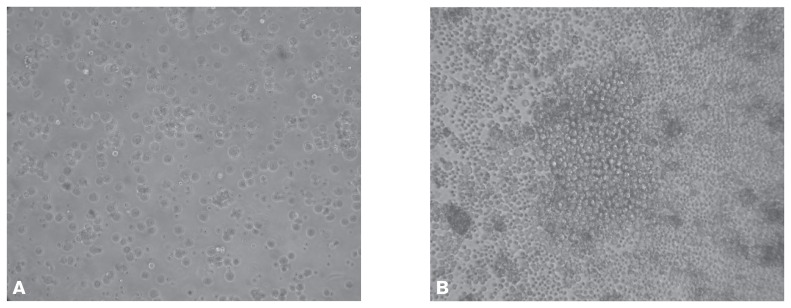
Monoclonal antibodies were produced as described previously (20,21) with some modifications. Briefly, BALB/C mice (male, 8 wk old) were injected subcutaneously with the gradient-purified whole CDV antigen of fraction 4. Splenic cells obtained from the mice were fused with SP2/0 myeloma cells with the use of polyethylene glycol 4000. The resulting hybridomas (Figure 2) were screened by ELISA, and those that produced CDV-specific monoclonal antibodies were subcloned 3 times from single cells by the limiting-dilution method. The purified CDV was coated on 96-well plates and then incubated with 100 μL of the IgG at different dilutions (1:100 to 1:102 400). Optical density was measured at 492 nm with a computer-interfaced microplate reader (Bio-Rad Laboratories, Hercules, California, USA). Of the 12 CDV-specific antibodies screened, 1 antibody, named 9C11, was selected for its strong immunoreactivity against CDV and little cross-reactivity with other proteins in immunoblots. The 9C11 cells were inoculated intraperitoneally into pristane-primed BALB/c mice, and IgG was isolated from the resulting ascites fluid by affinity chromatography with the HiTrap column.
Figure 2.
Development of hybridoma from spleen cells obtained from CDV-inoculated BALB/c mice and fused with SP2/0 myeloma cells. A — day 1. B — day 7. The magnification is × 200.
The ELISA procedures
The rabbit polyclonal IgG against CDV was diluted 1:500 in 50 mM carbonate–bicarbonate buffer, pH 9.6, and coated on 96-well plates. The plates were left at 4°C overnight, incubated for 60 min in PBS containing 3% bovine serum albumin (BSA), and then washed with PBS containing 0.1% Tween-20, pH7.4. A 50-μL aliquot of the purified CDV antigen and clinical samples was distributed into each well and the plate incubated at 37°C for 2 h. Each plate had CDV-positive and CDV-negative control antigens. The plates were washed and then incubated at 37°C for 1 h with 50 μL of the mouse monoclonal IgG against CDV diluted 1:1000 in PBS containing l% BSA. After being washed the plates were incubated at 37°C with goat IgG against rabbit antigen conjugated with horseradish peroxidase (HRP) diluted 1:5000 in PBS containing 1% BSA. The HRP activity on the immunoplate was detected with the use of O-phenylenediamine (12) and H2O2 as enzyme substrates. Color development was stopped with 2 M H2SO4 and the absorbance measured at 492 nm with the microplate reader. The results were considered positive if the absorbance was greater than that of the negative control. Titers were expressed as a reciprocal of the highest dilution of the sample showing a positive signal.
The dot ELlSA was done according to a previously described method (9) with minor modification. The nitrocellulose (NC) membrane strips were divided into squares 0.3 × 0.3 cm with a hard lead pencil, and 5-μL aliquots of rabbit polyclonal IgG against CDV diluted 1:500 in PBS were dotted on separate squares. The strips were allowed to dry, and then the protein-binding sites were blocked with a solution of 2% BSA in PBS. After a washing with PBS, the strips were cut into the squares and placed in the microwells. The remainder of the protocol was the same as for the ELISA described. A substrate solution of diaminobenzidine in 0.1 M Tris-HCl buffer, pH 7.4, with 0.01% H2O2 was used to color the NC membranes.
Determination of analytic specificity and sensitivity
The specificity of the dot ELISA was tested with the 20 supplied CDV strains isolated from dog species and the 10 supplied non-CDV virus strains (CPV, ICHV, and RV) along with 100 μL of PBS containing the purified CDV strains. Non-CDV viruses were used to eliminate false-positive results. No cross-reactivity was observed with any of the non-CDV viruses tested. The sensitivity of the dot ELISA was determined with the YJ-IV CDV strain diluted serially from 10 μg/50 μL to 1 ng/50 μL.
Clinical samples
A Yanbian University animal hospital provided 86 specimens (54 swabs of palpebral conjunctiva and 32 samples of blood lymphocytes) from 56 dogs suspected to have CD. The dogs demonstrated mainly acute and systemic clinical signs, such as fever, lack of appetite, vomiting, diarrhea, and dehydration. Conjunctival epithelial cells, obtained by vigorous swabbing with a cotton swab, were frozen and thawed in 1 mL of PBS, the suspensions were centrifuged at 2000 × g for 20 min, and the supernatant was stored at −20°C until tested. Blood lymphocytes were separated from 2 mL of peripheral blood containing heparin (100 units/mL) by centrifugation with Ficoll-Paque separation fluid. The samples were diluted from 1:5 to 1:80 with PBS for testing by ELISA and dot ELISA.
The palpebral conjunctival secretions and blood lymphocytes were inoculated onto Vero cells and incubated at 37°C for 2 d. When approximately 75% of the monolayer showed CPE the viruses were harvested by freezing and thawing the cells 3 times. Thereafter they were collected on carbon-coated grids by touching the grids against the samples. The grids were blot-dried by touching the edge of the grids to a filter paper. All of blood lymphocytes were then stained with 2% (w/v) sodium phosphotungstate, pH 7.0, for 2 min and observed with a JEOL JEM-1200EX electron microscope (JEOL, Peabody, Massachusetts, USA).
Statistical analysis
The results are expressed as means ± standard deviation (SD). Statistical analyses were carried out using the Student’s t-test. A P-value of less than 0.05 was considered statistically significant and less than 0.01 extremely significant.
Results
In purifying the CDV, sucrose-gradient centrifugation allowed the separation of CDV particles from cellular proteins that are responsible for cross-reactivity with the virus. Of the 6 fractions obtained, fraction 4 showed the greatest infectivity on Vero cell culture (105.0 TCID50); the infectivity of fractions 3 and 5 was 101.1 TCID50 and 101.3 TCID50, respectively (Figure 3). The protein concentration of fraction 4 was 1.36 mg/mL, whereas that of fractions 3 and 5 was 0.33 and 0.43 mg/mL, respectively. The purified CDV virions were morphologically identical to morbilliviruses by electron microscopy, and the virus diameter was approximately 200 nm. Fraction 4 was therefore used as the antigen for specific antibody production and for the dot ELISA.
Figure 3.
Infective titers of CDV purified by centrifugation on a sucrose gradient of 30%, 45%, and 60% (w/v) for 18 h at 54 000 × g. Six major fractions were collected and the 50% tissue culture infective dose (TCID50) determined.
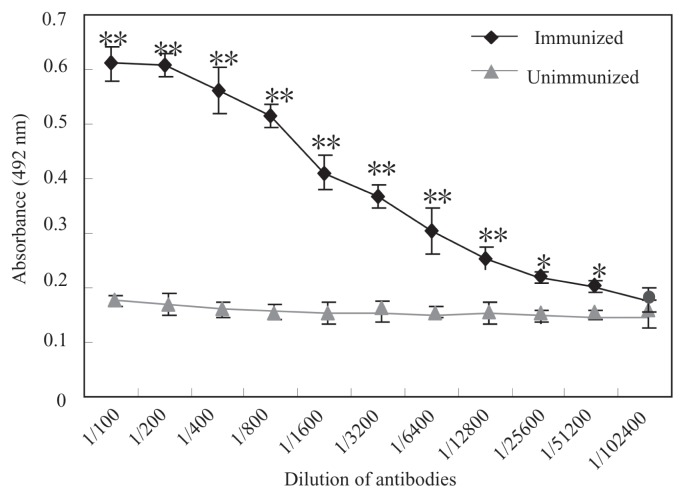
The titer of the polyclonal antibody against CDV was measured by ELISA as 1:102 400 (data not shown). Monoclonal antibody 9C11 was selected from among the 12 CDV-specific monoclonal antibodies screened by ELISA because of its strong immunoreactivity against CDV (Figure 4) and little cross-reactivity with other proteins in immunoblots; its titer was 1:51 200, suggesting its high sensitivity for recognizing the CDV protein.
Figure 4.
Titers of monoclonal anti-CDV IgG (9C11) as detected by enzyme-linked immunosorbent assay (ELISA). Purified CDV was coated on 96-well plates and then incubated with 100 μL of the IgG at different dilutions. Optical density was measured at 492 nm. A positive signal was not detected in serum from unimmunized mice. *P < 0.05; **P < 0.01.
Comparison of the results for dot ELISA and ELISA showed that 1:20 dilutions of samples, 1:1000 dilutions of monoclonal IgG against CDV, and 1:2000 dilutions of polyclonal IgG against CDV gave optimal results. The CDV detection limits of the dot ELISA and ELISA were 10 ng/50 μL and 40 ng/50 μL, respectively. Moreover, the dot ELISA showed no cross-reactivity with the other viruses: CPV, ICHV, and RV (Figure 5), indicating its high specificity.
Figure 5.
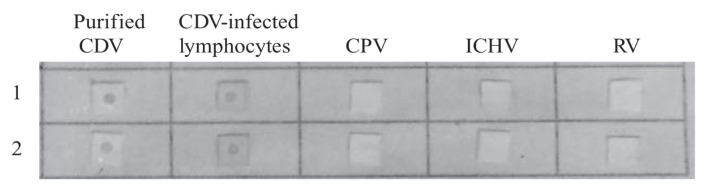
Specificity of sandwich-dot ELISA in detecting purified CDV and the CDV in CDV-infected blood lymphocytes and not cross-reacting with canine parvovirus (CPV) (strain JIN-C-4), canine hepatitis virus (ICHV) (strain TS-25), or rabies virus (RV) (strain RU-34). The experiment was performed twice.
Among the clinical specimens from 56 dogs suspected to have CD, the rates of detection of CDV antigen by the dot ELISA and the ELISA differed significantly (0.01 < P < 0.05): 91% and 81%, respectively, for the 32 blood-lymphocyte specimens and 88% and 75%, respectively, for the conjunctival-swab samples (Table I). Of the 22 blood-lymphocyte samples that were positive by dot ELISA in which CDV particles were sought by electron microscopy, CDV particles were observed in 21; 17 were positive and 5 negative by ELISA. The particles configurationally resembled morbilliviruses. The overall reliability of dot ELISA relative to electron microscopy was therefore 96%, with only 1 sample yielding false-positive results by dot ELISA.
Table I.
Detection by enzyme-linked immunosorbent assay (ELISA) and sandwich-dot ELISA of canine distemper virus in specimens from dogs with suspected infection
| Results of ELISAa | Dot-ELISA results;b Number of specimens | Total number of specimens | |||
|---|---|---|---|---|---|
|
| |||||
| Blood lymphocytes (n = 54) | Palpebral conjunctiva (n = 32) | ||||
|
|
|
||||
| Positive | Negative | Positive | Negative | ||
| Positive | 44 | 0 | 24 | 0 | 68 |
| Negative | 5 | 5 | 4 | 4 | 18 |
| Total | 49 | 5 | 28 | 4 | 86 |
Rate of positive results: 81% (44/54) for blood lymphocytes and 75% (24/32) for palpebral conjunctiva.
Rate of positive results: 91% (49/54) for blood lymphocytes and 88% (28/32) for palpebral conjunctiva, both rates significantly higher (0.01 < P < 0.05) than those for the ELISA.
Discussion
Monoclonal antibody testing is a powerful tool for the detection of CDV antigens (22). In recent outbreaks of CD in Yanbian, Jilin, China, cross-reaction with the YJ-IV strain of CDV was prominent. To develop a more sensitive test for rapid diagnosis, we referred to previous successes in the purification of CDV (14) and used sucrose-gradient centrifugation to isolate the fraction with greatest infectivity in Vero cell culture. We then prepared monoclonal and polyclonal antibodies against fraction 4 and used them in a dot ELISA to detect CDV. In this study the dot ELISA had greater sensitivity than the ELISA: the lowest limits of detection of purified CDV antigen were 10 ng/50 μL and 40 ng/50 μL, respectively.
The porous structure of the NC membrane provides a higher binding capacity than does the solid polystyrene surface of an ELISA well (9,17,18,23). In addition, no apparent difference in color intensity was observed on NC membranes freshly prepared or stored for 6 mo (data not shown). Accordingly, the use of such membranes could greatly facilitate the reproducibility and field applicability of the dot ELISA (17,18).
Because CDV has been shown to multiply in the monocytes and lymphocytes of the host (1,24) and can be detected with fluorescent antibody techniques in the cytoplasm of epithelial cells and neutrophils in conjunctival or genital smears (8), we used ELISA and dot ELISA to detect CDV antigen in blood lymphocytes and palpebral conjunctival secretions from dogs suspected of having CD. Our data showed that lymphocytes are the most suitable clinical specimens from live dogs, the rates of detection of CDV antigen being 91% for the dot ELISA and 81% for the ELISA. From a diagnostic viewpoint, one of the most reliable methods for the diagnosis of CD is isolation of CDV from affected animals or detection of CDV antigen in their tissues or cells (11,25). Comparison of the results of dot ELISA and electron microscopy for detection of CDV yielded a relative reliability of 96% for dot ELISA: only 1 of 21 samples had false-positive results.
In this study, using the YJ-IV CDV strain, we successfully established a monoclonal-antibody-based dot ELISA to detect CDV infection in dogs. This rapid test detected infection in clinical samples from different regions and years. Because the dot ELISA proved to be nearly as sensitive and specific as electron microscopy while being simpler and more rapid, it would be an adequate screening test for suspected CDV and useful for epidemiologic surveillance of CD infections in the field.
Acknowledgments
We thank Professor Yongkui Jin (Yanbian University, Jilin, China) for help with the electron microscopic analyses and Professor Yongchun Jin (Yanbian University) for the clinical samples. This work was supported by grants of the National Nature Science Foundation of China (30972675 and 31270864) and the Science and Technology Planning Project of Dalian City, China (2010J21DW011).
References
- 1.Appel MJ. Pathogenesis of canine distemper. Am J Vet Res. 1969;30:1167–1182. [PubMed] [Google Scholar]
- 2.Dorsey WA. Comments on canine distemper outbreak. J Am Vet Med Assoc. 2005;226:32. [PubMed] [Google Scholar]
- 3.Pawar RM, Raj GD, Gopinath VP, et al. Isolation and molecular characterization of canine distemper virus from India. Trop Anim Health Prod. 2011;43:1617–1622. doi: 10.1007/s11250-011-9880-7. [DOI] [PubMed] [Google Scholar]
- 4.Gemma T, Miyashita N, Shin YS, et al. Serological survey of canine distemper virus infection using enzyme-linked immunosorbent assay. J Vet Med Sci. 1995;57:761–763. doi: 10.1292/jvms.57.761. [DOI] [PubMed] [Google Scholar]
- 5.Litster A, Nichols J, Volpe A. Prevalence of positive antibody test results for canine parvovirus (CPV) and canine distemper virus (CDV) and response to modified live vaccination against CPV and CDV in dogs entering animal shelters. Vet Microbiol. 2012;157:86–90. doi: 10.1016/j.vetmic.2011.12.030. Epub 2011 Dec 30. [DOI] [PubMed] [Google Scholar]
- 6.Soma T, Ishii H, Hara M, et al. Detection of canine distemper virus antigen in canine serum and its application to diagnosis. Vet Rec. 2003;153:499–501. doi: 10.1136/vr.153.16.499. [DOI] [PubMed] [Google Scholar]
- 7.Waner T, Mazar S, Nachmias E, et al. Evaluation of a dot ELISA kit for measuring immunoglobulin M antibodies to canine parvovirus and distemper virus. Vet Rec. 2003;152:588–591. doi: 10.1136/vr.152.19.588. [DOI] [PubMed] [Google Scholar]
- 8.Fairchild GA, Wyman M, Donovan EF. Fluorescent antibody technique as a diagnostic test for canine distemper infection: Detection of viral antigen in epithelial tissues of experimentally infected dogs. Am J Vet Res. 1967;28:761–768. [PubMed] [Google Scholar]
- 9.Abdel-Moneim AS, El-Kady MF, Ladman BS, Gelb J., Jr S1 gene sequence analysis of a nephropathogenic strain of avian infectious bronchitis virus in Egypt. Virol J. 2006;3:78. doi: 10.1186/1743-422X-3-78. Epub 2006 Sep 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin Y, Mori T, Okita M, et al. Detection of canine distemper virus nucleocapsid protein gene in canine peripheral blood mononuclear cells by RT-PCR. J Vet Med Sci. 1995;57:439–445. doi: 10.1292/jvms.57.439. [DOI] [PubMed] [Google Scholar]
- 11.Potgieter LN, Ajidagba PA. Quantitation of canine distemper virus and antibodies by enzyme-linked immunosorbent assays using protein A and monoclonal antibody capture. J Vet Diagn Invest. 1989;1:110–115. doi: 10.1177/104063878900100203. [DOI] [PubMed] [Google Scholar]
- 12.An DJ, Kim TY, Song DS, et al. An immunochromatography assay for rapid antemortem diagnosis of dogs suspected to have canine distemper. J Virol Methods. 2008;147:244–249. doi: 10.1016/j.jviromet.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gemma T, Iwatsuki K, Shin YS, et al. Serological analysis of canine distemper virus using an immunocapture ELISA. J Vet Med Sci. 1996;58:791–794. doi: 10.1292/jvms.58.791. [DOI] [PubMed] [Google Scholar]
- 14.Bernard SL, Shen DT, Gorham JR. Antigen requirements and specificity of enzyme-linked immunosorbent assay for detection of canine IgG against canine distemper viral antigens. Am J Vet Res. 1982;43:2266–2269. [PubMed] [Google Scholar]
- 15.Di Francesco CE, Di Francesco D, Di Martino B, et al. Detection by hemi-nested reverse transcription polymerase chain reaction and genetic characterization of wild type strains of canine distemper virus in suspected infected dogs. J Vet Diagn Invest. 2012;24:107–115. doi: 10.1177/1040638711425700. [DOI] [PubMed] [Google Scholar]
- 16.Scagliarini A, Dal Pozzo F, Gallina L, et al. TaqMan based real time PCR for the quantification of canine distemper virus. Vet Res Commun. 2007;31(Suppl 1):261–263. doi: 10.1007/s11259-007-0020-9. [DOI] [PubMed] [Google Scholar]
- 17.He F, Soejoedono RD, Murtini S, Goutama M, Kwang J. Complementary monoclonal antibody-based dot ELISA for universal detection of H5 avian influenza virus. BMC Microbiol. 2010;10:330. doi: 10.1186/1471-2180-10-330. Epub 2010 Dec 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Domingues HG, Campalans J, Almeida RS, et al. Dot-enzyme linked immunosorbent assay as an alternative technique for the detection of bovine respiratory syncytial virus (BRSV) antibodies. Vet Res. 2002;33:397–404. doi: 10.1051/vetres:2002025. [DOI] [PubMed] [Google Scholar]
- 19.Del Puerto HL, Martins AS, Braz GF, et al. Vero cells infected with the Lederle strain of canine distemper virus have increased Fas receptor signaling expression at 15 h post-infection. Genet Mol Res. 2011;10:2527–2533. doi: 10.4238/2011.October.18.3. [DOI] [PubMed] [Google Scholar]
- 20.Saliki JT, Lehenbauer TW. Monoclonal antibody-based competitive enzyme-linked immunosorbent assay for detection of morbillivirus antibody in marine mammal sera. J Clin Microbiol. 2001;39:1877–1881. doi: 10.1128/JCM.39.5.1877-1881.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugiyama M, Minamoto N, Kinjo T, et al. Characterization of monoclonal antibodies against four structural proteins of rinderpest virus. J Gen Virol. 1989;70(Pt 10):2605–2613. doi: 10.1099/0022-1317-70-10-2605. [DOI] [PubMed] [Google Scholar]
- 22.Masuda M, Sato H, Kamata H, et al. Characterization of monoclonal antibodies directed against the canine distemper virus nucleocapsid protein. Comp Immunol Microbiol Infect Dis. 2006;29(2–3):157–165. doi: 10.1016/j.cimid.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Tsai SJ, Hutchinson LJ, Zarkower A. Comparison of dot immunobinding assay, enzyme-linked immunosorbent assay and immunodiffusion for serodiagnosis of paratuberculosis. Can J Vet Res. 1989;53:405–410. [PMC free article] [PubMed] [Google Scholar]
- 24.Krakowka S, Cockerell G, Koestner A. Effects of canine distemper virus infection on lymphoid function in vitro and in vivo. Infect Immun. 1975;11:1069–1078. doi: 10.1128/iai.11.5.1069-1078.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kai C, Ochikubo F, Okita M, et al. Use of B95a cells for isolation of canine distemper virus from clinical cases. J Vet Med Sci. 1993;55:1067–1070. doi: 10.1292/jvms.55.1067. [DOI] [PubMed] [Google Scholar]