Abstract
Background
The purpose of this study was to compare anatomic and visual changes in patients with lamellar macular hole undergoing pars plana vitrectomy with those in patients undergoing follow-up only.
Methods
In this retrospective consecutive case series study, we evaluated two groups of eyes, comprising 19 eyes from 19 patients with lamellar macular hole who underwent pars plana vitrectomy with internal limiting membrane peeling and 21 eyes from 21 patients with lamellar macular hole who had follow-up only. Corrected distance visual acuity (CDVA, in logMAR) and optical coherence tomography findings, including measurements of maximum diameter of lamellar defect and foveal thickness, and whether the inner segment/outer segment band was intact or not were documented at initial and follow-up examinations.
Results
At initial examination, mean CDVA was 0.54 logMAR in the study group and 0.51 logMAR in the control group, and 0.33 logMAR and 0.55 logMAR, respectively, on final examination. In the study group, postoperative optical coherence tomography images were found to be normalized in ten patients (52.6%), improved in six (31.5%), unchanged in two (10.5%), and to have progressed to full-thickness macular hole in one (5.2%) in the intervention group, while all patients in the control group were found to have deteriorated within the follow-up period between March 2004 and June 2010.
Conclusion
In patients with lamellar macular hole, combination treatment with pars plana vitrectomy and internal limiting membrane peeling appears to be effective, but further studies are required to establish new treatment modalities for patients who do not have a satisfactory outcome from treatment.
Keywords: lamellar macular hole, pars plana vitrectomy, internal limiting membrane peeling, outcome
Introduction
Lamellar macular hole (LMH) was first described by Gass in 1975 as a partial-thickness foveal defect caused by cystoid macular edema.1 Subsequently, it was defined as a macular lesion believed to occur through the abortive process of formation of a full-thickness macular hole.2,3 On biomicroscopy, LMH appears as a round or irregularly shaped, well circumscribed, reddish lesion, but clinical detection of LMH at an early stage may be difficult.4,5 Optical coherence tomography (OCT) has contributed greatly to the diagnosis of LMH.5,6 The criteria for diagnosis of LMH acquired by OCT images include foveal thinning with irregular contour, breaks in the inner layers of the fovea, intraretinal split, and absence of a full-thickness foveal defect with intact foveal photoreceptors.6 In many cases, visual acuity seems to remain at more than 20/40, and long-term follow-up in the clinic is sufficient for these patients.7
Although the pathogenesis of LMH remains unclear, one theory is that LMH is associated with contraction of the perifoveal epiretinal membrane and internal limiting membrane complex.6,8 In recent years, there has been increasing evidence that surgery may be beneficial in certain patients with LMH, and surgery is being seen increasingly as a reasonable option compared with observation in more symptomatic patients.8,9 To the best of our knowledge, no study has compared the results of a surgical approach versus observation in these patients. The aim of this study was to compare the anatomic and visual changes occurring in patients who underwent surgery and those who had follow-up only.
Materials and methods
The study included 40 eyes from 40 patients presenting with LMH between March 2004 and June 2010 at the retina clinic of the Haydapasa Training and Research Hospital in Istanbul. To confirm the diagnosis of LMH, each patient underwent OCT imaging (Stratus OCT™, Carl Zeiss Meditec Inc, Dublin, CA, USA) and fundus examination. The criteria defined by Witkin et al6 were taken as the reference for diagnosis of LMH by assessing OCT findings, ie, breaks in the inner layers of the fovea, irregular foveal contour and thinning, intraretinal splitting, and intact foveal photoreceptors. Differential diagnosis of LMH from macular pseudohole, pseudocyst, and full-thickness macular hole was performed using OCT. Patients with eye disease or blurred vision other than cataracts were excluded from the study. The maximum diameter of the lamellar defect and foveal thickness were measured, and the inner segment/outer segment (IS/OS) band was determined to be intact or not. Twenty-one control patients were followed up at 6-month intervals from March 2004 to February 2008. Only one surgeon performed the 23 gauge three-port pars plana vitrectomy (PPV) procedures. Patients were informed in writing of the objectives, methods, benefits, and risks of the study and understood that they were participating of their own free will. Institutional review board approval to conduct this study was obtained from the department of ophthalmology at our hospital.
Pars plana vitrectomy and epiretinal membrane peeling was performed in the study group. During surgery, the posterior hyaloid was completely separated from the retina after the core vitrectomy phase. Trypan blue solution (0.06%) was used for epiretinal membrane staining. An additional trypan blue injection was administered to visualize any remaining internal limiting membrane in the macular region. Fluid-air exchange was carried out and air in the vitreous cavity was replaced with 20% sulfur hexafluoride or 16% perfluoroethane.
After surgery, patients were advised to remain in a prone position for 3–6 days under the supervision of ward nurses during their hospital stay. They underwent a complete ophthalmologic examination, including OCT, preoperatively and on day 1, at week 1, and at months 1, 3, 6, and 12 after surgery. Visual acuity was measured as changes in logMAR values on the Snellen chart. The two groups were compared with regard to anatomic and visual changes.
Advanced cataracts, which do not allow for proper visualization of the posterior pole and internal limiting membrane peeling, were removed prior to vitrectomy in a separate operating session. In these patients, postoperative visual acuity was recorded as the initial corrected distance visual acuity (CDVA). Cataract cases that developed de novo during follow-up were also operated.
In the study group, measurements performed in the 12 months postoperatively were used for the statistical analyses and those of the last examination findings were used for the control group. One patient in the study group and three in the control group were excluded from the study because their follow-up was less than 12 months.
NCSS 2007 and PASS 2008 Statistical Software for Windows (Kaysville, UT, USA) was used for the statistical analysis. The Student’s t-test was used for descriptive statistical methods (mean, standard deviation, median), and was also used for comparisons among the quantitative data; the Mann–Whitney U-test was used to make comparisons between groups. Qualitative data were compared using Fisher’s exact test. P < 0.05 was considered to be statistically significant.
Results
The study included 40 eyes from 40 patients of mean age 68.88 ± 3.17 (58–77) years. There were 15 male (42.9%) and 20 female (57.1%) patients in the study (Table 1). The mean duration of follow-up in the study group was 17.58 ± 4.50 (12–28) months and in the control group was 21.62 ± 6.03 (12–35) months.
Table 1.
Clinical outcomes in the study and control groups
| Study group | Control group | P-valuea | |
|---|---|---|---|
| Patients (n) | 19 | 21 | – |
| Mean age | 69.26 ± 4.47 | 68.57 ± 4.38 | – |
| Mean CDVA | |||
| Initial | 0.54 ± 0.92 | 0.51 ± 1.0 | 0.537 |
| Final | 0.33 ± 0.54 | 0.55 ± 1.0 | 0.001** |
| P-valueb | 0.001** | 0.025* | – |
| Mean maximum LMH diameter (μm) | |||
| Initial | 1,168.31 ± 542.86 | 1,094.38 ± 552.81 | 0.672 |
| Final | Not measured | 1,221.81 ± 574.51 | – |
| P-valueb | – | 0.001** | – |
| Mean foveal thickness (μm) | |||
| Initial | 103.00 ± 37.45 | 90.05 ± 18.47 | 0.184 |
| Final | 195.83 ± 59.68 | 77.33 ± 21.13 | 0.001** |
| P-valueb | 0.001** | 0.001** | – |
Notes: Data are given as the mean ± standard deviation.
Independent samples test;
paired samples test;
P < 0.05;
P < 0.01.
Abbreviations: CDVA, corrected distance visual acuity; LMH, lamellar macular hole.
Mean initial CDVA did not differ significantly between the groups (P > 0.05). Mean CDVA, which was 0.54 ± 0.92 initially, improved to 0.33 ± 0.54 at the last examination in the study group (P < 0.001). However, in the control group it decreased from 0.51 ± 1.0 to 0.55 ± 1.0 (P = 0.025). A statistically significant difference was found for final CDVA between the two groups (P < 0.01). The findings for visual acuity were consistent with those for foveal thickness and an inverse correlation was detected (Table 1). The mean maximum diameter of the LMH was initially 1,168.31 ± 542.86 μm in the study group and 1,094.38 ± 552.81 μm in the control group. In the study group, the diameter of the LMH was not measured postoperatively because the defect often improves. However, at final examination, the diameter of the LMH in the control group was measured to be 1,221.81 ± 574.51 μm (Table 1). The mean initial foveal thickness was 103.00 ± 37.45 μm in the study group and 90.05 ± 18.47 μm in the control group, and at final examination was 195.83 ± 59.68 μm and 77.33 ± 21.13 μm, respectively (Table 1).
Five patients in the study group initially had defects in the IS/OS band, with three patients continuing to have defects postoperatively and two patients had little defects on the OCT images. However, there was no remarkable increase in visual acuity in patients whose defects were closed. Three patients in the control group had defects in the IS/OS band at initial examination. Two further patients developed damage to the IS/OS layer during follow-up, with a decrease in visual acuity of two Snellen lines in one patient and three Snellen lines in the other.
Three patients in the study group initially had cataract and five patients developed cataract de novo after PPV. Phacoemulsification was performed in these patients during the follow-up period.
Two patients in the study group developed full-thickness macular hole. In both cases, macular reattachment surgery was performed using gas tamponade (perfluoroethane). This treatment was successful in one case but the patient’s CDVA decreased by one Snellen line compared with initial CDVA, and in the other case, treatment was unsuccessful and the patient’s CDVA decreased by two Snellen lines compared with initial CDVA. In the control group, all cases were found to have deteriorated by the end of the follow-up period, with no improvement in full-thickness macular hole nor spontaneous closure of LMH. No complications occurred as a result of surgery. An OCT image of successful LMH surgery is shown in Figure 1.
Figure 1.
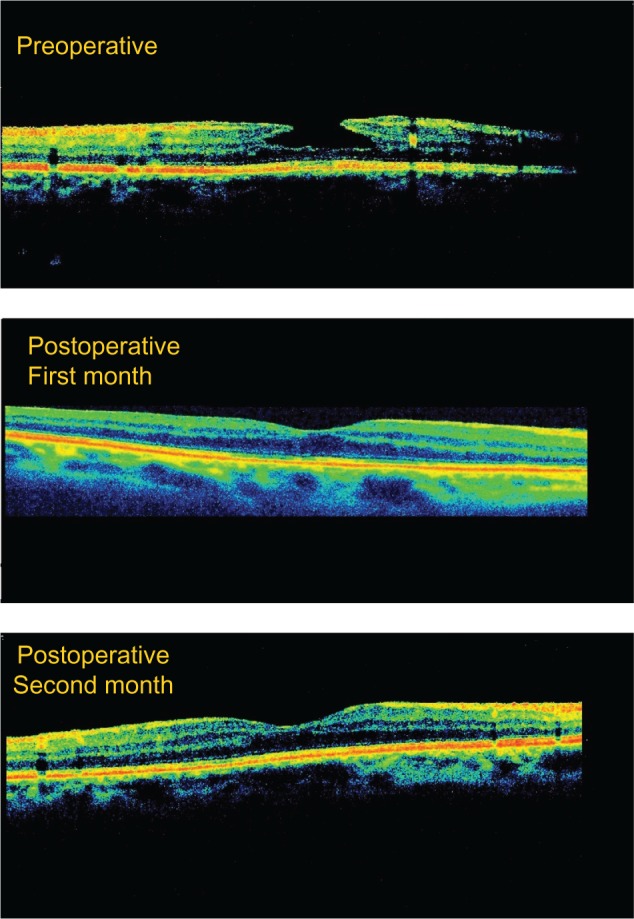
Typical optical coherence tomography image representative of successful lamellar macular hole surgery.
Discussion
Lamellar macular hole is an acquired disorder involving macular distortion and is characterized by intraretinal splitting, break in the inner fovea, irregular foveal contour, visual distortion, and loss of visual acuity.5 It is controversial whether surgical treatment for LMH is worthwhile. Patients with LMH generally have visual acuity of more than 20/40, so many physicians simply follow-up with these patients in the long-term without surgery.7 In recent years, a number of peer-reviewed journals have published the results of surgery for LMH.
Theodossiadis et al followed the natural course of LMH in 41 eyes from 41 patients for a mean of 37.1 months, and reported that CDVA decreased in 11 (22%) patients, and that foveal thickness decreased and LMH diameter increased.7 Casparis and Bovey8 noted that the foveal photoreceptor layer did not appear to be thinned or particularly irregular on OCT in more recent LMH, but in patients with long-term LMH, these tissues looked thin and irregular. In addition, there are case reports of patients who progressed from LMH to full-thickness macular hole.3,9 In contrast with these reports, Theodossiadis et al observed spontaneous closure of LMH in two patients 11 and 21 months after the baseline examination.10 In this study, mean CDVA decreased from 0.31 ± 0.10 to 0.28 ± 0.10 in 21 patients in the control group during a mean 21.62 ± 6.03 months of follow-up (P = 0.025).
Favorable results have been obtained after LMH surgery, but with a few exceptions. The first case of PPV performed for LMH was reported by Kokame et al.11 They performed PPV, membrane peeling, and fluid-air exchange in a patient with LMH and observed a normal foveal contour and foveal configuration on OCT, and an increase in CDVA. Hirakawa et al12 achieved an anatomic and functionally successful outcome in two patients undergoing PPV. An anatomic success rate of over 90% and a visual success rate of 63.3%–100% after PPV have been reported in eight case series, each containing between ten and 45 eyes.8,13–19 Garretson et al13 reported that 25 of 27 patients (93%) had improved vision after PPV with internal limiting membrane peeling and without gas tamponade for LMH. Other studies have reported visual improvement using gas tamponade (Engler et al14 in 10/10 cases; Michalewska et al15 in 24/26 cases; Figueroa et al16 in 9/12 cases; Parolini et al17 in 14/19 cases; Casparis and Bovey8 in 40/41cases; Lee et al18 in 19/30 cases; and Lee et al19 in 23/31 cases).
In this study, the initial mean CDVA improved from 0.29 ± 0.12 to 0.47 ± 0.23 at the 12-month examination in the 19 patients operated for LMH, and vision was improved in 14 of these cases. These visual improvements are comparable with those reported in other interventional studies.
In contrast with these studies, Witkin et al20 reported the surgical results for 16 patients who underwent PPV for LMH, including four patients whose outcomes they had already published.6 Visual improvement was achieved in five eyes and anatomic improvement in seven eyes. Two patients developed a full-thickness macular hole.
Physicians suggesting vitrectomy for LMH rely on the presence of perifoveal epiretinal membrane. Many studies have shown that patients with LMH also have epiretinal membrane.8,16,18,19 It is suggested in the literature that the pathogenesis of LMH is related to contraction of the perifoveal epiretinal membrane-internal limiting membrane complex.5,13 In addition, Michalewska et al21 demonstrated that macular pseudohole can progress to LMH following progressive epiretinal membrane contraction. Theodossiadis et al10 demonstrated spontaneous closure of LMH following resolution of epiretinal membrane with complete posterior vitreous detachment, suggesting the role of epiretinal membrane in the pathogenesis of LMH. In this study, epiretinal membrane was found in 14 of 21 eyes using Stratus OCT in the control group and in all 19 eyes during surgery in the study group. The authors of this study consider that Stratus OCT does not allow observation of epiretinal membrane and photoreceptor defects, which is a limiting factor in this report.
The presence of complete posterior vitreous detachment is a controversial topic in LMH. Androudi et al22 suggested that posterior vitreous detachment should be a diagnostic criterion for LMH, but the study by Garretson et al13 does not confirm that complete posterior vitreous detachment is always present. Wang et al23 have suggested that, in some cases of anomalous posterior vitreous detachment, the vitreous may remain attached to the optic disc. This vitreopapillary adhesion might be responsible for outward tangential traction of the epiretinal membrane that leads to retinal dehiscence and formation of LMH.23–25 In contrast, when there is complete vitreous detachment with no papillary adhesion, the epiretinal membrane would contract centripetally, as in a pseudohole.16 Romano et al26 proposed a new classification for the formation of a pseudomacular hole and LMH according to three different patterns whereby one of them has full-thickness posterior vitreous detachment and the others have vitreoschisis. The presence of vitreopapillary adhesion induces progression of anatomic changes up to the outer retinal layers, and worse functional results for vision. In this study, we had no information concerning the presence of vitreoschisis from OCT operative reports. This is another limitation of this paper.
Michalewska et al15 compared their findings with those in the literature; however, there was no study including a control group. Casparis and Bovey8 compared data (visual acuity, initial hole diameter, foveal thickness) from their series with those from an observational study by Theodossiadis et al7 that included data on the natural history of 41 patients with LMH. Recently, Romano et al26 reported the first comparative study of surgery versus observation for LMH. Ours is also a retrospective comparative study including observation for LMH in a control group. a retrospective comparative study including observation for LMH in a control group.
One of our goals was to compare the anatomic results between the two groups. In the study group, postoperative OCT images were found to be normalized in ten (52.6%) patients, improved in six (31.5%) patients, unchanged in two (10.5%) patients, and to have progressed to full-thickness macular hole in one (5.2%) patient. Mean foveal thickness in the control group decreased from 90.05 ± 18.47 μm to 77.33 ± 21.13 μm. Spontaneous closure of LMH or progression to full-thickness macular hole was not observed in the control group.
According to the results of our study and those in the literature, LMH is not an aggressive advancing disease per se. However, visual and anatomic deterioration could be seen during follow-up. In addition, a defect in the IS/OS band was found in three patients in the control group at initial examination and during follow-up, and two further patients developed damage to the IS/OS layer of the fovea. We attribute the persistent growth of this defect to continued shrinkage due to the epiretinal membrane which was not in the surrounding fovea. We believe that the decrease in macular thickness and the new defect that occurred at the IS/OS band resulted from physiologic deterioration and thinning of the retinal tissue because of becoming vulnerable to the ultraviolet ray exposure.
An increase in mean CDVA along with improved or normalized anatomic OCT findings has been seen in patients undergoing surgery for LMH, both in this study and in those reported in the literature. However, complications may occur during and after surgery. One of these is progression of LMH to full-thickness macular hole after surgery.6,13,16 Two patients in our study developed full-thickness macular hole. In both cases, macular reattachment surgery was performed. We do not know if there is a predilection for development of full-thickness macular hole in patients with LMH after surgery or not. The availability of this information would facilitate our decision to proceed to surgery. Certainly we believe that it is helpful to use expanding gas during surgery to prevent development of full-thickness macular hole postoperatively.
Patients with defects in the IS/OS band should be followed up for LMH because successful visual results cannot be obtained in these patients even if PPV is successful anatomically.15 In our study, five patients in the study group initially had defects in the IS/OS band; three continued to have defects postoperatively and two had minor defects on OCT. However, there was no remarkable increase in visual acuity in our cases, including those whose defects were closed. The reason why the IS/OS band became visible on OCT postoperatively may involve gliosis induced by peeling of the internal limiting membrane. Glial cells becoming swollen and moving between the photoreceptors may contribute to the intact appearance of the IS/OS line on OCT. However, visual acuity depends on the intercone distance.27 If the preoperative photoreceptor defect is greater, proliferating glial cells will form a large intercone distance. This helps to explain why visual acuity is not increased as expected in some cases although the IS/OS band seems intact on spectral-domain OCT.15
In conclusion, although PPV for LMH seems more likely to produce significantly better results than follow-up alone, further studies are needed to identify which patients should be treated with surgery or simply followed up.
Author contributions
HS, AE, and SS were involved in the design and conduct of the study, UC and AE in the preparation and review of the study, FBA and ED in the data collection, and AE in the statistical analysis. All authors contributed to the critical revision of the manuscript and approved the final proof to be published.
Disclosure
None of the authors has a financial or proprietary interest in any material or method mentioned in this work.
References
- 1.Gass JD. Lamellar macular hole: a complication of cystoid macular edema after cataract extraction: a clinicopathologic case report. Trans Am Ophthalmol Soc. 1975;73:231–250. [PMC free article] [PubMed] [Google Scholar]
- 2.Gass JDM. Vitreofoveal separation and lamellar hole formation. In: Gass JDM, editor. Stereoscopic Atlas of Macular Diseases: Diagnosis and Treatment. 4th ed. St Louis, MO: CV Mosby; 1997. [Google Scholar]
- 3.Takahashi H, Kishi S. Tomographic features of a lamellar macular hole formation and a lamellar hole that progressed to a full-thickness macular hole. Am J Ophthalmol. 2000;130:677–679. doi: 10.1016/s0002-9394(00)00626-7. [DOI] [PubMed] [Google Scholar]
- 4.Chen JC, Lee LR. Clinical spectrum of lamellar macular defects including pseudoholes and pseudocysts defined by optical coherence tomography. Br J Ophthalmol. 2008;92:1342–1346. doi: 10.1136/bjo.2007.133041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haouchine B, Massin P, Tadayoni R, Erginay A, Gaudric A. Diagnosis of macular pseudoholes and lamellar macular holes by optical coherence tomography. Am J Ophthalmol. 2004;138:732–739. doi: 10.1016/j.ajo.2004.06.088. [DOI] [PubMed] [Google Scholar]
- 6.Witkin AJ, Ko TH, Fujimoto JG, et al. Redefining lamellar holes and the vitreomacular interface: an ultrahigh-resolution optical coherence tomography study. Ophthalmology. 2006;113:388–397. doi: 10.1016/j.ophtha.2005.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Theodossiadis PG, Grigoropoulos VG, Emfietzoglou I, et al. Evolution of lamellar macular hole studied by optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2009;247:13–20. doi: 10.1007/s00417-008-0927-z. [DOI] [PubMed] [Google Scholar]
- 8.Casparis H, Bovey EH. Surgical treatment of lamellar macular hole associated with epimacular membrane. Retina. 2011;31:1783–1790. doi: 10.1097/IAE.0b013e31820a6818. [DOI] [PubMed] [Google Scholar]
- 9.Castro LC, Duker JS. Spontaneous progression of a long-standing lamellar macular hole into a full-thickness macular hole. Ophthalmic Surg Lasers Imaging. 2010;9:1–4. doi: 10.3928/15428877-20100215-54. [DOI] [PubMed] [Google Scholar]
- 10.Theodossiadis PG, Grigoropoulos VG, Emfietzoglou I, Nikolaidis P, Papathanasiou M, Theodossiadis GP. Spontaneous closure of lamellar macular holes studied by optical coherence tomography. Acta Ophthalmol. 2012;90:96–98. doi: 10.1111/j.1755-3768.2009.01745.x. [DOI] [PubMed] [Google Scholar]
- 11.Kokame GT, Tokuhara KG. Surgical management of inner lamellar macular hole. Ophthalmic Surg Lasers Imaging. 2007;38:61–63. doi: 10.3928/15428877-20070101-10. [DOI] [PubMed] [Google Scholar]
- 12.Hirakawa M, Uemura A, Nakano T, Sakamoto T. Pars plana vitrectomy with gas tamponade for lamellar macular holes. Am J Ophthalmol. 2005;140:1154–1155. doi: 10.1016/j.ajo.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 13.Garretson BR, Pollack JS, Ruby AJ, Drenser KA, Williams GA, Sarrafizadeh R. Vitrectomy for a symptomatic lamellar macular hole. Ophthalmology. 2007;115:884–886. doi: 10.1016/j.ophtha.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 14.Engler C, Schaal KB, Höh AE, Dithmar S. Surgical treatment of lamellar macular hole. Ophthalmologe. 2008;105:836–839. doi: 10.1007/s00347-008-1765-4. German. [DOI] [PubMed] [Google Scholar]
- 15.Michalewska Z, Michalewski J, Odrobina D, et al. Surgical treatment of lamellar macular holes. Graefes Arch Clin Exp Ophthalmol. 2010;248:1395–1400. doi: 10.1007/s00417-010-1400-3. [DOI] [PubMed] [Google Scholar]
- 16.Figueroa MS, Noval S, Contreras I. Macular structure on optical coherence tomography after lamellar macular hole surgery and its correlation with visual outcome. Can J Ophthalmol. 2011;46:491–497. doi: 10.1016/j.jcjo.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Parolini B, Schumann RG, Cereda MG, Haritoglou C, Pertile G. Lamellar macular hole: a clinicopathologic correlation of surgically excised epiretinal membranes. Invest Ophthalmol Vis Sci. 2011;52:9074–9083. doi: 10.1167/iovs.11-8227. [DOI] [PubMed] [Google Scholar]
- 18.Lee CS, Koh HJ, Lim HT, Lee KS, Lee SC. Prognostic factors in vitrectomy for lamellar macular hole assessed by spectral-domain optical coherence tomography. Acta Ophthalmol. 2012;90:597–602. doi: 10.1111/j.1755-3768.2012.02456.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee SJ, Jang SY, Moon D, Choi KS, Jung GY. Long-term surgical outcomes after vitrectomy for symptomatic lamellar macular holes. Retina. 2012;32:1743–1748. doi: 10.1097/IAE.0b013e3182551c3c. [DOI] [PubMed] [Google Scholar]
- 20.Witkin AJ, Castro LC, Reichel E, Rogers AH, Baumal CR, Duker JS. Anatomic and visual outcomes of vitrectomy for lamellar macular holes. Ophthalmic Surg Lasers Imaging. 2010;41:425–431. doi: 10.3928/15428877-20100426-04. [DOI] [PubMed] [Google Scholar]
- 21.Michalewski J, Michalewska Z, Dzięgielewski K, Nawrocki J. Evolution from macular pseudohole to lamellar macular hole-spectral domain OCT study. Graefes Arch Clin Exp Ophthalmol. 2011;249:175–178. doi: 10.1007/s00417-010-1463-1. [DOI] [PubMed] [Google Scholar]
- 22.Androudi S, Stangos A, Brazitikos PD. Lamellar macular holes: tomographic features and surgical outcome. Am J Ophthalmol. 2009;148:420–426. doi: 10.1016/j.ajo.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Wang MY, Nguyen D, Hindoyan N, Sadun AA, Sebag J. Vitreo-papillary adhesion in macular hole and macular pucker. Retina. 2009;29:644–650. doi: 10.1097/IAE.0b013e31819e0d92. [DOI] [PubMed] [Google Scholar]
- 24.Sebag J. Vitreoschisis. Graefes Arch Clin Exp Ophthalmol. 2008;246:329–332. doi: 10.1007/s00417-007-0743-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haouchine B, Massin P, Gaudric A. Foveal pseudocyst as the first step in macular hole formation: a prospective study by optical coherence tomography. Ophthalmology. 2001;108:15–22. doi: 10.1016/s0161-6420(00)00519-4. [DOI] [PubMed] [Google Scholar]
- 26.Romano MR, Vallejo-Garcia JL, Camesasca FI, Vinciguerra P, Costagliola C. Vitreo-papillary adhesion as a prognostic factor in pseudo- and lamellar macular holes. Eye. 2012;26:810–815. doi: 10.1038/eye.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandberg MA, Berson E. Visual acuity and cone spatial density in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1983;24:1511–1513. [PubMed] [Google Scholar]


