Abstract
For nearly a decade, thermoresponsive ophthalmic in situ gels have been recognized as an interesting and promising ocular topical delivery vehicle for lipophilic drugs. In this study, a series of thermosensitive copolymers, hyaluronic acid-g-poly(N-isopropylacrylamide) (HA-g-PNIPAAm), was synthesized, by coupling carboxylic end-capped PNIPAAm to aminated hyaluronic acid through amide bond linkages, and was used as a potential carrier for the topical ocular administration of cyclosporine A (CyA). The lower critical solution temperature of HA-g-PNIPAAm59 in aqueous solutions was measured as 32.7°C, which was not significantly affected by the polymer concentration. Moreover, HA-g-PNIPAAm59 microgels showed a high drug loading efficiency (73.92%) and a controlled release profile that are necessary for biomedical application. Transmission electron microscopy (TEM) and atomic force microscopy (AFM) observations showed that HA-g-PNIPAAm microgels were spherical in shape with homogeneous size. Based on the result of the eye irritation test, the HA-g-PNIPAAm microgels formulation was shown to be safe and nonirritant for rabbit eyes. In addition, HA-g-PNIPAAm microgels achieved significantly higher CyA concentration levels in rabbit corneas (1455.8 ng/g of tissue) than both castor oil formulation and commercial CyA eye drops. Therefore, these newly described thermoresponsive HA-g-PNIPAAm microgels demonstrated attractive properties to serve as pharmaceutical delivery vehicles for a variety of ophthalmic applications.
Keywords: thermosensitive microgels, ophthalmic drug delivery, hyaluronic acid, cyclosporine A
Introduction
Topical dosing of ophthalmic drugs to the eye is a widely accepted route of administration due to its convenience, ease of use, and noninvasiveness. Eye drops, suspensions, and ointments account for approximately 90% of commercially available ophthalmic formulations. Eye drops are the conventional approach for the treatment and diagnosis of ocular diseases, which allow accurate doses.1,2 However, this conventional system should not be considered an optimum formulation for the treatment of eye disorders, mainly due to the biopharmaceutical problems related to the special characteristics of the eye that restrict drug bioavailability.3 The eye is partially isolated from the remainder of the body by several types of barriers that impede the effective passage of many drugs, leading to limited absorption.4 Moreover, certain physiological processes might contribute to the poor efficacy of conventional drug formulations; for example, blinking and tear drainage through the lachrymal drainage system can reduce the residence time of topically applied molecules. Additionally, eye drops placed onto the ocular surface are washed away in less than 30 seconds. Altogether, less than 5% of topically administered drugs reach the intraocular tissues.5 As a result, concentrated solutions and frequent dosing are required for the instillation to achieve an adequate level of therapeutic effect, which might cause undesirable side effects due to systemic absorption of drugs through the nasolacrimal duct.6,7 In general, the major disadvantages of the conventional dosage forms are the extremely low bioavailability, short precorneal residence time, and rapid nasolacrimal drainage of the instilled drug.
Different strategies were carried out to improve the precorneal residence time and/or penetration ability of the active ingredient. The development of in situ gel systems has received considerable attention over the past few years. Major progress in the development of ophthalmic formulations has been achieved through ophthalmic gel technology, in the development of droppable gels, called in situ gels, which consist of certain polymers undergoing sol-gel phase transition by an induction of environment conditions, such as pH,8 specific ions,2 and temperature.9 In previous reports, diclofenac sodium in situ gel was shown to have potential as an alternative to the conventional diclofenac sodium eye drop.6 Microgels are crosslinked hydrogel particles that are confined to smaller dimensions. When the microgel particles are submicron sized, they are called nanogels. Previous studies have shown that microgels used as ocular drug delivery systems can increase drug bioavailability and decrease systemic side effects.10,11 Microgels derived from naturally occurring polysaccharides, such as hyaluronic acid (HA), chitosan, and chondroitin sulfate, have recently attracted a great deal of interest in drug delivery due to their high water content, biocompatibility, and functionality, as well as their biodegradation properties, low toxicity, abundance in nature, and low cost.12,13 HA, a linear anionic polysaccharide composed of repeating disaccharide units of D-glucuronic acid and N-acetyl-D-glucosamine, is mainly found in the vitreous body, and in a much lower concentration in the aqueous humor. Thus, ophthalmic products containing HA are used as vitreous replacement and for corneal protection in ocular surgeries.14 In addition, HA has been used as an excipient in contact lens products in order to improve their wettability and comfort.15 In previous reports, HA was suggested as effective treatment for dry eye symptoms, and thus has been used in artificial tears for the treatment of this syndrome.16
Biodegradable microgels capable of phase transition in response to external stimuli, such as temperature, represent another type of useful building block for ophthalmic applications. Microgel networks are able to host drug molecules with controlled release characteristics.17 Poly(N-isopropylacrylamide) (PNIPAAm) is a well-known thermosensitive polymer with a thermoreversible phase transition temperature of 32°C, which is close to human body surface temperature.18 However, the application of PNIPAAm polymer as an ocular drug delivery system may be restricted by its synthetic nature.19 This limitation has provided the motivation for developing biodegradable PNIPAAm-based polymers by conjugating the PNIPAAm with natural biodegradable polysaccharides such as chitosan, chondroitin sulfate, and HA. A prior study has demonstrated the usefulness of hyaluronic acid-g-poly(N-isopropylacrylamide) (HA-g-PNIPAAm) microgels as an injectable hydrogel for adipose tissue engineering.20 However, as far as we know, very little work has been done regarding the use of HA-g-PNIPAAm microgels for ophthalmic application.
Cyclosporine A (CyA) is a neutral, hydrophobic, cyclic peptide of eleven amino acids which can be isolated from several species of fungi.21,22 Over the past several years, numerous studies have shown the potential applications of CyA in ophthalmology.23,24 Due to its high hydrophobicity and poor aqueous solubility, CyA cannot be formulated into the common aqueous formulations, leading to a low absorption and bioavailability.25 To overcome these obstacles, various ophthalmic formulations have been developed to enhance CyA solubility and bioavailability.26 So far, only one ophthalmic emulsion, RESTASIS® (Allergan Inc, Irvine, CA, USA) has been approved by the US Food and Drug Administration for human use.27 However, this formulation is considered to be poorly tolerated and provides low ocular bioavailability. Thermosensitive in situ forming microgels are known to have good biocompatibility, high drug loading capacity, and controlled drug release characteristics compared with other ophthalmic preparations.28,29 Therefore, these microgels represent promising drug carriers for the ocular administration of hydrophobic drugs.
In this study, a microgel consisting of biocompatible HA and temperature-sensitive PNIPAAm was designed and synthesized by covalently bonding carboxylic end-capped PNIPAAm (PNIPAAm-COOH) to aminated hyaluronic acid (AHA) through amide formation. The resulting microgels would combine the unique properties of their component precursors, where the presence of PNIPAAm in microgels would confer a thermoresponsive capability to HA, while HA with its biological properties could improve the microgels’ biocompatibility. Therefore, HA-g-PNIPAAm microgels are expected to improve the penetration of the encapsulated CyA into ocular tissues with a good ocular tolerance, and to provide therapeutic concentrations of CyA in the precorneal area immediately. The aim of the present work was to evaluate the potential of an in situ gel-forming delivery system comprised of HA-g-PNIPAAm microgels as vehicles for enhanced ocular permeation of CyA compared to oil and CyA eye drops.
Materials and methods
Materials
Hyaluronic acid (HA; molecular weight ~1.1×10 kDa) was purchased from Freda Biochem Co, Ltd (Jinan, People’s Republic of China). N-isopropylacrylamide (NIPAAm), adipic dihydrazide (ADH), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC), azodiisobutyronitrile (AIBN), Mercaptopropionic acid (MPA), hydrochloric acid (HCl), sodium hydroxide (NaOH), dimethyl formamide (DMF), acetone, and n-hexane were obtained from Aladdin (Shanghai, People’s Republic of China). N-isopropylacrylamide (NIPAAm) was purified by recrystallization in ethyl acetate and n-hexane, respectively, before use.
Synthesis of HA-g-PNIPAAm
Synthesis of PNIPAAm-COOH
PNIPAAm-COOH was synthesized as described previously with some modifications (Figure 1A).30 In brief, 1 g of the recrystallized monomer NIPAAm and 0.02 g of azodiisobutyronitrile were dissolved in 25 mL of methanol in a three-neck flask, then 50 μL of MPA was added into the mixture. The reaction was bubbled with nitrogen atmosphere at 60°C for 24 hours (h). After the reaction was complete, the solvent was evaporated off. The crude product was vacuum-dried, then dissolved in acetone, followed by precipitation in excess cold n-hexane. The precipitate was carefully washed three times with cold n-hexane to remove unreacted products, followed by vacuum drying to get a slightly yellow powder, which was characterized by Fourier transform infrared spectroscopy (FT-IR).
Figure 1.
The synthesis route of (A) PNIPAAm-COOH,(B) AHA and (C) HA-g-PNIPAAm.
Abbreviations: AIBN, azobisisobutyronitrile; EDC, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride; PNIPAAm, poly(N-isopropylacrylamide); AHA, aminated hyaluronic acid; PNIPAAm-COOH, carboxylic end-capped PNIPAAm; HA-g-PNIPAAm, hyaluronic acid-g-poly(N-isopropylacrylamide).
Synthesis of AHA
AHA was synthesized in aqueous conditions following previously described procedures with slight modifications. First, HA (0.5 g) and hydrazide crosslinker (ADH, 10 g), dissolved in water, were reacted overnight with EDC at pH 4.75, which was adjusted and maintained by adding 0.1 M hydrochloric acid solution to the reaction mixture. In order to quench the reaction, 0.1 M sodium hydroxide was added to adjust the pH of the reaction mixture to 7.0. The resulting solution was dialyzed (molecular weight cut-off [MWCO] 3500) against 0.1 M NaCl, then a 25:75 ethanol-water mixture, and finally distilled water. The polymer solution was filtered through a 0.22 μm microporous membrane and lyophilized then stored at 4°C until further use. The structure of AHA was determined by 1H nuclear magnetic resonance spectroscopy (300MHz) spectra at ambient temperature using deuterium oxide as a solvent.
Synthesis of HA-g-PNIPAAm polymer
HA-g-PNIPAAm polymer was synthesized as described previously by grafting PNIPAAm-COOH onto an AHA chain (Figure 1C). We dissolved 0.1 g of AHA in 100 mL distilled water to make a solution. To graft the PNIPAAm-COOH onto AHA, PNIPAAm-COOH was dissolved in distilled water and incubated with EDC, kept in the refrigerator at 4°C for 48 h. As a catalyst, the quantity of EDC needed is 2.5 times that of PNIPAAm. The PNIPAAm-COOH/EDC solution was then added into the AHA solution with agitation, and the pH of the reaction mixture was adjusted to 6.0. The mixture was incubated at room temperature for 24 h before dialysis (MWCO 7000) for 3 days. The residue was freeze dried at −50°C to get HA-g-PNIPAAm polymer, which was kept at 4°C. To verify the HA-g-PNIPAAm polymer structure, proton nuclear magnetic resonance spectra were measured at ambient temperature using deuterium oxide as a solvent.
Characterization of HA-g-PNIPAAm
The average molecular weight of PNIPAAm-COOH was determined by two methods: end-group titration and rhodamine colorimetry. For the first method 0.5 g of the polymer was dissolved in 10 mL of water and then titrated with 0.01 M sodium hydroxide, in order to determine the concentration of the carboxyl end group. The average molecular weight (MW) was calculated by using the following equation: MW = m/n (m = weight of PNIPAAm titrated n = moles of PNIPAAm carboxyl end groups).
Rhodamine colorimetric method was carried out using rhodamine 6G as a chromogenic agent. Briefly, 10 mg of rhodamine 6G was dissolved in 0.1 M Na3PO4 buffer solution (pH = 11), followed by extraction with 100 mL of toluene. A red-orange color liquid was obtained as a chromogenic solution. PNIPAAm-COOH was dissolved in a certain volume of N,N-dimethyl formamide to obtain a test solution of 0.5 mg/mL concentration. The test solution was then mixed with an equal volume of the chromogenic solution, and placed in the dark for 30 minutes (min). The absorbance of the resulting solution was detected at 536 nm by UV-visible spectrophotometer. A series with known concentrations of acetic acid in dimethyl formamide was prepared to generate the calibration curve. The samples were then treated as described above, and subsequently analyzed by UV(ultra violet)-visible spectrophotometry. The carboxyl acid concentration in the test solution was determined from the standard curve, which was obtained by plotting the absorbance of samples containing the known concentrations of acetic acid.
The degree of substitution (DS) of HA-g-PNIPAAm was calculated from the following equation:
| [1] |
where WHA-g-PNIPAAm is the weight of freeze-dried graft copolymer and WHA and WPNIPAAm-COOH are the weights of HA and PNIPAAm-COOH in the feed, respectively.
Preparation of drug-free and CyA-loaded HA-g-PNIPAAm microgels
HA-g-PNIPAAm microgels were prepared by a simple sonication method in aqueous conditions. We dissolved 100 mg of HA-g-PNIPAAm in 10 mL double-distilled water, followed by ultrasonication for 30 min (Ultrasonic Cell Crusher, Beidi-II YJ, Nanjing, People’s Republic of China). The samples were then centrifuged at 3,500 rpm for 10 min to remove precipitation. The resulting homogeneous clear solution was stored overnight in a refrigerator at 4°C.
The loading of CyA into microgels was achieved by the incubation method.31 First, 2 mg/mL of blank microgels solution was prepared as described above, followed by the addition of a methanol solution of CyA (1.0 wt%) dropwise into the blank microgels solution with stirring. The mixture was then incubated at 4°C for 24, 48, and 72 h. CyA-loaded HA-g-PNIPAAm (CyA-HAPN) microgels were isolated by centrifugation at 15,000 rpm for 15 min. The resulting solution was dialyzed against the distilled water (MWCO 7000) for 8 h to remove the free CyA, followed by lyophilization.
Characterization of drug-free and CyA-HAPN microgels
Drug loading efficiency
CyA concentrations were measured by high-performance liquid chromatography (HPLC; Shimadzu LC-2010 system; Kyoto, Japan) equipped with a Lichrospher™ C18 column (5 mm particle size, 250 mm × 4.6 mm) at 60°C. The mobile phase consisted of acetonitrile, methanol, and water (57:25:18, v/v), with a flow rate of 1.0 mL/min. The detection wavelength was 210 nm, and the volume of the injected sample was 20 μL. The drug loading efficiency of microgels was calculated as follows:
| [2] |
Particle size and zeta potential measurement
The particle size and zeta potential were measured by dynamic light scattering (DLS) using a Malvern Zetasizer Nano-ZS90 (Malvern Instruments, Malvern, UK). All of the DLS measurements were performed at a scattering angle of 90°. The measurements were carried out with 2.0 wt% aqueous microgels solutions and repeated three times.
Osmotic pressure, surface tension, and pH measurements
Osmotic pressure, surface tension, and pH measurements of HA-g-PNIPAAm microgels were performed by OSMOMAT 030-D (Gonotec GmbH, Berlin, Germany), Dataphysics DC AT 21 (DataPhysics Instruments GmbH, Filderstadt, Germany), pHS-25B (Shanghai Dapu Instruments Co Ltd Shanghai, People’s Republic of China), respectively.
Lower critical solution temperature measurement
The lower critical solution temperature (LCST) was defined as the temperature at which the optical transmittance of the solution reduced to 50% of its original value. To examine the reversible thermoresponse, optical transmittance was measured using a controlled temperature circular program, increasing from 25°C–40°C at 0.5°C/min. The thermoresponsive phase transitions of the aqueous solutions of PNIPAAm-COOH and HA-g-PNIPAAm with different DS were measured with a UV/visible spectrometer (TU-1800; PERSEE, Beijing, People’s Republic of China) by monitoring the absorbance of 600 nm light beam through the aqueous solutions.
Viscosity measurement
HA-g-PNIPAAm microgels were dissolved in distilled water to form a solution with a concentration of 5 wt%. Viscosity at varied temperatures was measured by Viscometer (LVDV-III Ultra, Brookfield Engineering Laboratories, Middleboro, MA, USA). The microgel solution was transferred to the measuring cup, where the temperature was controlled by the circulating water. The samples were equilibrated at 5°C ± 0.5°C for 3 min, and then the microgels’ viscosity was measured. The test temperature ranged between 5°C–40°C, and the heating rate was set at 1°C/min. All experiments were repeated three times.
Morphological observation
The morphological observation was performed by transmission electron microscopy (TEM; H-600; Hitachi, Tokyo, Japan). Additionally, atomic force microscopy (AFM; Nano Scope IIIa; Veeco, Plainview, NY, USA) was used to characterize the surface morphology. The samples of AFM were created using the following process: one drop of properly diluted microgel suspension was placed on the surface of a clean glass wafer and air dried at room temperature. The samples were observed by AFM with a 5 μm scanner in tapping mode.
In vitro release
The in vitro drug release of CyA-HAPN microgels was investigated by the dialysis method. To start the release, 1 mL of HA-g-PNIPAAm59 (degree of substitution; 59) microgels formulation (containing 0.5 mg of CyA) was transferred into a dialysis bag with a membrane MWCO of 140 kDa, which was put into 200 mL of release medium (0.1 M phosphate buffer) and stirred at 100 rpm at 25°C (or 37°C). At predetermined time intervals, 1.5 mL aliquots of the release media were withdrawn and replaced with an equal volume of the fresh solution. The samples were filtered through 220 nm filters and then analyzed by HPLC.
In vivo eye irritation test
The in vivo eye irritation test of the CyA-HAPN microgels formulation and the commercial CyA eye drops were performed in two groups of 12 New Zealand White rabbits. The first group received 25 μL of the CyA-HAPN microgels formulation, which was instilled into the lower conjunctival sac of the rabbit’s right eye, while the left eye was kept as a control without manipulation. The second group received the same volume of commercial CyA eye drops, administered as for the first group. The test eye was observed at 0, 5, 10, and 30 min and 1, 6, 12, 24, 48, and 72 h for changes to the cornea, iris, conjunctiva, and chemosis compared to the control. The degree of eye irritation was scored following the modified Draize test.6,32
In vivo ocular distribution investigation of CyA
All animal experiments were conducted in full compliance with local, national, ethical, and regulatory principles for animal care. New Zealand White rabbits (weighing 2.5~3 kg), fed on standard laboratory diet at an ambient temperature and humidity in air-conditioned rooms were used for the present studies. Reference formulation for the in vivo studies was CyA castor oil solution. Animals were randomly divided into three groups as follows: Group 1 received CyA solution in castor oil; Group 2 received commercial CyA eye drops; and Group 3 received CyA-HAPN microgels. Each group received the same volume of different formulations (25 μL) containing 500 μg of CyA in six instillations at 10 min intervals, which were instilled topically in the center of the lower cul-de-sac of both eyes using a micropipette. During the instillation, the lower eyelid was pulled slightly away from the globe and returned into normal position immediately after instillation. Great care was taken not to irritate the eye or to touch the cornea. At 0.5, 1, 2, 4, 8, and 24 h following the last instillation of the formulations, ocular tear samples (2 μL) were collected into Eppendorf tubes (Eppendorf AG, Hamburg, Germany) using a capillary tube. Blood samples were taken immediately before sacrificing the animals with a sodium pentobarbital overdose. The conjunctiva, the cornea, the aqueous humor, and the iris/ciliary body were removed from each eye and placed into tubes. The volume of liquid samples and tissue weight were also determined.
Tear samples were directly diluted with 100 μL of methanol and vortexed for 5 min, and then centrifuged at 12,000 rpm for 10 min. The blood samples, aqueous humor samples, and ocular tissue homogenates were extracted by vortexing with 2.4 mL of n-hexane/ethyl ether (1:1, v/v) for 5 min, and centrifuged at 3,500 rpm for 5 min.33 Then 1.8 mL of the upper organic layers were transferred to the centrifuge tube and evaporated. The residues were dissolved in 150 μL of acetonitrile/water (1:1, v/v) by vortexing for 5 min; then 300 μL of n-hexane was added, followed by centrifugation at 3,500 rpm for 5 min. The supernatants were discarded and 300 μL of n-hexane was added again; the mixture was then vortexed for 5 min and centrifuged at 12,000 rpm for 10 min. Aliquots of the supernatants were analyzed by HPLC method.
Results and discussion
Synthesis and characterization of HA-g-PNIPAAm
The chemical compositions of NIPAAm and the synthesized PNIPAAm-COOH were confirmed by FT-IR measurements. In order to conjugate PNIPAAm with HA, the -COOH group provided by MPA was added to the structure of NIPAAm during its polymerization. As shown in Figure 2B, the absorption bands at the 1,714 cm−1 peak were assigned to the terminal carboxyl peak of PNIPAAm-COOH, and the typical amide I and II bands at 1,649 and 1,539 cm−1. The bending vibrations of isopropyl groups at 1,388 and 1,363 cm−1 were observed, respectively, in the spectrum of PNIPAAm-COOH. The telescopic vibration peaks of –CH2–, –CH3– were 2,970 cm−1, 2,938 cm−1, and 2,875 cm−1, respectively.
Figure 2.
FT-IR spectra (A) NIPAAm and (B) PNIPAAm-COOH recorded in KBr pellets.
Abbreviations: FT-IR, Fourier transform infrared spectroscopy; NIPAAm, N-isopropylacrylamide; PNIPAA m-COOH, carboxylic end-capped poly(N-isopropylacrylamide); KBr, potassium bromide.
Following the synthesis routes shown in Figure 1, HA-g-PNIPAAm was successfully obtained, as determined by the protein nuclear magnetic resonance (1H NMR) spectra, with evidence of proton peaks from the ADH residue (Figure 3B) and PNIPAAm-COOH residues (Figure 3C and D). Figure 3A shows the 1H NMR spectrum of HA; the peak at 2.0 ppm was assigned to the acetamido moiety of the N-acetyl-D-glucosamine residue of HA. The peaks between 2.2 ppm and 2.4 ppm refer to the integration of methylene protons of ADH. Seventy-six percent of D-glucuronic acid residues were modified by ADH.34 This was calculated from the ratio of the integrated value of the peak for the methyl proton of ADH residues at 1.62 ppm to that of the methyl proton of N-acetyl-D glucosamine residues at 2.00 ppm.
Figure 3.

1H NMR spectra of HA and AHA and HA-g-PNIPAAm in D2O. (A) was assigned to the acetamido moiety of the N-acetyl-D-glucosamine residue of HA; (B) referred to the integration of methylene protons of ADH; (C and D) were assigned to –ch3 and –Nh-groups of PNIPAAm-COOH.
Abbreviations: 1H NMR spectra; proton nuclear magnetic resonance; ADH, adipic dihydrazide; AHA, aminated hyaluronic acid; D2O, deuterium oxide; HA, hyaluronic acid; PNIPAAm, poly(N-isopropylacrylamide); PNIPAAm-COOH, carboxylic end-capped PNIPAAm.
A typical spectrum of HA-g-PNIPAAm presents both the resonance peaks from ADH and PNIPAAm, demonstrating the occurrence of the grafting reaction.20 As shown in the spectrum, the peaks at 1.1 ppm (Figure 3C) and 3.9 ppm (Figure 3D) are assigned to –CH3 and –NH-groups, respectively. The DS values of PNIPAAm controlled by the feed mole ratio of PNIPAAm-COOH are shown in Table 1. The results indicated that the DS increased from 23% to 59% with the increase in the feed ratio of PNIPAAm-COOH. The subscript numbers, such as 23, 48 and 59, represented the DS of PNIPAAm-COOH.
Table 1.
Characteristics of a series of HA-g-PNIPAAm microgels and CyA-HAPN microgels
| Samples | Feed ratiob | DSc | Size (nm) | Zeta potential (mv) | pH | Osmotic pressure (osmol · kg−1) |
|---|---|---|---|---|---|---|
| HA-g-PNIPAAm23a | 1:1 | 23 | 405.4 ± 4.7 | −26.08 ± 0.43 | 6.68 ± 0.22 | 0.268 ± 0.23 |
| CyA-HAPN23a | 387.8 ± 6.6 | −22.80 ± 0.54 | 6.44 ± 0.45 | 0.271 ± 0.32 | ||
| HA-g-PNIPAAm48a | 1:2 | 48 | 243.9 ± 3.4 | −24.52 ± 0.67 | 6.52 ± 0.19 | 0.301 ± 0.29 |
| CyA-HAPN48a | 222.6 ± 5.7 | −22.05 ± 0.21 | 6.13 ± 0.12 | 0.261 ± 0.12 | ||
| HA-g-PNIPAAm59a | 1:3 | 59 | 188.6 ± 5.2 | −23.99 ± 0.51 | 6.02 ± 0.16 | 0.289 ± 0.44 |
| CyA-HAPN59a | 165.8 ± 4.8 | −21.88 ± 0.06 | 6.17 ± 0.24 | 0.254 ± 0.39 |
Notes: Each value represents the mean ± SD (n = 3);
The number represents the degree of substitution of PNIPAAm-COOH;
molar feed ratio of PNIPAAm to sugar residues of HA polymer;
degree of substitution of PNIPAAm-COOH.
Abbreviations: CyA, cyclosporin A; CyA-HAPN, CyA-loaded HA-g-PNIPAAm microgels; DS, degree of substitution; HA, hyaluronic acid; HA-g-PNIPAAm, HA-g-PNIPAAm microgels; PNIPAAm, poly(N-isopropylacrylamide); SD, standard deviation.
Characterization of drug-free and CyA-HAPN microgels
Drug loading efficiency
Previous reports have described different structures of PNIPAAm-based core-shell particles with hydrophobic groups as core and hydrophilic groups as shell.36,37 In the PNIPAAm microgels system, there exists a hydrophilic/hydrophobic balance in the NIPAAm unit resulting from the amide group (hydrophilic) and isopropyl group (hydrophobic) regions of the PNIPAAm (Figure 4). As described previously, when the temperature was lower than LCST, the strong H-bonding between the hydrophilic groups and water outweighs the unfavorable free energy related to the exposure of hydrophobic groups to water, leading to a good polymer solubility in water; when the temperature was higher than LCST, the hydrogen bonds are overwhelmed by the hydrophobic interactions between the hydrophobic groups, and osmotic pressure played a major role in the drug release process.38
Figure 4.

Schematic representation of the HA-g-PNIPAAm microgels assembly and drug release process.
Notes: HA-g-PNIPAAm polymers were synthesized in the first step. Then HA-g-PNIPAAm microgels were prepared by a simple sonication method in aqueous condition. The loading of CyA into microgels was achieved by incubation method. When the temperature was lower than LCST, HA-g-PNIPAAm polymers had a good solubility in water; and when the temperature was higher than LCST, HA-g-PNIPAAm polymers exhibited temperature-sensitive properties, and osmotic pressure played a major role in the drug release process.
Abbreviations: CyA, cyclosporin A; HA, hyaluronic acid; LCST, lower solution critical temperature; PNIPAAm, poly(N-isopropylacrylamide); PNIPAAm-COOH, carboxylic end-capped PNIPAAm; T, temperature.
In the present study, CyA was chosen as a model drug to be incorporated into HA-g-PNIPAAm microgels. As shown in Table 2, the drug loading efficiency of HA-g-PNIPAAm59 increased (from 53.47 to 73.92) when the incubation time was increased (from 24 h to 72 h). In addition, the drug loading efficiency increased with the increase of DS, and the highest value was obtained from HA-g-PNIPAAm59 microgels. Such a high drug loading efficiency was considered the result of the hydrophobic interactions between CyA and the hydrophobic region (PNIPAAm) of the HA-g-PNIPAAm microgels.
Table 2.
Drug loading efficiency of various CyA-HAPN microgels
| Incubation time (h) | Drug loading efficiency
|
||
|---|---|---|---|
| HA-g-PNIPAAm23a | HA-g-PNIPAAm48a | HA-g-PNIPAAm59a | |
| 24 | 34.26 | 42.59 | 53.47 |
| 48 | 43.07 | 43.79 | 61.12 |
| 72 | 53.58 | 58.20 | 73.92 |
Note:
The number represents the degree of substitution of PNIPAAm-COOH.
Abbreviations: CyA-HAPN microgels, CyA-loaded HA-g-PNIPAAm microgels; h, hours HA, hyaluronic acid; PNIPAAm, poly(N-isopropylacrylamide); CyA, cyclosporin A.
Physico-chemical properties
The main physico-chemical characteristics of CyA-HAPN microgels are summarized in Table 1. The mean particle size of HA-g-PNIPAAm microgels was analyzed by DLS in an aqueous solution. It was found that the mean particle size of HA-g-PNIPAAm microgels decreased from 405 to 188 nm as the DS of PNIPAAm increased, suggesting a more compact hydrophobic inner core was formed due to the increasing of hydrophobic chains. In addition, the particle size of drug-loaded microgels was slightly smaller than those of blank microgels, suggesting that the addition of a hydrophobic drug could induce the microgels to become more compact by the hydrophobic interaction between CyA and the hydrophobic segments of the microgels. In addition, HA-g-PNIPAAm microgels exhibited a negative surface charge. The zeta potential of HA and AHA were −83.63 mv and −35.65 mv, respectively. By grafting PNIPAAm to HA, the zeta potential of HA-g-PNIPAAm microgels was increased owing to the introduction of carboxyl groups in PNIPAAm.35
The osmotic pressure of HA-g-PNIPAAm microgels was determined by the freezing-point method. As shown in Table 1, the osmotic pressure values of different samples were within the normal intraocular pressure range (0.205–0.513 osmol · kg-1). Moreover, the pH values of different formulations were within the range of ocular physiological pH (5.0–9.0).
Thermal behavior
Generally, the temperature at which the swelling degree of the sample decreases dramatically (the optical transmittance reduced to 50%) is regarded as the LCST.39 Figure 5 shows the temperature-dependent absorbance changes of different polymer solutions at 2 wt% and 5 wt% concentrations. The LCST of HA-g-PNIPAAm microgels was around 33°C which was closer to that of ocular surface temperature (34°C). During the heating process, a temperature change from 25°C to 37°C resulted in a dramatic increase of absorbance due to temperature-driven solution-to-gel transition. The approximate gel formation temperatures of PNIPAAm-COOH and HA-g-PNIPAAm59 microgels at 2 wt% were 32.3°C ± 0.2°C and 32.7 ± 0.3, respectively. At 5 wt% concentration, HA-g-PNIPAAm59 had a slightly higher sol-to-gel phase transition temperature (33.0°C ± 0.3°C) during the heating process in comparison to PNIPAAm-COOH (32.2°C ± 0.2°C). In the PNIPAAm microgels system, there exists a hydrophilic/hydrophobic balance in the NIPAAm unit resulting from the amide group (hydrophilic) and isopropyl group (hydrophobic) regions of the PNIPAAm.40 Similar to the PNIPAAm micro-gels, the HA-g-PNIPAAm microgels became swollen at temperatures below the LCST, but underwent a deswelling process when the temperature was increased. Thus, this increase in the LCST is attributed to the increase of hydrophilic HA.
Figure 5.
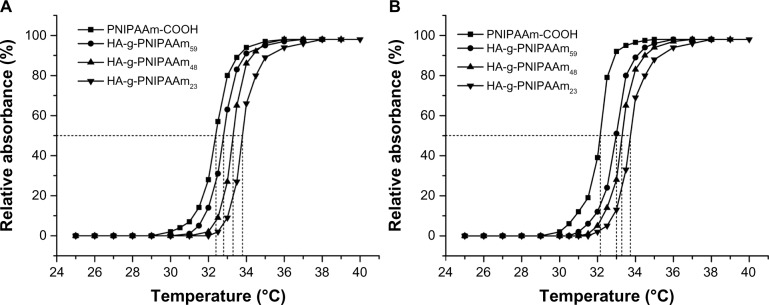
Phase transition behavior of HA-g-PNIPAAm microgels at (A) 2 wt% and (B) 5 wt%.
Abbreviation: HA-g-PNIPAAm, hyaluronic acid-g-poly(N-isopropylacrylamide).
Viscosity analysis
The effect of shear rate on viscosity of three kinds of HA-g-PNIPAAm microgels with different DS at 25°C was investigated. As shown in Figure 6A, the microgels’ viscosity was markedly decreased when the shear rate was increased, and then it flattened. Moreover, the shear thinning phenomenon became more obvious with the increase in HA-g-PNIPAAm particle size. At 25°C, the microgels were in a swollen state due to the hydrogen bonding between water and microgel particles, which maintained a certain moisture content and allowed the polymer chains to freely extend outward. At high shear rate values, the polymer chains were no longer in a free-stretched state, suggesting a shear thinning behavior of the microgels fluid. These results suggested that HA-g-PNIPAAm microgels showed a non-Newtonian behavior, which was indicated by a higher viscosity at lower shear rates and lower viscosity at higher shear rates. Therefore, the bioavailability of the ocular formulation could be improved even at relatively high viscosity, since the eyelids can still blink freely with a good biological tolerance and without discomfort.
Figure 6.

The influence of (A) shear rate and (B) temperature on the microgels’ viscosity. The subscript numbers represent the degree of substitution of PNIPAAm-COOH.
Abbreviation: HA-g-PNIPAAm, hyaluronic acid-g-poly(N-isopropylacrylamide).
The sol-to-gel transition behaviors were further illustrated by monitoring the microgels’ viscosity as a function of temperature (Figure 6B). From 5°C to 31°C, the microgels’ viscosity was decreasing with the increase of temperature owing to the role of hydrogen bonding between the microgels and water, while they disappeared between the microgels particles. An abrupt increase in viscosity was observed at around 32°C, meaning that the phase transition process occurred to form the microgels network. This increase in viscosity would be beneficial to extend the drug residence time in the eye, leading to an improved drug bioavailability.
Morphology
Based on the above results, HA-g-PNIPAAm59 exhibited the highest DS and drug loading efficiency. Therefore, the HA-g-PNIPAAm59 was selected for further analysis. TEM and AFM images showed that the drug-loaded HA-g-PNIPAAm59 microgels were almost spherical in shape, and the size was about 150 nm (Figure 7). The TEM image was further enlarged (Figure 7B) to clearly identify the shape and the morphological structure of the drug-loaded HA-g-PNIPAAm59 microgels. The appearance indicates an obvious structure with PNIPAAm (the black region) as the core and HA (the pale region) as the shell. The representative AFM image of HA-g-PNIPAAm59 particles (Figure 7C and D) showed that the microgels were also almost spherical in shape, and the size was about 145 nm. However, the microgels size obtained by TEM and AFM were found to be smaller than those obtained by DLS, which might be attributed to the sample preparation methods, due to the shrinkage of the hydrophilic shell of microgels during air-drying and the contribution of hydrodynamic diameter in DLS measurements.
Figure 7.
(A) TEM image of CyA-HAPN59 microgels. (B) Enlargement of (A). (C) 2D and (D) 3D AFM images of CyA-HAPN59 microgels.
Abbreviations: AFM, atomic force microscopy; CyA-HAPN59 microgels, CyA-loaded HA-g-PNIPAAm59 microgels; TEM, transmission electron microscopy; HA, hyaluronic acid; PNIPAAm, poly(N-isopropylacrylamide); CyA, cyclosporin A.
In vitro release of CyA
In vitro CyA release was assayed from HA-g-PNIPAAm microgels with different DS using PBS as the release medium. The cumulative amounts of CyA released from the CyA-HAPN59 microgels as a function of time are shown in Figure 8. The drug release of CyA-HAPN59 microgels formulation was relatively fast in the initial 12 h, and followed by sustained release profiles. It was also found that there were significant differences between the release behaviors of the drug-loaded microgels at the two investigated temperatures. The cumulative drug release amounts at 37°C within 48 h were significantly higher than that at 25°C. It was anticipated that CyA molecules trapped in the hydrophobic core could gradually diffuse into the hydrophilic shell and then diffuse out of the shell to be released. When heated above LCST, the complex was dissociated, leading to a drug release of CyA due to the coil-to-globule transition of the HA-g-PNIPAAm copolymer. As described previously, when the temperature was lower than LCST, drug release was mainly based on the microgels’ swelling spread; when the temperature was higher than LCST, osmotic pressure played a major role in the drug release process.41
Figure 8.

Accumulative release profiles of CyA-HAPN microgels at predetermined temperatures.
Note: Each value represents the mean ± SD (n = 3).
Abbreviations: CyA-HAPN microgels, CyA-loaded HA-g-PNIPAAm microgels; SD, standard deviation; HA, hyaluronic acid; PNIPAAm, poly(N-isopropylacrylamide); CyA, cyclosporin A; h, hours.
Eye irritation test
The results of the eye irritation test of the CyA-HAPN microgels formulation and the commercial CyA eye drops studied in New Zealand White rabbits are shown in Table 3. CyA-HAPN microgels did not irritate rabbits’ eyes, as seen in the total score of an eye irritation assessment, equaling to 0. As shown in Figure 9, there was a significant difference in the irritant effect between the two formulations. Compared to the commercial CyA eye drops group, the eyes of the rabbits in the group with CyA-HAPN microgels looked normal. The CyA-HAPN microgels were well tolerated by the rabbits, and no macroscopic signs of irritation, redness, or other toxic effects were observed. Therefore, CyA-HAPN microgels can be used as a safe formulation for ophthalmic use.
Table 3.
Score obtained from eye irritation assessment of CyA-HAPN microgels and commercial CyA eye drops in New Zealand White rabbits
| Lesion | Score for each lesion | Score obtained from assessment
|
|
|---|---|---|---|
| CyA-HAPN microgels | Commercial CyA eye drops | ||
| A-conjuctival edema (chemosis) | |||
| No swelling | 0 | 0 | 1 |
| Any swelling | 1 | ||
| Prominent swelling along with partial lid eversion | 2 | ||
| Swelling with half-closed lids | 3 | ||
| Swelling with totally closed lids | 4 | ||
| B-redness in conjunctiva | |||
| Absent | 0 | 0 | 0 |
| Abnormal conjunctival injections | 1 | ||
| More diffuse and deeper hyperemia, separate vessels cannot be seen easily | 2 | ||
| Diffuse and dense hyperemia | 3 | ||
| C-secretion | |||
| Absent | 0 | 0 | 1 |
| Any abnormal secretion | 1 | ||
| Secretion leading to wet eye lashes closer to lids | 2 | ||
| Secretion leading to wet lids and whole periorbital area | 3 | ||
| D-corneal opacity | |||
| Absent | 0 | 0 | 0 |
| Scattered or diffused areas-detail of the iris discernible | 1 | ||
| Easy discernible, transparent areas, detail of the iris slightly darkened | 2 | ||
| Opalescent areas, no details of the iris discernible, size of the pupil barely discernible | 3 | ||
| Opaque cornea, iris not discernible | 4 | ||
| E-iris involvement | |||
| Absent | 0 | 0 | 0 |
| Pronounced deep folds, congestion, deep swelling, circumcorneal injection, the iris still reacts to light | 1 | ||
| No response, hemorrhage, marked destruction | 2 | ||
| Total score | 0 | 2 | |
Abbreviations: CyA, cyclosporin A; CyA-HAPN microgels, CyA-loaded HA-g-PNIPAAm microgels; HA, hyaluronic acid; PNIPAAm, poly(N-isopropylacrylamide).
Figure 9.

Eye irritation assessment profiles of CyA-HAPN microgels (A) and CyA eye drops (B) in rabbits.
Abbreviations: CyA-HAPN microgels, CyA-loaded HA-g-PNIPAAm microgels; HA, hyaluronic acid; PNIPAAm, poly(N-isopropylacrylamide); CyA, cyclosporin A.
Distribution of CyA in ocular tissues
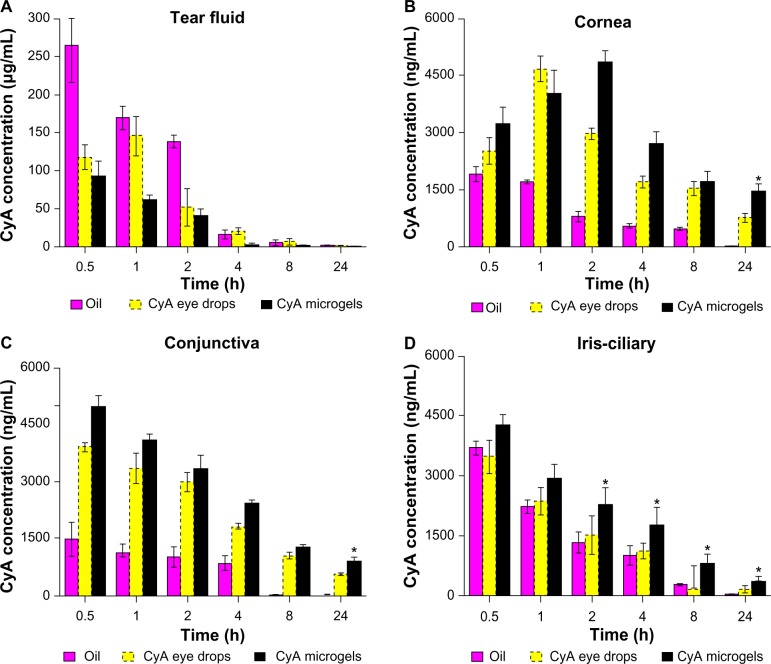
The topical application of CyA to the eye has been studied in an attempt to reduce the risk of systemic toxicity while minimizing its local therapeutic potential. The biodistribution of CyA in rabbit eye tissues is shown in Figure 10. One of the major problems encountered with the topical delivery of ophthalmic drugs is the rapid and extensive precorneal loss caused by the drainage and high tear fluid turnover. As shown in Figure 10A, the CyA concentration in the tear fluid of the microgels group was lower than that of other formulations at all the time points.
Figure 10.
Biodistribution of CyA in rabbit eyes after topical administration of CyA solution in castor oil, CyA eye drops, and CyA-HAPN microgels (500 μg CyA). (A) Tear fluid; (B) cornea; (C) conjunctiva; (D) iris/ciliary.
Notes: Data are presented as mean ± standard deviation (n = 3). *Differs from oil solution and commercial eye drops, P < 0.05.
Abbreviations: CyA, cyclosporin A; CyA-HAPN microgels, CyA-loaded HA-g-PNIPAAm microgels; HA, hyaluronic acid; PNIPAAm, poly(N-isopropylacrylamide); h, hours.
The corneal concentrations of CyA following the topical administration of the three formulations are shown in Figure 10B. CyA solution in castor oil resulted in a very low corneal uptake for the 24 h period. A burst release of CyA was observed within the first hour with the commercial CyA eye drops, followed by a rapid elimination. However, the corneal concentration of CyA, which was obtained with the CyA-HAPN59 microgels formulation at 24 h after topical administration, was 1455.8 ng/g of tissue. Moreover, CyA-HAPN microgels showed the highest concentration levels and the slowest elimination from the corneal tissues at several time points. This might be attributed to the bioadhesive HA polymer leading to a significant increase in the corneal uptake of CyA.
Figure 10C shows the conjunctival concentrations of CyA obtained with different formulations. The mean conjunctival CyA concentrations during 24 h ranged between 0–1467.49 ng/g of tissue for Group 1, 3882.05–557.28 ng/g of tissue for Group 2, and 4941.13–887.28 ng/g of tissue for Group 3. After 24 h of topical administration, the conjunctival concentration of CyA in the CyA-HAPN microgels group was significantly higher than that of castor oil solution and commercial CyA eye drops (P < 0.05). In addition, the mean conjunctival CyA concentration of castor oil solution was the lowest of all the formulations at all time points. These results suggested that HA-g-PNIPAAm microgels could deliver CyA to the conjunctival tissue and might have a slightly increased tendency to penetrate into the conjunctiva.
CyA-HAPN microgels were broadly distributed in eye tissues, including the iris–ciliary body. As shown in Figure 10D, the iris–ciliary body concentration levels of CyA were significantly increased compared to the castor oil solution and commercial CyA eye drops at several intervals (P < 0.05). Moreover, the blood concentrations of CyA after topical application were below 10 ng/mL (data not shown), indicating limited absorption and diffusion of CyA into the bloodstream, which could effectively avoid the systemic side effects.
This study clearly proved that the HA-g-PNIPAAm microgels were able to accumulate in rabbit eye tissues and release CyA, allowing the drug to work effectively. In a previous study, topical application of CyA at a concentration of 50–300 ng/g of ocular tissue was shown to suppress inflammatory and immune responses.42 In the present study, HA-g-PNIPAAm microgels formulations achieved high CyA levels in rabbit corneas (1455.8 ng/g of tissue) during the first 24 h, which were significantly higher than those from commercial CyA eye drops (P < 0.05). Furthermore, the prolonged precorneal retention time of HA-g-PNIPAAm microgels would provide an intimate contact between the drug and ocular surface tissues, resulting in a high penetration of the drug into eye tissues. This remarkable enhancement of CyA concentrations in the periocular tissues after topical administration could be explained by the use of the temperature-sensitive HA-g-PNIPAAm polymer, which is believed to increase the ocular bioavailability and extend the retention time by the corneal and conjunctival surfaces.43
Conclusion
Currently, there are only a few studies on ocular formulations with promising results. Within this study, the thermosensitive microgels carrier modified by poly(N-isopropylacrylamide) was evaluated as a promising carrier for ocular drug delivery in vitro and in vivo. The thermosensitive HA-g-PNIPAAm microgels were shown to be safe and nonirritant while displaying high drug loading ability. In addition, the in vivo distribution evaluation indicated that HA-g-PNIPAAm microgels achieved significantly higher CyA concentration levels in rabbit corneas (1455.8 ng/g of tissue) than both the castor oil formulation and commercial CyA eye drops. Therefore, the thermosensitive HA-g-PNIPAAm microgels could be promising carriers for the topical administration of lipophilic drugs.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No 81173006), the Project Program of State Key Laboratory of Natural Medicines, China Pharmaceutical University (No JKGQ201107), Jiangsu Natural Science Foundation of China (SBK201322347), Qing Lan Project, and Jiangsu Overseas Research and Training Program for University Prominent Young and Middle-aged Teachers and Presidents.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Calvo P, Vila-Jato JL, Alonso MJ. Evaluation of cationic polymer-coated nanocapsules as ocular drug carriers. Int J Pharm. 1997;153(1):41–50. [Google Scholar]
- 2.Ludwig A. The use of mucoadhesive polymers in ocular drug delivery. Adv Drug Deliv Rev. 2005;57(11):1595–1639. doi: 10.1016/j.addr.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Nanjawade BK, Manvi FV, Manjappa AS. In situ-forming hydrogels for sustained ophthalmic drug delivery. J Control Release. 2007;122(2):119–134. doi: 10.1016/j.jconrel.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev. 2006;58(11):1131–1135. doi: 10.1016/j.addr.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 5.Keister JC, Cooper ER, Missel PJ, Lang JC, Hager DF. Limits on optimizing ocular drug delivery. J Pharm Sci. 1991;80(1):50–53. doi: 10.1002/jps.2600800113. [DOI] [PubMed] [Google Scholar]
- 6.Asasutjarit R, Thanasanchokpibull S, Fuongfuchat A, Veeranondha S. Optimization and evaluation of thermoresponsive diclofenac sodium ophthalmic in situ gels. Int J Pharm. 2011;411(1–2):128–135. doi: 10.1016/j.ijpharm.2011.03.054. [DOI] [PubMed] [Google Scholar]
- 7.Mahmoud AA, El-Feky GS, Kamel R, Awad GE. Chitosan/sulfobutylether-β-cyclodextrin nanoparticles as a potential approach for ocular drug delivery. Int J Pharm. 2011;413(1–2):229–236. doi: 10.1016/j.ijpharm.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 8.Wu C, Qi H, Chen W, Chen W, Huang C, et al. Preparation and evaluation of a Carbopol/HPMC-based in situ gelling ophthalmic system for puerarin. Yakugaku Zasshi. 2007;127(1):183–191. doi: 10.1248/yakushi.127.183. [DOI] [PubMed] [Google Scholar]
- 9.Dumortier G, Grossiord JL, Agnely F, Chaumeil JC. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm Res. 2006;23(12):2709–2728. doi: 10.1007/s11095-006-9104-4. [DOI] [PubMed] [Google Scholar]
- 10.Yenice I, Mocan MC, Palaska E, et al. Hyaluronic acid coated poly-epsilon-caprolactone nanospheres deliver high concentrations of cyclosporine A into the cornea. Exp Eye Res. 2008;87(3):162–167. doi: 10.1016/j.exer.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Moya-Ortega MD, Alves TF, Alvarez-Lorenzo C, et al. Dexamethasone eye drops containing γ-cyclodextrin-based nanogels. Int J Pharm. 2013;441(1–2):507–515. doi: 10.1016/j.ijpharm.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, Jiao Y, Wang Y, Zhou C, Zhang Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv Drug Deliv Rev. 2008;60(15):1650–1662. doi: 10.1016/j.addr.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Oh JK, Lee DI, Park JM. Biopolymer-based microgels/nanogels for drug delivery applications. Prog Polym Sci. 2009;34(12):1261–1282. [Google Scholar]
- 14.Oh EJ, Park K, Kim KS, et al. Target specific and long-acting delivery of protein, peptide, and nucleotide therapeutics using hyaluronic acid derivatives. J Control Release. 2010;141(1):2–12. doi: 10.1016/j.jconrel.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Fonn D. Targeting contact lens induced dryness and discomfort: what properties will make lenses more comfortable. Optom Vis Sci. 2007;84(4):279–285. doi: 10.1097/OPX.0b013e31804636af. [DOI] [PubMed] [Google Scholar]
- 16.Stuart JC, Linn JG. Dilute sodium hyaluronate (Healon) in the treatment of ocular surface disorders. Ann Ophthalmol. 1985;17(3):190–192. [PubMed] [Google Scholar]
- 17.Kettel MJ, Dierkes F, Schaefer K, Moeller M, Pich A. Aqueous nanogels modified with cyclodextrin. Polymer. 2011;52(9):1917–1924. [Google Scholar]
- 18.Yang HW, Chen JK, Cheng CC, Kuo SW. Association of poly(N-isopropylacrylamide) containing nucleobase multiple hydrogen bonding of adenine for DNA recognition. Appl Surf Sci. 2013;271:60–69. [Google Scholar]
- 19.Liu W, Huang Y, Liu H, Hu Y. Composite structure of temperature sensitive chitosan microgel and anomalous behavior in alcohol solutions. J Colloid Interface Sci. 2007;313(1):117–121. doi: 10.1016/j.jcis.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 20.Tan H, Ramirez CM, Miljkovic N, Li H, Rubin JP, Marra KG. Thermosensitive injectable hyaluronic acid hydrogel for adipose tissue engineering. Biomaterials. 2009;30(36):6844–6853. doi: 10.1016/j.biomaterials.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bloch P, Tamm C, Bollinger P, Petcher TJ, Weber HP. Pseurotin, a new metabolite of Pseudeurotium ovalis Stolk having an unusual hetero-spirocyclic system. Helv Chim Acta. 1976;59(1):133–137. doi: 10.1002/hlca.19760590114. [DOI] [PubMed] [Google Scholar]
- 22.Kahan BD. Cyclosporin A: a new advance in transplantation. Tex Heart Inst J. 1982;9(3):253–266. [PMC free article] [PubMed] [Google Scholar]
- 23.Hingorani M, Calder VL, Buckley RJ, Lightman S. The immunomodulatory effect of topical cyclosporin A in atopic keratoconjunctivitis. Invest Ophthalmol Vis Sci. 1999;40(2):392–399. [PubMed] [Google Scholar]
- 24.Tang-Liu DD, Acheampong A. Ocular pharmacokinetics and safety of ciclosporin, a novel topical treatment for dry eye. Clin Pharmacokinet. 2005;44(3):247–261. doi: 10.2165/00003088-200544030-00003. [DOI] [PubMed] [Google Scholar]
- 25.Francis MF, Piredda M, Winnik FM. Solubilization of poorly water soluble drugs in micelles of hydrophobically modified hydroxypropylcellulose copolymers. J Control Release. 2003;93(1):59–68. doi: 10.1016/j.jconrel.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Gökçe EH, Sandri G, Eğrilmez S, Bonferoni MC, Güneri T, Caramella C. Cyclosporine a-loaded solid lipid nanoparticles: ocular tolerance and in vivo drug release in rabbit eyes. Curr Eye Res. 2009;34(11):996–1003. doi: 10.3109/02713680903261405. [DOI] [PubMed] [Google Scholar]
- 27.Lallemand F, Felt-Baeyens O, Besseghir K, Behar-Cohen F, Gurny R. Cyclosporine A delivery to the eye: a pharmaceutical challenge. Eur J Pharm Biopharm. 2003;56(3):307–318. doi: 10.1016/s0939-6411(03)00138-3. [DOI] [PubMed] [Google Scholar]
- 28.Cao Y, Zhang C, Shen W, Cheng Z, Yu LL, Ping Q. Poly(N-isopropylacrylamide)-chitosan as thermosensitive in situ gel-forming system for ocular drug delivery. J Control Release. 2007;120(3):186–194. doi: 10.1016/j.jconrel.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Hsiue GH, Hsu SH, Yang CC, Lee SH, Yang IK. Preparation of controlled release ophthalmic drops, for glaucoma therapy using thermosensitive poly-N-isopropylacrylamide. Biomaterials. 2002;23(2):457–462. doi: 10.1016/s0142-9612(01)00127-2. [DOI] [PubMed] [Google Scholar]
- 30.Gao C, Möhwald H, Shen J. Thermosensitive poly(allylamine)-g-poly(N-isopropylacrylamide): synthesis, phase separation and particle formation. Polymer. 2005;46(12):4088–4097. [Google Scholar]
- 31.Chai S, Zhang J, Yang T, Yuan J, Cheng S. Thermoresponsive microgel decorated with silica nanoparticles in shell: Biomimetic synthesis and drug release application. Colloids Surf A Physicochem Eng Asp. 2010;356(1):32–39. [Google Scholar]
- 32.Bozdağ S, Gümüş K, Gümüş O, Unlü N. Formulation and in vitro evaluation of cysteamine hydrochloride viscous solutions for the treatment of corneal cystinosis. Eur J Pharm Biopharm. 2008;70(1):260–269. doi: 10.1016/j.ejpb.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 33.Zhou L, Tan D, Theng J, Lim L, Liu YP, Lam KW. Optimized analytical method for cyclosporin A by high-performance liquid chromatography-electrospray ionization mass spectrometry. J Chromatogr B Biomed Sci Appl. 2001;754(1):201–207. doi: 10.1016/s0378-4347(00)00608-3. [DOI] [PubMed] [Google Scholar]
- 34.Yeo Y, Highley CB, Bellas E, et al. In situ cross-linkable hyaluronic acid hydrogels prevent post-operative abdominal adhesions in a rabbit model. Biomaterials. 2006;27(27):4698–4705. doi: 10.1016/j.biomaterials.2006.04.043. [DOI] [PubMed] [Google Scholar]
- 35.Chen JP, Cheng TH. Preparation and evaluation of thermo-reversible copolymer hydrogels containing chitosan and hyaluronic acid as injectable cell carriers. Polymer. 2009;50(1):107–116. [Google Scholar]
- 36.Lin CL, Chiu WY, Lee CF. Thermal/pH-sensitive core-shell copolymer latex and its potential for targeting drug carrier application. Polymer. 2005;46(23):10092–10101. [Google Scholar]
- 37.Yi F, Zheng S. Effect of hydrophobic polystyrene microphases on temperature-responsive behavior of poly(N-isopropylacrylamide) hydrogels. Polymer. 2009;50(2):670–678. [Google Scholar]
- 38.Zhao H, Chen H, Li Z, Su W, Zhang Q. The synthesis of temperature-sensitive PMMA-coating PNIPAM particles via a rapid microwave-assisted polymerization. Eur Polym J. 2006;42(9):2192–2198. [Google Scholar]
- 39.Hao Y, Peng J, Li J, Zhai M, Wei G. An ionic liquid as reaction media for radiation-induced grafting of thermosensitive poly (N-isopropylacrylamide) onto microcrystalline cellulose. Carbohydr Polym. 2009;77(4):779–784. [Google Scholar]
- 40.Khan A. Preparation and characterization of N-isopropylacrylamide/acrylic acid copolymer core-shell microgel particles. J Colloid Interface Sci. 2007;313(2):697–704. doi: 10.1016/j.jcis.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 41.Wang M, Fang Y, Hu D. Preparation and properties of chitosan-poly(N-isopropylacrylamide) full-IPN hydrogels. React Funct Polym. 2001;48:215–221. [Google Scholar]
- 42.Kaswan RL. Intraocular penetration of topically applied cyclosporine. Transplant Proc. 1988;20(2 Suppl 2):650–655. [PubMed] [Google Scholar]
- 43.Kobayashi J, Okano T. Thermoresponsive thin hydrogel-grafted surfaces for biomedical applications. React Funct Polym. 2013;73(7):939–944. [Google Scholar]