Abstract
Background
Image-derived input function (IDIF) from carotid arteries is an elegant alternative to full arterial blood sampling for brain PET studies. However, a recent study using blood-free IDIFs found that this method is particularly vulnerable to patient motion. The present study used both simulated and clinical [11C](R)-rolipram data to assess the robustness of a blood-based IDIF method (a method that is ultimately normalized with blood samples) with regard to motion artifacts.
Methods
The impact of motion on the accuracy of IDIF was first assessed with an analytical simulation of a high-resolution research tomograph using a numerical phantom of the human brain, equipped with internal carotids. Different degrees of translational (from 1 to 20mm) and rotational (from 1 to 15°) motions were tested. The impact of motion was then tested on the high-resolution research tomograph dynamic scans of three healthy volunteers, reconstructed with and without an online motion correction system. IDIFs and Logan-distribution volume (VT) values derived from simulated and clinical scans with motion were compared with those obtained from the scans with motion correction.
Results
In the phantom scans, the difference in the area under the curve (AUC) for the carotid time–activity curves was up to 19% for rotations and up to 66% for translations compared with the motionless simulation. However, for the final IDIFs, which were fitted to blood samples, the AUC difference was 11% for rotations and 8% for translations. Logan-VT errors were always less than 10%, except for the maximum translation of 20 mm, in which the error was 18%. Errors in the clinical scans without motion correction appeared to be minor, with differences in AUC and Logan-VT always less than 10% compared with scans with motion correction.
Conclusion
When a blood-based IDIF method is used for neurological PET studies, the motion of the patient affects IDIF estimation and kinetic modeling only minimally.
Keywords: image-derived input function, neuroreceptor tracers, PET
Introduction
Image-derived input function (IDIF) from carotid arteries is an elegant alternative to full arterial blood sampling in brain PET studies. However, IDIF is technically very challenging [1]. One of the most important challenges is partial volume effect correction, because the carotid diameter (5mm) is close to the spatial resolution of modern PET cameras. Perhaps most difficult is the correction of the whole-blood time–activity curve for the metabolite fraction. Indeed, most tracers have variable amounts of radiometabolites. Even if partial volume effect is reliably corrected, the resulting vascular time–activity curve would be that of the whole-blood radioactivity, and PETcameras cannot distinguish the parent compound from its radiometabolites.
A recent study underscored another potential limitation of this technique: its vulnerability to patient motion [2]. Indeed, carotid regions of interest (ROIs) are usually defined over the early frames – the only ones in which the carotids are easily visible – and then copied to subsequent frames. However, if the patient moves during the scan, the carotid ROIs would fall outside the anatomical carotid, causing IDIF estimation errors. Using a noninvasive IDIF method, Mourik et al. [2] showed that even a small amount of patient motion (about 5mm translation or 6° rotation) was associated with large underestimation and overestimation in the final distribution volume (VT) values.
It is, however, our contention that vulnerability to patient motion also depends on the IDIF technique used. IDIF methods can be broadly classified as blood based (i.e. when IDIFs are ultimately scaled using one or more blood samples) [3–5] or blood free (which are completely noninvasive and rely only on the measurement of pixel activity in the images) [6–8]. Although blood-free methods are obviously more attractive from a clinical point of view, they are also more vulnerable to image artifacts and to variations in reconstruction parameters, filtering, and scatter correction [1].
The aim of this study was to use both simulated and clinical brain [11C](R)-rolipram data to assess the impact of motion on kinetic modeling when the input function is obtained with a blood-based IDIF technique.
Methods
Image-derived input function method
The IDIF method used in the present study was originally proposed for [18F]-FDG by Chen et al. [3]. Carotid and surrounding regions were manually drawn on the summed PET frames obtained during the first 2min and then copied to all following frames.
The image-derived whole-blood carotid signal was represented as a linear combination of the radioactivity from blood and surrounding tissue using the formula
| (1) |
where Ccarotid is the concentration of radioactivity seen in the carotid region in the PET image; Cwb is the concentration of radioactivity measured in whole blood sampled from the radial artery; RC is the recovery coefficient; Csurround is the radioactivity in the surrounding tissues; and SP is the spill-in coefficient. RC and SP were estimated with linear least square fitting of Ccarotid, Cwb, and Csurround in Eq. (1), with Cwb measured at 6, 20, 60, and 90 min. A variation of Eq. (1) has been described, which postulates that SP=1−RC [9]. However, this approach could not be implemented in the present study because RC+SP was not generally equal to 1.
In the present study, the IDIF method included the additional step of estimating the [11C](R)-rolipram parent fraction [10]. The concentration of [11C](R)-rolipram was measured in the same four blood samples (6, 20, 60, and 90 min) used to estimate RC and SP. The concentrations of [11C](R)-rolipram for the entirety of the scan were estimated by fitting a monoexponential function to the [11C](R)-rolipram/whole-blood ratios at the four time points and then multiplying the resulting curve by the partial-volume and spill-over corrected whole-blood IDIF. Therefore, the VT errors found in the present study incorporate not only the errors due to the whole-blood IDIF calculation but also those due to estimation of the parent fraction. Errors due to estimation of the parent fraction are, however, minimal. For the three subjects in the present study, the Logan-VT differences using the estimated (with a monoexponential function) and measured (with high-performance liquid chromatography analysis at each time point) parent fractions were 2.1, 2.4 and 3.2%, respectively. Obviously, these differences are not affected by patient movement and therefore do not affect the comparison between the scans with and without motion.
Simulated positron emission tomography scans
As in a previous study [11], we used a MRI-based numerical phantom of the human brain [12] into which a set of internal carotids (average diameter=5mm) were added. The phantom is composed of 19 anatomic labels, including carotids, frontal, temporal, parietal, and occipital gray matter, white matter, basal ganglia, bones, and soft tissues. For each label, a time–activity curve was defined, obtained by averaging the time–activity curves of the corresponding regions of 12 [11C](R)-rolipram test studies (see below). The time–activity curve used as an input for the simulation of the carotid labels was considered as the reference arterial whole-blood curve. The fraction of unchanged parent was obtained from the average parent plasma concentration of the clinical scans. The simulated time–activity curves for each region were used as input for an analytic fast simulator [13], which we upgraded to simulate high-resolution research tomography (HRRT) studies with a realistic detector resolution model for this machine [14]. This simulator takes into account true, scattered, and random coincidence detector efficiencies. Noisy sinograms were generated using the same framing used in the clinical studies. By calibrating the true, random, and scatter events and noise-equivalent count rates of the phantoms with those of the clinical data, we obtained a realistic noise level. The sinograms were then reconstructed with the OP-OSEM algorithm, with 16 subsets and 20 iterations. The voxel size of the reconstructed images was 1.22×1.22×1.22mm. An image-based point spread function model was used during the reconstruction in both forward and back projection, with an isotropic and stationary 3D kernel given by the formula
| (2) |
where σ1 = 0.9 mm, σ2 = 2.5 mm, and ρ = 0.07 [15].
Simulated motion in the phantom studies
As in the study by Mourik et al. [2], two types of motion were added to the simulated scan. First, different degrees of rotation in the z-axis (napping) of 1, 3, 5, 7, 10, 12, and 15° were applied. Axial inferior movements of 1, 3, 5, 7, 10, 12, 15, and 20 mm were then simulated. These are the most commonly seen movements when the head is constrained by a head holder, and their amplitude corresponds to those observed in clinical practice [2,16,17]. Motion begins from the 10th minute and continues until the end of the scan. This is a realistic assumption for two reasons: (a) movement most often occurs late in the course of the scan [2]; (b) carotid ROIs are defined in the early summed frames and then copied to all the subsequent frames. Therefore, as regards IDIF, movement is only relative to the early frames in which the ROIs are defined. Accordingly, the present study simulated a motion of gradually increasing amplitude that reached a maximum in the last few frames, as follows:
frames obtained between 10 and 30 min: 25% of maximum motion;
frames obtained between 30 and 50 min: 50% of maximum motion;
frames obtained between 50 and 70 min: 75% of maximum motion; and
frames obtained between 70 min and the end of the scan: maximum motion.
Clinical positron emission tomography scans
The clinical PET scans were chosen from a series of 24 [11C](R)-rolipram scans (12 test–retest scans) conducted in healthy volunteers as part of a previous protocol [10]. All scans were acquired using the HRRT (Siemens Medical Solutions, Knoxville, Tennessee, USA) for 120 min in 33 frames, except in the case of one patient who underwent a 90-min scan. Because VT values are stably estimated within ~90 min of image acquisition [10], the present study analyzed only the dynamic scans up to the frame that begins at 90 min – that is, six frames of 30 s each, followed by 3×60, 2×120, and 17×300 s. All PET images were corrected for attenuation and scatter [18]. During the acquisition, blood samples (1ml each) were drawn from the radial artery at 15-s intervals until 150 s, followed by drawing of 3ml samples at 3, 4, 6, 8, 10, 15, 20, 30, 40, and 50 min and 4.5 ml samples at 60, 75, and 90 min. Whole-blood activity, the fraction of unchanged radioligand in plasma, and the plasma/whole-blood ratio were calculated [19,20].
Motion in the clinical studies
Head motion during each scanning session was monitored using a Polaris Vicra Optical Tracking System (NDI, Waterloo, Ontario, Canada) [16], which is based on infrared tracking of four retroreflective spheres attached to the patient’s head. The recorded movement is then used to correct for motion during image reconstruction (see below).
Using the Polaris motion information, we selected the scans with the highest movement and reconstructed them without motion correction. In the Polaris information, movements are recorded into translations in the x, y, and z axes expressed in millimeters, and rotations are recorded in the x, y, and z axes expressed in degrees. We calculated the average of the absolute values for each of these six parameters over the duration of the scan and selected all the scans having the highest mean value for each parameter.
Magnetic resonance imaging
To identify brain regions, MRIs were obtained for all patients using a 1.5T GE Signa scanner (GE Healthcare, Piscataway, New Jersey, USA). Three sets of axial images were acquired parallel to the anterior commissure–posterior commissure line with a spoiled gradient recalled sequence of TR/TE/flip angle=12.4 ms/5.3 ms/20°, voxel size=0.94×0.94×1.2mm, and matrix=256×256. These three MRI sets were realigned and averaged and then coregistered to the PET images (see below).
Image analysis
PET data were reconstructed on a 256×256 matrix with a pixel size of 1.22×1.22×1.23mm. Motion correction was performed, event by event, by applying the Polaris information during reconstruction using a MOtion-compensated List-mode OSEM Algorithm with Resolution-recovery [18]. The average MR image from the three acquisitions for each patient (see above) was coregistered to the summed PET image using SPM5 (Wellcome, Department of Cognitive Neurology, London, UK). Both MR and all PET images were spatially normalized to a standard anatomic space (Montreal Neurological Institute space) based on transformation parameters from the MR images. Preset volumes of interest were positioned on the spatially normalized MR images to overlie the thalamus (12.6 cm3), caudate (5.6 cm3), putamen (6.5 cm3), cerebellum (51.2 cm3), and frontal (27.2 cm3), parietal (26.6 cm3), lateral temporal (25.0 cm3), occipital (31.2cm3), anterior cingulate (7.5 cm3), and medial temporal (14.3 cm3) cortices. Image analysis was performed using PMOD (PMOD Technologies Ltd, Zurich, Switzerland).
Comparison of image-derived input functions
For both simulated and clinical data, scans with and without motion were compared in terms of: (a) the estimated input functions, and (b) the mean Logan-VT, obtained by averaging the VT of each volume of interest. For both the carotid time–activity curves and the IDIFs, the area under the curve (AUC) obtained from the scans with motion were compared with the values obtained from the scans without motion.
Notably, when a patient moves, the movement affects not only the estimation of IDIF but also the accuracy of the brain time–activity curves. However, to isolate the impact of motion on any calculation of IDIF, we applied both sets of IDIF data to the same brain time–activity curves obtained from the scans without motion. It should also be noted that, in case of patient movement, VT errors are mainly due to the mislocation of IDIF and not due to errors in the brain time–activity curves [2].
Results
Image-derived input function calculations
Phantoms
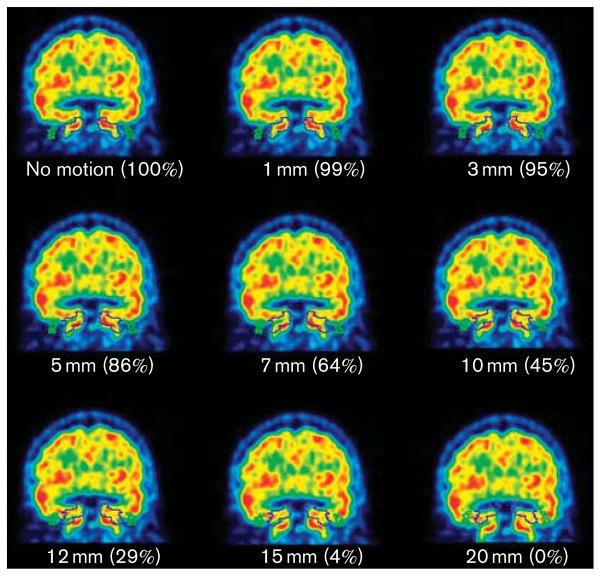
Figures 1 and 2 demonstrate that even with large movements the carotid ROIs always overlapped, at least partially, with the anatomical carotids of the phantoms. The only exceptions occurred in the frames with the largest movements (i.e. 15° rotation and 20mm translation), in which the carotids were just outside the ROIs.
Fig. 1.
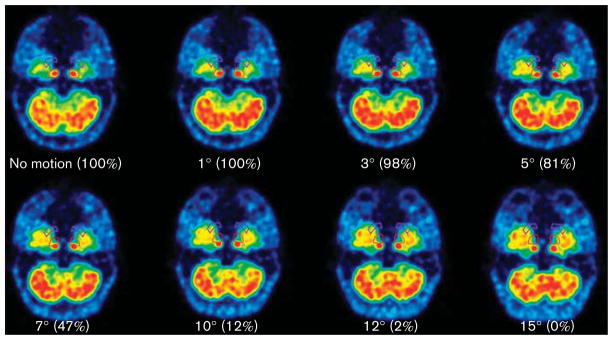
Transaxial views of the phantom at different rotation degrees. These are the summed early frames of the dynamic scan (the only ones in which carotids are clearly visible), rotated with the same amplitude as the last frames of each simulation. The round ROIs are for the carotids, and the comma-shaped ROIs are for the background activity. The numbers in parentheses represent the percentage of the phantom carotids inside the ROIs for each rotation degree. The carotids were completely outside the ROIs only for the maximum rotation. ROI, region of interest.
Fig. 2.
Coronal views of the phantom for different translations. The numbers in parentheses represent the percentage of the phantom carotids inside the ROIs for each translation. The only translation in which the ROIs did not touch the carotids was 20 mm. ROI, region of interest.
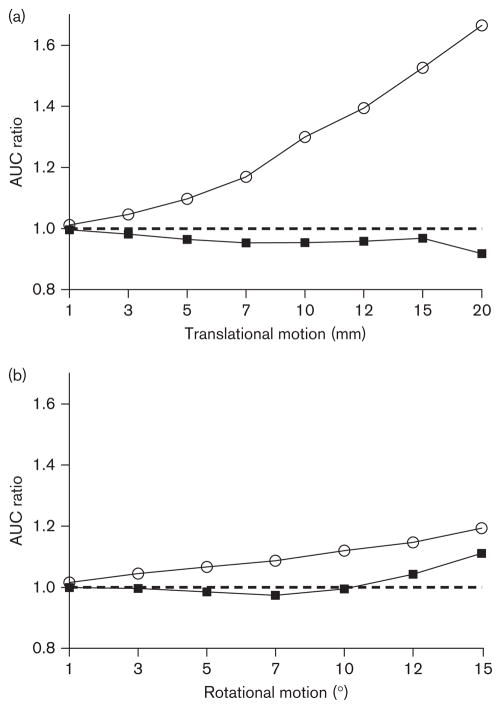
When the carotid ROIs were not well located in the scans with motion, this translated into AUC errors as compared with the scans without motion. As the magnitude of translation and rotation increased, the AUC ratio for the carotid time–activity curves increased progressively (Fig. 3). This effect was more visible for translational motions than for rotations. The AUC difference after 10min (when the phantom began moving) to the end of the scan was up to 19% for rotations and up to 67% for translations.
Fig. 3.
AUC ratios of the input functions between the scans without motion correction and the scan with motion correction for different movements of translation (a) and rotation (b). Open symbols (○) represent the carotid time–activity curves, and closed symbols (■) represent the IDIFs. As the movement increased, AUC values of the carotid time–activity curves progressively diverged from those obtained with motion correction. The carotid curves (○) were then fitted with blood samples. Therefore, the AUC values of the IDIFs (■) were much more similar to those without motion correction, with a ratio closer to 1. AUC, area under the curve; IDIF, image-derived input function.
It is important to note that these carotid time–activity curves are ultimately fitted with blood samples to obtain a partial-volume-corrected IDIF, according to the method of Chen and colleagues. Therefore, the AUCs of the final IDIFs are more similar to the AUCs without motion, with a ratio much closer to 1 (Fig. 3). For the IDIFs, the AUC difference after 10 min was only up to 11% for 15° rotation and up to 8% for 20mm translation.
Humans
Polaris information was available for 20 of the 24 scans. Among these 20 scans, three were selected and reconstructed with and without motion correction. These three scans had the highest average motions for each of the Polaris parameters (i.e. three translations and three rotations in the x, y, and z axes). Motion in the clinical scans was relatively mild compared with motion simulated in the phantom. The highest average translation (in the z-axis) and the highest average rotation (in the x-axis) were both seen in the same patient (Fig. 4) and were 11.3mm and 11.3°, respectively. For all other scans, all motion parameters were below 10mm and 10°.
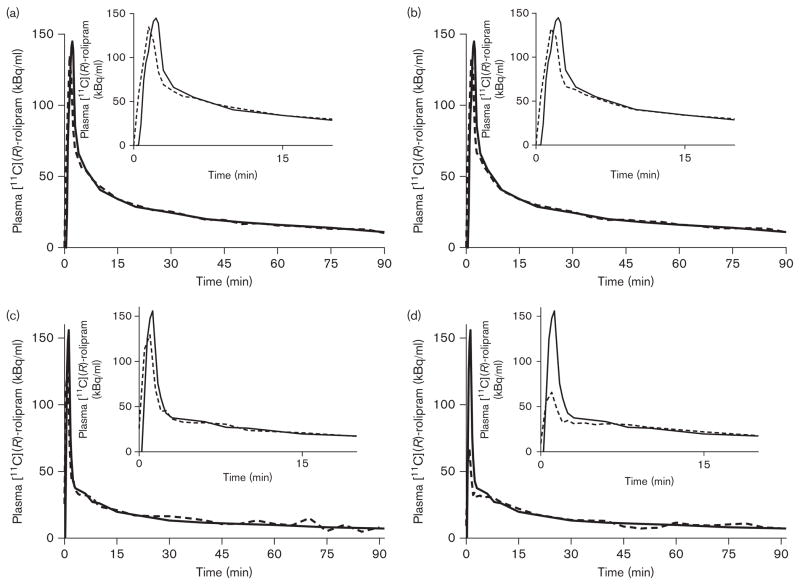
Fig. 4.
Two of the three clinical scans in this series. In the first scan, the curves with (a) and without (b) motion correction were virtually identical. This scan also had the highest recorded movement. In this patient the peak was perfectly estimated, which is not typically the norm. For another patient, there was only a minor underestimation of the peak in the motion-corrected scan, and the VT error for this scan was 0.1% (c). However, in the scan without motion correction (d), the peak was poorly underestimated and appears as about one-third of the reference peak. Nevertheless, the VT error was only 8.2%. Typically, errors in peak estimation are not very important when using graphical analyses, such as Patlak or Logan, because they mainly rely on the area under the curve of the input function. In contrast, compartmental modeling is less reliable when used with IDIF, because the individual rate constants are more sensitive to the shape of the input function and would be poorly estimated [1].
Few differences were visible in the IDIFs between the scans with motion and those without motion. The most important changes were seen in the peaks; the tails, which were fitted to four blood samples, were almost identical for each patient (Fig. 4). As compared with the scan reconstructed with motion correction, the AUC differences in both the carotid time–activity curves and the IDIFs were very similar and always less than 10%.
Effect of motion on kinetic analysis
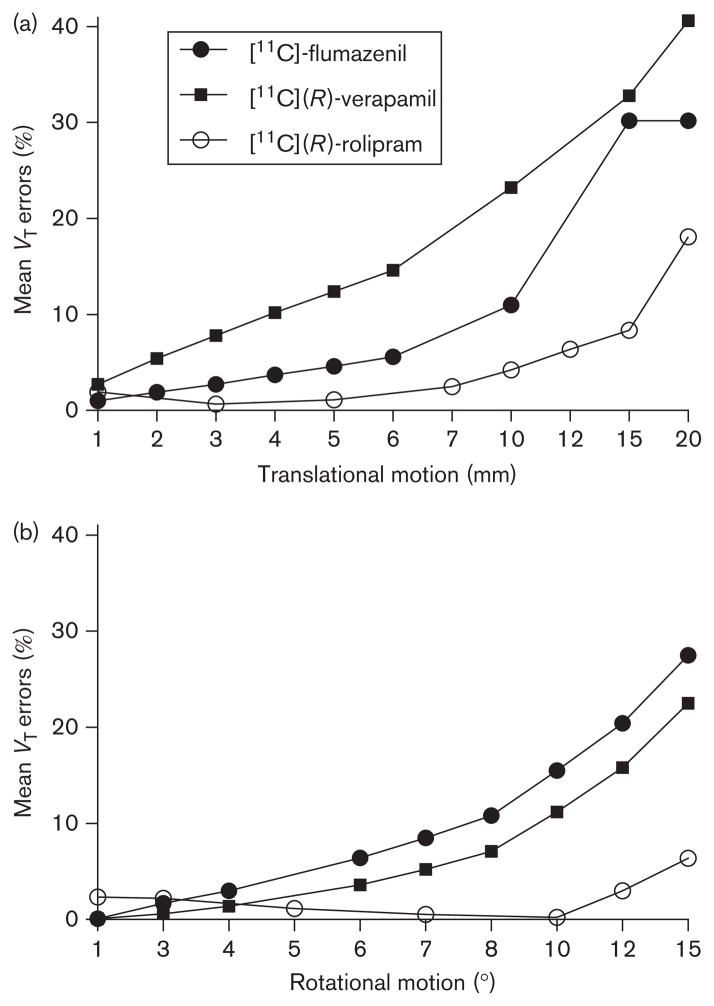
The estimate of Logan-VT values proved to be remarkably accurate even for large movements. In the phantom, the Logan-VT error in the scan without motion was 2%, and the VT error with the maximum rotation of 15° was only 6%. For the maximum translation of 20 mm, the error was 18%. For the second highest translation (15mm) the error was 8% (Fig. 5). No significant differences in VT estimates were observed between clinical scans with and without motion correction. The rate of errors was always less than 10% in both sets of scans. For the three patients evaluated, as compared with the VT values obtained with arterial sampling, VT errors were 0.1, 0.5, and 5.2% with motion correction and became 8.2, 0.8, and 4.6% without motion correction.
Fig. 5.
VT errors as a function of translational (a) and rotational (b) motions. The [11C](R)-rolipram (○) data are from the present study. The values for [11C](R)-verapamil (■) and [11C]-flumazenil (●) are from [2]. VT errors increase as motion increases. The amount of error observed with the blood-based method ([11C](R)-rolipram) is smaller than the number of errors observed with the blood-free method ([11C](R)-verapamil and [11C]-flumazenil).
Discussion
Using a blood-based IDIF method, this study showed that Logan-VT values could be accurately estimated even in the case of significant patient movement during image acquisition. These results contrasted with those of Mourik et al. [2], who found that even small patient motion led to important underestimation or overestimation of VT values.
Notably, this discrepancy in the results is due to the different IDIF methods used. Mourik et al. [7] defined the carotid ROIs by selecting in early frames the four hottest pixels per plane, usually located at the center of the vessel. This technique is well validated [7,21,22] and indeed among the most reliable [11,23]. Moreover, this method is particularly attractive because the hot pixels are considered free of partial-volume effects; thus, calibration with blood samples is not necessary for [11C]-flumazenil or [11C](R)-verapamil scans [7,22]. However, the association between very small ROIs and lack of scaling with blood samples makes this method more sensitive to motion artifacts. Even small movements suffice to dislodge the tiny ROIs from the central part of the carotid and, as a consequence, the small four-pixel ROIs would record a different value. This incorrect value cannot be further corrected because the carotid time–activity curve is not calibrated with blood samples. Therefore, the errors would directly affect VT measurement.
In contrast, the method used here – first proposed by Chen et al. [3] – has two important advantages, despite the fact that it is invasive. First, it uses large carotid ROIs, which can be larger than the carotid itself (Fig. 1). This means that the carotid is rarely completely outside the ROI, even in the case of relatively large patient movements. Nevertheless, when movement occurs, the value inside the ROI may change considerably. However, the carotid time–activity curves are not taken directly as an input function but are instead fitted with blood samples (which is the reason why large ROIs can be used), thus bringing the new value in line with the radioactivity level measured in the blood.
To understand the impact of ROI size, it is beneficial to compare the time–activity curves obtained using the method of Mourik et al. [2] with those obtained in the present study using the method of Chen and colleagues. Mourik and colleagues showed that ROI positioning became problematic when there was a translation of at least 4mm or a rotation of at least 3°. Moreover, for movements of 10mm or 6° the carotid ROIs were largely positioned outside the carotid and within the brain. This translated into large differences, frame-wise, between the IDIF without motion and the IDIF with motion. For [11C]-flumazenil, maximum differences were up to 205 and 239% for rotation and translation motion, respectively; for [11C](R)-verapamil differences were up to 91 and 154% for rotation and translation, respectively. When these IDIFs were used for kinetic modeling, important VT errors were found even for scans with small motion. The maximum VT errors were between 20 and 30% for maximum rotations and between 30 and 40% for maximum translations (depending on the tracer) [2].
In comparison, the large ROIs used in the present study were completely outside the phantom carotids only in the frames with the highest motion (i.e. 15° and 20mm). Therefore, the AUC differences between the carotid time–activity curves with and without motion were small. The AUC difference after 10 min (when the phantom began moving) was up to 19% for rotations (maximum frame-wise difference 42%) and up to 67% for translations (maximum frame-wise difference 100%). Notably, however, in the method of Chen and colleagues the carotid time–activity curve is ultimately fitted to blood samples; therefore, numerical variations due to motion are largely corrected. Indeed, the IDIF AUC difference was only 11% for 15° rotation (maximal difference 45%) and 8% for 20mm translation (maximal difference 60%). As a result, VT estimates were very robust. The maximum rotation of 15° engendered a VT error of only 6%, and the maximum translation of 20mm yielded an error of 18%. For the second highest translation (15mm) the error reduced to 8%.
In view of the results obtained with the phantoms, the accurate results found in the clinical scans were not unexpected. Indeed, the amount of movement in the clinical scans was less important than that in simulated scans, with a maximum average translation and motion of about 11mm and 11°, respectively. As a result, only small differences were observed in both IDIF values and VT estimates between the scans with and without motion correction. Moreover, in real clinical scans, movements are seldom unidirectional. Instead, the carotid often moves around a central spot. Because the method of Chen and colleagues uses large ROIs that encompass the whole vessel, it is likely that the carotids never completely left the ROIs. In fact, compared with motionless scans, the AUC differences in both the carotid curves and the IDIFs were minor.
In addition to testing the effect of motion in a real-world situation, the clinical scans were also key to evaluating the impact of two artifacts that were not simulated in the phantoms: (a) intraframe motion and (b) attenuation correction. With regard to the former, clinical scans are affected by intraframe – as opposed to solely interframe – motion. Intraframe motion blurs the carotid by spreading its activity over a larger area. With the method of Chen and colleagues, however, the large ROIs would still comprise most of the activity, and fitting with blood samples would correct whatever variation of the ROI value was due to intraframe movement. Notably, intraframe movement cannot be properly corrected using frame-by-frame techniques but only with a real-time event-based system, which is available in only a minority of PET centers.
As regards the latter artifact, when movement occurs, carotid time–activity values would also be misestimated because of erroneous attenuation correction. However, differences due to attenuation correction are likely to be minor, as the density of the blood inside the carotid and that of the surrounding soft tissues is practically the same. Furthermore, fitted with blood samples, minor differences in carotid time–activity values would not translate into significant differences in the final IDIF.
In the clinical scans acquired for the present study, patients’ heads were only loosely constrained by a Velcro strip, because motion correction relied principally on the online system. Therefore, it is likely that the amount of motion seen in these scans when reconstructed without motion correction is higher than what would typically be seen when heads are fixed with a thermoplastic mask. However, these were all healthy individuals and it is possible that the amplitude of movement would be greater in patients with neurological conditions, such as Parkinson’s disease or Alzheimer’s disease. It is important to note that the magnitude of the movements usually seen in such patients is comparable to that of our simulation [2,16,17].
It should also be noted that for the present study we used high-resolution HRRT images. Due to the higher spatial resolution (~2.5mm), movements have a greater impact on IDIF than when using a standard resolution machine (~6mm), because carotid arteries are sampled by smaller voxels and also tend to be delineated by smaller ROIs.
Finally, we would like to make it clear that it is not our intention to demonstrate that motion correction is unnecessary. Motion correction should always be performed, as it improves kinetic modeling results when using blood-free IDIF methods as well [2]. However, most of the time an online motion correction system is not available and patients are constrained only with a thermoplastic mask or with Velcro strips. Therefore, a certain amount of movement is unavoidable and cannot be completely corrected by standard postacquisition realignment. In that case, blood-based IDIF methods may be recommended.
In our opinion, IDIF techniques have many drawbacks. They work with only a limited number of tracers and they rarely allow avoiding an arterial line [1]. However, vulnerability to motion is a drawback that can be minimized using a blood-based IDIF method.
Conclusion
When a blood-based IDIF method is used for neurological PET studies, the motion of the patient affects IDIF estimation and kinetic modeling only minimally.
Acknowledgments
The authors gratefully acknowledge the support of the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH). The authors thank Thada Shantalaxmi for help with the HRRT scan reconstructions. Ioline Henter provided invaluable editorial assistance.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Zanotti-Fregonara P, Chen K, Liow JS, Fujita M, Innis RB. Image-derived input function for brain PET studies: many challenges and few opportunities. J Cereb Blood Flow Metab. 2011;31:1986–1998. doi: 10.1038/jcbfm.2011.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mourik JE, Lubberink M, Lammertsma AA, Boellaard R. Image derived input functions: effects of motion on tracer kinetic analyses. Mol Imaging Biol. 2011;13:25–31. doi: 10.1007/s11307-010-0301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen K, Bandy D, Reiman E, Huang SC, Lawson M, Feng D, et al. Noninvasive quantification of the cerebral metabolic rate for glucose using positron emission tomography, 18F-fluoro-2-deoxyglucose, the Patlak method, and an image-derived input function. J Cereb Blood Flow Metab. 1998;18:716–723. doi: 10.1097/00004647-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Guo H, Renaut RA, Chen K. An input function estimation method for FDG-PET human brain studies. Nucl Med Biol. 2007;34:483–492. doi: 10.1016/j.nucmedbio.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanabria-Bohorquez SM, Maes A, Dupont P, Bormans G, de Groot T, Coimbra A, et al. Image-derived input function for [11C]flumazenil kinetic analysis in human brain. Mol Imaging Biol. 2003;5:72–78. doi: 10.1016/s1536-1632(03)00046-5. [DOI] [PubMed] [Google Scholar]
- 6.Croteau E, Lavallee E, Labbe SM, Hubert L, Pifferi F, Rousseau JA, et al. Image-derived input function in dynamic human PET/CT: methodology and validation with (11)C-acetate and (18)F-fluorothioheptadecanoic acid in muscle and (18)F-fluorodeoxyglucose in brain. Eur J Nucl Med Mol Imaging. 2010;37:1539–1550. doi: 10.1007/s00259-010-1443-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mourik JE, Lubberink M, Klumpers UM, Comans EF, Lammertsma AA, Boellaard R. Partial volume corrected image derived input functions for dynamic PET brain studies: methodology and validation for [11C]flumazenil. Neuroimage. 2008;39:1041–1050. doi: 10.1016/j.neuroimage.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 8.Su KH, Wu LC, Liu RS, Wang SJ, Chen JC. Quantification method in [18F]fluorodeoxyglucose brain positron emission tomography using independent component analysis. Nucl Med Commun. 2005;26:995–1004. doi: 10.1097/01.mnm.0000184999.81203.5c. [DOI] [PubMed] [Google Scholar]
- 9.Cook GJ, Lodge MA, Marsden PK, Dynes A, Fogelman I. Non-invasive assessment of skeletal kinetics using fluorine-18 fluoride positron emission tomography: evaluation of image and population-derived arterial input functions. Eur J Nucl Med. 1999;26:1424–1429. doi: 10.1007/s002590050474. [DOI] [PubMed] [Google Scholar]
- 10.Zanotti-Fregonara P, Zoghbi SS, Liow JS, Luong E, Boellaard R, Gladding RL, et al. Kinetic analysis in human brain of [11C](R)-rolipram, a positron emission tomographic radioligand to image phosphodiesterase 4: a retest study and use of an image-derived input function. Neuroimage. 2011;54:1903–1909. doi: 10.1016/j.neuroimage.2010.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zanotti-Fregonara P, Liow JS, Fujita M, Dusch E, Zoghbi SS, Luong E, et al. Image-derived input function for human brain using high resolution PET imaging with [C](R)-rolipram and [C]PBR28. Plos One. 2011;6:e17056. doi: 10.1371/journal.pone.0017056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zubal IG, Harrell CR, Smith EO, Rattner Z, Gindi G, Hoffer PB. Computerized three-dimensional segmented human anatomy. Med Phys. 1994;21:299–302. doi: 10.1118/1.597290. [DOI] [PubMed] [Google Scholar]
- 13.Comtat C, Kinahan P, Defrise M, Michel C, Townsend D. Simulating whole-body PET scanning with rapid analytical methods. IEEE Nuclear Science Symposium Conference Record; 24–30 October 1999; Seattle, Washington, USA. 1999. [Google Scholar]
- 14.Dusch E, Comtat C, Trebossen R. Simulation-based evaluation of OSEM reconstruction bias on low activity PET data for the HRRT scanner. IEEE Nuclear Science Symposium Conference Record (NSS/MIC); 24 October to 1 November; Orlando, Florida, USA. 2009. pp. 2770–2773. [Google Scholar]
- 15.Comtat C, Sureau F, Sibomana M, Hong I, Sjoholm N, Trebossen R. Image based resolution modeling for the HRRT OSEM reconstructions software. IEEE Nuclear Science Symposium Conference Record; 19–25 October 2008; Dresden, Germany. 2008. pp. 4120–4123. [Google Scholar]
- 16.Bloomfield PM, Spinks TJ, Reed J, Schnorr L, Westrip AM, Livieratos L, et al. The design and implementation of a motion correction scheme for neurological PET. Phys Med Biol. 2003;48:959–978. doi: 10.1088/0031-9155/48/8/301. [DOI] [PubMed] [Google Scholar]
- 17.Rahmim A, Dinelle K, Lidstone S, Blinder S, Cheng J, Topping G, et al. Impact of accurate motion-corrected statistical reconstruction on dynamic PET kinetic parameter estimation. IEEE Nuclear Science Sympoium Conference Record; 26 October to 3 November 2007; Honolulu, Hawaii. 2007. pp. 2697–2704. [Google Scholar]
- 18.Carson RE, Barker WC, Liow J-S, Yao R, Thada S, Zhao Y, et al. List-mode reconstruction for the HRRT. J Nucl Med. 2004;45:105P. [Google Scholar]
- 19.Fujita M, Zoghbi SS, Crescenzo MS, Hong J, Musachio JL, Lu J-Q, et al. Quantification of brain phosphodiesterase 4 in rat with (R)-[11C]rolipram-PET. Neuroimage. 2005;26:1201–1210. doi: 10.1016/j.neuroimage.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Zoghbi SS, Shetty HU, Ichise M, Fujita M, Imaizumi M, Liow JS, et al. PET imaging of the dopamine transporter with 18F-FECNT: a polar radiometabolite confounds brain radioligand measurements. J Nucl Med. 2006;47:520–527. [PubMed] [Google Scholar]
- 21.Mourik JE, van Velden FH, Lubberink M, Kloet RW, van Berckel BN, Lammertsma AA, Boellaard R. Image derived input functions for dynamic high resolution research tomograph PET brain studies. Neuroimage. 2008;43:676–686. doi: 10.1016/j.neuroimage.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 22.Mourik JE, Lubberink M, Schuitemaker A, Tolboom N, van Berckel BN, Lammertsma AA, Boellaard R. Image-derived input functions for PET brain studies. Eur J Nucl Med Mol Imaging. 2009;36:463–471. doi: 10.1007/s00259-008-0986-8. [DOI] [PubMed] [Google Scholar]
- 23.Zanotti-Fregonara P, Fadaili el M, Maroy R, Comtat C, Souloumiac A, Jan S, et al. Comparison of eight methods for the estimation of the image-derived input function in dynamic [(18)F]-FDG PET human brain studies. J Cereb Blood Flow Metab. 2009;29:1825–1835. doi: 10.1038/jcbfm.2009.93. [DOI] [PubMed] [Google Scholar]