Abstract
Fatty acid composition of adipose tissue (AT) is an established long-term biomarker for fatty acid (FA) intake and status, but AT samples are not easily available. Nonesterified FA composition in plasma (pNEFA) may be a good indicator of AT FA composition, because pNEFA are mainly generated by AT lipolysis. We investigated the correlation of 42 pNEFA and subcutaneous as well as visceral AT FA in 27 non-diabetic women with a median BMI of 36 kg/m2 (Q0.25: 25 kg/m2; Q0.75: 49 kg/m2). Close correlations of pNEFA and AT FA were found for odd-chain FA (15∶0 r = 0.838 and 0.862 for subcutaneous and visceral AT, respectively) and omega-3 FA (22∶6 r = 0.719/0.535), while no significant or low correlations were found for other FA including 18∶1 (r = 0.384/0.325) and 20∶4 (r = 0.386/0.266). Close correlations of pNEFA and AT FA were found for essential fatty acids, like 18∶2 (r = 0.541/0.610) and 20∶5 (r = 0.561/0.543). The lower correlation for some pNEFA species with AT FA indicates that the variation of most pNEFA is significantly affected by other FA sources and flux of FA to tissue, in addition to release from AT. A relevant influence of BMI on the level of correlation was shown for saturated FA. NEFA analysis in fasted plasma can serve as a virtual AT biopsy for some FA, and as a biomarker for intake of dairy products and sea fish.
Introduction
Fatty acid (FA) composition of adipose tissue (AT) is a well-accepted biomarker for the assessment of long-term dietary FA intake, considered to be superior to dietary records and food frequency questionnaires [1]. Percentage contributions of FA in AT, representing intake of dairy products, fish or fish oil are highly correlated to dietary intake [2], [3]. While essential polyunsaturated FA (PUFA) show a close relationship between dietary intake and AT content, saturated FA (SFA) and monounsaturated FA (MUFA) are less closely correlated [4], presumably because these FA are derived from both diet and endogenous synthesis [5]. Nevertheless, SFA and MUFA in AT are of importance as biomarkers for various disease risks [3]. Alterations of AT fatty acid composition appear to play a crucial role in the development of insulin resistance and diabetes [6], [7], [8].
Although AT is a biomarker for the intake of FA and reflects FA metabolism, routine determination of AT composition is not practical due to the invasive nature of sample collection via biopsies, particularly in larger clinical trials or in vulnerable populations such as children. During fasting, AT lipolysis releases nonesterified fatty acids into plasma (pNEFA). Thus, pNEFA could provide a valuable surrogate marker for AT FA composition. Some studies indicate a close correlation between pNEFA and AT FA content for some FA [1]. To our knowledge, only Yli-Jama et al. investigated the relationship of AT and pNEFA for a large number of 27 FA in a sizable group of patients with myocardial infarction and of controls [9]. They reported widely differing coefficients of correlation between AT and pNEFA for individual FA.
So far, most studies of the relationship between pNEFA and AT focused on specific metabolic steps, e.g. mobilization [10] or (re-)uptake of pNEFA by adipocytes [11]. Mobilization studies were mostly performed in-vitro [12], in animals with induced lipolysis [13] or using venous-arterial differences of human AT, which indicated a preferential mobilization of PUFA [14], [15]. These approaches do not reflect the relation of AT and pNEFA FA percentages, because the pNEFA pool is affected by a complex interaction of AT lipolysis [10], reincorporation of NEFA into AT triacylglycerols (TAG) [16], uptake of pNEFA by peripheral tissues, oxidation rates of individual FA [17], intracellular metabolism [18] and contribution of pNEFA derived from plasma TAG or phospholipid hydrolysis [19].
Since there is limited information on relationship between pNEFA and AT FA composition, the objective of this study is to explore the relationship of fatty acid composition of pNEFA, visceral AT and subcutaneous AT in subjects with differing BMI. We used a sensitive and precise LC-MS/MS method [20] enabling the quantification of more than 40 FA, including very long-chain fatty acids (VLCFA), saturated and unsaturated odd-chain fatty acids and C24 intermediates of the endogenous n-3 docosahexaenoic acid and n-6 docosapentaenoic acid synthesis.
Materials and Methods
Ethics Statement
The study protocol was approved by the Ethical Committee of the University of Leipzig Medical Center. Written informed consent was obtained from all subjects.
Subjects
Participants were recruited at the University of Leipzig (Department for Internal Medicine, Division for Endocrinology and Nephrology). Fatty acid composition was investigated in 27 donors of paired visceral omental (vAT) and subcutaneous abdominal adipose tissue (sAT) samples, who underwent abdominal surgery for weight reduction (sleeve gastrectomy or Roux en Y gastric bypass), cholecystectomy, or explorative laparotomy. All subjects had a stable weight, defined as the absence of fluctuations of >2% of body weight for at least 3 months before surgery. Intake of any medication affecting glucose and lipid metabolism were defined as exclusion criteria. Adipose tissue was taken during surgery and immediately frozen in liquid nitrogen. Plasma samples were taken after prolonged fasting of more than 12 h on the day of surgery before anaesthetization. Blood samples were collected into refrigerated EDTA containing tubes and centrifuged; subsequently the plasma was aliquoted and stored at −80°C.
All subjects were Caucasian, female and non-diabetic with a mean age of 55±14 years (M±SD). Diabetes was excluded by fasted glucose and HbA1c analysis. The BMI of the study participants was not normally distributed and ranged from normal weight to extremely obese with a median BMI of 36 kg/m2 (Q0.25: 25 kg/m2; Q0.75: 49 kg/m2).The group consisted of 8 normal-weighted (BMI<25 kg/m2, age: 60±15 years), 4 over-weighted (BMI: 25 to 30 kg/m2, age: 64±9 years), 7 obese (BMI: 30 to 40 kg/m2, age: 56±15 years) and 8 morbidly obese (BMI>40 kg/m2, age: 44±9 years) patients.
Methods
All used chemicals were obtained in highest purity available (suppliers: Merck, Darmstadt, Germany; Promochem, Wesel, Germany; Fluka Sigma-Aldrich Chemistry GmbH, Steinheim, Germany).
Plasma samples
Sample preparation for pNEFA analysis was performed as previously reported [20]. Briefly, 20 µl of plasma were mixed with 200 µl isopropanol (containing 2 mg/100 ml uniformly 13C-labelled palmitic acid) in a 96-deepwell plate. After centrifugation the supernatant was transferred into a 96-well plate for LC-MS/MS analysis.
Adipose tissue samples
Homogenisation of adipose tissue samples (about 50 mg) was performed in 2 ml Biozym Cryovials (Biozym Scientific GmbH, Oldendorf, Germany) containing glass pellets and 1000 µl chloroform∶methanol (CHCl3∶MeOH 2∶1, +5 g/l butylated hydroxytoluene) for 2×30 seconds at 7134× g with a MagNA Lyser Instrument (Roche Diagnostics, Mannheim, Germany).
After cell lysis the vials were centrifuged for 10 min at 2330× g (room temperature). 10 µl of the supernatant were used for hydrolysis of total lipids according to Pettinella et al. [21]. The aliquot was dissolved in 850 µl CHCl3∶MeOH (1∶8) and after the addition of 150 µl KOH (40%) hydrolysis was performed in a nitrogen atmosphere at 60°C for 30 min. After cooling to room temperature, 700 µl phosphate buffer (pH = 7.4, 50 mM) and 300 µl HCl (2.5 mM) were added. Extraction was performed with 2×2000 µl hexane∶diethylether (1∶1, v/v). The upper layer was taken off, dried under nitrogen flow and FA redissolved in 500 µl isopropanol for LC/MS-MS.
In agreement with previous observations [1], [22], separation of dissolved AT lipids by TLC and subsequent quantification by gas chromatography revealed that TAG contributed more than 97% to total lipids. Therefore, extracts were directly hydrolysed without further purification.
Liquid Chromatography and ESI-MS/MS
LC-MS/MS analysis was performed as previously reported [20]. Briefly, an UPLC diphenyl column (Pursuit UPS Diphenyl, 1.9 µm, 100 mm×3.0 mm; Varian, Darmstadt, Germany) was used for chromatographic separation at 40°C with an Agilent 1200 SL series HPLC system (Waldbronn, Germany). The injection volume was set to 10 µL for plasma samples and to 2 µl for AT samples with an eluent flow rate of 700 µL/min. Equilibration was performed for 2.5 min with 45% of eluent A (water containing 5 mM ammonium acetate, 2.1 mM acetic acid) and 55% of eluent B (acetonitrile with 20% isopropanol), eluent B was linearly increased to 95% at a duration of 4 min and was held constant for 3 min.
A hybrid triple quadrupole mass spectrometer (4000 QTRAP, AB Sciex, Darmstadt, Germany) operating in negative ESI mode was coupled to the HPLC system [20]. Collision energy was optimized for each fatty acid individually to obtain signals well above the quantification limit, but within the linear response range.
With the analytical method applied, fatty acids are separated according to chain length and number of double bonds, but not according to position of double bonds.
Statistical analysis
Data analysis was performed with Microsoft Excel 2010 (Microsoft Inc., Redmond, WA) and “R: A language and environment for statistical computing” [23]. FA levels in pNEFA, sAT and vAT are presented as molar percentages of total FA analysed unless explicitly stated otherwise. Most fatty acid percentages were not normally distributed according to histograms, QQ plots and Anderson-Darling tests, results are given as median and interquartile ranges. Spearman's rho statistics were used to estimate rank-based correlation coefficients (r). Since the p-value does not reflect the strength of the relationship but strongly depends on sample number, p-values for correlation analyses are only mentioned in the corresponding figure and not substance for the interpretation of results. Wilcoxon signed rank tests were performed with a limit for statistical significance of <0.001068 due to multiple testing. The significance limit was calculated according to the Sidak correction [24]:
| (1) |
With a global significance level αglobal of 0.05 for n = 48 (42 FA+6 sum parameters).
To evaluate influence of BMI on the correlation of AT and pNEFA, two quantile regression models were implemented to describe variance of AT FA percentages with pNEFA only or with BMI and pNEFA using the R package “quantreg” [25]. The two models were compared by calculated R-squared (r2) and ANOVA.
To predict the FA percentages of 15∶0, 17∶0 and 22∶6 which were strongly correlated between compartments and were normally distributed in the 3 compartments, the “glm” function with multiple linear regression (MLR) and leave-one-out cross-validation (CV) of the R package “boot” was utilized [26]. Model 1 was established using pNEFA and BMI for AT FA prediction, while model 2 used pNEFA only.
The model comparisons were performed with ANOVA. Absolute prediction error and adjusted r2 were estimated to compare different models. Absolute prediction error for every FA was calculated as the mean absolute differences of analysed and predicted values after CV validation.
Results
42 fatty acids were analysed in sAT, vAT and plasma samples with good precision (CV<20%).
Fatty acid composition
Both adipose tissues and pNEFA contained high concentrations of MUFA, dominated by 18∶1, followed by SFA and PUFA (Table1).
Table 1. Fatty acid composition of plasma nonesterified fatty acids (pNEFA), subcutaneous adipose tissue (sAT) and visceral adipose tissue (vAT).
| FA | pNEFA | sAT | vAT |
| 12∶0 * | 0.312% [0.263%,0.450%] | 1.076% [0.812%,1.402%] | 1.258% [1.027%,1.642%] |
| 12∶1 * | 0.065% [0.051%,0.083%] | 0.207% [0.137%,0.251%] | 0.236% [0.206%,0.339%] |
| 14∶0 | 1.934% [1.710%,2.198%] | 5.442% [4.894%,6.646%] | 6.020% [5.134%,6.447%] |
| 14∶1 | 0.226% [0.181%,0.334%] | 0.785% [0.681%,0.862%] | 0.837% [0.742%,0.924%] |
| 15∶0 | 0.400% [0.339%,0.519%] | 0.846% [0.673%,0.963%] | 0.808% [0.675%,0.956%] |
| 16∶0 | 26.659% [24.985%,28.172%] | 22.152% [21.326%,22.797%] | 21.548% [21.029%,22.588%] |
| 16∶1 | 5.504% [4.584%,6.559%] | 8.740% [7.281%,10.514%] | 9.378% [7.895%,10.089%] |
| 16∶2 | 0.050% [0.040%,0.060%] | 0.109% [0.100%,0.122%] | 0.110% [0.100%,0.121%] |
| 17∶0 | 0.445% [0.403%,0.503%] | 0.545% [0.469%,0.609%] | 0.528% [0.427%,0.625%] |
| 17∶1 | 0.383% [0.347%,0.407%] | 0.459% [0.434%,0.508%] | 0.471% [0.438%,0.493%] |
| 17∶2 | 0.009% [0.008%,0.012%] | 0.021% [0.020%,0.023%] | 0.021% [0.019%,0.023%] |
| 18∶0 | 7.819% [6.967%,8.823%] | 5.264% [4.478%,6.403%] | 5.428% [4.706%,6.108%] |
| 18∶1 | 40.367% [39.445%,41.434%] | 36.294% [35.178%,36.809%] | 35.949% [35.556%,36.500%] |
| 18∶2 | 9.959% [9.333%,11.329%] | 10.649% [9.791%,10.874%] | 10.437% [9.543%,11.006%] |
| 18∶3 | 1.392% [1.148%,1.769%] | 1.717% [1.567%,2.021%] | 1.683% [1.425%,1.968%] |
| 18∶4 | 0.017% [0.013%,0.022%] | 0.038% [0.033%,0.047%] | 0.044% [0.039%,0.056%] |
| 19∶0 | 0.040% [0.037%,0.051%] | 0.05% [0.041%,0.059%] | 0.062% [0.045%,0.071%] |
| 19∶1 | 0.166% [0.149%,0.193%] | 0.263% [0.223%,0.296%] | 0.277% [0.231%,0.302%] |
| 19∶2 | 0.014% [0.012%,0.016%] | 0.021% [0.019%,0.022%] | 0.019% [0.018%,0.022%] |
| 20∶0 * | 0.044% [0.038%,0.058%] | 0.193% [0.152%,0.276%] | 0.258% [0.240%,0.350%] |
| 20∶1 | 0.478% [0.409%,0.509%] | 1.364% [1.125%,1.556%] | 1.399% [1.230%,1.583%] |
| 20∶2+ | 0.220% [0.197%,0.231%] | 0.500% [0.452%,0.533%] | 0.444% [0.400%,0.512%] |
| 20∶3+ | 0.230% [0.205%,0.262%] | 0.576% [0.444%,0.629%] | 0.396% [0.358%,0.511%] |
| 20∶4+ | 0.682% [0.534%,0.729%] | 0.868% [0.703%,0.907%] | 0.654% [0.557%,0.714%] |
| 20∶5+ | 0.107% [0.078%,0.160%] | 0.178% [0.120%,0.218%] | 0.130% [0.094%,0.161%] |
| 22∶0 * | 0.015% [0.011%,0.023%] | 0.021% [0.012%,0.031%] | 0.033% [0.028%,0.048%] |
| 22∶1 * | 0.042% [0.025%,0.049%] | 0.129% [0.076%,0.186%] | 0.193% [0.127%,0.253%] |
| 22∶2 * | 0.008% [0.006%,0.009%] | 0.015% [0.012%,0.018%] | 0.018% [0.016%,0.020%] |
| 22∶3 | 0.010% [0.009%,0.013%] | 0.030% [0.023%,0.039%] | 0.031% [0.024%,0.039%] |
| 22∶4+ | 0.124% [0.108%,0.134%] | 0.361% [0.250%,0.395%] | 0.266% [0.225%,0.316%] |
| 22∶5+ | 0.222% [0.189%,0.254%] | 0.450% [0.362%,0.505%] | 0.350% [0.295%,0.405%] |
| 22∶6 | 0.498% [0.350%,0.598%] | 0.392% [0.285%,0.458%] | 0.317% [0.234%,0.386%] |
| 24∶0 * | 0.020% [0.016%,0.027%] | 0.012% [0.008%,0.019%] | 0.021% [0.015%,0.029%] |
| 24∶1 * | 0.045% [0.037%,0.054%] | 0.016% [0.011%,0.031%] | 0.031% [0.021%,0.045%] |
| 24∶2 * | 0.005% [0.004%,0.006%] | 0.003% [0.002%,0.004%] | 0.004% [0.003%,0.006%] |
| 24∶3 * | 0.001% [0.001%,0.001%] | 0.001% [0.000%,0.001%] | 0.001% [0.001%,0.001%] |
| 24∶4 * | 0.005% [0.004%,0.006%] | 0.003% [0.002%,0.003%] | 0.004% [0.003%,0.005%] |
| 24∶5 * | 0.006% [0.005%,0.007%] | 0.008% [0.006%,0.009%] | 0.010% [0.007%,0.013%] |
| 24∶6 * | 0.004% [0.003%,0.005%] | 0.006% [0.005%,0.009%] | 0.008% [0.006%,0.011%] |
| 26∶0 * | 0.002% [0.001%,0.003%] | 0.003% [0.002%,0.004%] | 0.005% [0.003%,0.006%] |
| 26∶1 * | 0.003% [0.003%,0.005%] | 0.002% [0.001%,0.004%] | 0.003% [0.002%,0.006%] |
| 26∶2 * | 0.002% [0.002%,0.002%] | 0.001% [0.001%,0.001%] | 0.001% [0.001%,0.002%] |
| SFA | 37.559% [35.820%,39.735%] | 36.429% [33.455%,38.296%] | 36.591% [34.518%,38.109%] |
| MUFA | 47.405% [46.673%,49.373%] | 48.300% [46.207%,49.412%] | 48.638% [47.261%,50.094%] |
| PUFA+ | 13.697% [12.640%,15.359%] | 15.952% [14.694%,16.552%] | 14.804% [13.923%,16.606%] |
| n3+ | 2.237% [1.898%,2.656%] | 2.812% [2.618%,3.048%] | 2.593% [2.448%,2.788%] |
| n6+ | 11.298% [10.627%,13.205%] | 13.125% [12.244%,13.813%] | 12.463% [11.835%,13.511%] |
| (n6-18∶2)+ | 1.504% [1.302%,1.636%] | 2.790% [2.273%,3.001%] | 2.136% [1.952%,2.475%] |
Fatty acid (FA) percentage concentrations (mol%) of plasma nonesterified fatty acids (pNEFA), subcutaneous adipose tissue (sAT) and visceral adipose tissue (vAT) are given as median and interquartile range.
*- significantly higher in vAT compared to sAT, +- significantly lower in vAT compared to sAT; Wilcoxon test adjusted for multiple testing with Sidak correction: significance is assumed with p<0.001068 achieving a global significance level of 0.05.
The most abundant FA in the studied compartments were 18∶1, 16∶0 and 18∶2, followed by 18∶0 in plasma and 16∶1 in AT.
Significant differences in FA composition between both sites of AT were mainly found for VLCFA which were higher in vAT, and some PUFA with significantly higher contribution to sAT (Table1).
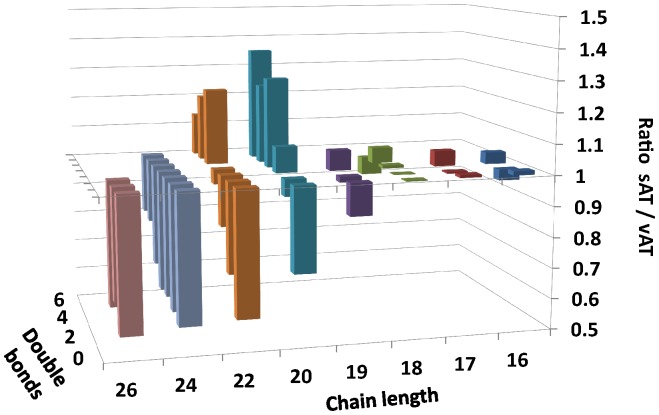
Plotting the ratio of sAT to vAT percentages versus carbon chain-length and number of double bonds indicated a clear trend for preferential incorporation of highly unsaturated FA into sAT, while longer carbon chain FA tended to be higher in vAT (Figure1).
Figure 1. Fatty acid (FA) composition of adipose tissue sites.
Ratio of FA percentages in subcutaneous adipose tissue (sAT) and visceral adipose tissue (vAT) by chain length and number of double bonds.
No significant difference between sAT and vAT was found for medium- and long-chain SFA (14∶0,16∶0,18∶0), MUFA (14∶1,16∶1,18∶1,20∶1) and the sums of SFA and MUFA as well as odd-chain fatty acids (15∶0,17∶0,17∶1,17∶2,19∶0,19∶1,19∶2) and some PUFA.
While most FA were not significantly different between sAT and vAT, most pNEFA differed significantly from sAT, vAT or both tissues. The abundant FA 16∶0, 18∶0 and 18∶1 were significantly higher in pNEFA, compared to sAT and vAT (p<1E-07). As a consequence of the relative excess of these FA in pNEFA, percentages of most other FA were lower in pNEFA compared to AT, with two exceptions.
22∶6 had a significantly higher content in pNEFA compared to both AT, and some VLCFA (24∶1, 24∶2, 24∶3, 24∶4, 26∶2) were higher in pNEFA compared to sAT, although not statistically significant compared to vAT.
20∶4 was significantly lower in pNEFA than in sAT (p = 2.09E-4), but tended to be higher than in vAT. No significant difference was found for 18∶2, 24∶0, 26∶1 and the sum of all MUFA in pNEFA in comparison to both AT.
Correlation of fatty acid compositions
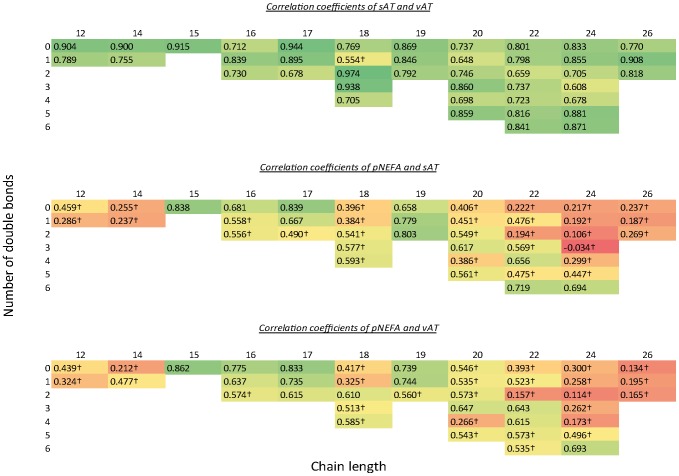
Most FA were closely correlated (r>0.7) between sAT and vAT, except some lower concentrated FA and 18∶1 which showed the lowest correlation coefficient (r = 0.554) (Figure2).
Figure 2. Correlation of pNEFA and AT sites.
Correlation coefficients (r) of plasma nonesterified fatty acids (pNEFA), subcutaneous adipose tissue (sAT) and visceral adipose tissue (vAT). FA are arranged by chain length and double bond number. Colour gradient with red (r = 0), yellow (r = 0.5) and green (r = 1). † p>0.001068.
While most FA correlated strongly between both AT, the correlation of pNEFA and AT fatty acid percentages varied widely between FA.
16∶0 showed close correlations between pNEFA and the AT fatty acid percentages, while 16∶1, 18∶0, 18∶2 and 18∶3 had less close correlations. For the most highly concentrated 18∶1, the correlation coefficient of pNEFA with sAT was only 0.35 and 0.33 with vAT.
Odd-chain fatty acids 15∶0, 17∶0, 17∶1, 19∶0, 19∶1 and 19∶2 showed high correlation coefficients above 0.7. Also 22∶6 (r = 0.719/0.535) and 24∶6 (r = 0.694/0.693) had high correlation coefficients between pNEFA and sAT as well as vAT. In contrast, medium-chain FA correlated less between NEFA and both AT sites. Most VLCFA showed weak correlation between plasma and AT fatty acid percentages. R-values tended to increase with unsaturation, as 24∶5 (r = 0.447/0.496) and 24∶6 (r = 0.694/0.693) were the only pNEFA with more than 22 carbon atoms correlating highly with sAT and vAT.
There was a general trend for a stronger correlation of PUFA compared to their saturated and monounsaturated counterparts, except for 20∶4 which showed a low correlation to sAT (r = 0.386) and vAT (r = 0.266).
Influence of BMI on correlation of fatty acid compositions
Quantile regression models were established for all FA analysed to determine the influence of BMI on the relation of AT fatty acid composition and pNEFA (Table2).
Table 2. Quantile regression models.
| FA | vAT | sAT | ||||
| ∼NEFA (r2) | ∼NEFA+BMI (r2) | p_value | ∼NEFA (r2) | ∼NEFA+BMI (r2) | p_value | |
| 12∶0 | 0.232 | 0.401 | 0.035 | 0.201 | 0.356 | 0.021 |
| 12∶1 | 0.303 | 0.348 | 0.710 | 0.247 | 0.414 | 0.036 |
| 14∶0 | 0.157 | 0.468 | 0.006 | 0.150 | 0.397 | 0.006 |
| 14∶1 | 0.437 | 0.492 | 0.013 | 0.221 | 0.316 | 0.229 |
| 15∶0 | 0.612 | 0.676 | 0.066 | 0.609 | 0.657 | 0.002 |
| 16∶0 | 0.571 | 0.613 | 0.437 | 0.531 | 0.544 | 0.191 |
| 16∶1 | 0.382 | 0.402 | 0.858 | 0.331 | 0.338 | 0.352 |
| 16∶2 | 0.426 | 0.504 | 0.176 | 0.291 | 0.285 | 0.555 |
| 17∶0 | 0.763 | 0.772 | 0.578 | 0.798 | 0.788 | 0.567 |
| 17∶1 | 0.533 | 0.560 | 0.165 | 0.483 | 0.518 | 0.034 |
| 17∶2 | 0.258 | 0.377 | 0.069 | 0.161 | 0.263 | 0.003 |
| 18∶0 | 0.304 | 0.528 | 0.085 | 0.203 | 0.289 | 0.246 |
| 18∶1 | 0.190 | 0.460 | 0.039 | 0.198 | 0.280 | 0.386 |
| 18∶2 | 0.700 | 0.721 | 0.128 | 0.604 | 0.649 | 0.219 |
| 18∶3 | 0.288 | 0.282 | 0.820 | 0.249 | 0.258 | 0.553 |
| 18∶4 | 0.320 | 0.490 | 0.191 | 0.453 | 0.527 | 0.246 |
| 19∶0 | 0.486 | 0.622 | 0.091 | 0.396 | 0.476 | 0.137 |
| 19∶1 | 0.610 | 0.598 | 0.454 | 0.663 | 0.650 | 0.012 |
| 19∶2 | 0.312 | 0.298 | 0.318 | 0.471 | 0.489 | 0.811 |
| 20∶0 | −0.034 | 0.605 | 0.001 | −0.036 | 0.541 | 0.000 |
| 20∶1 | 0.237 | 0.274 | 0.398 | 0.166 | 0.288 | 0.281 |
| 20∶2 | 0.386 | 0.284 | 0.576 | 0.465 | 0.449 | 0.891 |
| 20∶3 | 0.463 | 0.528 | 0.108 | 0.333 | 0.569 | 0.027 |
| 20∶4 | −0.011 | 0.056 | 0.048 | 0.038 | 0.357 | 0.003 |
| 20∶5 | 0.225 | 0.242 | 0.147 | 0.230 | 0.351 | 0.182 |
| 22∶0 | −0.017 | 0.468 | 0.000 | −0.003 | 0.507 | 0.004 |
| 22∶1 | 0.280 | 0.462 | 0.000 | 0.244 | 0.383 | 0.168 |
| 22∶2 | −0.013 | 0.207 | 0.088 | 0.007 | 0.121 | 0.242 |
| 22∶3 | 0.241 | 0.186 | 0.891 | 0.268 | 0.256 | 0.885 |
| 22∶4 | 0.661 | 0.677 | 0.550 | 0.634 | 0.625 | 0.669 |
| 22∶5 | 0.288 | 0.292 | 0.787 | 0.102 | 0.088 | 0.563 |
| 22∶6 | 0.416 | 0.438 | 0.441 | 0.534 | 0.536 | 0.695 |
| 24∶0 | 0.029 | 0.499 | 0.000 | 0.015 | 0.526 | 0.023 |
| 24∶1 | −0.041 | 0.378 | 0.002 | −0.067 | 0.327 | 0.019 |
| 24∶2 | 0.001 | 0.333 | 0.006 | −0.015 | 0.197 | 0.318 |
| 24∶3 | 0.005 | 0.216 | 0.008 | −0.082 | −0.018 | 0.466 |
| 24∶4 | 0.087 | 0.247 | 0.174 | 0.143 | 0.221 | 0.643 |
| 24∶5 | 0.448 | 0.554 | 0.015 | 0.371 | 0.455 | 0.238 |
| 24∶6 | 0.624 | 0.682 | 0.090 | 0.601 | 0.622 | 0.006 |
| 26∶0 | −0.001 | 0.466 | 0.000 | 0.000 | 0.461 | 0.002 |
| 26∶1 | 0.010 | 0.386 | 0.002 | −0.008 | 0.296 | 0.115 |
| 26∶2 | 0.000 | 0.387 | 0.009 | −0.011 | 0.303 | 0.073 |
Quantile regression models were established to predict visceral (vAT) and subcutaneous adipose tissue (sAT) by plasma nonesterified fatty acids (pNEFA)±BMI. For model comparison, ANOVA was performed whereas presented p-values indicate significant more explanation of variance with model containing pNEFA and BMI.
For some SFA, regression models including BMI in addition to pNEFA explained significantly more variance as well as in sAT and vAT. The saturated VLCFA 20∶0, 22∶0, 24∶0 and 26∶0 showed the highest relative improvement of r2 between models with and without BMI.
A better regression for both AT, including BMI in the regression model also appears for 12∶0, 14∶0, 15∶0, 20∶0, 20∶4 and 24∶1. Significantly more variance of vAT was explained for 14∶1, 18∶0, 18∶1, 19∶0, 22∶1, 22∶2, 24∶2, 24∶3, 24∶5 and 26∶1 by inclusion of BMI into the model. For 12∶1, 17∶1, 19∶1 and 20∶3, the inclusion of BMI improved r2 in sAT.
Regarding the r2 values for both models, the model with pNEFA only explained more than 50% of variance in both AT for 15∶0, 16∶0, 17∶0, 18∶2, 19∶1, 22∶4 and 24∶6, in sAT for 22∶6 and in vAT for 22∶6. The inclusion of BMI additionally explained more than 50% of variance in both AT for 20∶0, 20∶3 and 24∶0, in sAT for 17∶1, 18∶4 and 22∶0 and in vAT for 16∶2, 19∶0 and 24∶5.
For the strongly correlated odd-chain FA 15∶0 and 17∶0 and 22∶6, two MLR models were tested to estimate sAT and vAT fatty acid composition from pNEFA (Table3).
Table 3. Multiple linear regression models.
| vAT | sAT | |||||
| Prediction error | p (model comparison) | Adjusted R-squared | Prediction error | p (model comparison) | Adjusted R-squared | |
| MLR model 1 (pNEFA,BMI) | ||||||
| 15∶0 | 1.06E-03 | 0.046 | 0.652 | 9.84E-04 | 0.073 | 0.629 |
| 17∶0 | 5.79E-04 | 0.318 | 0.754 | 4.95E-04 | 0.718 | 0.786 |
| 22∶6 | 8.10E-04 | 0.378 | 0.403 | 6.44E-04 | 0.706 | 0.498 |
| MLR model 2 (pNEFA) | ||||||
| 15∶0 | 1.15E-03 | - | 0.610 | 1.08E-03 | - | 0.595 |
| 17∶0 | 5.56E-04 | - | 0.753 | 4.70E-04 | - | 0.793 |
| 22∶6 | 7.95E-04 | - | 0.407 | 6.19E-04 | - | 0.516 |
Multiple linear regression models (MLR) were established to estimate fatty acid composition of subcutaneous (sAT) and visceral adipose tissue (vAT) from plasma nonesterified fatty acids (pNEFA) and/or BMI.
Validation was performed using leave-one-out cross-validation with absolute prediction errors, adjusted r squared and model comparison with ANOVA of model 1 and 2.
Both CV prediction error and adjusted r squared were similar between the models. Prediction quality was improved by including BMI only for 15∶0. ANOVA analysis showed no significant difference between the two models for 17∶0 and 22∶6, but MLR model 1 (including pNEFA & BMI) was significantly better compared to model 2 for 15∶0. However, regarding the slight difference of prediction error and adjusted R squared for 15∶0, it is also possible to establish model 2 for this FA.
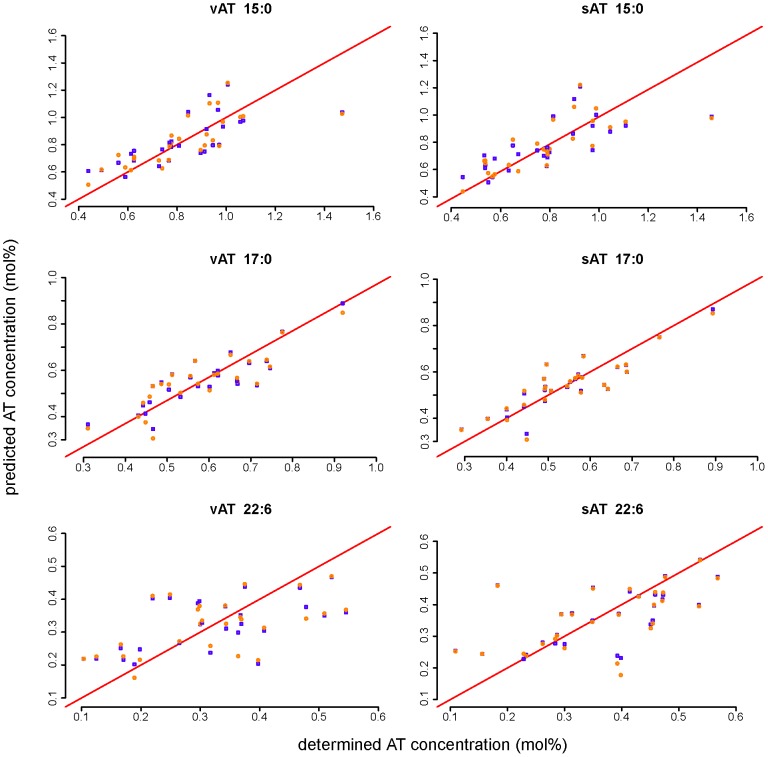
The strong correlation of these FA percentages in pNEFA and AT was apparent from scatter plots of AT fatty acid percentages predicted from pNEFA and actually analysed values in AT (Figure3). The values were close to the bisecting line without influence of the MLR model. This was true for correlations with sAT and vAT.
Figure 3. Regression models for AT FA and pNEFA.
Comparison of analysed subcutaneous (sAT) and visceral adipose tissue (vAT) fatty acid composition and predicted AT fatty acid composition by using different multiple linear regression models with pNEFA (blue squares) or pNEFA+BMI (orange circles).
Discussion
The present study shows a wide variation of the coefficients of correlation between fatty acid percentages of adipose tissue and nonesterified fatty acids in plasma.
Of importance, close correlations between pNEFA and AT were found for FA known to be markers of specific dietary intakes, i.e. odd-chain FA (markers for dairy fat intake) and omega3 (n-3) LC-PUFA (markers for fish intake).
The content of 15∶0 in TAG of AT is a marker for the intake of dairy products in women [27] and men [2]. Similarly, total 15∶0 in plasma and erythrocytes is used as a biomarker for dairy fat intake [28]. Nonesterified FA were not previously analysed in these or other studies on serum markers [28], [29]. Considering the strong correlation of 15∶0 and other odd-chain FA percentages in AT and pNEFA, we assume that pNEFA reflect dairy intake similar to other serum markers. Hence, 15∶0 in pNEFA could provide an alternative biomarker for habitual dairy food intake in epidemiological studies. Certain odd-chain fatty acids are also found in marine fish with up to 2.2% of 15∶0 in keeled mullet [30], while bovine milk contains about 0.9% [31].
A close correlation between dietary intake and FA percentages in AT has been well established for n-3 FA [3]. Our observation of a high correlation coefficient of 0.719 for the n-3 FA 22∶6 between pNEFA and sAT is in line with previous findings of Leaf et al. (r = 0.87) [32] and Yli-Jama et al. (r = 0.572) [9] and is further underlined by the good validation parameters of the MLR models for these FA including only pNEFA percentage as independent parameter. The similarly high correlation coefficients of 20∶5 and 24∶6 indicate a generally strong correlation of n-3 FA between AT and pNEFA. Long chain n-3 FA 20∶5 and 22∶6 share a low rate of β-oxidation in peroxisomes [17], [33]; therefore, pNEFA concentrations of individual subjects are only slightly affected by fatty acid oxidation. This agrees with the higher percentages of 22∶6 in pNEFA compared to AT.
The strong correlation of 22∶6 and other PUFA was related to higher AT mobilization rates by Yli-Jama et al. [9]. PUFA are preferentially mobilized from AT in in-vitro studies with adipocytes [12], in in-vivo studies in animals [13] and in humans [15]. The mobilization rates were not determined in the presented study, but there seems to be a general trend towards relatively high correlation coefficients for PUFA in accordance with their previously mentioned higher mobilization rates.
In contrast to other PUFA, pNEFA 20∶4 showed a low correlation with AT. This confirms the observation of Yli-Jama [9]. Low correlations for 20∶4 have also been observed between diet and AT [3], [4] as well as between glycerophospholipids from cheek cells, erythrocytes and plasma [34]. Moreover, correlation between sAT and vAT is lower for 20∶4 than for most other FA (Figure2). Although a high mobilization rate of 1.6 has been previously found for 20∶4 [12], its plasma concentration might be even stronger affected by its conversion to eicosanoids, as this fatty acid is typically the major precursor for eicosanoids [35] and 20∶4-dervied eicosanoids are formed at a faster rate than 20∶5-derived eicosanoids [18].
Another trend, contributing to the variety of the correlation pattern, was found in physicochemical properties. Increasing chain-length and increasing saturation negatively impact correlation coefficients. In previous studies, this has been ascribed to lower mobilization as well as lower clearance of SFA and MUFA, especially of 18∶0 [15]. The influence of flux to the different tissues may be confirmed by the significantly lower percentages of medium-chain FA 12∶0, 12∶1, 14∶0 and 14∶1, as these FA have a higher flux to tissue due to direct transport through cell membrane and rapid mitochondrial oxidation independently of the carnitine transport system [36].
Many metabolic processes besides AT TAG lipolysis affect pNEFA variation, such as lipolysis of lipoprotein bound TAG by LPL [37] and release or exchange of fatty acids of lipoprotein or membrane phospholipids [19]. Thus, the contribution by non AT lipolysis and cellular FA uptake for beta-oxidation and synthesis of prostaglandins may be regulated differently between FA species and individuals. As a result, the observed correlation between pNEFA and AT decreases.
Nevertheless, AT is the dominating source for pNEFA generation and mainly determines concentration as well as percentages of pNEFA, as evidenced by the similarity of molar percentages of highly concentrated FA. The close relation between pNEFA and AT is supported by strong correlation of desaturase indices [38]. High correlations for the ratio of 16∶1/16∶0, 18∶1/18∶0 (stearoyl-desaturase, respectively) and 20∶4/20∶3 (delta-5-desaturase) were found between pNEFA and sAT. The strong correlation of stearoyl-desaturase indices was confirmed in the presented study (data not shown).
In contrast to the wide variation of correlation coefficients between pNEFA and AT, correlation between sAT and vAT FA was strong for the majority of FA species, although significant differences in FA composition between the two sites of AT were found. This demonstrates that strong correlations do not necessarily depend on very similar concentrations, but stable pools with slow relative turnover rates support the establishment of close relations between tissues.
The higher amount of PUFA in sAT is in contrast to a previous study finding no significant differences of PUFA between AT sites [39]. But it agrees with the observation that subcutaneous fat is softer than deeper fat and that vAT contains more SFA compared to sAT [39].
In previous studies sAT [40], [41] or vAT [39] have been claimed to be the major source of pNEFA, while our correlation analyses did not point towards a clearly higher contribution of sAT or vAT. Remarkably, BMI has a FA specific influence on correlation. Explanation of variance of FA percentages in AT was significantly improved by including BMI additionally to pNEFA into quantile regression models only for SFA and 20∶4. Thus, with increasing BMI and increasing fat mass the correlation of SFA content between pNEFA and AT increased which may be a result of a higher contribution of FA release from AT to the pNEFA pool as well as a relatively lower flux of these pNEFA to tissue.
Eventually, the lower flux to tissue is the result of decreased β-oxidation. In obese mice lower hepatic carnitine levels were found compared to controls [42] resulting in an insufficient β-oxidation. Additionally, the lower flux to tissue and elevated pNEFA levels may result in an increased (re-)uptake of pNEFA to AT which strengthen the correlation of SFA between pNEFA and AT in obese subjects [43].
Thus, in highly obese patients a relation between SFA in pNEFA and AT FA composition can be supposed, while there is a less close relationship in normal weight subject. The findings of the present study for odd-chain FA and n-3 FA were confirmed in a correlation analysis only including the 8 normal-weight subjects (data not shown) and seem to be valid for normal weight as well as obese subjects.
A limitation of our study is that no information about habitual diet of the participants was available, as fat content of the diet may influence the specific uptake into sAT or vAT [44] and the fatty acid composition of the diet, e.g. n3/n6 ratio, affects metabolic fatty acid deposition and AT metabolism [10]. Since none of the participants followed a specific diet and they varied widely in respect to age and BMI, we can assume a wide variation of dietary preferences.
We only studied abdominal sAT, but FA uptake and composition in sAT have been found to differ between sites of sAT [45]. Abdominal sAT is most frequently collected in clinical trials and upper-body fat contributes significantly to pNEFA [14], thus, our results should be of relevance and reflect the major effects. Since our study was accomplished to determine the suitability of pNEFA as surrogate markers for AT composition, we point out that only women were studied and considering the influence of gender on sAT and vAT metabolism [46], further studies are needed to expand our results to males or infants. The strong correlation of desaturase indices in Swedish men [38] hypothesizes similar results of FA correlation in male subjects.
Conclusion
Plasma nonesterified fatty acids are derived mainly from adipose tissue TAG hydrolysis, but additional processes, such as oxidation or prostaglandin synthesis seem to affect their flux and consequently the variation of pNEFA.
We demonstrate that nonesterified odd-chain FA in plasma reflect AT contents very well and thus seem a suitable alternative to AT biopsies for the estimation of long-term status and dietary habits. Fish intake and n-3 status of adipose tissue are reflected by pNEFA 22∶6.
Acknowledgments
We are grateful to Arne Dietrich (Department of Surgery, University of Leipzig), who performed the adipose tissue biopsies. We thank Martina Weber (Division of Metabolic and Nutritional Medicine, Dr. von Hauner Children's Hospital, University of Munich) who supported the statistical data analysis. The data presented are part of the Ph.D. thesis accomplished by Christian Hellmuth at the Medical Faculty of the Ludwig- Maximilians-University of Munich.
Funding Statement
This work was supported financially by the “Kompetenznetz Adipositas” (“Competence Network for Adiposity”) funded by the German Federal Ministry of Education and Research (FKZ, grant 01GI0826 and FKZ, grant 01GI1128). Further support by the Commission of the European Communities, within the seventh Framework Programme, NUTRIMENTHE, grant FP7-212652, and by the Munich Center of Health Sciences (McHealth) is gratefully acknowledged. This manuscript does not necessarily reflect the views of the Commission and in no way anticipates the future policy in this area. This work was additionally supported by a grant from Deutsche Forschungsgemeinschaft (DFG) for the Clinical Research group “Atherobesity” KFO 152 (project BL 833/1-1) (MB). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hodson L, Skeaff CM, Fielding BA (2008) Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 47: 348–380. [DOI] [PubMed] [Google Scholar]
- 2. Wolk A, Furuheim M, Vessby B (2001) Fatty acid composition of adipose tissue and serum lipids are valid biological markers of dairy fat intake in men. J Nutr 131: 828–833. [DOI] [PubMed] [Google Scholar]
- 3. Baylin A, Kabagambe EK, Siles X, Campos H (2002) Adipose tissue biomarkers of fatty acid intake. Am J Clin Nutr 76: 750–757. [DOI] [PubMed] [Google Scholar]
- 4. Garland M, Sacks FM, Colditz GA, Rimm EB, Sampson LA, et al. (1998) The relation between dietary intake and adipose tissue composition of selected fatty acids in US women. Am J Clin Nutr 67: 25–30. [DOI] [PubMed] [Google Scholar]
- 5. Summers LK, Barnes SC, Fielding BA, Beysen C, Ilic V, et al. (2000) Uptake of individual fatty acids into adipose tissue in relation to their presence in the diet. Am J Clin Nutr 71: 1470–1477. [DOI] [PubMed] [Google Scholar]
- 6. Garaulet M, Hernandez-Morante JJ, Tebar FJ, Zamora S (2011) Relation between degree of obesity and site-specific adipose tissue fatty acid composition in a Mediterranean population. Nutrition 27: 170–176. [DOI] [PubMed] [Google Scholar]
- 7. Caron-Jobin M, Mauvoisin D, Michaud A, Veilleux A, Noel S, et al. (2012) Stearic acid content of abdominal adipose tissues in obese women. Nutr Diabetes 2: e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Decsi T, Molnar D, Koletzko B (1998) The effect of under- and overnutrition on essential fatty acid metabolism in childhood. Eur J Clin Nutr 52: 541–548. [DOI] [PubMed] [Google Scholar]
- 9. Yli-Jama P, Haugen TS, Rebnord HM, Ringstad J, Pedersen JI (2001) Selective mobilisation of fatty acids from human adipose tissue. Eur J Intern Med 12: 107–115. [DOI] [PubMed] [Google Scholar]
- 10. Raclot T (2003) Selective mobilization of fatty acids from adipose tissue triacylglycerols. Prog Lipid Res 42: 257–288. [DOI] [PubMed] [Google Scholar]
- 11. Ali AH, Koutsari C, Mundi M, Stegall MD, Heimbach JK, et al. (2011) Free fatty acid storage in human visceral and subcutaneous adipose tissue: role of adipocyte proteins. Diabetes 60: 2300–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raclot T, Langin D, Lafontan M, Groscolas R (1997) Selective release of human adipocyte fatty acids according to molecular structure. Biochem J 324 (Pt 3): 911–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Conner WE, Lin DS, Colvis C (1996) Differential mobilization of fatty acids from adipose tissue. Journal of Lipid Research 37: 290–298. [PubMed] [Google Scholar]
- 14. Frayn KN, Humphreys SM (2012) Metabolic characteristics of human subcutaneous abdominal adipose tissue after overnight fast. Am? J? Physiol Endocrinol Metab 302: E468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Halliwell KJ, Fielding BA, Samra JS, Humphreys SM, Frayn KN (1996) Release of individual fatty acids from human adipose tissue in vivo after an overnight fast. Journal of Lipid Research 37: 1842–1848. [PubMed] [Google Scholar]
- 16. Koutsari C, Mundi MS, Ali AH, Jensen MD (2012) Storage rates of circulating free fatty acid into adipose tissue during eating or walking in humans. Diabetes 61: 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sprecher H, Chen Q (1999) Polyunsaturated fatty acid biosynthesis: a microsomal-peroxisomal process. Prostaglandins Leukot Essent Fatty Acids 60: 317–321. [DOI] [PubMed] [Google Scholar]
- 18. Kremmyda LS, Tvrzicka E, Stankova B, Zak A (2011) Fatty acids as biocompounds: their role in human metabolism, health and disease: a review. part 2: fatty acid physiological roles and applications in human health and disease. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 155: 195–218. [DOI] [PubMed] [Google Scholar]
- 19. Mittendorfer B, Liem O, Patterson BW, Miles JM, Klein S (2003) What does the measurement of whole-body fatty acid rate of appearance in plasma by using a fatty acid tracer really mean? Diabetes 52: 1641–1648. [DOI] [PubMed] [Google Scholar]
- 20. Hellmuth C, Weber M, Koletzko B, Peissner W (2012) Nonesterified fatty acid determination for functional lipidomics: comprehensive ultrahigh performance liquid chromatography-tandem mass spectrometry quantitation, qualification, and parameter prediction. Anal Chem 84: 1483–1490. [DOI] [PubMed] [Google Scholar]
- 21. Pettinella C, Lee SH, Cipollone F, Blair IA (2007) Targeted quantitative analysis of fatty acids in atherosclerotic plaques by high sensitivity liquid chromatography/tandem mass spectrometry. J? Chromatogr? B? Analyt Technol Biomed Life Sci 850: 168–176. [DOI] [PubMed] [Google Scholar]
- 22. HIRSCH J, FARQUHAR JW, AHRENS EH, PETERSON ML, STOFFEL W (1960) Studies of Adipose Tissue in Man. The American Journal of Clinical Nutrition 8: 499–511. [DOI] [PubMed] [Google Scholar]
- 23. Teegala SM, Willett WC, Mozaffarian D (2009) Consumption and health effects of trans fatty acids: a review. J AOAC Int 92: 1250–1257. [PubMed] [Google Scholar]
- 24. Sidak Z (1967) Rectangular Confidence Regions for the Means of Multivariate Normal Distributions. Journal of the American Statistical Association 62: 626–633. [Google Scholar]
- 25.Roger Koenker (2013). quantreg: Quantile Regression R package version 4.96.
- 26. Watson AD (2006) Thematic review series: systems biology approaches to metabolic and cardiovascular disorders. Lipidomics: a global approach to lipid analysis in biological systems. J Lipid Res 47: 2101–2111. [DOI] [PubMed] [Google Scholar]
- 27. Wolk A, Vessby B, Ljung H, Barrefors P (1998) Evaluation of a biological marker of dairy fat intake. Am J Clin Nutr 68: 291–295. [DOI] [PubMed] [Google Scholar]
- 28. Sun Q, Ma J, Campos H, Hu FB (2007) Plasma and erythrocyte biomarkers of dairy fat intake and risk of ischemic heart disease. Am J Clin Nutr 86: 929–937. [DOI] [PubMed] [Google Scholar]
- 29. Brevik A, Veierod MB, Drevon CA, Andersen LF (2005) Evaluation of the odd fatty acids 15:0 and 17:0 in serum and adipose tissue as markers of intake of milk and dairy fat. Eur J Clin Nutr 59: 1417–1422. [DOI] [PubMed] [Google Scholar]
- 30. Ozogul Y, Ozogul F, Cicek E, Polat A, Kuley E (2009) Fat content and fatty acid compositions of 34 marine water fish species from the Mediterranean Sea. Int J Food Sci Nutr 60: 464–475. [DOI] [PubMed] [Google Scholar]
- 31.Mansson HL (2008) Fatty acids in bovine milk fat. Food Nutr Res 52. [DOI] [PMC free article] [PubMed]
- 32. Leaf DA, Connor WE, Barstad L, Sexton G (1995) Incorporation of dietary n-3 fatty acids into the fatty acids of human adipose tissue and plasma lipid classes. The American Journal of Clinical Nutrition 62: 68–73. [DOI] [PubMed] [Google Scholar]
- 33. Sprecher H, Chen Q, Yin FQ (1999) Regulation of the biosynthesis of 22:5n-6 and 22:6n-3: a complex intracellular process. Lipids 34 Suppl: S153–156. [DOI] [PubMed] [Google Scholar]
- 34. Klingler M, Demmelmair H, Koletzko B, Glaser C (2011) Fatty acid status determination by cheek cell sampling combined with methanol-based ultrasound extraction of glycerophospholipids. Lipids 46: 981–990. [DOI] [PubMed] [Google Scholar]
- 35. Calder PC (2011) Fatty acids and inflammation: the cutting edge between food and pharma. Eur? J? Pharmacol 668 Suppl 1: S50–58. [DOI] [PubMed] [Google Scholar]
- 36. Papamandjaris AA, MacDougall DE, Jones PJ (1998) Medium chain fatty acid metabolism and energy expenditure: obesity treatment implications. Life Sci 62: 1203–1215. [DOI] [PubMed] [Google Scholar]
- 37. Teusink B, Voshol PJ, Dahlmans VE, Rensen PC, Pijl H, et al. (2003) Contribution of fatty acids released from lipolysis of plasma triglycerides to total plasma fatty acid flux and tissue-specific fatty acid uptake. Diabetes 52: 614–620. [DOI] [PubMed] [Google Scholar]
- 38. Warensjo E, Rosell M, Hellenius ML, Vessby B, De Faire U, et al. (2009) Associations between estimated fatty acid desaturase activities in serum lipids and adipose tissue in humans: links to obesity and insulin resistance. Lipids Health Dis 8: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Garaulet M, Perez-Llamas F, Perez-Ayala M, Martinez P, de Medina FS, et al. (2001) Site-specific differences in the fatty acid composition of abdominal adipose tissue in an obese population from a Mediterranean area: relation with dietary fatty acids, plasma lipid profile, serum insulin, and central obesity. Am J Clin Nutr 74: 585–591. [DOI] [PubMed] [Google Scholar]
- 40. Karpe F, Dickmann JR, Frayn KN (2011) Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes 60: 2441–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lafontan M, Langin D (2009) Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res 48: 275–297. [DOI] [PubMed] [Google Scholar]
- 42. Kim HJ, Kim JH, Noh S, Hur HJ, Sung MJ, et al. (2011) Metabolomic analysis of livers and serum from high-fat diet induced obese mice. J Proteome Res 10: 722–731. [DOI] [PubMed] [Google Scholar]
- 43. Xie B, Waters MJ, Schirra HJ (2012) Investigating potential mechanisms of obesity by metabolomics. J Biomed Biotechnol 2012: 805683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Votruba SB, Mattison RS, Dumesic DA, Koutsari C, Jensen MD (2007) Meal fatty acid uptake in visceral fat in women. Diabetes 56: 2589–2597. [DOI] [PubMed] [Google Scholar]
- 45. Malcom GT, Bhattacharyya AK, Velez-Duran M, Guzman MA, Oalmann MC, et al. (1989) Fatty acid composition of adipose tissue in humans: differences between subcutaneous sites. Am J Clin Nutr 50: 288–291. [DOI] [PubMed] [Google Scholar]
- 46. Jensen MD (2002) Adipose tissue and fatty acid metabolism in humans. J R Soc Med 95 Suppl 42: 3–7. [PMC free article] [PubMed] [Google Scholar]