Abstract
Physical exercise has been shown to increase brain volume and improve cognition in randomized trials of non-demented elderly. Although greater social engagement was found to reduce dementia risk in observational studies, randomized trials of social interventions have not been reported. A representative sample of 120 elderly from Shanghai, China was randomized to four groups (Tai Chi, Walking, Social Interaction, No Intervention) for 40 weeks. Two MRIs were obtained, one before the intervention period, the other after. A neuropsychological battery was administered at baseline, 20 weeks, and 40 weeks. Comparison of changes in brain volumes in intervention groups with the No Intervention group were assessed by t-tests. Time-intervention group interactions for neuropsychological measures were evaluated with repeated-measures mixed models. Compared to the No Intervention group, significant increases in brain volume were seen in the Tai Chi and Social Intervention groups (p < 0.05). Improvements also were observed in several neuropsychological measures in the Tai Chi group, including the Mattis Dementia Rating Scale score (p = 0.004), the Trailmaking Test A (p = 0.002) and B (p = 0.0002), the Auditory Verbal Learning Test (p = 0.009), and verbal fluency for animals (p = 0.01). The Social Interaction group showed improvement on some, but fewer neuropsychological indices. No differences were observed between the Walking and No Intervention groups. The findings differ from previous clinical trials in showing increases in brain volume and improvements in cognition with a largely non-aerobic exercise (Tai Chi). In addition, intellectual stimulation through social interaction was associated with increases in brain volume as well as with some cognitive improvements.
Keywords: Cognition, exercise, intervention studies, magnetic resonance imaging, pilot study, Tai Chi
INTRODUCTION
Most [1–7], but not all [8–11], longitudinal epidemiologic studies have shown a decreased risk of incident dementia or Alzheimer’s disease (AD) in individuals who exercise regularly. Other studies have examined the effects of physical exercise on cognitive decline, with most demonstrating beneficial effects [12–17]. Observational studies [18, 19] as well as randomized trials [20, 21] have provided evidence that physical activity, particularly that leading to improved cardiorespiratory fitness, is associated with increased brain tissue volumes. In the most recently published trial [21], increases in hippocampal volume and improved performance on a spatial memory test in non-demented elders were reported to be associated with supervised walking three times per week for one year.
Observational cohort studies also have shown that greater social engagement is associated with a lower risk of incident dementia [22–25]. However, no randomized trials have been conducted to assess the effect of social interventions on the risk of dementia, cognitive decline, or changes in brain volume.
In the present study, a population-based sample of 120 non-demented older residents living independently in a geographically-defined area of Shanghai, China was randomized to one of four groups, including aerobic (Walking) and non-aerobic (Tai Chi) exercise, Social Interaction, and No Intervention. Each of the intervention groups met three times per week for 40 weeks. Changes in magnetic resonance imaging (MRI)-determined whole brain volume from the beginning to the end of the intervention period and performance on a battery of neuropsychological tests at baseline, 20 weeks, and 40 weeks were assessed. On the basis of published findings showing growth of brain volume with a physical exercise intervention [20], we hypothesized that the Walking and Tai Chi exercise groups would demonstrate increases in brain volume when compared with the No Intervention group. We further hypothesized that those who walked faster would benefit more than those who walked slower.
MATERIALS AND METHODS
Participants
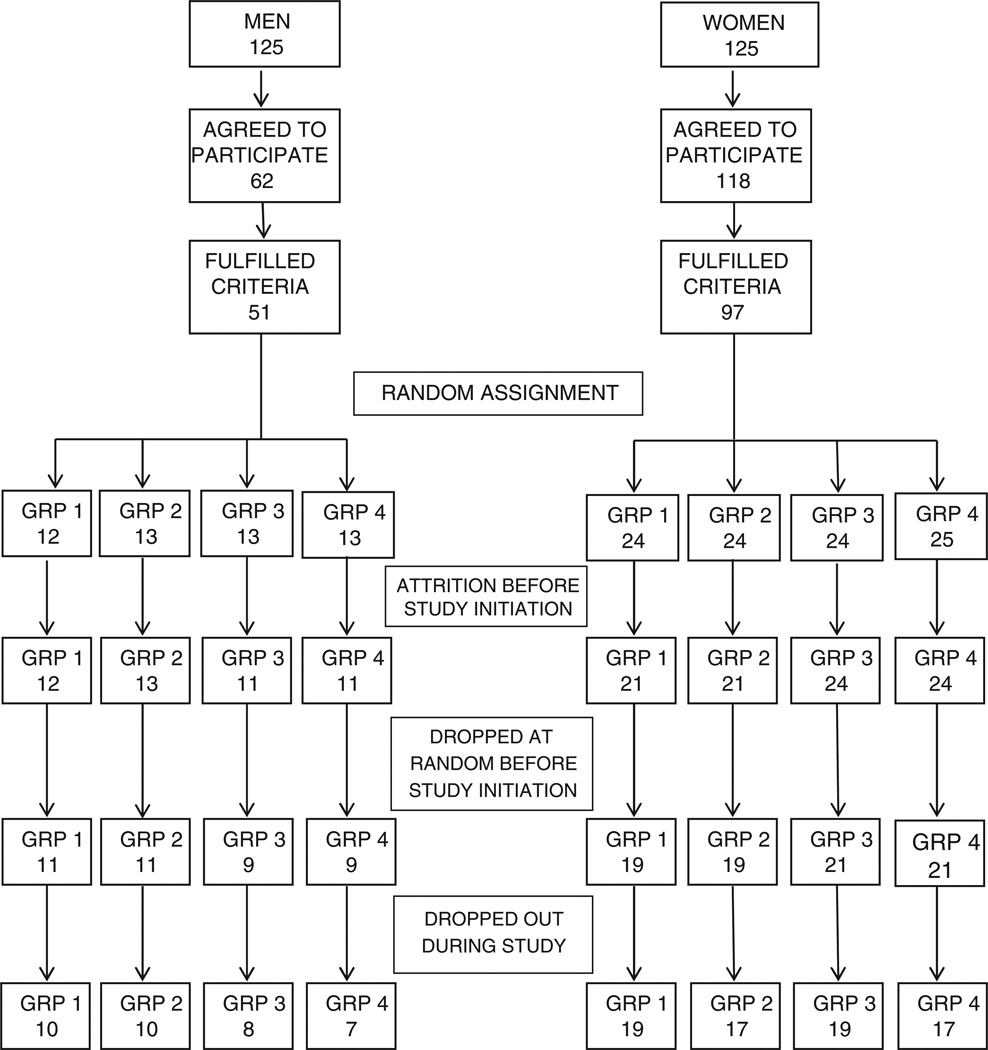
Participants were selected from a random sample of 250 residents, 125 men and 125 women aged 60–79, based on a government-maintained census name list for a specific neighborhood within the Jingansi Temple Community of Shanghai. The neighborhood was located within walking distance from the park where two of the three interventions were conducted. The name list, which is updated each year, includes the name, gender, birthdate, address, and telephone number of every resident, permitting selection of a random sample of individuals in the appropriate age range. Individuals in the random sample were visited in their homes by medical personnel from Huashan Hospital to ascertain their willingness to participate and to assess fulfillment of inclusion and exclusion criteria. Inclusion criteria included either gender and ages 60–79. Exclusion criteria included history of stroke, Parkinson’s disease or other neurologic disease; inability to walk unassisted for two kilometers or maintain balance with feet side-by-side or semitandem for 10 seconds each (to exclude subjects who could not participate in the intervention programs); education-adjusted Chinese Mini-Mental Examination scorec <26 (to exclude individuals with dementia or moderate cognitive impairment); cardiovascular or musculoskeletal conditions that would be contraindicated for the intervention programs; contraindications for MRI; diagnosis of any illness that would preclude participation in the full study; and regular vigorous exercise or Tai Chi practice. All subjects had to agree to participate in any of the four intervention groups if enrolled.
From the original random sample, 62 men and 118 women (72%) agreed to participate (Fig. 1). Of these, 51 men and 97 women met inclusion and exclusion criteria and were enrolled in the study. Those enrolled were assigned to one of four intervention groups (group 1: Tai Chi, group 2: Walking, group 3: Social Interaction, and group 4: No Intervention) using stratified randomization to assure approximately equal numbers of men and women in each intervention arm. Eleven individuals refused further participation after enrollment, but prior to initiation of the study. To assure approximately equal numbers of men and women in each of the four arms with a final sample size of 120, three individuals were selected randomly from enrolled men and women and dropped from group 1, four from group 2, five from groups 3 and 4. The final sample consisted of 11 men and 19 women in groups 1 and 2 and 9 men and 21 women in groups 3 and 4.
Fig. 1.
Study flowchart showing enrollment, random assignment, and attrition.
The study was approved by Huashan Hospital, Fudan University in Shanghai, China, and the University of South Florida Institutional Review Boards. We obtained written informed consent from all participants.
Staggered start design
To accommodate scheduling of MRI scans, a staggered start design was employed with each of the four groups beginning their intervention 3 weeks after the previous group.
Examinations
Prior to beginning an intervention, participants were given a neuropsychological battery and received an initial MRI scan. After 19–21 weeks of intervention, a second neuropsychological battery was given. After 40 weeks, participants were given a third neuropsychological battery and second MRI scan. At baseline, a questionnaire was administered to all participants to ascertain their participation in physical exercise activities over the preceding 5 years. Prior to intervention, subjects also were given a timed walk test that assessed the average time to walk 3 meters over three trials.
Intervention arms
Tai Chi
Participants assigned to this group met with a Tai Chi master and assistant three times per week in the morning in Jing An Park or at a nearby gymnasium depending on weather conditions. Each session included 20 min of warm-up exercises (lower back and hamstring stretching, gentle calisthenics, and balance training), 20 min of Tai Chi practice, and 10 min of cool-down exercises.
Walking
Participants assigned to this group met with two group leaders three times per week in the morning in Jing An Park and were encouraged to walk quickly around a 400 meter circular route. Each session consisted of 10 min of warm-up stretching, 30 min of brisk walking, and 10 min of cool-down exercises. Prior to the first session, each participant was given a pedometer with their name on it. They were asked to take 100 steps and the sensitivity and positioning of the pedometer was adjusted to assure accurate measurement. At the termination of each session, the pedometers were collected and the number of steps taken by each participant at that session recorded. A record of the number of steps taken for every participant at each session over the 40 week period was maintained. The Walking group exercised in a different part of the park from the Tai Chi group, so there was little communication between these two intervention groups.
Social interaction
Participants assigned to this group met with a group leader and an assistant for 1 h three times a week in the morning at the neighborhood community center. Although direction was initially given regarding subjects for discussion, the participants decided on their own to organize and select topics themselves. Discussion was extremely lively, and this group has continued to meet on their own for more than two years after termination of the formal study.
No intervention
The fourth group received no intervention. Contact was maintained by phone during the intervention period to reduce dropout. Participants were called four times during the 40 weeks intervention period by the study coordinator.
Neuropsychological battery
The neuropsychological battery included the WAIS-R Digit Span [26], Bell Cancellation Test [27], Rey-Osterrieth Complex Figure [28] (copying and recall), Stroop Test [29], Chinese Auditory Verbal Learning Test [30], Category Verbal Fluency Test, WAIS-R Similarities Test [26], Trail-Making Test [31], Clock-Drawing Test [32], Boston Naming Test [33], and Mattis Dementia Rating Scale [34]. Psychometrists meeting national certification criteria in China, who were trained by the study neuropsychologist (QHG), administered the battery.
MRI acquisition and analysis
MRIs were acquired with a 1.5T GE scanner. To quantify whole brain volume, an Axial-oblique 3D Fast Spoiled Gradient Recalled Echo (SPGR) sequence was obtained with the following characteristics: TE: 2.9 ms (min), TR: 9 ms (min), Flip angle: 15°, Slice thickness: 1.5 mm, Slice spacing: 0.0 mm, Number of slices: 128, NEX: 2, FOV: 25 cm × 25 cm, Matrix: 256 × 256, Bandwidth: 15.63 KHz, Phase FOV: 1.00, Freq direction: A/P, Options: Increased image dynamic range: On (CV User 2:40.00, CV User 4: 8.00), Scan time: 7 min, 33 s.
MR data collected in Shanghai were transmitted to Dr. DeCarli’s laboratory at the University of California/Davis for post-image analysis. Segmentation of gray matter, white matter, and cerebrospinal fluid was performed on native space T1 SPGR images by an in-house computer program using Bayesian maximal-likelihood EM computation [35]. Tissue probabilities used a combination of Gaussian intensity distributions combined with a Markov Random Field (MRF) component for modeling the tissue classification of voxel neighborhoods. Two in-house enhancements included 1) automatic initialization of the EM step via a high dimensional B-spline warp in which template-based tissue probability maps are fitted to the native T1 SPGR images; and 2) edge detection to dictate the appropriate neighborhood clique structure of the MRF for locations in homogeneous tissue or at tissue boundaries.
Statistical analyses
White and gray matter volumes were summed and divided by total intracranial volume to obtain normalized whole brain volume. Groups were compared at baseline using ANOVA (with post-hoc comparisons using Tukey’s Studentized Range Test) for continuous variables and chi square for discrete variables. Comparison of changes in brain volumes in groups 1–3 versus group 4 were assessed by t-tests. Time-intervention group interactions for neuropsychological measures were assessed using a repeated-measures mixed modeling procedure (S AS PROC MIXED) with an intent-to-treat design. In addition, the Walking group was stratified into two groups based on the median number of steps taken per week by participants, and the repeated-measures mixed modeling procedure was employed to compare the effects of the intervention on neuropsychological performance in these two groups.
Multiple linear regression was used to assess the association between the mean number of steps taken per week across the intervention period in walking group participants and differences in scores on the neuropsychological tests from baseline to final assessment, adjusted for walking speed at baseline, age, gender, and education.
All analyses were performed using SAS 9.2 (SAS, Cary, NC).
RESULTS
Of the 180 people who agreed to participate, 32 were excluded for not meeting study exclusion criteria. Of these, 13 participated in regular vigorous exercise or Tai Chi, 7 had a history of stroke, 9 had problems walking two kilometers, 9 failed to maintain balance for 10 s with feet in a semi-tandem position, and 10 had Mini-Mental State Exam scores less than 26. There was overlap in reasons for exclusion, so the total number of exclusion reasons recorded exceeded 32. Thirteen participants out of 120 (11%) did not complete the study (Fig. 1). One participant was lost from the Tai Chi group, three each from the Walking and Social Intervention groups, and six from the No Intervention group. Comparison of dropouts with completers demonstrated no significant differences in gender (χ2 = 1.24, p = 0.27), age (t = −0.04, p = 0.97) or total score on the Mattis Dementia Rating Scale at baseline (t = 1.41, p = 0.16).
Comparisons of participants in the four groups at baseline showed no significant differences across or between groups in percent female, mean age and education, mean time spent exercising in the previous 5 years, percent current smokers, mean Mattis Dementia Rating Scale score, mean number of words recalled after a 10-minute interference filled delay on the Auditory Verbal Learning Test, mean number of correct targets on the Stroop Color Word Test and mean normalized whole brain volume (Table 1).
Table 1.
Demographic, lifestyle, neuropsychological and brain volume variables by intervention group at baseline. No significant differences (p < 0.05) were seen across or between intervention groups
| Intervention | Tai chi (n = 30) |
Walking (n = 30) |
Social (n = 30) |
No intervention (n = 30) |
χ2,F | p |
|---|---|---|---|---|---|---|
| Gender (% female) | 63.3 | 63.3 | 70.0 | 70.0 | 1.03 | 0.80 |
| Age (mean, SD) | 67.3 (5.3) | 67.8(5.0) | 67.9(6.5) | 68.2 (6.5) | 0.13 | 0.94 |
| Education (y) (mean, SD) | 11.8(2.6) | 10.9(3.9) | 11.4(3.3) | 12.5(3.8) | 1.05 | 0.37 |
| Time spent exercising past 5 y (hours/week) (mean, SD) | 2.9 (0.6) | 2.9 (0.6) | 2.7 (0.7) | 2.9 (0.9) | 0.44 | 0.72 |
| Current smoking (%) | 10 | 10 | 10 | 6.7 | 0.30 | 0.96 |
| Mattis Dementia Rating Scale score at baseline (mean, SD) | 134.6(6.0) | 137.3(11.2) | 138.5(7.9) | 140.0(5.4) | 2.35 | 0.08 |
| Verbal delayed memory at baseline* (mean, SD) | 4.6(3.6) | 6.2(2.9) | 6.6(3.4) | 6.4(3.3) | 2.29 | 0.08 |
| Stroop Color Word Test score at baseline (mean, SD) | 43.3(6.2) | 46.2(2.6) | 46.5 (2.6) | 45.0 (6.7) | 2.63 | 0.06 |
| Normalized whole brain volume at baseline (mean, SD) | 0.77(0.01) | 0.78 (0.02) | 0.77 (0.02) | 0.78 (0.02) | 1.19 | 0.32 |
Number of words freely recalled out of 12 on Auditory Verbal Learning Test after 10 minute interference delay.
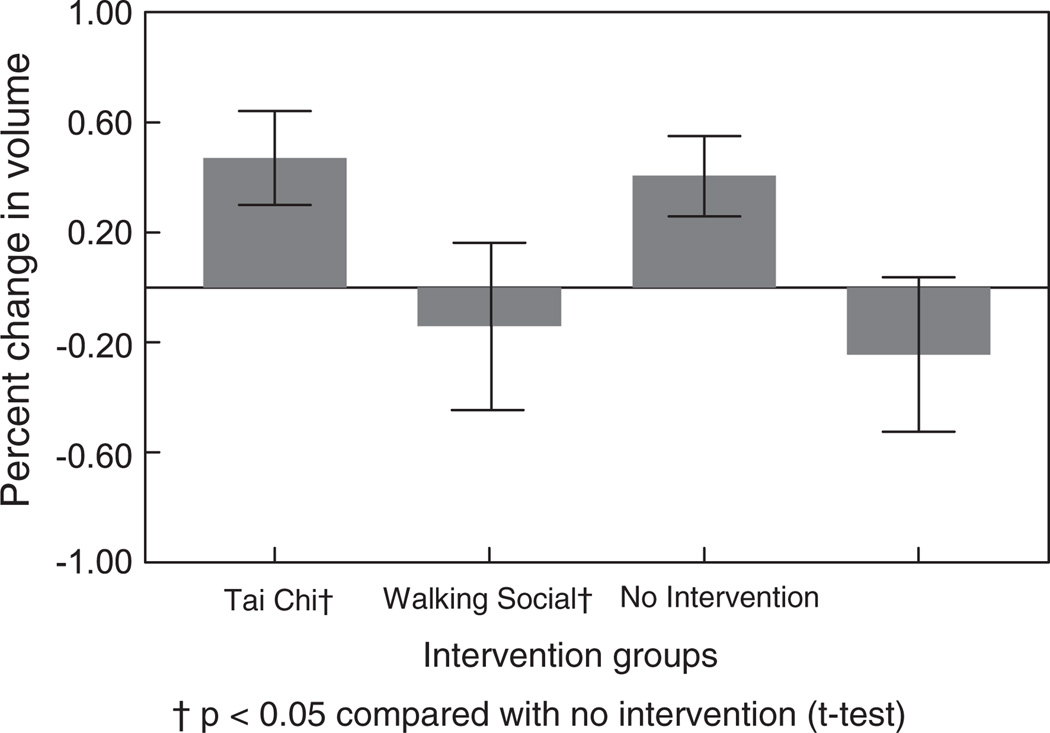
Figure 2 and Table 2 show the mean percentage change in normalized whole brain volume in the four intervention groups. Increases in brain volume were statistically significant (p < 0.05) in both the Tai Chi (t = 2.28) and Social Intervention (t =2.03) groups when compared with the No Intervention group.
Fig. 2.
Percent change in normalized brain volume (mean, SEM) in four intervention groups from baseline to 40 weeks.
Table 2.
Differences in neuropsychological test scores and in whole brain volume percent between 40 week follow-up and baseline
| Difference between 40 weeks of intervention and baseline | Tai Chi | Walking | Social | No intervention |
|---|---|---|---|---|
| WAIS digit span (forward) | −0.28(1.03) | 0.07 (0.92) | 0.33(1.18) | 0.48(1.08) |
| WAIS digit span (backward) | 0.41(1.43) | 0.59(1.04) | 0.48(1.25) | 0.22(1.28) |
| Bell cancellation test | −0.14(0.99) | 0.48 (0.80) | 0.11(0.97) | 0.30(0.93) |
| Rey Figure (copying) | 1.03(4.03) | −0.63 (2.68)† | 0.81 (4.42) | 1.00(2.45) |
| Rey Figure (recall) | −0.31(22.81) | 4.71 (4.29) | 3.44(6.51) | 3.44(4.89) |
| Stroop Test (word) | 0.17(0.38) | 0.58(1.06) | 0.33 (0.62) | 0.48(1.31) |
| Stroop Test (color) | 1.03(2.16) | 1.15(1.92) | 0.96(1.63) | 1.26(1.96) |
| Stroop Test (color-word) | 3.07(6.41) | 1.12(3.17) | 0.96 (2.62) | 2.74(5.87) |
| Auditory Verbal Learning Test (immed. recall) | 2.86(2.22) | 2.42 (2.64) | 3.44(2.01)* | 2.48(2.21) |
| Auditory Verbal Learning Test (delayed recall) | 4.48(3.17)* | 3.96(3.89) | 3.69(3.26) | 3.26(3.22) |
| Auditory Verbal Learning | ||||
| Test (delayed recognition) | 4.66(3.27)‡ | 3.16(4.50) | 3.38(3.26) | 2.65(3.20) |
| Category Verbal Fluency (animals) | −0.38(4.18)† | −1.81(3.52) | −0.59(3.14)† | −2.91(4.40) |
| WAIS Similarities | 2.43(3.91) | 1.00(2.98) | 2.44(3.91) | 1.48(2.87) |
| Trails A Time (seconds) | −11.17(17.47)‡ | 2.08(18.08) | −4.11(18.37)* | 4.24(16.63) |
| Trails B Time (seconds) | −38.21 (65.35)‡ | 0.33(65.51) | 3.00(61.96) | 16.90(62.40) |
| Clock drawing test | 1.52(4.18) | 1.29(5.74) | 3.00(5.59) | 2.14(4.39) |
| Boston Naming Test (correct names) | 1.76(2.01) | 1.15(3.13) | 1.30(2.09) | 1.17(3.38) |
| Mattis Dementing Rating Scale (total score) | 4.59(6.73)‡ | 0.41 (8.48) | 2.15(7.23) | 0.00(5.98) |
| Mattis attention score | 0.48 (0.99)† | 0.19(0.68) | 0.15(0.99) | −0.04(0.71) |
| Mattis initiation score | 1.06(4.03)‡ | −1.07(2.85) | −0.22(3.74) | −1.26(2.91) |
| Mattis construction score | −0.14(0.58) | −0.13(1.39) | 0.04(0.71) | 0.13(1.39) |
| Mattis conceptualization score | 1.52(3.25) | 0.74 (5.40) | 1.19(2.95) | 0.52(2.29) |
| Mattis memory score | 1.66(2.14)† | 0.81 (1.86) | 1.00(1.57) | 0.65(1.75) |
| Whole brain volume (% of total intracranial volume) | 0.47 (0.86)† | −0.15(1.39) | 0.41 (0.70)† | −0.24(1.26) |
All differences are shown as mean (standard deviation). For neuropsychological measures, significance levels of time-by-group interactions from mixed models are shown as
p<0.l
p < 0.05
p < 0.01.
For whole brain volume percent, †indicates P<0.05 for t-test comparison of intervention group with no intervention group. All significant changes represent improvements, except for Rey Figure (copying) in the Walking group.
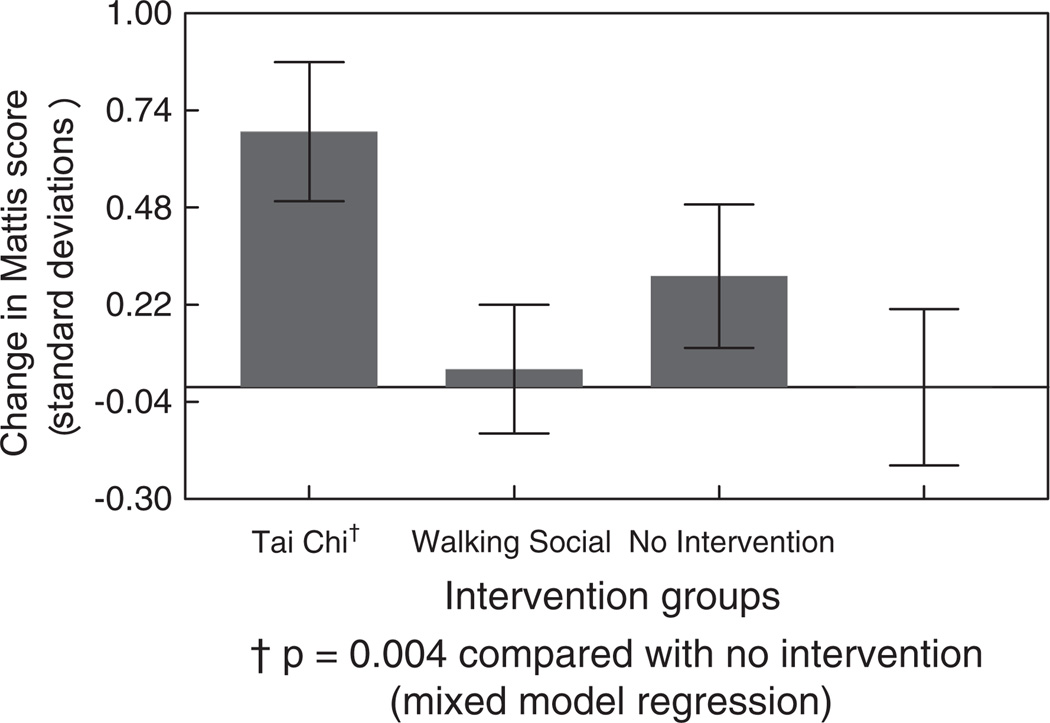
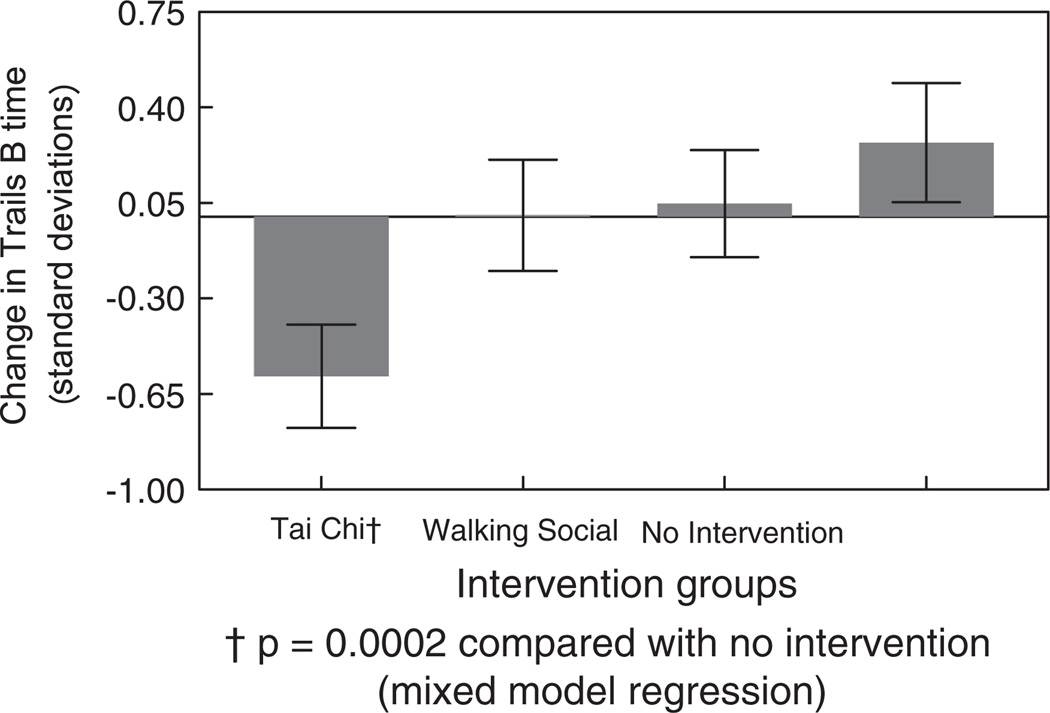
Results of the repeated-measures mixed-effect models across the three neuropsychological assessments are shown in Table 2, with significance levels indicated for time-by-group interactions. Improvements were evident on several measures in the Tai Chi group, including the Mattis Dementia Rating Scale total score (time × group interaction (TGI): t = 2.98, p = 0.004), Trailmaking Test forms A and B (Form A TGI: t = −3.26, p = 0.002; Form B TGI: t = −3.93, p = 0.0002), delayed recognition on the Auditory Verbal Learning Test (TGI: t = 2.66, p = 0.009), verbal fluency for animals (TGI: t = 2.60, p = 0.0l) and several components of the Mattis Dementia Rating Scale, including the Initiation score (TGI: t = 2.85,p = 0.005), Attention score (TGI: t = 2.44, p = 0.02), and Memory score (TGI: t = 2.37, p = 0.02). The Social Interaction group showed improvement on verbal fluency for animals (TGI: t = 2.49, p = 0.01) as well as trends for improvement (p < 0.10) on time to complete Trails A and recall after the third learning trial of the Auditory Verbal Learning Test. No significant effects or trends were observed in the Walking group as a whole when compared with the No Intervention group.
Figures 3 and 4 compare the mean change scores in the Mattis Dementia Rating Scale and time to complete the Trailmaking Test, Form B in the four groups, contrasting the results at 40 weeks with those at baseline.
Fig. 3.
Change in Mattis Dementia Rating Scale score (mean, SEM) in four intervention groups from baseline to 40 weeks.
Fig. 4.
Change in Trailmaking Test - Form B time (mean, SEM) in four intervention groups from baseline to 40 weeks.
Although no significant differences were apparent between the Walking group and the No Intervention group, when the Walking group was stratified into slow and fast walkers (above and below the median number of steps taken per week), fast walkers experienced slightly less brain tissue loss than slow walkers (0.02% vs. 0.3%, t = 0.43, p = 0.67). Repeated-measures mixed effect models showed significantly better performance in the fast walkers compared to the slow walkers over the three evaluations on the Stroop Color-Word Test (TGI: t = 2.39, p = 0.02) and on delayed recall (TGI: t =3.07, p = 0.005) and recognition (TGI: t = 2.37, p = 0.03) from the Chinese Auditory Verbal Learning Test. For each additional 1,000 steps per week in 90 min of walking (adjusted for baseline average walk speed, age, gender, and education), WAIS-R Similarities Score increased almost 2 points (p = 0.02) and two more targets were identified in the Stroop Color Word Interference Test (p = 0.04).
DISCUSSION
Compared with those in the no-intervention group, participants in the Tai Chi and Social Interaction groups showed significant increases in total brain volume over the intervention period as well as improvements on several neuropsychological measures. No statistically significant changes in brain volume or neuropsychological performance were seen in the Walking group participants compared to the no-intervention group. However, additional analyses showed that among the walkers, those who walked faster had improved scores on some cognitive tests compared to slower walkers.
Although we found increases in brain volume and improvements in performance on cognitive tests as a result of interventions, our findings differed from those of previous investigators, who reported such changes as a result of aerobic exercise, including walking. The largest and most consistent changes we observed were in the group practicing Tai Chi with smaller changes in the Social Interaction group who met three times a week to engage in lively intellectual discussion. The lack of an effect in the Walking group was unexpected. However, evaluation of the number of steps taken per minute in this group suggested that the pace adopted by the participants (median number of steps per minute =128) was relatively slow. Those who walked faster experienced greater gains on cognitive tests, suggesting that more vigorous aerobic exercise may be necessary to have a beneficial effect. Erickson and coauthors [21] used a paradigm where subjects monitored their heart rate continuously and were encouraged to attain certain target rates, whereas our participants were asked to walk around a circular course at their own speed. It is likely that the additional exercise experienced by the Walking group was small in relation to their usual walking, which in a city like Shanghai is a principal method of transportation. Walking for transportation may have put participants over the threshold for seeing an effect. A possible threshold effect of this type was reported in an observational study of exercise and dementia, where individuals who were more fit at baseline failed to show an association between exercise and dementia risk, whereas those who were less fit showed significant benefits from exercise [7].
The finding that a presumably less aerobic form of exercise, Tai Chi, had the greatest effect on brain growth and cognitive performance was unexpected, although modest gains in aerobic fitness have been demonstrated in clinical trials comparing Tai Chi participants to no intervention [36]. Tai Chi, which has been described as a type of moving meditation [37], requires continuous and sustained attention to maintenance of posture. Although advanced practitioners may be able to carry out the forms without much mental involvement, novices like those in the present study would require sustained attention. The higher level of intellectual involvement in this activity in comparison to walking around a circular course may have been a factor in leading to the disparity of results.
Although social interaction through animated discussion involves little physical activity, it too led to increases in brain volume and to improvement on a few cognitive tests. Observational cohort studies have shown that greater social engagement is associated with lower risk of dementia [22–25], although this may reflect in part reverse causation in which social disengagement is an early sign of impending dementia [24]. To our knowledge, no previous trials have been performed with a social activity intervention. The present findings are consistent with both increased brain growth and limited improvement on cognitive measures related to executive function.
Mechanisms by which exercise may contribute to enhanced cognition and brain growth include improved cerebral blood flow and oxygen delivery [38] as well as upregulation of neurotrophins, including brain-derived neurotrophic factor (BDNF), insulin-like growth factor-1, vascular endothelial growth factor, and neurotrophin granulocyte colony stimulating factor [39,40]. The most well-studied brain growth factor, BDNF, has been shown to have neuroprotective effects and to promote cell proliferation in the hippocampus and frontal cortex in addition to stimulating neurite outgrowth and synaptic plasticity [41, 42]. Although neurogenesis diminishes with age, exercise can reverse this decline [43]. In animals, blockade of BDNF action in the hippocampus eliminates the benefits of exercise on cognitive function [41], supporting the important mediating role of this neurotrophin. A recent clinical trial [21] in which human volunteers were randomized to an aerobic exercise program showed that exercise-related increases in BDNF were associated with increases in both left and right hippocampal volumes. Although most studies have focused on the effects of physical exercise on upregulation of BDNF expression, increased expression of this neurotrophin has been documented in environmentally-enriched animals [44]. The degree to which social, intellectual, and physical stimulation is responsible for this increased expression is unclear, but it suggests that expression of BDNF and other neurotrophins may be upregulated by social as well as intellectual stimulation.
Strengths of the present study include the population-based nature of the sample as well as provision of the first data relating to effectiveness of a social intervention on increases in brain volume and cognitive performance. Previous intervention studies have utilized volunteer subjects recruited through a variety of methods, limiting external validity. The present study was one of the first to assess the effectiveness of interventions in a population-based sample, a necessary step to understand how interventions are likely to affect the general population. The finding of increases in brain volume and performance on selected cognitive measures in the group engaged in stimulating social interaction suggests that mental as well as physical exercise may lead to similar benefits. This view is reinforced by the strong findings in the group practicing Tai Chi, which provides both mental and physical involvement.
Limitations included small sample size, which restricted the statistical power to examine differences between intervention groups. Even with this limitation, a consistent pattern of statistically significant findings emerged with changes in both total brain volume and performance on cognitive tests over the 8-month intervention period. However, the small sample size reduced the ability to examine in detail the associations between brain volume and cognitive changes. A further limitation was the restriction of MRI volumetric analysis to whole brain volume. Associations between changes in volume in specific brain regions, e.g., the hippocampus and the frontal lobe, and associated cognitive measures could provide important insight into the effects of particular interventions. For example, the recent report by Erickson and his coworkers [21] showed a significant association between hippocampal volume change and change in spatial memory performance.
Comparisons of the groups at baseline (Table 1) showed lower neuropsychological performance in the Tai Chi group, including lower scores on delayed free recall. Examination of the distribution of scores on this test revealed that eight of the Tai Chi group had scores of 0 or 1 compared with five in the other three groups. Because amnestic MCI was not an exclusion criterion for this study, it is possible that by chance more individuals with this condition were assigned to the Tai Chi group. With larger sample sizes, differential distributions of this type would be less likely. It may also be useful to stratify on poor performance on selected neuropsychological tests when randomly assigning subjects to groups.
It is also unclear how our findings would generalize to populations in other communities and countries. Although there is no reason to conclude that Chinese elders would respond differently from other elders, studies of similar interventions in Western populations are needed. This study was the first to show brain growth and cognitive improvement with Tai Chi as well as a social interaction intervention. These findings should be considered preliminary until replicated in an independent sample. In addition, the magnitude of brain growth demonstrated was relatively small. A longer duration study with increased sample size will be necessary to more clearly evaluate the potential of interventions to reverse brain atrophic processes and maintain cognitive abilities.
ACKNOWLEDGMENTS
This study was supported by a grant from the Johnnie B. Byrd, Sr. Alzheimer’s Center & Research Institute.
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=1204).
REFERENCES
- 1.Yoshitake T, Kiyohara Y, Kato I, Ohmura T, Iwamoto H, Nakayama K, Ohmori S, Nomiyama K, Kawano H, Ueda K. Incidence and risk factors of vascular dementia and Alzheimer’s disease in a defined elderly Japanese population: The Hisayama study. Neurology. 1995;45:1161–1168. doi: 10.1212/wnl.45.6.1161. [DOI] [PubMed] [Google Scholar]
- 2.Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- 3.Lindsay J, Laurin D, Verreault R, Hèbert R, Helliwell B, Hill GB, McDowell I. Risk factors for Alzheimer’s disease: A prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156:445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 4.Abbott RD, White LR, Ross GW, Masaki KH, Curb JD, Petro-vitch H. Walking and dementia in physically capable elderly men. JAMA. 2004;292:1447–1453. doi: 10.1001/jama.292.12.1447. [DOI] [PubMed] [Google Scholar]
- 5.Podewils LJ, Guallar E, Kuller LH, Fried LP, Lopez OL, Carlson M, Lyketsos CG. Physical activity, APOE genotype, and dementia risk: Findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639–651. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 6.Rovio S, Kåreholt I, Helkala EL, Viitanen M, Winblad B, Tuomilehto J, Soininen H, Nissinen A, Kivipelto M. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4:705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 7.Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, Kukull W. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 8.Wilson RS, Mendes De Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, Bennett DA. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- 9.Verghese J, Lipton RB, Katz MJ, Hall CB, Derby CA, Kuslan-sky G, Ambrose AF, Sliwinski M, Buschke H. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348:2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- 10.Broe GA, Creasey H, Jorm AF, Bennett HP, Casey B, Waite LM, Grayson DA, Cullen J. Health habits and risk of cognitive impairment and dementia in old age: A prospective study on the effects of exercise, smoking and alcohol consumption. Aust N Z J Public Health. 1998;22:621–623. doi: 10.1111/j.1467-842x.1998.tb01449.x. [DOI] [PubMed] [Google Scholar]
- 11.Yamada M, Kasagi F, Sasaki H, Masunari N, Mimori Y, Suzuki G. Association between dementia and midlife riskfactors: The Radiation Effects Research Foundation Adult Health Study. J Am GeriatrSoc. 2003;51:410–414. doi: 10.1046/j.1532-5415.2003.51117.x. [DOI] [PubMed] [Google Scholar]
- 12.Schuit AJ, Feskens EJ, Launer LJ, Kromhout D. Physical activity and cognitive decline, the role of the apolipoprotein e4 allele. Med Sci Sports Exerc. 2001;33:772–777. doi: 10.1097/00005768-200105000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: Women who walk. Arch Intern Med. 2001;161:1703–1708. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- 14.Barnes DE, Yaffe K, Satariano WA, Tager IB. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. J Am Geriatr Soc. 2003;51:459–465. doi: 10.1046/j.1532-5415.2003.51153.x. [DOI] [PubMed] [Google Scholar]
- 15.Richards M, Hardy R, Wadsworth ME. Does active leisure protect cognition? Evidence from a national birth cohort. Soc Sci Med. 2003;56:785–792. doi: 10.1016/s0277-9536(02)00075-8. [DOI] [PubMed] [Google Scholar]
- 16.Lytle ME, Vander Bilt J, Pandav RS, Dodge HH, Ganguli M. Exercise level and cognitive decline: The MoVIES project. Alzheimer Dis Assoc Disord. 2004;18:57–64. doi: 10.1097/01.wad.0000126614.87955.79. [DOI] [PubMed] [Google Scholar]
- 17.Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292:1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 18.Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58:176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- 19.Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, White SM, Wojcicki TR, McAuley E, Kramer AF. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 21.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer A. Exercise training increases size of hippocampus and improves memory. Proc Nat Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang HX, Karp A, Winblad B, Fratiglioni L. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: A longitudinal study from the Kungsholmen project. Am J Epidemiol. 2002;155:1081–1087. doi: 10.1093/aje/155.12.1081. [DOI] [PubMed] [Google Scholar]
- 23.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protectF against dementia. Lancet Neurol. 2004;3:343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- 24.Saczynski JS, Pfeifer LA, Masaki K, Korf ES, Laurin D, White L, Launer LJ. The effect of social engagement on incident dementia: The Honolulu-Asia Aging Study. Am J Epidemiol. 2006;163:433–440. doi: 10.1093/aje/kwj061. [DOI] [PubMed] [Google Scholar]
- 25.Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: A longitudinal cohort study. Lancet Neurol. 2006;5:406–412. doi: 10.1016/S1474-4422(06)70417-3. [DOI] [PubMed] [Google Scholar]
- 26.Wechsler D. WAIS-R Manual: Wechsler Adult Intelligence Scale-Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- 27.Gauthier L, Dehaut F, Joanette Y. The bells test: A quantitative and qualitative test for visual neglect. Int J Clin Neuropsychol. 1989;11:49–54. [Google Scholar]
- 28.Rey A. L-examen psychologique dans les cas d’encephalopathie traumatique. Arch Psychologie. 1941;28:286–340. [Google Scholar]
- 29.Golden CJ. Stroop Color and Word Test. Chicago: Stoelting Company; 1978. [Google Scholar]
- 30.Guo Q, Zhao Q, Chen M, Ding D, Hong Z. A comparison study of mild cognitive impairment with 3 memory tests among Chinese individuals. Alzheimer Dis Assoc Disord. 2009;23:253–259. doi: 10.1097/WAD.0b013e3181999e92. [DOI] [PubMed] [Google Scholar]
- 31.Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Motor Skills. 1958;8:271–276. [Google Scholar]
- 32.Rouleau I, Salmon DP, Butters N, Kennedy C, McGuire K. Quantitative and qualitative analyses of clock drawings in Alzheimer’s and Huntington’s disease. Brain Cogn. 1992;18:70–87. doi: 10.1016/0278-2626(92)90112-y. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea & Fibiger; 1983. [Google Scholar]
- 34.Mattis S. Dementia Rating Scale (DRS) Odessa, FL: Psychological assessment resources; 1988. [Google Scholar]
- 35.Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incomplete data via the EM algorithm. J Roy Stat Soc B Meth. 1977;39:1–38. [Google Scholar]
- 36.Lan C, Lai JS, Chen SY, Wong MK. 12-month Tai Chi training in the elderly: its effect on health fitness. Med Sci Sports Exercise. 1998;30:345–351. doi: 10.1097/00005768-199803000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Jin P. Efficacy of Tai Chi, brisk walking, meditation, and reading in reducing mental and emotional stress. J Psy-chosomat Res. 1992;36:361–370. doi: 10.1016/0022-3999(92)90072-a. [DOI] [PubMed] [Google Scholar]
- 38.Rogers RL, Meyer JS, Mortel KF. After reaching retirement age physical activity sustains cerebral perfusion and cognition. J Am Geriatr Soc. 1990;38:123–128. doi: 10.1111/j.1532-5415.1990.tb03472.x. [DOI] [PubMed] [Google Scholar]
- 39.Kramer AF, Erickson KI. Capitalizing on cortical plasticity: Influence of physical activity on cognition and brain function. Trends Cogn Sci. 2007;11:342–348. doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Flöel A, Ruscheweyh R, Krüger K, Willemer C, Winter B, Völker K, Lohmann H, Zitzmann M, Mooren F, Breitenstein C, Knecht S. Physical activity and memory functions: Are neurotrophins and cerebral gray matter the missing link? Neuroimage. 2010;49:2756–2763. doi: 10.1016/j.neuroimage.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 41.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 42.Cotman CW, Berchtold NC. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 43.Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol Aging. 2006;27:1505–1513. doi: 10.1016/j.neurobiolaging.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 44.Young D, Lawlor PA, Leone P, Dragunow M, During MJ. Environmental enrichment inhibits spontaneous apo-tosis, prevents seizures and is neuroprotective. Nat Med. 1999;5:448–453. doi: 10.1038/7449. [DOI] [PubMed] [Google Scholar]