Abstract
Focal cartilage defects reduce the ability of articular cartilage to resist mechanical loading and provide lubrication during joint motion. The limitations in current surgical treatments have motivated the use of biocompatible scaffolds as a future treatment option. Here we describe a second-generation, macroporous, polyvinyl alcohol (PVA) scaffold with independently tunable morphological and mechanical properties. The compressive moduli of the PVA scaffold increased with increasing polymer concentration and applied compressive strain, with values in the range for human articular cartilage (HA >1000 kPa, EY >500 kPa). Scaffolds also possessed strain-dependent permeability and Poisson’s ratio. The interconnected macroporous network was found to facilitate chondrocyte seeding and proliferation through the scaffold over 1 week in culture. Overall, these promising characteristics demonstrate the potential of this macroporous scaffold for future studies in focal cartilage defect repair.
Localized damage to articular cartilage alters its mechanical properties and impairs its ability to provide lubrication and distribute mechanical loads during joint motion (Gratz, et al., 2009). These focal cartilage defects can result in debilitating pain and the size of the defects can progressively increase over time, leading to extensive osteoarthritis in the joint (Heir, et al., 2009; Davies-Tuck, et al., 2008). Though current surgical treatments such as microfracture, mosaicplasty, and autologous chondrocyte transplantation can relieve pain and restore joint function in the short-term (≤ 2 years), recent studies have reported deteriorating clinical outcomes at longer follow-up times (>5 years post-operatively) (Solheim, et al., 2010; Moseley, et al., 2010; Mithoefer, et al., 2009). The limitations of current treatments have motivated the development of implantable scaffolds to encourage cartilage regeneration as a treatment for focal cartilage defects.
Our laboratory developed a non-biodegradable porous scaffold intended to repair focal cartilage defects. Our concept was that the scaffold would have a porous structure conducive to integration with the host tissue via cell migration into the scaffold and matrix generation at the interface, while the non-biodegradable component would provide both short-term and long-term mechanical stability in the defect site. Our polymer of choice for this concept was polyvinyl alcohol (PVA) hydrogel because of its biocompatibility (Maher, et al., 2007) and prior use in FDA approved medical devices such as contact lenses, wound dressings, orthopaedic devices, and drug delivery vehicles (Hyon, et al., 1994; Bourke, et al., 2003; Kobayashi, et al., 2001; Yamagata, et al., 1979). The polymerization of PVA is readily achieved via freeze-thaw cycling of aqueous PVA solutions, which introduces physical cross-links in the monomer via hydrogen bonding, polymer crystallization, and phase separation (Stauffer, et al., 1992). We previously developed a method of manufacture to allow control over the composition and the mechanical properties of the scaffold that also facilitated cell viability and matrix generation (Scholten, et al., 2011). However, the pores generated using this first generation method of manufacture were dependent on the release of dichloromethane during the polymerization process, which resulted in a variable and at times non-interconnected pore structure. In this paper, we present an improved, second generation method of manufacture of a PVA scaffold intended to provide enhanced control over porosity and pore interconnectivity for ease of cell-seeding, while retaining our ability to control scaffold mechanical properties.
To create the macroporous PVA scaffolds, surgical gelatin sponges (Ethicon – Johnson & Johnson, Somerville, NJ) were saturated in deionized water and then incubated in a graded series of PVA (Sigma Aldrich, St. Louis, MO) solutions made with deionized water until reaching either 10% or 20% wt/vol PVA concentration. PVA-soaked sponges were frozen to −20°C for 20 hours and thawed at 25°C for 4 hours and this freeze-thaw process was repeated 4 additional times. The PVA sponges were subsequently frozen for storage at −20°C and thawed for final processing at a later time, resulting in 6 total freeze-thaw cycles at which the PVA mechanical properties plateau (Holloway, et al., 2011). Disks (Ø5mm diameter) were cored out of the PVA sponges with biopsy punches and cut to a thickness of 2.5 mm on a freezing stage sledge microtome to ensure parallel flat surfaces. The disks (Ø5 × 2.5mm) were then thawed and digested for 14 hours with 500 units/ mL of collagenase (Type II, Worthington Biochemical Corp., Lakewood, NJ) at 37°C to completely remove the gelatin sponge. The resulting macroporous PVA scaffolds were washed under gentle agitation 3× for 30 minutes in deionized water to remove the collagenase solution and equilibrated in phosphate buffered saline (PBS) under gentle agitation for 30 minutes prior to analysis.
Environmental scanning electron micrographs of the hydrated scaffolds using a Quanta 600 microscope (FEI, Hillsboro, OR) showed that both 10% and 20% PVA scaffolds (n=6 per concentration group) possessed a randomly oriented, fibrous appearance with numerous interconnected, open pores (Figure 1A, only 10% PVA shown). To quantify the morphology, images were taken at 5 different positions along the surface and processed in ImageJ (NIH, Bethesda, Maryland) to determine pore size and apparent porosity for each individual scaffold. Given the skew in the pore size data, a non-parametric Wilcoxon sum-rank test was performed to compare data between concentration groups and a parametric t-test was performed to compare the apparent porosity data (α=0.05). There was no significant difference in pore diameter (10% PVA average pore diameter: 15.7 µm, range 8–304 um; 20% PVA average 17.5 µm, range of 7.5–410 um; p>0.05) or in apparent porosity (10% PVA: 81 ± 5%, 20% PVA 74 ± 6%; p>0.05) between the 10% and 20% PVA scaffolds indicating that the scaffold morphologies were statistically similar.
Figure 1.
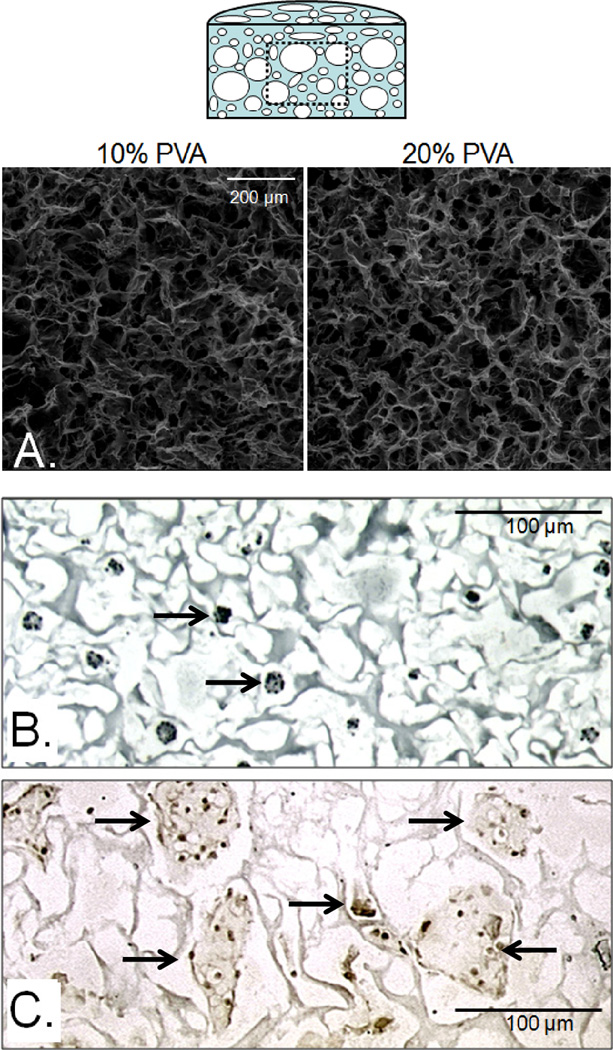
Representative coronal (cross-sectional) images of the central regions of the scaffolds (top schematic). Environmental scanning micrographs of the central region of 10% and 20% macroporous PVA scaffolds (A) revealed a fibrous, randomly oriented morphology with pores large enough for cell migration. Through a dehydration/rehydration process, chondrocytes were seeded throughout the scaffold (B, arrows, hematoxylin) and were found to remain viable and proliferate (C, arrows, BrdU immunohistochemistry) over 1 week in culture.
Confined and unconfined compressive testing (n=5 per concentration group) using methods previously described (Scholten, et al., 2011) found that both 10% and 20% PVA scaffolds possessed strain-dependent aggregate (confined) modulus (HA), Young’s (unconfined) modulus (EY), permeability (k), and Poisson’s ratio (υ) (Table 1). Scaffolds were tested in unconfined compression with applied strains (ε) from 10% – 70% of the original scaffold height. After unconfined tests, scaffolds were allowed to recover in PBS for 30 minutes to their original heights and then tested in confined compression up to ε = 70% for 10% PVA scaffolds and ε = 60% for 20% PVA scaffolds (due to limits of the load cell). Data from both mechanical tests were curve-fit (Jurvelin, et al., 1997; Mow, 1980) to determine k and υ values. Material properties were compared using two-way ANOVA with Scheffe post-hoc tests (α=0.05) and dependent variables being HA, EY, k, υ and the independent variables being scaffold polymer concentration and ε. Both the HA and EY for scaffolds increased significantly with applied strains of greater than or equal to 40%, with 20% PVA scaffolds having significantly higher values at all tested strains compared to 10% PVA scaffolds (p<0.05). The Poisson’s ratio increased from ~0.20 for both 10% and 20% scaffolds to ~0.40 at 60% applied strain. Permeability decreased by several orders of magnitude with increasing strain. No significant differences in k or υ existed between the scaffold groups.
Table 1.
Macroporous PVA scaffolds exhibited concentration and strain-dependent material properties.
| 10% PVA | ||||
|---|---|---|---|---|
| Strain | HA (kPa) | EY (kPa) | υ | k (m4/ N s) |
| 10% | 13.20 ± 1.55 | 11.67 ± 0.70 | 0.20 ± 0.04 | 1.88E-11 ± 6.20E-12 |
| 20% | 13.90 ± 0.97 | 11.70 ± 0.77 | 0.24 ± 0.02 | 1.56E-11 ± 9.80E-12 |
| 30% | 18.16 ± 2.08 | 15.09 ± 1.97 | 0.25 ± 0.01 | 1.99E-11 ± 5.75E-12 |
| 40% | 50.34 ± 12.64 † | 36.54 ± 8.67 † | 0.31 ± 0.01 † | 1.42E-14 ± 6.45E-15 † |
| 50% | 112.87 ± 15.38 † | 75.61 ±12.83 † | 0.33 ± 0.01 ‡ | 5.48E-16 ± 5.59E-17 † |
| 60% | 378.99 ± 64.48† | 194.83 ± 27.17 † | 0.38 ± 0.01 † | 1.12E-16 ± 7.61E-17 § |
| 70% | 2165.81 ± 254.58 † | 711.75 ± 33.42 † | 0.43 ± 0.01 † | 2.34E-16 ± 9.10E-17 § |
| 20% PVA | ||||
| Strain | HA (kPa)* | EY (kPa)* | υ | k (m4/ N s) |
| 10% | 66.15 ± 7.62 | 58.36 ± 3.49 | 0.21 ± 0.04 | 1.22E-11 ± 4.10E-12 |
| 20% | 73.66 ± 2.72 | 60.45 ± 3.50 | 0.25 ± 0.04 | 1.41E-11 ± 7.70E-12 |
| 30% | 95.24 ± 16.59 | 75.17 ± 10.16 | 0.27 ± 0.02 | 1.23E-11 ± 6.10E-12 |
| 40% | 253.67 ± 66.45 † | 184.34 ± 46.49 † | 0.31 ± 0.01 | 1.33E-14 ± 1.32E-15† |
| 50% | 559.06 ± 79.74 † | 374.74 ± 66.65 † | 0.33 ± 0.01 † | 7.83E-16 ± 9.26E-17 § |
| 60% | 1694.52 ± 157.01 † | 827.11 ± 33.64 † | 0.39 ± 0.01 † | 3.12E-16 ± 1.02E-16 § |
| 70% | X | 1807.32 ± 51.22 † | X | X |
Increased concentration resulted in increased compressive aggregate and Young’s moduli (HA and EY respectively, *p<0.05 vs. 10% PVA), with no effect on Poisson’s ratio (υ) or hydraulic permeability (k). For both PVA concentration scaffolds, HA, EY, and υ increased with applied strain whereas k decreased with applied strain. Values of HA and EY at 60–70% strain are in the range of human articular cartilage (Armstrong, et al., 1982; Athanasiou, et al., 1994).
p<0.05 vs. previous strains,
p<0.05 vs. 10 – 30% strains,
p<0.05 vs. 10 – 40% strains,
X – Compressive testing was not performed due to limits of load cell
The strain-dependent changes in the material properties of the scaffold are likely due to the collapse of the macropores at higher compressive strains. Indeed, at low strains, the compressive moduli and Poisson’s ratio of the macroporous scaffold were lower than reported values for non-macroporous PVA scaffolds whereas the permeability was several orders of magnitude higher (Stauffer, et al., 1992; Scholten, et al., 2011; Fromageau, et al., 2007; Urayama, et al., 1993). With increasing strain and collapse of the macropores, these values approach those reported in the literature for non-macroporous PVA hydrogels. The highest measures values for HA and EY for 10% PVA (HA: 2165.81±254.56 kPa, EY: 711.75±33.42 kPa; @ ε = 70%) and 20% PVA (HA: 1694.52±157.01 kPa @ ε = 60%, EY: 1807.32±51.22 kPa @ ε = 70%) are in the range of reported values for distal and proximal femoral articular cartilage in humans (HA: ~1000 kPa, EY: ~500 kPa) (Armstrong, et al., 1982; Athanasiou, et al., 1994; Jurvelin, et al., 2003). As all scaffolds recovered to their original dimensions after testing and exhibited elastic (linear) stress-strain behavior during the compression ramps, there is no evidence from the mechanical testing that any plastic deformation is occurring within the scaffold at strains up to 60–70%. However, direct visualization under high power EM is needed to confirm the presence/absence of micro-damage within the scaffold. In addition, at the higher strains, the values of k and υ derived from the models may not be accurate. Further direct measurements of these parameters are planned.
To test the ease of cell seeding and ability of chondrocytes to survive in the scaffold, a new batch of 10% PVA scaffolds (Ø5×2.5mm, n=10) were created and after the final washing steps in deionized water, the scaffolds were disinfected in 100% EtOH for 30 minutes. The ethanol was removed, the scaffolds dried under vacuum and heat for 2–3 hours, and placed in 24 well plates. Then, 200 µL of a cell suspension containing 20 × 106 juvenile bovine chondrocytes/mL in Advanced DMEM (Invitrogen, Carlsbad, CA) culture media was pipetted into the well around the scaffold. The scaffolds quickly absorbed the cell suspension and began to rehydrate. After the initial absorption, an additional 600 µL of cell suspension was added, 200µL at a time, until the scaffold appeared to be fully rehydrated. The scaffolds were incubated at 37°C and 5% CO2 for 30 minutes to allow for cell attachment and settling. Immediately after this seeding protocol, a subset of scaffolds (n=5) were fixed in 10% neutral buffered formalin, processed and embedded, and stained with hematoxylin. Cross-sectional/coronal images (schematic in Figure 1) of hematoxylin stained scaffolds showed chondrocytes were present through the scaffold after seeding (Figure 1B). The remaining scaffolds (n=5) were cultured for 7 days in ADMEM media and then incubated for 24 hours in 10 µM BrdU, a molecule incorporated into cells during DNA synthesis and proliferation. All seeded chondrocytes were positive for BrdU (Figure 1C), indicating that the cells were actively synthesizing DNA and proliferating within the scaffold.
The main goal in creating this second-generation scaffold was to incorporate a controllable, interconnected, macroporous network in a non-biodegradable PVA scaffold while retaining an ability to control scaffold mechanical properties. Our previous method of manufacture relied on the effervescence of dichloromethane (DCM) out of the scaffold during the freeze-thawing process to create macropores (Scholten, et al., 2011). However, precise control over pore interconnectivity was not possible; moreover injection of cells into the scaffold was the only method to ensure cell seeding, which resulted in a non-uniform distribution of cells. The improved technique presented in this paper creates a macroporous interconnected porous network that can be easily rehydrated with a cell suspension for uniform seeding. Our results show that by increasing PVA concentration, we can increase the scaffold mechanical properties (specifically modulus) without affecting scaffold porosity or pore diameter. This would imply that the scaffold mechanical properties and morphology are independently controlled using this method. Compared to articular cartilage, the values for HA, EY, and k for both 10% PVA and 20% PVA at high compressive strains approximate the properties of articular cartilage found in humans (Armstrong, et al., 1982; Athanasiou, et al., 1994; Jurvelin, et al., 2003). This implies that scaffold material properties can be modified by increasing polymer concentration and/or over-sizing the scaffold such that with an applied pre-strain, the scaffold elicits higher mechanical properties than at low strain. In principle, we could use any other degradable materials to generate the pore network such as polyglyolic acid (Freed, et al., 1994) or polylactic acid (Cao, et al., 1997). Controlled weaving of the porogenic material (Moutos, et al., 2007) would allow for the creation of directed channels if such a need arise or if this were found to present some advantage.
Regarding cell-polymer interactions, though the histology confirmed that the seeded chondrocytes were actively synthesizing DNA, the organization of the cells into clusters implies the lack of cell-attachment sites on the PVA surface. This lack of cell-attachment would also impact the seeding efficiency of the scaffold and the potential for fluid flow or mechanical forces to induce cell loss. With further time in culture, secreted extracellular matrix molecules may adsorb to the polymer surface facilitating chondrocyte attachment to the scaffold. Alternatively, the PVA surface itself may be modified to covalently bond to an attachment protein such as fibronectin to promote cell attachment (Nuttelman et al, 2001), which is currently being researched by our laboratory to address these limitations. In conclusion, we have demonstrated a novel method of manufacture of an interconnected, macroporous non-degradable PVA scaffold, capable of facilitating cell migration, the mechanical properties and morphology of which can be controlled.
ACKNOWLEDGMENTS
This research was supported by the NIH (TL1RR024998 - KW Ng, Core Center AR046121, Research Facilities Improvement Program C06-RR12538-01), Clark Foundation, Kirby Foundation, Leo Rosner Foundation, and the Russell Warren Chair for Tissue Engineering.
Footnotes
ETHICAL CONSIDERATIONS
No approval was necessary from IACUC or any institutional ethics committee for this work.
CONFLICT OF INTEREST
There are no financial conflicts of interest to report with this research. Complete conflict of interest forms will be submitted when this manuscript is accepted, as per author guidelines.
REFERENCES
- Gratz KR, Wong BL, Bae WC, Sah RL. The effects of focal articular defects on cartilage contact mechanics. J Orthop Res. 2009;27:584–592. doi: 10.1002/jor.20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heir S, Nerhus TK, Rotterud JH, Loken S, Ekeland A, Engebretsen L, Aroen A. Focal Cartilage Defects in the Knee Impair Quality of Life as Much as Severe Osteoarthritis: A Comparison of Knee Injury and Osteoarthritis Outcome Score in 4 Patient Categories Scheduled for Knee Surgery. Am J Sports Med. 2009 doi: 10.1177/0363546509352157. [DOI] [PubMed] [Google Scholar]
- Davies-Tuck ML, Wluka AE, Wang Y, Teichtahl AJ, Jones G, Ding C, Cicuttini FM. The natural history of cartilage defects in people with knee osteoarthritis. Osteoarthritis Cartilage. 2008;16:337–342. doi: 10.1016/j.joca.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Solheim E, Hegna J, Oyen J, Austgulen OK, Harlem T, Strand T. Osteochondral autografting (mosaicplasty) in articular cartilage defects in the knee: results at 5 to 9 years. Knee. 2010;17:84–87. doi: 10.1016/j.knee.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Moseley JB, Jr, Anderson AF, Browne JE, Mandelbaum B, Micheli LJ, Fu F, Erggelet C. Long-term durability of autologous chondrocyte implantation : A multicenter, observational study in U.S. patients. Am J Sports Med. 2010;38:238–246. doi: 10.1177/0363546509348000. [DOI] [PubMed] [Google Scholar]
- Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37:2053–2063. doi: 10.1177/0363546508328414. [DOI] [PubMed] [Google Scholar]
- Maher SA, Doty SB, Torzilli PA, Thornton S, Lowman AM, Thomas JD, Warren R, Wright TM, Myers E. Nondegradable hydrogels for the treatment of focal cartilage defects. J Biomed Mater Res A. 2007;83:145–155. doi: 10.1002/jbm.a.31255. [DOI] [PubMed] [Google Scholar]
- Hyon SH, Cha WI, Ikada Y, Kita M, Ogura Y, Honda Y. Poly(vinyl alcohol) hydrogels as soft contact lens material. J Biomater Sci Polym Ed. 1994;5:397–406. doi: 10.1163/156856294x00103. [DOI] [PubMed] [Google Scholar]
- Bourke SL, Al-Khalili M, Briggs T, Michniak BB, Kohn J, Poole-Warren LA. A photo-crosslinked poly(vinyl alcohol) hydrogel growth factor release vehicle for wound healing applications. AAPS PharmSci. 2003;5:E33. doi: 10.1208/ps050433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Toguchida J, Oka M. Development of polyvinyl alcohol-hydrogel (PVA-H) shields with a high water content for tendon injury repair. J Hand Surg [Br] 2001;26:436–440. doi: 10.1054/jhsb.2001.0581. [DOI] [PubMed] [Google Scholar]
- Yamagata S, Handa H, Taki W, Yonekawa Y, Ikada Y, Iwata H. Experimental nonsuture microvascular anastomosis using a soluble PVA tube and plastic adhesive. J Microsurg. 1979;1:208–215. doi: 10.1002/micr.1920010307. [DOI] [PubMed] [Google Scholar]
- Stauffer SR, Peppast NA. Poly(vinyl alcohol) hydrogels prepared by freezing-thawing cyclic processing. Polymer. 1992;33:3932–3936. [Google Scholar]
- Scholten PM, Ng KW, Joh K, Serino LP, Warren RF, Torzilli PA, Maher SA. A semi-degradable composite scaffold for articular cartilage defects. J Biomed Mater Res A. 2011 doi: 10.1002/jbm.a.33005. e-publication Feb 11 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway JL, Spiller KL, Lowman AM, Palmese GR. Analysis of the in vitro swelling behavior of poly(vinyl alcohol) hydrogels in osmotic pressure solution for soft tissue replacement. Acta Biomater. 2011;7:2477–2482. doi: 10.1016/j.actbio.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Jurvelin JS, Buschmann MD, Hunziker EB. Optical and mechanical determination of Poisson's ratio of adult bovine humeral articular cartilage. Journal of Biomechanics. 1997;30:235. doi: 10.1016/s0021-9290(96)00133-9. [DOI] [PubMed] [Google Scholar]
- Fromageau J, Gennisson JL, Schmitt C, Maurice RL, Mongrain R, Cloutier G. Estimation of polyvinyl alcohol cryogel mechanical properties with four ultrasound elastography methods and comparison with gold standard testings. IEEE Trans Ultrason Ferroelectr Freq Control. 2007;54:498–509. doi: 10.1109/tuffc.2007.273. [DOI] [PubMed] [Google Scholar]
- Urayama K, Takigawa T, Masuda T. Poisson's ratio of poly(vinyl alcohol) gels. Macromolecules. 1993;26:3092–3096. [Google Scholar]
- Armstrong CG, Mow VC. Variations in the intrinsic mechanical properties of human articular cartilage with age, degeneration, and water content. J Bone Joint Surg Am. 1982;64:88–94. [PubMed] [Google Scholar]
- Athanasiou KA, Agarwal A, Dzida FJ. Comparative study of the intrinsic mechanical properties of the human acetabular and femoral head cartilage. J Orthop Res. 1994;12:340–349. doi: 10.1002/jor.1100120306. [DOI] [PubMed] [Google Scholar]
- Jurvelin JS, Buschmann MD, Hunziker EB. Mechanical anisotropy of the human knee articular cartilage in compression. Proc Inst Mech Eng H. 2003;217:215–219. doi: 10.1243/095441103765212712. [DOI] [PubMed] [Google Scholar]
- Freed LE, Vunjak-Novakovic G, Biron RJ, Eagles DB, Lesnoy DC, Barlow SK, Langer R. Biodegradable polymer scaffolds for tissue engineering. Biotechnology (N Y) 1994;12:689–693. doi: 10.1038/nbt0794-689. [DOI] [PubMed] [Google Scholar]
- Cao Y, Vacanti JP, Paige KT, Upton J, Vacanti CA. Transplantation of chondrocytes utilizing a polymer-cell construct to produce tissue-engineered cartilage in the shape of a human ear. Plast Reconstr Surg. 1997;100:297–302. doi: 10.1097/00006534-199708000-00001. discussion 303–4. [DOI] [PubMed] [Google Scholar]
- Moutos FT, Freed LE, Guilak F. A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nat Mater. 2007;6:162–167. doi: 10.1038/nmat1822. [DOI] [PubMed] [Google Scholar]


