Abstract
Small vessel (SV) and large vessel (LV) brain infarcts are distinct pathologies. Using a homebound elderly sample, the numbers of either infarct subtypes were similar between those apolipoprotein E4 allele (ApoE4) carriers (n = 80) and noncarriers (n = 243). We found that the higher the number of SV infarcts, but not LV infarcts, a participant had, the higher the activity of substrate V degradation in serum especially among ApoE4 carriers (β=+0.154, SE = 0.031, P < .0001) after adjusting for the confounders. Since substrate V degradation could be mediated by insulin-degrading enzyme (IDE) or/and angiotensin-converting enzyme (ACE), but no relationship was found between SV infarcts and specific ACE activities, blood IDE may be a useful biomarker to distinguish the brain infarct subtypes. Insulin-degrading enzyme in blood may also imply an important biomarker and a pathological event in Alzheimer disease through SV infarcts in the presence of ApoE4.
Keywords: small vessel brain infarct, apolipoprotein E4 allele, substrate V degradation
Introduction
Small vessel (SV) and large vessel (LV) infarcts have different locations in the brain and are believed to represent distinctive pathological processes.1 Small vessel infarcts are caused by lipohyalinosis of small subcortical vessels and are generally related to chronic hypertension, and LV infarcts are usually caused by LV stenosis, artery-to-artery or cardiac embolism, and hemodynamic mechanisms.2 Despite the distinction in the brain locations and infarct size, there is a lack of studies examining the blood biochemistry or biomarkers of individual infarct subtypes.
Apolipoprotein E4 allele (ApoE4) is a genetic risk factor for both Alzheimer disease (AD) and vascular diseases including stroke, diabetes, hypertension, and atherosclerosis.3,4 Apolipoprotein E4 allele facilitates the formation of cerebral amyloid angiopathy (CAA) in patients with AD5,6 and in the amyloid precursor protein (APP) transgenic mice.7 A probable etiology for cerebral infarcts is through the deposits of amyloid-β peptide (Aβ) in cerebrovasculature to form CAA.8,9 Both insulin-degrading enzyme (IDE) and angiotensin-converting enzyme (ACE) are involved in the vascular pathology and mediate the degradation of Aβ, the major component of AD pathology and CAA.10,11 Using fluorogenic substrate V, which is a substrate of both IDE and ACE, our recent study has demonstrated the existence of IDE and ACE in human serum.12 The goal of this study was to examine the association between brain infarct subtypes determined by MRI and the biomarkers including ApoE4 allele, substrate V degradation, and ACE-specific activities in a cross-sectional homebound elderly population.
Methods
Study Sample
We studied a subgroup of 341 from a population based study, the nutrition, aging, and memory in the elderly (NAME) study.13 These subjects underwent clinical evaluation by physicians and had a brain magnetic resonance imaging (MRI) in the hospital. The NAME study was based on the clients of 4 home care agencies in the city of Boston. Anyone receiving home care services is registered with 1 of these agencies, if he or she lives in the city of Boston, has an annual income <$18 890, and needs home care service. All homebound elderly individuals aged 60 and older receiving services from these agencies were invited to participate in the study. Of all eligible participants, 66% enrolled in the study and gave informed consent approved by the Institutional Review Board of Tufts University New England Medical Center.14 Those with Mini-Mental State Examination (MMSE) ≤10 or verbal IQ <75 were not eligible to continue in the study. In total, 1262 participants completed the demographic and neuropsychological evaluation during the home visits and were asked whether they would be willing to participate in the second phase of the study, which involved going to the hospital and undergoing examination by the physicians and undergoing a brain MRI. The data of participants who came to the hospital were compatible with the whole study sample.13
Brain Magnetic Resonance Imaging
Magnetic resonance imaging scans were performed on a 1.5 T magnet (Siemens' Symphony; Islin, New Jersey). All participants had the following imaging protocol: (1) Intermediate and T2-weighted conventional spin-echo axial images, (2) fluid attenuation inversion recovery (FLAIR) axial images, and (3) magnetization prepared rapid acquisition gradient echo (MPRAGE) coronal 3D images with a section thickness of 1.5 mm.15
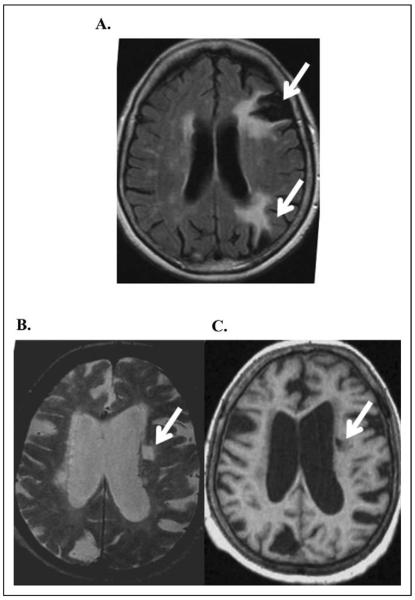
All MRI scans were evaluated by a board-certified neuroradiologist for the presence and number of brain infarcts. Large vessel infarcts were defined as infarcts in a major vascular territory or subcortical infarcts larger than 1.5cm in size.15 An infarct of any size in a cortical location was considered to be a manifestation of LV disease.16 Small vessel infarcts were defined as a focal subcortical brain lesion between 3 mm and 1.5cm in size, hyperintense on T2-weighted images, and hypointense on T1-weighted images (Figure 1).
Figure 1.
Large vessel infarcts and small vessel infarcts. A large vessel infarct was defined as an infarct in a vascular territory extending to the cortex or a subcortical infarct larger than 1.5 cm in size. Large vessel infarcts in left frontal and parietal regions are illustrated by arrows (A). A small vessel infarct was defined as an infarct in subcortical region which is high signal on T2-weighted (B) and low signal on T1-weighted images (C) and was between 3 mm and 1.5 cm in size. A small vessel infarct in the left periventricular white matter is illustrated by arrows (B and C).
Characterizing Proteases Activities Mediating Aβ Degradation in Human Serum
Substrate V, Mca-RPPGFSAFK (Dnp)-OH,17 is a synthetic short peptide, that serves as a substrate for IDE and ACE in serum12 and becomes fluorogenic after being degraded. To characterize the protease activity in human serum, we used 10 μmol/L fluorogenic substrate V to incubate with different amounts of serum in the presence of phosphate-buffered saline (PBS) at 37° C for various periods of time. Fluorometry with excitation at 320 nm and emission at 405 nm was used to measure the fluorescent intensity, and the relative fluorencent units (RFU) was indicated. To prove that this substrate V assay could be used to evaluate Aβ degradation mediated by these proteases, we preincubated serum with 10ng/mL synthetic Aβ1-40 at 37° C for 3 hours followed by adding substrate V and the continuation of incubation to examine the inhibition of substrate V degradation by Aβ.
ApoE Genotyping and Plasma Aβ Measurments
A 244-bp fragment of the ApoE gene including the 2 polymorphic sites was amplified by polymerase chain reaction (PCR) using a robotic Thermal Cycler (ABI 877, Perkin-Elmer/Applied Biosystems, Bedford, MA, USA), using oligonucleotide primers F4 (5′-ACAGAATTCGCCCCGGCCTGGTACAC-3′) and F6 (5′-TAAGCTTGGCACGGCTGTCCAAGGA-3′). The PCR products were digested with 5 units of Hha I and the fragments were separated by electrophoresis on 8% polyacrylamide nondenaturing gel. The specific allelic fragments were E2, E3, and E4. Apolipoprotein E4 allele was defined by E4/4, E3/4, or E2/4.18
The sandwich Aβ enzyme-linked immunosobent assay (ELISA) was used to measure plasma Aβ40 and Aβ4219,20 with some modifications. In brief, NUNC Maxisorb immunoassay plates were coated with 0.3 μg/well of 2G3 (anti-Aβ40) and 21F12 (anti-Aβ42) antibodies individually in PBS overnight at 4° C. Plates were subsequently blocked with Block ACE (Serotec Ltd, Raleigh, NC, USA) for 2 hours at room temperature. The plates were briefly washed with PBS-Tween (PBS-T). Samples were loaded on the plates and incubated overnight at 4° C. After washing the plates with PBS-T twice, they were then incubated with a biotinylated monoclonal anti-N terminus Aβ antibody (3D6B) for 2 hours. Then, Streptavidin-conjugated alkaline phosphatase (Promega, Madison, WI, USA) was added and incubated for another 1.5 hours. Finally, signal was amplified by adding 100 μL alkaline phosphatase fluorescent substrate (Promega) into each well. Fluorescence was measured with excitation at 444 nm and emission at 555 nm (VICTOR3, Perkin-Elmer). Detection limits were 3.125 pg/mg for both Aβ 1-40 and 1-42.
Clinical Diagnoses of Dementia
The diagnosis of dementia was based on the Diagnostic and Statistical Manual Manual of Mental Disorders (Fourth Edition; DSM-IV) criteria. The National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) guidelines21 were used to determine whether the criteria were met for a diagnosis of possible or probable AD. Possible AD was marked by differences in clinical features and course in addition to the MRI imaging data so that the etiology of dementia is not certain for AD compared with probable AD. We combined possible and probable AD to the AD category in this study. Vascular dementia was defined as having dementia after a clinical history of stroke. Body mass index (BMI) was measured and calculated as body mass (in kg) divided by the squared height (in m2).
Statistical Analysis
Statistical analysis was performed using SAS (version 9.1). Participants were divided according to absence or presence of infarct subtypes or categorized according to the number of infarct subtype: absence of infarct (0), 1 infarct (1), and more than 1 infarct (2). Mean ± standard deviation (SD) and t test or analysis of variance (ANOVA) were used for the variables with a normal distribution, and median (Q1, Q3) and Wilcoxon rank sum test or Kurskal-Wallis test were used for the variables with a skewed distribution. The chi-square test was used to compare proportions for binary end points. Linear regression was used to examine the associations between substrate V degradation as an outcome and the infarct subtypes or the interaction between a infarct subtype and ApoE4 allele while adjusting for potential confounders including age, gender, and BMI. For all analyses, the 2-sided significant level of .05 was used.
Results
A total of 323 homebound elderly individuals from the NAME study were used for this study analysis. These participants came to the hospital to have a brain MRI and had the data on the brain infarct subtypes and the ApoE4 allele.13 In total, 8% (n = 27) had LV infarcts, 18% (n = 60) had SV infarcts, and 2% (n = 8) had both LV and SV infarcts on brain MRI (Figure 1). The average age of this study sample was 73.3 ± 8.3 (mean ± SD) years old, and 73% were females. The sample was multiethnic with 62% white, 35% African American, and 3% other ethnicities. In total, 76% of the participants completed a high school education or above.
Of the homebound elderly individuals, 25% participants were ApoE4 carriers (n = 80). We first examined whether there was an association between ApoE4 allele and the infarct subtypes. Table 1 shows that there were no differences in the demographic information except ethnicity between those with and without ApoE4 allele. The numbers of cases with either infarct subtypes in addition to the peripheral vascular diseases including cardiovascular disease, diabetes, and hypertension in those with and without ApoE4 allele did not show statistical differences. We compared the serum activities of Aβ-degrading proteases between the ApoE4 carriers and ApoE4 noncarriers. Again, there were no differences of substrate V degradation, ACE N-domain, and ACE C-domain activities between the presence and the absence of ApoE4.
Table 1.
Demographic and Medical Status of Nonapolipoprotein E4 Alleleand Apolipoprotein E4 Carriers in the Homebound Elderly Populationa
| Demographic Data | ApoE4 + N = 80 | ApoE4 − N = 243 | P Values |
|---|---|---|---|
| Age, year, mean ± SD | 72.0 ± 7.7 | 73.6 ± 8.4 | .16 |
| High school graduate and above, n/total (%) | 60/78 (76.9%) | 182/243 (74.9%) | .48 |
| BMI, kg/m2, mean ± SD | 31.5 ± 6.9 | 30.5 ± 8.1 | .12 |
| African Americans, n/total (%) | 38/80 (47.5%) | 71/242 (29.3%) | .04 |
| Female, n/total (%) | 55/80 (68.8%) | 187/243 (73.3%) | .44 |
| MMSE score, , mean ± SD | 25.3 ± 3.3 | 25.8 ± 3.0 | .18 |
| Cardiovascular disease, n/total (%) | 26/76 (34.2%) | 90/238 (37.8%) | .57 |
| Diabetes, n/total (%) | 30/80 (37.5%) | 69/243 (28.4%) | .13 |
| Hypertension, n/total (%) | 67/79 (84.8%) | 199/239 (83.3%) | .75 |
| ACE inhibitor use, n/total (%) | 34/80 (42.5%) | 86/243 (35.4%) | .25 |
| Cerebral infarct subtypes and serum Aβ-degrading proteases | |||
| Large vessel infarct, n/total (%) | |||
| N = 0 | 71/80 (88.8%) | 217/243 (89.3%) | .40 |
| N = 1 | 4/80 (5.0%) | 18/243 (7.4%) | |
| N > 1 | 5/80 (6.3%) | 8/243 (3.3%) | |
| Small vessel infarct, n/total (%) | |||
| N = 0 | 64/80 (80.0%) | 193/243 (79.4%) | .69 |
| N = 1 | 9/80 (11.3%) | 22/243 (9.1%) | |
| N > 1 | 7/80 (8.8%) | 28/243 (11.5%) | |
| Substrate V degradation, Mean ± SD | 0.94 ± 0.17 | 0.93 ± 0.14 | .40 |
| ACE N-domain Activity, Mean ± SD | 0.65 ± 0.25 | 0.65 ± 0.24 | .74 |
| ACE C-domain Activity, Mean ± SD | 0.49 ± 0.34 | 0.56 ± 0.33 | .17 |
Abbreviations: ACE, angiotensin-converting enzyme; ApoE4, apolipoprotein E4 allele; BMI, body mass index; MMSE, Mini-Mental State Examination; SD, standard deviation.
Mean ± SD with t test or n/total (%) with chi-square (χ2 test) are presented. P values for statistical significance are shown.
We then studied whether there were associations between the infarct subtypes and the Aβ-degrading activities in blood. Using the multivariate linear regression model, serum substrate V degradation as an outcome was positively associated with the number of SV infarcts (β = +.031, SE = 0.013, P = .02), but not with of SV infarcts, (β = +.031, SE = 0.013, P = .02), but not with the number of LV infarcts, after adjusting for age, sex, ethnicity, BMI, and the ApoE4 allele (Table 2). Adding the vascular diseases into the model did not affect these relationships (Model II). Although there were high percentages of diabetes (32%), taking aspirin (42%) and statin (45%) in the homebound elderly population, these variables were not associated with substrate V degradation and did not affect the relationship between SV infarcts and substrate V degradation in serum (data not shown).
Table 2.
Linear Multivariable-Adjusted Correlates of Substrate V Degradation as an Outcome and Each Infarct Subtypea
| Model I Substrate V Degradation (n = 304) |
Model II Substrate V Degradation (n = 290) |
Model III Substrate V Degradation (n = 290) |
||||
|---|---|---|---|---|---|---|
| β Estimate (SE) | P Value | β Estimate (SE) | P Value | β Estimate (SE) | P Value | |
| Age, years | −.001 (0.001) | .29 | −.001 (0.001) | .42 | −.001 (0.001) | .47 |
| Female | +.017 (0.020) | .39 | +.023 (0.021) | .28 | +.020 (0.020) | .32 |
| White | +.002 (0.006) | .67 | +.003 (0.006) | .62 | +.003 (0.006) | .56 |
| BMI | −.001 (0.001) | .78 | −.001 (0.001) | .43 | −.001 (0.001) | .46 |
| ApoE4 | +.009 (0.020) | .67 | −.013 (0.020) | .53 | −.027 (0.022) | .22 |
| ACE inhibitor use | +.009 (0.018) | .61 | +.004 (0.020) | .84 | +.001 (0.019) | .95 |
| LV infarct (0, 1, >1) | −.006 (0.019) | .75 | −.009 (0.020) | .64 | −.014 (0.019) | .45 |
| SV infarct (0, 1, >1) | +.031 (0.013) | .02 | +.029 (0.014) | .04 | +.0001 (0.019) | .99 |
| Cardiovascular disease | – | – | +.015 (0.019) | .43 | +.015 (0.019) | .43 |
| Diabetes | – | – | +.036 (0.020) | .08 | +.029 ((0.020) | .14 |
| Hypertension | – | – | +.030 (0.027) | 27 | +.021 (0.026) | .42 |
| ApoE4*SV infarct | – | – | – | – | +.158 (0.034) | <.0001 |
Abbreviations:ACE, angiotensin-converting enzyme; ApoE4, apolipoprotein E4 allele; BMI, body mass index; SV, small vessel.
Model I: multivariate linear regression adjusting for age, gender, race, BMI, ApoE4, and ACE inhibitor use to study the relationship between LV and SV infarcts and substrate V degradation as an outcome.Model II: Model I plus the vascular diseases including cardiovascular disease, diabetes, and hypertension.Model III: Model II plus ApoE4*SV infarct = interaction of ApoE4 allele and SV infarct.
Further, we found that serum substrate V degradation as an outcome was significantly associated with the interaction between ApoE4 and the number of SV infarcts (β = +.158, SE = 0.034, P < .0001; (model III in Table 2), but not the interaction between ApoE4 and the number of LV infarcts (data not shown). Consistently, the association between increased substrate V degradation and the number of SV infarcts was only found among the ApoE4 carriers (β = +.154, SE = 0.031, P < .0001), but not among the ApoE4 non-carriers (β = +.001, SE = 0.014, P = .94; Table 3). In the presence of ApoE4, the higher the number of SV infarcts, the higher the average serum activity of substrate V degradation (Mean ± SD: no SV infarct = 0.90 ± 0.14, 1 SV infarct = 0.99 ± 0.09, and more than 1 SV infarct = 1.67 ± 0.35, P = .004; Table 4). In the absence of ApoE4, there was no difference in substrate V degradation between those with and without SV infarcts. When ACE N-terminal- and C-terminal-specific substrates were also used, we did not find any associations between the ACE activities and SV infarcts regardless of the ApoE4 genotype.
Table 3.
Linear Multivariable-Adjusted Correlates of Substrate V Degradation as an Outcome and Each Infarct Subtype Among Those Apolipoprotein E4 Allele Carriers Versus Noncarriers
| ApoE4 Carriers Substrate V Degradation (n = 74) |
ApoE4 nonCarriers Substrate V Degradation (n = 229) |
|||
|---|---|---|---|---|
| β Estimate (SE) | P Value | β Estimate (SE) | P Value | |
| Age, years | −.001 (0.003) | .63 | −.001 (0.002) | .28 |
| Female | +.063 (0.045) | .17 | −.004 (0.022) | .84 |
| White | +.012 (0.013) | .34 | −.001 (0.006) | .95 |
| BMI | −.002 (0.003) | .48 | −.001 (0.001) | .48 |
| ACE inhibitor use | +.039 (0.037) | .30 | +.003 (0.020) | .87 |
| LV infarct (0, 1, >1) | −.015 (0.037) | .70 | −.008 (0.022) | .71 |
| SV infarct (0, 1, >1) | +.154 (0.031) | <.0001 | +.001 (0.014) | .94 |
Abbreviations:ACE, angiotensin-converting enzyme; ApoE4, apolipoprotein E4 allele; BMI, body mass index; SD, standard deviation; SV, small vessel.
Table 4.
Comparisons of Substrate V Degradation Versus ACE-Specific Activities in Different Infarct Subgroupsa
| ApoE4 Carriers | SV infarct = 0, n = 64 | SV infarct = 1, n = 9 | SV infarct >1, n = 7 | P Values |
|---|---|---|---|---|
| Substrate V degradation, mean ± SD | 0.90 ± 0.14 | 0.99 ± 0.09 | 1.67 ± 0.35 | .004 |
| ACE N-domain Activity, Mean ± SD | 0.71 ± 0.23 | 0.43 ± 0.25 | 0.67 ± 0.23 | .02 |
| ACE C-domain Activity, Mean ± SD | 0.50 ± 0.33 | 0.40 ± 0.41 | 0.52 ± 0.32 | .56 |
|
| ||||
| ApoE4 nonCarriers | SV infarct = 0, n = 194 | SV infarct = 1, n = 22 | SV infarct >1, n = 28 | P Values |
|
| ||||
| Substrate V degradation, mean ± SD | 0.94 ± 0.15 | 0.92 ± 0.11 | 0.94 ± 0.14 | .88 |
| ACE N-domain Activity, Mean ± SD | 0.66 ± 0.25 | 0.61 ± 0.18 | 0.63 ± 0.19 | .63 |
| ACE C-domain Activity, Mean ± SD | 0.57 ± 0.33 | 0.49 ± 0.34 | 0.51 ± 0.33 | .48 |
Abbreviations:ACE, angiotensin-converting enzyme; ANOVA, analysis of variance; ApoE4, apolipoprotein E4 allele; SD, standard deviation; SV, small vessel.
Participants were divided into subgroups according to ApoE4 carriers versus ApoE4 noncarriers and further the numbers of small vessel (SV) infarcts they had. Mean ± SD with ANOVA test are presented. P values for statistical significance are shown for the comparisons among the SV infarct subgroups in those with and without ApoE4 allele.
Discussion
Large vessel and SVinfarcts are different pathologies in the brain, and our study was aimed to search for the biomarkers to distinguish these 2 cerebrovascular subtypes. While we did not find any relationship between ApoE alleles and either infarct subtype, using substrate V we found that the activity of serum Aβ-degrading proteases was associated with SV infarcts only in the presence of ApoE4. Both IDE and ACE in serum can degrade substrate V12; however, using ACE-specific substrates the serum ACE activities were not in association with SV infarcts. Thus, our data suggest that serum IDE, but not ACE, could be a biomarker for SV infarct in the brain and may suggest some pathological process in AD.
Insulin-degrading enzyme22,23 degrades Aβ, the major component of AD pathology and the cerebrovascular lesion CAA5,6 Small vessel infarct pathology might have reactively increased IDE activity in serum (Table 2). Consistently, CAA is not frequently observed in subcortical vessels where an SV infarct is located24,25 but often seen in leptomeningeal and cortical arteries. The Aβ1-40, which has vascular toxicity,3,7,26–28 is a major substrate of IDE, and the impared Aβ clearance by IDE is thought to lead to its accumulation in the vessel wall to cause CAA.6,29–31 Despite that ACE32,33 also degrades Aβ, and 1 study shows that mean plasma ACE is elevated in multiple lacunar infarction,34 our study did not find any relationship between serum ACE activity and either infarct subytpes in the brain (Table 4). This might be explained by the common usage of ACE inhibitor in the elderly individuals (Table 1).
Apolipoprotein E4 allele is the major risk factor of late onset AD.35 The association between SV infarcts and increased IDE Aβ-degrading activities only among the ApoE4 carriers (Table 4) may imply an importan pathological event in AD. Vascular diseases including stroke, diabetes, hypertension, and atherosclerosis increase the risk of AD.36 The APP transgenic mice have increased susceptibility to ischemic brain damage.37 It has been shown that the APP transgenic mice when in the background of ApoE knockout present with significantly increased ischemic volume38 and reduce the formation of CAA38 compared to the APP transgenic mice in the presence of ApoE. Apolipoprotein E4 allele favors the formation of CAA compared with ApoE37 and is the major genetic risk factor of AD through enhancing Aβ aggregation and inhibiting Aβ degradation.24 Cross-sectionally, we did not find a difference in AD rates between those with LV and SV infarcts; however, both LV and SV infarcts have been reported to be associated with AD in the longitudinal study.39
This study has limitations. Without a longitudinal study, we cannot conclude that this assay can show a causal relationship between cerebral infarct subtypes and substrate V degradation. We did not have direct evidence that IDE degraded substrate V is associated with SV infarcts and also could not relate either infarct subtype with CAA in this study. Nevertheless, we found that Aβ catabolism by IDE in blood illustrated by substrate V degradation may be a differential biomarker for cerebral infarct subtypes. It is possible that different cerebral pathologies, LV and SV infarcts, induce Aβ-degrading proteases differently in the presence of ApoE4. Our findings warrant prospective studies to examine whether peripheral substrate V degradation specifically mediated by IDE is related to each brain pathology, and predicts the onset of cognitive decline in this and other populations.
Conclusions
Small vessel infarcts, but not LV infarcts, were positively associated with substrate V degradation especially in the presence of ApoE4. As ApoE4 is the major risk factor of AD, IDE in blood may imply an important biomarker and a pathological event in AD through SV infarcts in the presence of ApoE4.
Acknowledgment
We thank the NAME study staff and the Boston home care agencies for their hardwork and acquisition of participants, and Xian Adiconis from Dr Jose Ordovas's laboratory for her characterization of ApoE alleles.
Funding The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from NIA, AG-022476 for W.Q.Q and AG-21790 for I.R. Support was also provided through the General Clinical Research Center funded by the National Center for Research Resources of the NIH under grant no. MO1-RR00054.
Footnotes
Declaration of Conflicting Interests The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Rovira A, Grive E, Rovira A, Alvarez-Sabin J. Distribution territories and causative mechanisms of ischemic stroke. Eur Radiol. 2005;15(3):416–426. doi: 10.1007/s00330-004-2633-5. [DOI] [PubMed] [Google Scholar]
- 2.de Jong G, Kessels F, Lodder J. Two types of lacunar infarcts: further arguments from a study on prognosis. Stroke. 2002;33(8):2072–2076. doi: 10.1161/01.str.0000022807.06923.a3. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg SM, Rebeck GW, Vonsattel JP, Gomez-Isla T, Hyman BT. Apolipoprotein E epsilon 4 and cerebral hemorrhage associated with amyloid angiopathy. Ann Neurol. 1995;38(2):254–259. doi: 10.1002/ana.410380219. [DOI] [PubMed] [Google Scholar]
- 4.Utter S, Tamboli IY, Walter J, et al. Cerebral small vessel disease-induced apolipoprotein E leakage is associated with Alzheimer disease and the accumulation of amyloid beta-protein in perivascular astrocytes. J Neuropathol Exp Neurol. 2008;67(9):842–856. doi: 10.1097/NEN.0b013e3181836a71. [DOI] [PubMed] [Google Scholar]
- 5.Jellinger KA. Prevalence and impact of cerebrovascular lesions in Alzheimer and lewy body diseases. Neurodegener Dis. 2010;7(1–3):112–115. doi: 10.1159/000285518. [DOI] [PubMed] [Google Scholar]
- 6.Thal DR, Griffin WS, de Vos RA, Ghebremedhin E. Cerebral amyloid angiopathy and its relationship to Alzheimer's disease. Acta Neuropathol. 2008;115(6):599–609. doi: 10.1007/s00401-008-0366-2. [DOI] [PubMed] [Google Scholar]
- 7.Fryer JD, Simmons K, Parsadanian M, et al. Human apolipoprotein E4 alters the amyloid-beta 40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an amyloid precursor protein transgenic model. J Neurosci. 2005;25(11):2803–2810. doi: 10.1523/JNEUROSCI.5170-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenberg SM, Vonsattel JP. Diagnosis of cerebral amyloid angiopathy. Sensitivity and specificity of cortical biopsy. Stroke. 1997;28(7):1418–1422. doi: 10.1161/01.str.28.7.1418. [DOI] [PubMed] [Google Scholar]
- 9.Vasilevko V, Passos GF, Quiring D, et al. Aging and cerebrovascular dysfunction: contribution of hypertension, cerebral amyloid angiopathy, and immunotherapy. Ann N Y Acad Sci. 2010;1207:58–70. doi: 10.1111/j.1749-6632.2010.05786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selkoe DJ. Biochemistry and molecular biology of amyloid beta-protein and the mechanism of Alzheimer's disease. Handb Clin Neurol. 2008;89:245–260. doi: 10.1016/S0072-9752(07)01223-7. [DOI] [PubMed] [Google Scholar]
- 11.Thal DR, Griffin WS, Braak H. Parenchymal and vascular Abeta-deposition and its effects on the degeneration of neurons and cognition in Alzheimer's disease. J Cell Mol Med. 2008;12(5B):1848–1862. doi: 10.1111/j.1582-4934.2008.00411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Z, Zhu H, Fang GG, et al. Characterization of insulin degrading enzyme and other amyloid-beta degrading proteases in human serum: a role in Alzheimer's disease? J Alzheimers Dis. 2012;29(2):329–340. doi: 10.3233/JAD-2011-111472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott TM, Peter I, Tucker KL, et al. The Nutrition, Aging, and Memory in Elders (NAME) study: design and methods for a study of micronutrients and cognitive function in a homebound elderly population. Int J Geriatr Psychiatry. 2006;21(6):519–528. doi: 10.1002/gps.1503. [DOI] [PubMed] [Google Scholar]
- 14.Qiu WQ, Sun X, Selkoe DJ, et al. Depression is associated with low plasma Abeta42 independently of cardiovascular disease in the homebound elderly. Int J Geriatr Psychiatry. 2007;22(6):536–542. doi: 10.1002/gps.1710. [DOI] [PubMed] [Google Scholar]
- 15.Scott TM, Tucker KL, Bhadelia A, et al. Homocysteine and B vitamins relate to brain volume and white-matter changes in geriatric patients with psychiatric disorders. Am J Geriatr Psychiatry. 2004;12(6):631–638. doi: 10.1176/appi.ajgp.12.6.631. [DOI] [PubMed] [Google Scholar]
- 16.Bryan RN, Wells SW, Miller TJ, et al. Infarctlike lesions in the brain: prevalence and anatomic characteristics at MR imaging of the elderly–data from the Cardiovascular Health Study. Radiology. 1997;202(1):47–54. doi: 10.1148/radiology.202.1.8988191. [DOI] [PubMed] [Google Scholar]
- 17.Lan X, Xu J, Kiyota T, Peng H, Zheng JC, Ikezu T. HIV-1 reduces A{beta}-degrading enzymatic activities in primary human mononuclear phagocytes. J Immunol. 2011;186(12):6925–6932. doi: 10.4049/jimmunol.1100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lahoz C, Osgood D, Wilson PW, Schaefer EJ, Ordovas JM. Frequency of phenotype-genotype discrepancies at the apolipo-protein E locus in a large population study. Clin Chem. 1996;42(11):1817–1823. [PubMed] [Google Scholar]
- 19.Sun X, Sato S, Murayama O, et al. Lithium inhibits amyloid secretion in COS7 cells transfected with amyloid precursor protein C100. Neurosci Lett. 2002;321(1–2):61–64. doi: 10.1016/s0304-3940(01)02583-6. [DOI] [PubMed] [Google Scholar]
- 20.Fukumoto H, Tennis M, Locascio JJ, Hyman BT, Growdon JH, Irizarry MC. Age but not diagnosis is the main predictor of plasma amyloid beta-protein levels. Arch Neurol. 2003;60(7):958–964. doi: 10.1001/archneur.60.7.958. [DOI] [PubMed] [Google Scholar]
- 21.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 22.Kurochkin IV, Goto S. Alzheimer's beta-amyloid peptide specifically interacts with and is degraded by insulin degrading enzyme. FEBS Lett. 1994;345(1):33–37. doi: 10.1016/0014-5793(94)00387-4. [DOI] [PubMed] [Google Scholar]
- 23.Qiu WQ, Walsh DM, Ye Z, et al. Insulin-degrading enzyme regulates extracellular levels of amyloid beta-protein by degradation. J Biol Chem. 1998;273(49):32730–32738. doi: 10.1074/jbc.273.49.32730. [DOI] [PubMed] [Google Scholar]
- 24.Alonzo NC, Hyman BT, Rebeck GW, Greenberg SM. Progression of cerebral amyloid angiopathy: accumulation of amyloid-beta40 in affected vessels. J Neuropathol Exp Neurol. 1998;57(4):353–359. doi: 10.1097/00005072-199804000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Revesz T, Holton JL, Lashley T, et al. Sporadic and familial cerebral amyloid angiopathies. Brain Pathol. 2002;12(3):343–357. doi: 10.1111/j.1750-3639.2002.tb00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura S, Tamaoka A, Sawamura N, et al. Carboxyl end-specific monoclonal antibodies to amyloid beta protein (A beta) subtypes (A beta 40 and A beta 42(43)) differentiate A beta in senile plaques and amyloid angiopathy in brains of aged cynomolgus monkeys. Neurosci Lett. 1995;201(2):151–154. doi: 10.1016/0304-3940(95)12160-9. [DOI] [PubMed] [Google Scholar]
- 27.Urmoneit B, Prikulis I, Wihl G, et al. Cerebrovascular smooth muscle cells internalize Alzheimer amyloid beta protein via a lipoprotein pathway: implications for cerebral amyloid angiopathy. Lab Invest. 1997;77(2):157–166. [PubMed] [Google Scholar]
- 28.Herzig MC, Winkler DT, Burgermeister P, et al. Abeta is targeted to the vasculature in a mouse model of hereditary cerebral hemorrhage with amyloidosis. Nat Neurosci. 2004;7(9):954–960. doi: 10.1038/nn1302. [DOI] [PubMed] [Google Scholar]
- 29.Morelli L, Llovera R, Gonzalez SA, et al. Differential degradation of amyloid beta genetic variants associated with hereditary dementia or stroke by insulin-degrading enzyme. J Biol Chem. 2003;278(26):23221–23226. doi: 10.1074/jbc.M300276200. [DOI] [PubMed] [Google Scholar]
- 30.Morelli L, Llovera RE, Mathov I, et al. Insulin-degrading enzyme in brain microvessels: proteolysis of amyloid {beta} vasculotropic variants and reduced activity in cerebral amyloid gangiopathy. J Biol Chem. 2004;279(53):56004–56013. doi: 10.1074/jbc.M407283200. [DOI] [PubMed] [Google Scholar]
- 31.Nicoll JA, Yamada M, Frackowiak J, Mazur-Kolecka B, Weller RO. Cerebral amyloid angiopathy plays a direct role in the pathogenesis of Alzheimer's disease. Pro-CAA position statement. Neurobiol Aging. 2004;25(5):589–597. doi: 10.1016/j.neurobiolaging.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Hu J, Igarashi A, Kamata M, Nakagawa H. Angiotensin-converting enzyme degrades Alzheimer amyloid beta-peptide (A beta); retards A beta aggregation, deposition, fibril formation; and inhibits cytotoxicity. J Biol Chem. 2001;276(51):47863–47868. doi: 10.1074/jbc.M104068200. [DOI] [PubMed] [Google Scholar]
- 33.Hemming ML, Selkoe DJ. Amyloid beta-protein is degraded by cellular angiotensin-converting enzyme (ACE) and elevated by an ACE inhibitor. J Biol Chem. 2005;280(45):37644–37650. doi: 10.1074/jbc.M508460200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brenner D, Labreuche J, Pico F, et al. The renin-angiotensinaldosterone system in cerebral small vessel disease. J Neurol. 2008;255(7):993–1000. doi: 10.1007/s00415-008-0816-8. [DOI] [PubMed] [Google Scholar]
- 35.Strittmatter WJ, Saunders AM, Schmechel D, et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90(5):1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cechetto DF, Hachinski V, Whitehead SN. Vascular risk factors and Alzheimer's disease. Expert Rev Neurother. 2008;8(5):743–750. doi: 10.1586/14737175.8.5.743. [DOI] [PubMed] [Google Scholar]
- 37.Zhang F, Eckman C, Younkin S, Hsiao KK, Iadecola C. Increased susceptibility to ischemic brain damage in transgenic mice over-expressing the amyloid precursor protein. J Neurosci. 1997;17(20):7655–7661. doi: 10.1523/JNEUROSCI.17-20-07655.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koistinaho M, Kettunen MI, Holtzman DM, Kauppinen RA, Higgins LS, Koistinaho J. Expression of human apolipoprotein E downregulates amyloid precursor protein-induced ischemic susceptibility. Stroke. 2002;33(7):1905–1910. doi: 10.1161/01.str.0000020124.61998.bc. [DOI] [PubMed] [Google Scholar]
- 39.Jellinger KA. The pathology of ischemic-vascular dementia: an update. J Neurol Sci. 2002;203–204:153–157. doi: 10.1016/s0022-510x(02)00282-4. [DOI] [PubMed] [Google Scholar]