Abstract
The use of novel biomarkers to detect incident acute kidney injury (AKI) in the critically ill is hindered by heterogeneity of injury and the potentially confounding effects of prevalent AKI. Here we examined the ability of urine NGAL (NGAL), L-type Fatty Acid Binding Protein (L-FABP), and Cystatin C to predict AKI development, death, and dialysis in a nested case-control study of 380 critically ill adults with an eGFR over 60 ml/min/1.73 m2. One-hundred thirty AKI cases were identified following biomarker measurement and were compared to 250 controls without AKI. Areas under the receiver-operator characteristic curves (AUC-ROCs) for discriminating incident AKI from non-AKI were 0.58(95%CI: 0.52-0.64), 0.59(0.52-0.65), and 0.50(0.48-0.57) for urine NGAL, L-FABP, and Cystatin C, respectively. The combined AUC-ROC for NGAL and L-FABP was 0.59(56-0.69). Both urine NGAL and L-FABP independently predicted AKI during multivariate regression; however, risk reclassification indices were mixed. Neither urine biomarker was independently associated with death or acute dialysis [NGAL hazard ratio 1.35(95%CI: 0.93-1.96), L-FABP 1.15(0.82-1.61)] though both independently predicted the need for acute dialysis [NGAL 3.44(1.73-6.83), L-FABP 2.36(1.30-4.25)]. Thus, urine NGAL and L-FABP independently associated with the development of incident AKI and receipt of dialysis but exhibited poor discrimination for incident AKI using conventional definitions.
Introduction
Acute Kidney Injury (AKI) frequently complicates critical illness and strongly associates with a dismal prognosis.1, 2 Efforts to improve care are limited by the inability to provide timely or accurate diagnosis, mechanistic insight, or prognostic information.3 Emerging biological markers with improved specificity and sensitivity for tubular injury have shown promise for addressing these limitations.4 However, validation studies across different clinical settings have demonstrated varied ability to predict the development of AKI or clinical outcomes. More robust performance has been observed in anticipated injury settings such as cardiac surgery or nephrotoxic exposures.5-7
Although likely to benefit from timely and informative injury markers, the critically ill present unique challenges that hinder both their study and application.8, 9 For example, patients with critical illness often already have AKI upon presentation, a finding that can be undiscoverable as pre-admission creatinine data is often missing. In addition, single marker studies are unlikely to account for the biological heterogeneity underlying different injury subtypes observed in this population. We hypothesized that measurement of multiple biologically distinct urine injury markers would improve diagnostic and prognostic performance in critically ill adults compared to any single marker alone. We also hypothesized that performance of biomarkers to detect incident AKI would improve beyond previously described results in the critically ill after minimizing the potential confounding effects of unrecognized prevalent AKI and underlying chronic kidney disease (CKD).10-12 To test these hypotheses, we examined the individual and additive utility of urine Neutrophil Gelatinase-Associated Lipocalin (uNGAL) (inflammation/iron trafficking),13 urine L-type Fatty Acid Binding Protein (uL-FABP) (lipid peroxidation),14 and urine Cystatin C (uCysC) (proximal tubule metabolism)15 to predict the development of incident AKI and predict dialysis and death in a large nested case-control study of critically ill adults without overt CKD.
Results
Subject Characteristics
Patients were selected from the previously described Validation of biomarkers for Acute Lung Injury Diagnosis (VALID) study.11, 16 Biomarker measurement occurred at two time points: study enrollment and 48 hours later (Figure 1). Cases were identified by acute increase of 0.3 mg/dl or 50% increase in serum creatinine following biomarker measurement. Controls not meeting injury criteria were selected randomly and paired with cases to approximate a total AKI case:non-AKI control ratio of 1:2 for each time interval (see methods). Table 1 is the summary of data taken at enrollment or from the time of biomarker measurement stratified according to whether patients went on to develop AKI (N=130) and patients who did not develop AKI (N=250); 3 patients with AKI and 5 non-AKI controls did not have urine available for measurement. A trend towards older age, male gender, higher diabetes prevalence, injury severity (SAPS and modified APACHE II scores), and sepsis status was observed in the AKI group, which did not reach statistical significance. A higher proportion of patients in the AKI group came from the surgical ICU than in the non-AKI group. No differences were observed in median (IQR) serum creatinine values [0.90(0.72-1.15) vs. 0.90(0.71-1.04) mg/dl, p=0.46] or eGFR measurements [88.1(71.3-108.1) vs. 89.8(75.7-112.2) ml/min/1.73 m2, p=0.57] at the time of biomarker measurement between AKI and non-AKI patients, respectively. Figure 2 shows the separation of serum creatinine values between patients who developed AKI and those that did not over the ensuing 48 hours following biomarker measurement. Patients with AKI during the first 48 hours after biomarker measurement were further staged according to the maximal stage reached over 7 days using AKIN criteria in the following distribution: Stage I (n=93, 73.2%), Stage II (n=18, 14.2%), and Stage III (n=16, 12.6%). Patients with AKI had a higher 7-day peak median (IQR) serum creatinine 1.54(1.20-2.24) versus 1.10(0.90-1.39) mg/dl and were more likely to die (31% versus 11%) than non-AKI controls, p<0.001.
Figure 1. Study Scheme.
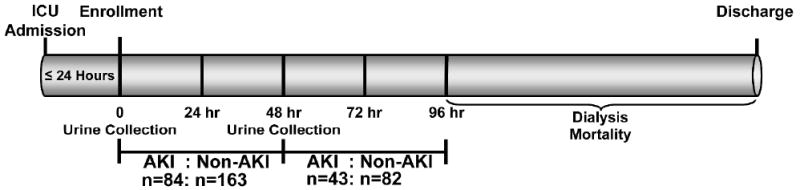
Table 1.
| Variable | AKI N=(127) | No AKI (N=245) | P Value |
|---|---|---|---|
| Age | 49(40-63) | 51(36-61) | 0.57 |
| Gender (%F) | 39(31%) | 88(36%) | 0.32 |
| Ethnicity (%non-white) | 20(16%) | 32(13%) | 0.54 |
| Diabetes Mellitus | 31 (24%) | 49(20%) | 0.33 |
| Modified APACHE II | 24(19-27) | 22(18-27) | 0.25 |
| SAPS II | 49(37-58) | 49(37-58) | 0.69 |
| Sepsis* | 53(42%) | 81(33%) | 0.10 |
| Patient Location | 0.001 | ||
| Surgical ICU | 36(28%) | 45(18%) | |
| Medical ICU | 52(41%) | 79(32%) | |
| Trauma ICU | 35(28%) | 118(48%) | |
| Cardiac ICU | 4(3%) | 3(1%) | |
| Creatinine at Study Enrollment (mg/dl) | 0.90 (0.74-1.15) | 0.91 (0.70-1.08) | 0.89 |
| Estimated GFR at Study Enrollment (ml/min/1.73m2) | 87(73-109) | 87(74-109) | 0.99 |
| uNGAL* (ng/mg urine creatinine) | 63(24-232) | 41(16-118) | 0.004 |
| uL-FABP* (ng/mg urine creatinine) | 177.2 (61.2-545.5) | 94.7(41.5-271.2) | 0.003 |
| uCysC* (ng/mg urine creatinine) | 100.0(49.1-310.8) | 102.5(46.2-367.0) | 0.87 |
| Urine Creatinine* | 98.6(53.6-160.1) | 101.4(58.8-151.0) | 0.87 |
| Peak Serum Creatinine During Hospitalization | 1.54(1.20-2.24) | 1.10(0.90-1.39) | <0.001 |
| Died | 39(31%) | 28(11%) | <0.0013 |
Variables recorded at the time of biomarker measurement. All other variables are recorded at enrollment unless otherwise indicated.
Figure 2. Box-plot of Changes in Serum Creatinine Between AKI Cases and non-AKI Controls.
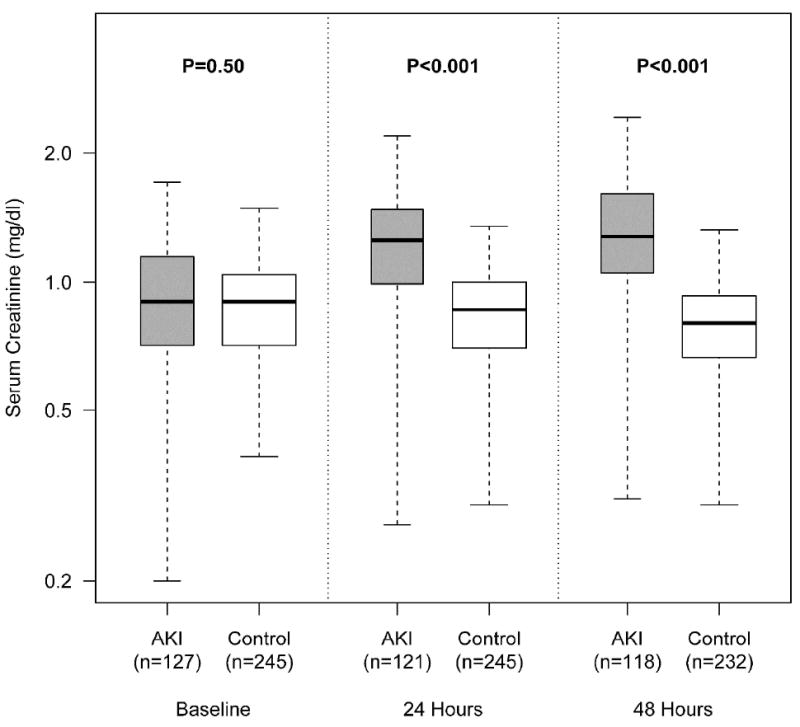
The first, second, and third panels represent the baseline serum creatinine, the highest serum creatinine within 24 hours after biomarker measurement, and the highest serum creatinine within 24-48 hours of biomarker measurement, respectively. Creatinine measurements were ordered by point of care personnel. P values <0.05 denote statistical significance.
Individual and Combined Biomarker Utility for Early Discrimination of AKI Status
Table 1 and Figure 3 describe uNGAL, uL-FABP, and uCysC levels grouped according to AKI status. Adjusting for urine creatinine, patients who went on to develop AKI within 48 hours had higher median levels of uNGAL [63(IQR: 24-232) vs. 41(IQR: 16-118) ng/mg, p=0.004] and uL-FABP [177.2(IQR: 61.2-545.5) vs. 94.7(IQR: 41.5-271.2) ng/mg, p=0.003] than non-AKI controls. No differences in median uCysC levels were observed between patients developing AKI [100(IQR: 49.1-310.8)] ng/mg and not developing AKI within 48 hours [102.5(IQR: 46.2-367.0)] ng/mg, p=0.87. A moderate and significant statistical correlation was noted between uNGAL and uL-FABP(Spearman ρ=0.56,p<0.01).
Figure 3. Box-plot of Levels of Urine NGAL and L-FABP Grouped by AKI versus Non-AKI Status.
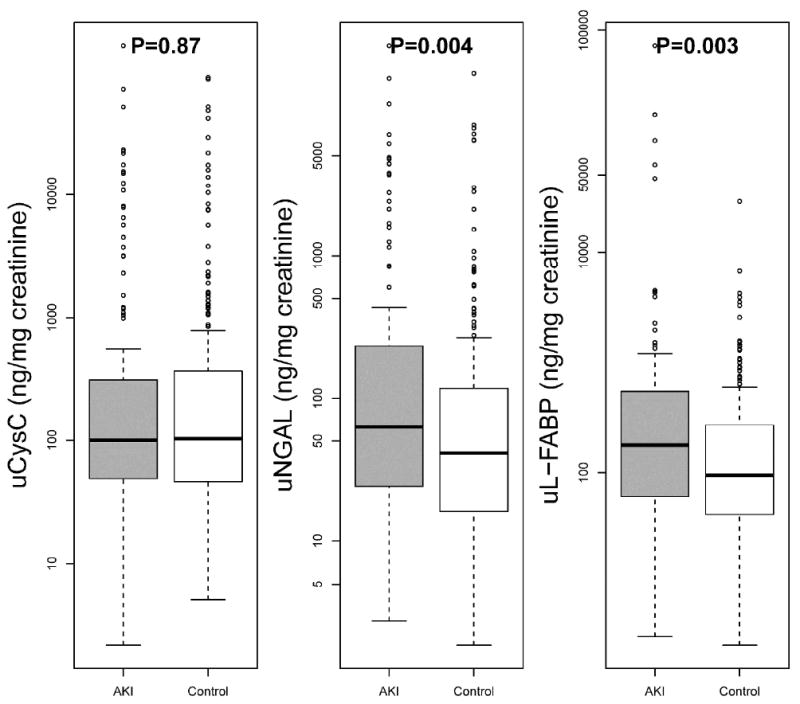
Levels are adjusted for urine creatinine values. P values <0.0273 denote statistical significance
The areas under the receiver-operating characteristic curve (AUC-ROC) for uNGAL, uL-FABP, and uCysC for diagnosis of AKI were 0.58(95%CI: 0.52-0.64), 0.59(95%CI: 0.52-0.65), and 0.51(95%CI: 0.48-0.57) respectively. (Figure 4a) As uCysC levels were not different between patients who developed and did not develop AKI, only uNGAL and uL-FABP were included in further analysis. The combined AUC using both uNGAL and uL-FABP for detection of AKI over the next 48 hours of 0.59(95%CI: 0.56-0.69). Discrimination was improved between patients with more severe injury (combined AKIN II and III) versus no injury with AUC-ROCs for uNGAL, uL-FABP, or both biomarkers of 0.69(95%CI: 0.59-0.77), 0.65(95%CI: 0.55-0.74) and 0.69(95%: 0.65-0.82), respectively. (Figure 4b)
Figure 4.
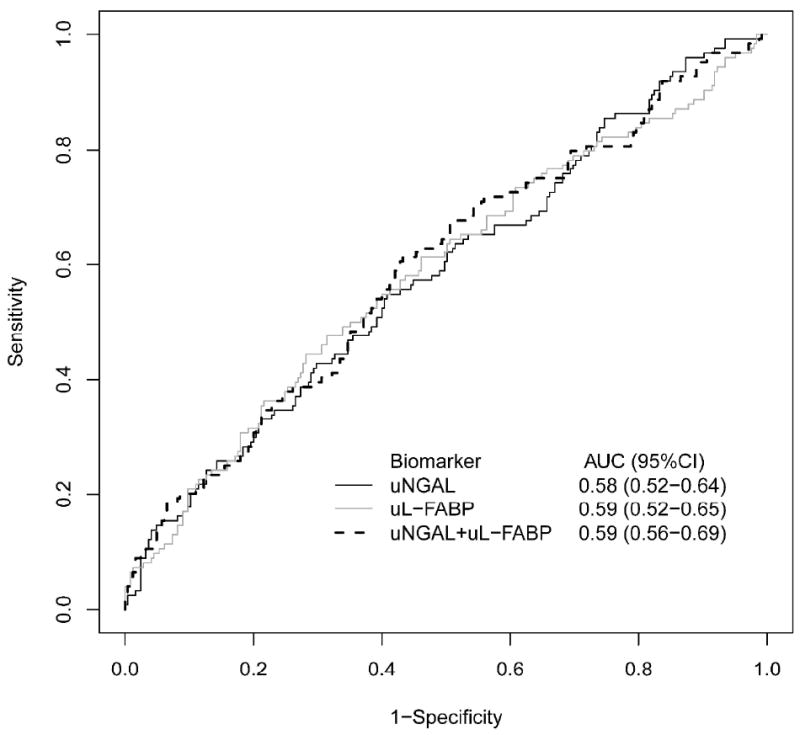
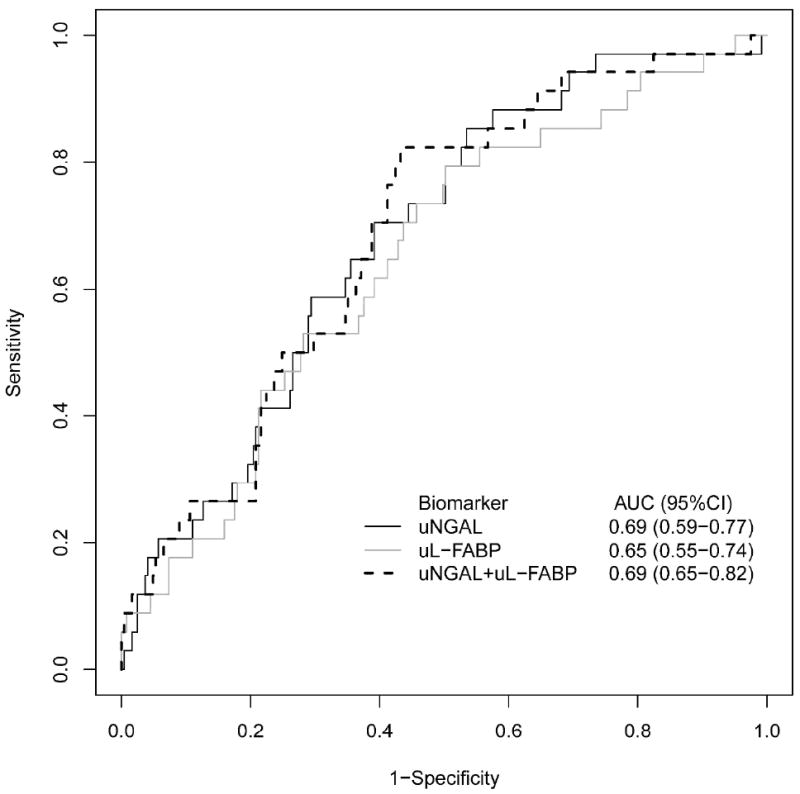
a. Area Under the Receiver Operating Curves for Urine NGAL, L-FABP, and Both Biomarkers Combined for Discriminating Any Future AKI from non-AKI Status.
b. Area Under the Receiver Operating Curves for Urine NGAL, L-FABP, and Both Biomarkers Combined for Discriminating Future AKIN Stage II and III from non-AKI Status.
The incremental benefit in discriminative performance conferred by biomarkers relative to a pre-specified clinical model was also tested. The a priori selected variables in the clinical model included age, modified APACHE II score, serum creatinine, sepsis status, and patient location. The AUC-ROC for the clinical model alone was 0.63(95%CI: 0.59-0.71). The AUC-ROCs with the addition of uNGAL, uL-FABP, or both biomarkers to the clinical predictive model were 0.65(95%CI: 0.61-0.73), 0.65(95%CI: 0.61-0.73), and 0.65(95%CI: 0.62-0.73), respectively.
Among the more severely injured (AKIN II and III), the AUC-ROC for the clinical model (modified APACHE II score, serum creatinine, and sepsis) for distinguishing severe injury from non-injury improved to 0.68(95%CI: 0.59-0.78). The addition of uNGAL, uL-FABP, or both biomarkers to the clinical predictive model increased the AUC-ROCs to 0.72(95%CI: 0.63-0.82), 0.73(95%CI: 0.64-0.82), and 0.73(95%CI: 0.66-0.83), respectively.
Biomarker Associations with the Risk of Developing AKI
Associations between biomarker levels and the risk of developing AKI were tested using multivariable logistic regression. After adjusting for a priori selected clinical predictors including age, modified APACHE II score, serum creatinine at biomarker measurement, the presence of sepsis, and ICU type, an increase in the inter-quartile range for both uNGAL and uL-FABP independently associated with the development of AKI with Odds Ratios of 1.40(95%CI: 1.05-1.87, p=0.02) and 1.51(95%CI: 1.11-2.06, p=0.001), respectively. The potential for these markers to improve risk prediction beyond a clinical model alone was further assessed using category free Net Reclassification Improvement (NRI) and the Integrated Discrimination Improvement (IDI). (Table 2) Total NRI and IDI values for uNGAL were 18.3%(95%CI: -3.3-39.9, p=0.10) and 0.0158(95%CI: 0.0030-0.0290, p=0.018). NRI and IDI values for uL-FABP were 24.8%(95%CI: 3.2-46.4, p=0.024) and 0.0190(95%CI: 0.003-0.0350, p=0.017). When both markers were combined, NRI and IDI were 19.2%(95%CI: -2.4-40.8, p=0.081) and 0.0230(95%CI: 0.005-0.040, p=0.010), respectively.
Table 2.
Category-Free Net Reclassification Index and Integrated Discrimination Index for Individual and Combined Biomarker Levels Added to the Clinical Model for AKI*
| uNGAL | uL-FABP | Combined | ||
|---|---|---|---|---|
|
|
||||
| NRI | AKI reclassified to higher risk | 50.80% | 52.40% | 47.60% |
|
| ||||
| AKI reclassified to lower risk | 49.20% | 47.60% | 52.40% | |
|
| ||||
| Non-AKI reclassified to lower risk | 58.40% | 60.00% | 62.00% | |
|
| ||||
| Non-AKI reclassified to higher risk | 41.60% | 40.00% | 38.00% | |
|
| ||||
| Total Category-Free Net Reclassification Improvement | 18.30% (95%CI: -3.3-39.9) p=0.096 | 24.80% (95%CI: 3.2-46.4) p=0.024 | 19.20% (95%CI: -2.4-40.8) p=0.081 | |
|
| ||||
| IDI | IDI Events | 0.0105 (95%CI: 0.0004-0.0337) | 0.01262 (95%CI: 0.0012-0.0385) | 0.0150 (95%CI: 0.0036-0.0448) |
|
| ||||
| IDI Nonevents | 0.0053 (95%CI: 0.0002-0.0165) | 0.0064 (95%CI:0.0006-0.0186) | 0.0076 (95%CI:0.0020-0.0217) | |
|
| ||||
| IDI Total | 0.0158 (95%CI: 0.0030-0.0290) p=0.018 | 0.0190 (95%CI: 0.003-0.0350) p=0.017 | 0.0230 (95%CI: 0.005-0.040) p=0.010 | |
P values <0.0273 denote statistical significance. All the 95% confidence interval for events IDI and non-events IDI were calculated using bootstrapped method
When AKIN II and III were used to define AKI, an increase in the inter-quartile range for uNGAL and uL-FABP remained associated with developing severe AKI after adjusting for modified APACHE II score, serum creatinine at biomarker measurement, and sepsis status with ORs for uNGAL and uL-FABP of 1.55(95%CI: 1.00-2.39, p=0.05) and 1.92(95%CI: 1.18-3.11, p=0.01), respectively. NRI and IDI values for uNGAL were 21.8%(95%CI: -14.1-57.7, p=0.23) and 0.015(95%CI: -0.008-0.037, p=0.22), respectively. NRI and IDI values for uL-FABP were 24.9%(95%CI: -11.0-61.0, p=0.17) and 0.033 (95%CI: -0.006-0.075, p=0.09).
Individual Biomarker and Clinical Outcomes in Patients with AKI
Among patients with AKI, ten received dialysis and 38 patients died within 28 days following enrollment. Patients experiencing the composite outcome of death or dialysis (N=46) had significantly higher levels of uNGAL than those who did not [116(IQR: 41-1493) vs. 50(IQR: 21-130) ng/mg, p=0.006)]. No statistically significant differences in uL-FABP levels were observed in patients who reached composite outcome than in those who did not [181.1(IQR: 81.3-929.6) vs. 177.2(58.9-452.2) ng/mg, p=0.199](Figure 5). Exploratory component analyses revealed that both uNGAL and uL-FABP levels were higher in those who required dialysis (n=10) than in those who did not, [838(IQR: 148-4243) vs. 60(IQR: 22-158) ng/mg], p=0.007)] and [510.9(IQR: 218.6-1016.3) vs. 152.9(IQR: 57.1-496.5), p=0.021, respectively. No statistically significant differences in biomarker levels between those that died or survived were observed [NGAL: 85(IQR: 37-612) vs. 60(IQR: 24-151) ng/mg, p=0.191)] [uL-FABP: 146.7(IQR: 50.6-872.2) vs. 200.9(IQR: 64.8-505.8) ng/mg, p=0.996).
Figure 5. Box-plot of Levels of Urine NGAL and L-FABP Grouped According to Patients whether Patients Experienced the Composite Outcome of Dialysis or Mortality.
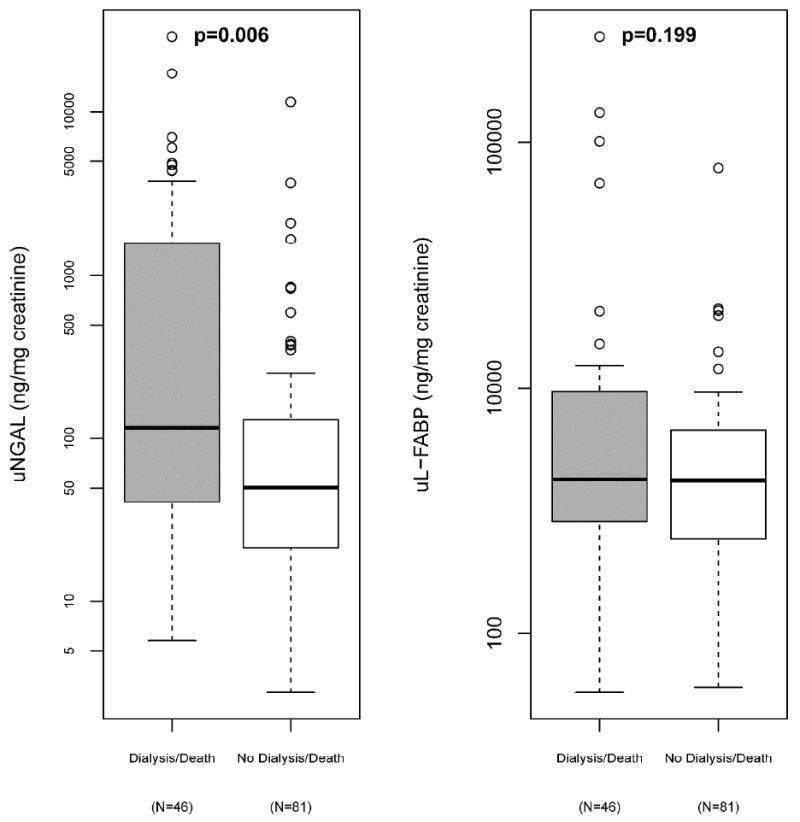
P values <0.0273 denote statistical significance.
Separate Cox regression models to examine the association between the risk of the developing the composite outcome were created for uNGAL and uL-FABP and were adjusted for modified APACHE II score, sepsis status at enrollment, and creatinine levels at biomarker measurement (Table 3). Hazard ratios describing the association between biomarker levels and the risk of developing the composite outcome for uNGAL and uL-FABP after adjusting for clinical covariates were HR 1.35(95%CI: 0.93-1.96, p=0.12) and uL-FABP HR 1.15(95%CI: 0.82-1.61), p=0.43, respectively. Exploratory analyses revealed that both markers were independently associated with the risk for acute dialysis after adjusting for serum creatinine at biomarker measurement [uNGAL HR 3.44 (95%CI: 1.73-6.83), p=0.004],[uL-FABP HR 2.36(1.30-4.25), p=0.005]. Neither biomarker was independently associated with time to mortality after adjusting for APACHE II score and sepsis status.
Table 3.
Cox Regression Model for the Composite Outcome of Dialysis or Death Within 28 Days*
| Outcome | uNGAL | uL-FABP | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P Value | HR | 95%CI | P Value | |
| Dialysis Following Enrollment (N=10)a | 3.44 | 1.73-6.83 | 0.004 | 2.36 | 1.30-4.25 | 0.005 |
| 28-day mortality (N=38)b | 1.11 | 0.73-1.68 | 0.62 | 0.95 | 0.65-1.40 | 0.80 |
| 28-day dialysis or mortality (N=46)c | 1.35 | 0.93-1.96 | 0.12 | 1.15 | 0.82-1.61 | 0.43 |
Biomarkers were log10 transformed. Hazard Ratio (HR) are represented for interquartile range difference of biomarkers concentrations. Separate cox proportional hazards regression models for uNGAL, and uL-FABP were adjusted for:
Serum creatinine
APACHE II, sepsis
modified APACHE II, sepsis, and serum creatinine
P values <0.0273 denote statistical significance
Sensitivity Analyses
We performed two separate sensitivity analyses. The first required patients to meet AKI criteria for at least 48-72 hours from the time of initial diagnosis. Sixty-one (61) of the original 127 cases survived long enough and had creatinine data available to meet these met these criteria. Median(IQR) levels of uNGAL for patients developing AKI and those not developing AKI were 80.5(32.8-372.5) ng/mg and 41.3(16.0-117.8) ng/mg, p<0.001, respectively. Median(IQR) levels of uL-FABP for patients developing AKI and those not developing AKI were 218.0(81.2-801.5) ng/mg and 94.7(41.5-271.2) ng/mg, p<0.001, respectively. Discrimination showed modest improvement with areas under the receiver-operator characteristic curves (AUC-ROCs) values for distinguishing subsequent AKI from non-AKI status of 0.66(95%CI: 0.56-0.73), 0.64(95%CI: 0.57-0.71), and 0.55(95%CI: 0.50-0.63) for uNGAL, uL-FABP, and uCysC, respectively.
The second sensitivity analysis restricted analysis to patients with sepsis at the time of biomarker measurement. A total of 53 patients who subsequently developed AKI were compared with 81 non-AKI patients. Median(IQR) uNGAL levels in sepsis patients developing AKI and not developing AKI were 139.7(58.5-598.2) ng/mg and 91.7(38.7-313.6), p=0.04, respectively. Median uL-FABP levels in sepsis patients developing AKI and not developing AKI were 197.0(67.0-803.6) ng/mg and 124.1(53.1-411.7) ng/mg, p=0.15, respectively. Discrimination was similar to the parent analysis with AUC-ROC values of 0.59(95%CI: 0.51-0.67) for uNGAL and 0.61(95%CI: 0.53-0.68) for uL-FABP, respectively.
Discussion
We hypothesized that biologically distinct biomarkers would be robust for the detection of incident AKI in a heterogeneous group of critically ill adults without overt CKD and provide important prognostic information. Despite these allowances, no significant differences in uCysC levels were observed between patients who did or did not develop AKI and both uNGAL and uL-FABP did not reliably discriminate between those who did and did not subsequently develop AKI. Both uNGAL and uL-FABP independently predicted AKI in a multivariate regression model, however, risk reclassification indices were mixed with only uL-FABP showing modest improvements in both IDI and NRI. Both markers also independently predicted subsequent dialysis but neither independently associated with the composite outcome of death or dialysis.
The examination and application of AKI biomarkers in the critically ill is frequently complicated by unrecognized prevalent AKI. This poses potential problems if biomarker elevation is only transient or AKI versus CKD status cannot be verified due to unknown or unavailable baseline serum creatinine values. The latter can result in misclassification of disease status, particularly if patients whose serum creatinine values do not continue to rise are misclassified as non-AKI controls. As much of the initial justification for the need of biomarkers has focused on achieving a more timely diagnosis, our study design attempted to minimize the confounding effects of undiagnosed prevalent AKI. In addition, widespread adoption of incrementally smaller changes in serum creatinine to define AKI may carry lower specificity, particularly among those with CKD, further challenging the interpretation of results.17 We previously demonstrated in subgroup analyses that discrimination of disease status by biomarker candidates improves among patients with higher eGFR.11, 16 Despite both the expansion of these subgroup analyses to a formal case-control study and reducing the impact of prevalent AKI or CKD on biomarker expression or AKI diagnosis, a substantial robustness in the diagnostic performance of uNGAL and uL-FABP was not observed.
These findings illustrate important challenges faced in biomarker studies focused on “early detection”.18 Even when the timing of injury can be pinpointed, emerging data from large validation cohorts suggest “early” diagnostic utility for AKI using candidate markers may be limited when confirmed using current creatinine-based criteria.19, 20 Whether results reflect the inherent performance limitations of the creatinine standard, regardless of what threshold is applied, or poor performance of the biomarkers themselves for diagnostic purposes remains unclear. As performance did improve modestly when defining AKI using persistent or more severe stages of injury, one possible explanation is that current creatinine-based criteria to meet the minimum criteria for injury may be less useful for discerning between temporary hemodynamic changes versus true parenchymal injury among patients with sufficient renal reserve. However, whether such patients are truly less susceptible to tubular injury or simply require a more severe insult to meet diagnostic criteria remains unknown. Of note, much of the observed NRI improvement was driven by reclassification of non-AKI patients to a lower risk category suggesting that lower biomarker levels may be informative in patients without AKI, though the mean change in the predicted risk by the IDI was small. The latter may be due to the ability of the clinical model to identify patients at low risk for injury. In contrast, the interpretation of higher biomarker levels for diagnosis using a creatinine standard remains difficult.
These findings highlight the need for further detailed studies that examine how clinically relevant outcomes develop in patients in which biomarker and creatinine data both agree and disagree on injury status. Indeed, a recent NIDDK workshop held to determine the optimal approach to clinical trials of AKI highlighted a need to better establish the relationship between biomarkers and hard clinical endpoints before recommending their use as surrogate short-term outcomes.21 A multi-center study among adults emergency room patients demonstrated that biomarkers were not superior to serum creatinine in distinguishing intrinsic from prerenal injury.22 While these findings were likely partially due to this outcome being creatinine-based, a key finding of recent studies in other settings was that biomarker data was able to predict mortality or dialysis in those both with and without serum creatinine elevation.22, 23
As the overarching goal of this area of research is to better phenotype AKI and its clinical impact, it is likely that the simultaneous incorporation of both functional and injury markers will be required. Examining clinically relevant outcomes in populations where underlying disease prevalence is higher, such as those already meeting conventional diagnostic thresholds for AKI or recently proposed criteria to suggest that early injury maybe be occurring may better reveal the purported utility of novel injury markers.8 Our exploratory analysis failed to demonstrate an independent association of either uNGAL or uL-FABP with the composite outcome of dialysis or death. However, these data were taken before established AKI where mortality, an outcome governed by factors besides kidney damage, predominated the composite outcome. Both markers independently associated with the need for acute dialysis, however, sample size was limited and results should be considered exploratory in nature.
Recognizing that a single biomarker is unlikely to reflect the multiple pathways (e.g., inflammatory, ischemic, nephrotoxic, oxidative stress) involved in the generation of AKI in a broadly selected patient population, we attempted to improve diagnostic performance by leveraging distinct markers. Urine Cystatin C is a member of cysteine protease family produced by all nucleated cells, filtered freely at the glomerulus, and metabolized by the proximal tubule.15, 24 We hypothesized that tubular injury would hamper tubular metabolism and increase urine levels relative to patients without ongoing injury. Unfortunately, no differences were observed between AKI and non-AKI patients and early diagnostic performance was subsequently poor. This is in-line with other recently published data and may be partially explained by the offsetting effect of diminished filtration during evolving injury.25, 26 NGAL is a 25-kD protein of the lipocalin family that modulates local iron channeling and serves as a growth and differentiation factor for renal tubular epithelia.13, 27 Increased expression in the proximal, and to a lesser extent, distal renal tubule during ischemic injury has provided a rationale for its use as an early biomarker of AKI. L-type fatty acid-binding protein (L-FABP) is a 14-kD protein that participates in fatty acid trafficking and as a protective cellular antioxidant against reactive lipids generated during hypoxic injury. Shedding of uL-FABP in the urine from the proximal tubule has been demonstrated in various AKI settings including septic shock.28 Despite their biological diversity, the combination of uNGAL and uL-FABP did not substantially improve diagnostic performance or risk prediction. One potential explanation may be found in the partial correlation observed between these markers suggesting the possibility of a shared mechanism of injury or similar thresholds for expression urine. The failure of correlated markers to provide independent value in other disease states has been previously demonstrated and highlights the need to further determine the degree of biological or statistical correlation between markers in different injury settings.29
Strengths of the study include the use of a large, well-phenotyped, and diverse ICU-population unlikely to be confounded by prevalent injury or CKD. As discussed previously, significant limitations include the use of serum creatinine elevations as a reference standard to define AKI, which, to date, remains the most feasible reference standard available. As patient selection for this case-control study was based on AKI versus non-AKI status, studies relating to other clinical outcomes should be considered exploratory. In addition, the lack of serial measurements within a given 48-hour period limits a more accurate detailing of the temporal profiles of these markers.
In summary, uNGAL and uL-FABP were both independently associated with the development of AKI and the need for dialysis in a critically ill population without prevalent kidney injury and may add incremental information to current risk prediction tools. However, both markers had only modest utility for discriminating incident injury from non-injury using current conventional definitions. Future studies the relative contribution of both conventional functional markers along with injury markers to inform the risk of poor clinical outcomes in patients early in the course of injury is warranted.
Materials and Methods
Patients
A nested case-control study was performed with patients from the Validation of biomarkers for Acute Lung Injury Diagnosis (VALID) study.11 A portion of patient data in this study has been previously reported.11 In brief, VALID is a single-center, multi-ICU prospective cohort study with a total enrollment of 2550 patients whose primary objective is to discover and validate new and existing protein biomarkers to diagnose organ injury including to, but not limited to, Acute Lung Injury and Acute Kidney Injury. All adult (≥18 years of age) patients admitted to one of four ICUs (Medical, Cardiac, Surgical, Trauma) at Vanderbilt University Medical Center (VUMC) who were eligible were enrolled within 24 hours of ICU admission. Patients were excluded from the parent study if they had chronic lung disease requiring oxygen supplementation, pulmonary fibrosis, experienced a cardiac arrest prior to enrollment, had transfer orders written or anticipated within 4 hours, died or were discharged within 48 hours of ICU admission, were admitted for uncomplicated overdose, or were in the ICU for more than 3 days prior to enrollment. Secondary exclusion criteria for this study included patients with known renal transplant or history of chronic dialysis. To limit the effects of prevalent AKI occurring before admission, patients were required to have an eGFR at enrollment of > 60 ml/min/1.73 m2 as estimated using the abbreviated Modification of Diet and Renal Disease (MDRD) equation and not experience a 0.3 mg/dl or 50% increase in serum creatinine between hospital admission and study enrollment.17 The study protocol and consent forms were approved by the Vanderbilt University Medical Center Human Subjects Institutional Review Board prior to study initiation and were in accordance with the Declaration of Helsinki.
Clinical Data Collection
Demographic and physiological data were collected at the time of enrollment. APACHE Il30 and SAPS Il31 were calculated at the time of ICU admission. The presence of the systemic inflammatory response syndrome (SIRS), sepsis, or severe sepsis was determined on a daily basis according to the American College of Chest Physicians/Society of Critical Care Medicine Consensus definition.32 Details on Vasopressor use, nephrotoxin exposure, blood product transfusion, twenty-four hour urine output, and fluid balance were collected during the ensuing 72-hours. Patients were followed prospectively until hospital discharge and ICU and hospital length of stay, and hospital mortality were recorded. The VALID database has been cross-referenced to the Social Security Death Index to allow for longitudinal determination of mortality. The Social Security Death Index is 88.2% sensitive for death for the general population.33 Patients without a recorded death in the death index were considered alive at 28 days after the study enrollment.
Biosample Collection and Definitions
Urine biomarker measurement occurred at two points: study enrollment and at 48 hours later. Serum creatinine was measured per clinical care for critically ill patients. Cases were defined by an acute increase of 0.3 mg/dl or 50% in serum creatinine measured at any point within 48 hours of biomarker measurement. For patients with multiple measurements within a given 24-hour period, the highest value was used to determine AKI or non-AKI status. For the first time interval, the serum creatinine closest to the first biomarker measurement was used as the baseline value. Patients without AKI following the first biomarker measurement were allowed to become potential cases following the 2nd biomarker measurement. For the second time period, the lowest of either the serum creatinine closest to the 2nd biomarker measurement or the enrollment value was used as the baseline value. The rationale for the latter was to keep the threshold for an increase in serum creatinine required to meet injury criteria consistent. Controls not meeting injury criteria were selected randomly and paired with cases for a total AKI case:non-AKI control ratio of 1:2 for each time interval. Lastly, as lower eGFR is a potent risk factor for AKI,34 we frequency-matched cases:controls according by eGFR groups at the time of biomarker measurement of >90 ml/min/1.73 m2 and 60-90 ml/min/1.73 m2. Glomerular filtration rate was estimated by the abbreviated Modification of Diet and Renal Disease (MDRD) equation [GFR (mL/min/1.73 m2) = 186 × (SCr)-1.154 × (Age)-0.203 × (0.742 if female) × (1.210 if African-American)].35
Laboratory Data Collection
Urine samples were collected the morning of enrollment and 48 hours later from the proximal meter reservoir of the Foley catheter, immediately placed on ice, pipetted into 400 microliter aliquots and frozen at -80°C within 1 hour of collection. Urine NGAL, L-FABP, and Cystatin C levels were measured in urine using the Enzo Life Sciences (Plymouth Meeting, PA,) Hycult Biotech (Plymouth Meeting, PA,) and R & D Systems (Minneapolis, MN) Enzyme-linked immunosorbent assay kits. Samples were run in duplicate and lab personnel were blinded to the injury status of each patient. Each ELISA kit underwent an additional in-lab validation for measurement in human urine. In brief, recombinant protein standards supplied by the ELISA manufacturer, at various concentrations were spiked into normal, control urine. After subtracting the concentration of the analyte of the unspiked control from the recovered values in the spiked samples, we determined that there was good correlation between the spiked and recovery concentrations within the standard curve for each analyte. The mean intra-assay coefficient of variations in our laboratory for uNGAL, uL-FABP, and uCysC were 3.1%, 5.6%, and 2.9%, respectively. Urine creatinine was measured at the RenaLab Clinical Core lab performed using the Jaffe enzymatic method (Roche Diagnostics, Inc.)
Statistical Analysis
Patient characteristics were described as medians with interquartile range [IQR] for continuous variables and compared using the Wilcoxon rank sum test. Categorical variables were expressed as proportions and compared using the Pearson χ2. Biomarker values were adjusted for volume status by dividing by the urine creatinine value. The ability of biomarkers to discriminate between AKI and non-AKI using creatinine criteria over the ensuing 48 hours was determined using Receiver Operating Characteristic (ROC) curves providing sensitivity and specificity at different cutoff values to detect AKI and the area under the curve (AUC). To assess the independent predictive ability of uNGAL and uL-FABP relative to a priori specified predictors of AKI such as age, modified APACHE II score, the presence of sepsis, serum creatinine at the time of biomarker measurement, and ICU location, multivariable logistic regression modeling was used. A modified APACHE II score was calculated based on the total APACHE II score minus the points derived from the serum creatinine value to allow for adjustment of creatinine as a separate covariate when these variables were simultaneously included in a multivariable regression. Adjusted effects of biomarkers were presented as odds ratios with 95% confidence intervals showing their contribution to the existing clinical predictors.
The value of each biomarker for predicting clinical outcomes beyond AKI was evaluated using a Cox Proportional Hazards model for 3 different outcomes: (1) the need for inpatient acute dialysis within 28 days of measurement (2) death within 28 days (3) and the composite end point of death or needing dialysis within 28 days. The biomarker value used was the single measurement at the beginning of the 48-hour window each AKI or non-AKI patient was selected from. Patients who were discharged alive without dialysis were considered as not having dialysis and being alive at 28 days because of the low likelihood of outpatient dialysis initiation within 28 days. To minimize overfitting,36 the dialysis models were adjusted for APACHE score only, the 28-day mortality model was adjusted for APACHE and sepsis status, and the composite outcome model was adjusted for APACHE, sepsis status and serum creatinine. The category-free net reclassification improvement (NRI) was calculated as a measure to estimate any overall improvement in reclassification of patients when biomarker data is added to clinical prediction variables. For combined biomarkers, we included a cross-product term between two biomarkers along with their main effect variables to reflect any interaction effect and overcome multicolinearity.37 Both biomarkers were mean-centered. For analysis related to biomarkers, the critical P value for considering association significant was determined using the false discovery rate,38, 39 a multiple test correction procedure that better accounts for correlated tests and balances type I and II error better than Bonferroni or other family-wise error rate corrections. The statistical software package R version 2.15.0 (www.r-project.org) and SAS version 9 were used for analyses.
Acknowledgments
This study is supported by NIH Grant UO1 HL081332 and K24 HL103836 from the National Heart, Lung and Blood Institute; K24 DK62849 from the National Institute of Diabetes, Digestive and Kidney Diseases; and Clinical Translational Science Award 1UL-1RR024975 from the National Center for Research Resources. EDS is supported by Grant K23 DK088964-02 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Dislosures
EDS reports being a consultant for Alere, Inc.
References
- 1.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 2.Bagshaw SM, Uchino S, Bellomo R, et al. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol. 2007;2:431–439. doi: 10.2215/CJN.03681106. [DOI] [PubMed] [Google Scholar]
- 3.Himmelfarb J, Ikizler TA. Acute kidney injury: changing lexicography, definitions, and epidemiology. Kidney Int. 2007;71:971–976. doi: 10.1038/sj.ki.5002224. [DOI] [PubMed] [Google Scholar]
- 4.Siew ED, Ware LB, Ikizler TA. Biological markers of acute kidney injury. Journal of the American Society of Nephrology : JASN. 2011;22:810–820. doi: 10.1681/ASN.2010080796. [DOI] [PubMed] [Google Scholar]
- 5.Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson MA, Vaidya VS, Waikar SS, et al. Urinary liver-type fatty acid-binding protein predicts adverse outcomes in acute kidney injury. Kidney Int. 2010;77:708–714. doi: 10.1038/ki.2009.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koyner JL, Bennett MR, Worcester EM, et al. Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int. 2008;74:1059–1069. doi: 10.1038/ki.2008.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein SL, Chawla LS. Renal angina. Clin J Am Soc Nephrol. 2010;5:943–949. doi: 10.2215/CJN.07201009. [DOI] [PubMed] [Google Scholar]
- 9.Siew ED, Matheny ME, Ikizler TA, et al. Commonly used surrogates for baseline renal function affect the classification and prognosis of acute kidney injury. Kidney international. 2010;77:536–542. doi: 10.1038/ki.2009.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nejat M, Pickering JW, Walker RJ, et al. Rapid detection of acute kidney injury by plasma cystatin C in the intensive care unit. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2010;25:3283–3289. doi: 10.1093/ndt/gfq176. [DOI] [PubMed] [Google Scholar]
- 11.Siew ED, Ware LB, Gebretsadik T, et al. Urine neutrophil gelatinase-associated lipocalin moderately predicts acute kidney injury in critically ill adults. J Am Soc Nephrol. 2009;20:1823–1832. doi: 10.1681/ASN.2008070673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doi K, Negishi K, Ishizu T, et al. Evaluation of new acute kidney injury biomarkers in a mixed intensive care unit. Critical care medicine. 2011;39:2464–2469. doi: 10.1097/CCM.0b013e318225761a. [DOI] [PubMed] [Google Scholar]
- 13.Mori K, Lee HT, Rapoport D, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115:610–621. doi: 10.1172/JCI23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto T, Noiri E, Ono Y, et al. Renal L-type fatty acid--binding protein in acute ischemic injury. J Am Soc Nephrol. 2007;18:2894–2902. doi: 10.1681/ASN.2007010097. [DOI] [PubMed] [Google Scholar]
- 15.Grubb A, Nyman U, Bjork J, et al. Simple cystatin C-based prediction equations for glomerular filtration rate compared with the modification of diet in renal disease prediction equation for adults and the Schwartz and the Counahan-Barratt prediction equations for children. Clinical chemistry. 2005;51:1420–1431. doi: 10.1373/clinchem.2005.051557. [DOI] [PubMed] [Google Scholar]
- 16.Siew ED, Ikizler TA, Gebretsadik T, et al. Elevated urinary IL-18 levels at the time of ICU admission predict adverse clinical outcomes. Clinical journal of the American Society of Nephrology : CJASN. 2010;5:1497–1505. doi: 10.2215/CJN.09061209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McIlroy DR, Wagener G, Lee HT. Neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery: the effect of baseline renal function on diagnostic performance. Clinical journal of the American Society of Nephrology : CJASN. 2010;5:211–219. doi: 10.2215/CJN.04240609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waikar SS, Betensky RA, Emerson SC, et al. Imperfect gold standards for kidney injury biomarker evaluation. Journal of the American Society of Nephrology : JASN. 2012;23:13–21. doi: 10.1681/ASN.2010111124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parikh CR, Coca SG, Thiessen-Philbrook H, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. Journal of the American Society of Nephrology : JASN. 2011;22:1748–1757. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parikh CR, Devarajan P, Zappitelli M, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. Journal of the American Society of Nephrology : JASN. 2011;22:1737–1747. doi: 10.1681/ASN.2010111163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molitoris BA, Okusa MD, Palevsky PM, et al. Design of clinical trials in AKI: a report from an NIDDK workshop. Trials of patients with sepsis and in selected hospital settings. Clinical journal of the American Society of Nephrology : CJASN. 2012;7:856–860. doi: 10.2215/CJN.12821211. [DOI] [PubMed] [Google Scholar]
- 22.Nickolas TL, Schmidt-Ott KM, Canetta P, et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. Journal of the American College of Cardiology. 2012;59:246–255. doi: 10.1016/j.jacc.2011.10.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haase M, Devarajan P, Haase-Fielitz A, et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. Journal of the American College of Cardiology. 2011;57:1752–1761. doi: 10.1016/j.jacc.2010.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newman DJ, Thakkar H, Edwards RG, et al. Serum cystatin C: a replacement for creatinine as a biochemical marker of GFR. Kidney Int Suppl. 1994;47:S20–21. [PubMed] [Google Scholar]
- 25.Endre ZH, Pickering JW, Walker RJ, et al. Improved performance of urinary biomarkers of acute kidney injury in the critically ill by stratification for injury duration and baseline renal function. Kidney international. 2011;79:1119–1130. doi: 10.1038/ki.2010.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Royakkers AA, Korevaar JC, van Suijlen JD, et al. Serum and urine cystatin C are poor biomarkers for acute kidney injury and renal replacement therapy. Intensive care medicine. 2011;37:493–501. doi: 10.1007/s00134-010-2087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mori K, Nakao K. Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney Int. 2007;71:967–970. doi: 10.1038/sj.ki.5002165. [DOI] [PubMed] [Google Scholar]
- 28.Doi K, Noiri E, Maeda-Mamiya R, et al. Urinary L-type fatty acid-binding protein as a new biomarker of sepsis complicated with acute kidney injury. Critical care medicine. 2010;38:2037–2042. doi: 10.1097/CCM.0b013e3181eedac0. [DOI] [PubMed] [Google Scholar]
- 29.Gerszten RE, Accurso F, Bernard GR, et al. Challenges in translating plasma proteomics from bench to bedside: update from the NHLBI Clinical Proteomics Programs. Am J Physiol Lung Cell Mol Physiol. 2008;295:L16–22. doi: 10.1152/ajplung.00044.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 31.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 32.Bone RC, Sibbald WJ, Sprung CL. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest. 1992;101:1481–1483. doi: 10.1378/chest.101.6.1481. [DOI] [PubMed] [Google Scholar]
- 33.Schisterman EF, Whitcomb BW. Use of the Social Security Administration Death Master File for ascertainment of mortality status. Popul Health Metr. 2004;2:2. doi: 10.1186/1478-7954-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grams ME, Astor BC, Bash LD, et al. Albuminuria and estimated glomerular filtration rate independently associate with acute kidney injury. J Am Soc Nephrol. 2010;21:1757–1764. doi: 10.1681/ASN.2010010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 36.Peduzzi P, Concato J, Feinstein AR, et al. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. Journal of clinical epidemiology. 1995;48:1503–1510. doi: 10.1016/0895-4356(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 37.Cohen J, Coehn P, West SG, Aiken LS. Applied Multiple Regression/Correlation Analaysis for the Behavioral Sciences. 3. Mahwah, NJ: Lawrence Erlbaum Associates; 2003. [Google Scholar]
- 38.Benjamini YYD. The Control of the False Discovery Rate in Multiple Testing Under Dependency. Annals of Statistics. 2001;29:1165–1188. [Google Scholar]
- 39.Krasner MS, Epstein RM, Beckman H, et al. Association of an educational program in mindful communication with burnout, empathy, and attitudes among primary care physicians. JAMA : the journal of the American Medical Association. 2009;302:1284–1293. doi: 10.1001/jama.2009.1384. [DOI] [PubMed] [Google Scholar]


