Abstract
Chemotherapy-induced cognitive changes have been an increasing concern among cancer survivors. Using adjuvant treatment for breast cancer as the prototype, this manuscript reviews research from neuropsychological, imaging, genetic, and animal model studies that have examined the clinical presentation and potential mechanisms for cognitive changes associated with exposure to chemotherapy. An impressive body of research supports the hypothesis that a subgroup of patients is vulnerable to post-treatment cognitive changes, although not exclusively related to chemotherapy. Further, imaging and animal model studies are providing accumulating evidence for putative mechanisms for chemotherapy-induced cognitive change. Models of aging are also reviewed in support of the proposal that cognitive changes associated with cancer and cancer treatments can be viewed in the context of factors that affect the trajectory of normal aging.
Introduction
References to cognitive changes associated with chemotherapy can be found dating back to the 1980s (1); however, serious scientific attention was not paid to the topic until the mid-90s (2–4). Post-treatment cognitive changes frequently include problems in attention, concentration, working memory and executive function. This review will use cognitive changes associated with adjuvant treatment for breast cancer as an illustrative example since the bulk of the research has been conducted in this area. Further, the relevance of viewing this research within the context of models of aging will be explored.
To date, 21 longitudinal studies of breast cancer patients (5–25) that include pre-and post-treatment assessments have been reported and the majority of studies (16) have found evidence for post-treatment cognitive change in a subgroup of individuals, while 5 studies reported negative findings (10, 14, 16, 20–21). Estimates of the prevalence of post-treatment cognitive change vary among studies, likely due to differences in patient populations, assessment instruments used, criteria for defining change, and other aspects of study methods. Many investigators site the incidence of post-treatment cognitive problems as ranging from 15–25% (26), although percentages as high as 61% have been reported (5).
Inclusion of pretreatment assessments revealed an unanticipated result in that studies have found that 20–30% of breast cancer patients have lower than expected cognitive performance based on age and education prior to receiving adjuvant treatment (e.g., 27–28). Interestingly, lower than expected level of performance does not appear to be related to psychological factors (depression or anxiety), fatigue, or surgical factors (e.g., type and length of general anesthesia) (27). Two, non-mutually exclusive hypotheses have been proposed to explain this finding (29): 1) The biology of cancer (e.g., an inflammatory response triggering neurotoxic cytokines) may contribute to lower than expected cognitive performance and/or 2) Common risk factors for the development of both breast cancer and mild cognitive changes over years may exists (e.g., poor DNA repair mechanisms have been linked to risk of cancer and neurodegenerative disorders).
Further, the assumption that cognitive changes were due to chemotherapy exposure alone has also been questioned as evidence emerged suggesting that the combination of chemotherapy and endocrine therapy or endocrine therapy alone may cause cognitive change (30). Initial examination of this issue produced mixed results; however, most studies were not powered to adequately examine the independent effects of endocrine therapy. Schilder et al (31) conducted neuropsychological assessments in the context of a longitudinal study examining patients not treated with chemotherapy, who were randomized to treatment with tamoxifen or exemestane. They demonstrated that patients treated with tamoxifen, but not exemestane experienced cognitive change compared to healthy controls. Early investigators assumed that they were studying the effects of chemotherapy; however, most breast cancer patients receive multi-modality treatment; surgery with exposure to general anesthesia, radiation therapy, and endocrine therapy in addition to chemotherapy. This in combination with the evidence for pretreatment cognitive problems led Hurria and colleagues to propose the phrase “cancer and cancer treatment associated cognitive change” more accurately describes this phenomenon (32).
Risk Factors
The finding that only a subgroup of patients experience persistent post-treatment cognitive decline, leads logically to the examination of risk factors for cognitive change. Age is a well established risk factor for cognitive decline, and researchers have speculated that older adults may be more vulnerable to cognitive side effects of cancer treatments. Cognitive reserve, which represents innate and developed cognitive capacity (influenced by education, occupational attainment, and lifestyle) has also been associated resiliency (high) or vulnerability (low) to cognitive decline following various brain insults. Support for an interaction of age, cognitive reserve and exposure to chemotherapy as risk factors for cognitive decline has been reported (19); older patients with lower levels of pretreatment cognitive reserve exposed to chemotherapy demonstrated significantly reduced performance on post-treatment processing speed. Exploratory analyses conducted by Schilder et al (31) also revealed that, in older breast cancer patients (>65), tamoxifen had a larger effect on more cognitive domains suggesting an age-dependency of the impact of tamoxifen on cognitive functioning.
Genetic factors have also been examined as potential risk factors for cognitive decline. Apolipoprotein E (ApoE) is a complex glycolipoprotein that facilitates the uptake, transport, and distribution of lipids and plays an important role in neuronal repair and plasticity after injury. The human E4 allele has been associated with a variety of disorders with prominent cognitive dysfunction including healthy individuals with memory difficulties, Alzheimer’s disease, and poor outcomes in stroke and traumatic brain injury (33). Ahles et al. (34) evaluated the relationship of the ApoE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. The results demonstrated that survivors with at least one E4 allele scored significantly lower in the visual memory and spatial ability domains, with a trend to score lower in the psychomotor domain, as compared to survivors who did not carry an E4 allele.
Small et al. (35) studied catechol-o-methyl transferase (COMT) which influences the metabolic breakdown of catecholamines through the methylation of dopamine (DA). The valine version (val allele) is approximately four times as active as the methionine version of the gene (met allele). Individuals homozygous for the val allele presumably metabolize DA much more rapidly (i.e., have lower levels of DA) than those with the met allele. Therefore, COMT is a major modulator of dopaminergic tone in the frontal cortex. These researchers found that breast cancer patients who had the COMT-Val allele and were treated with chemotherapy performed more poorly on tests of attention, verbal fluency and motor speed as compared to COMT-Met homozygotes.
Other genetic factors that have been suggested as potential candidates for increasing risk for chemotherapy-induced cognitive change include genes that regulate DNA repair (e.g., X-ray repair cross complementing protein 1, XRCC1; Meiotic recombination 11 homolog A, MRE11A), cytokine regulation (e.g., Interleukin 1, IL1; IL6; tumor necrosis factor alpha TNF-alpha), neurotransmitter activity (e.g., BDNF), and blood brain barrier efficiency (e.g., multidrug resistance 1, MDR1; organic anion transporting polypeptide, OATP). However, no studies to date have directly examined 7 the relationship between these genes and chemotherapy-induced cognitive dysfunction (29).
Inconsistencies in the Pattern of Results
Several studies have not found evidence for cognitive changes associated with chemotherapy or other treatments. This inconsistent pattern of results may be related to variability in study design and choice of comparison groups. Two of the studies compared patients treated with chemotherapy to patients treated with endocrine therapy, but did not include a healthy control group (14, 21). However, both chemotherapy and endocrine treated patients may experience cognitive change, although through different mechanisms, which may explain the lack of group differences. Further, the pattern of post-treatment cognitive deficits may be influenced by sample characteristics like age and cognitive reserve. Therefore, if a study population consists of young, highly educated (one proxy for cognitive reserve) patients, then one might expect less evidence of post-treatment cognitive deficits as compared to a study that includes older, less educated individuals. Two of the studies with negative findings included cancer patients with a mean age in the 40s (16, 20). Further, in modest size studies, the mix of patients with the vulnerable alleles of genes like APOE and COMT can vary significantly.
Imaging Studies
Several cross-sectional, post-treatment studies utilizing magnetic resonance imaging (MRI) have documented reductions in gray matter, primarily in frontal structures and hippocampus, and white matter integrity in cancer survivors treated with chemotherapy (36–40), although negative results have been reported (41). Longitudinal studies have reported similar results: 1) decreased gray matter density in bilateral frontal, temporal (including hippocampus), and cerebellar regions and right thalamus at one month post-chemotherapy with only partial recovery at one year post-chemotherapy in several structures, contrasted with no significant changes in gray matter over time in the no chemotherapy cancer group or the healthy controls (42) and 2) Decreased frontal, parietal, and occipital white matter integrity in chemotherapy exposed patients with no changes in either no chemotherapy or healthy controls at post-treatment (43).
Cross-sectional studies of cancer survivors utilizing functional imaging techniques including functional MRI (fMRI) (44–47) and functional positron emission tomography (fPET) (48) have demonstrated areas of decreased activation during performance of a cognitive task in survivors exposed to chemotherapy as compared to controls in areas similar to the structural differences described above. McDonald et al (49) conducted a longitudinal study utilizing fMRI and found frontal lobe hyperactivation to support a working memory task prior to treatment, decreased activation one month post-chemotherapy, and a return to pretreatment hyperactivation at one year post-treatment. A similar pattern was seen in patients treated with endocrine therapy. Interestingly, two other studies have reported over-activation during a memory task prior to treatment in cancer patients compared to healthy controls, consistent with the reports of neuropsychological deficits at pretreatment (50–51). One interpretation is that pretreatment over activation represents an attempt to compensate for pretreatment decreases in brain resources; however, over years, patients lose the ability for compensatory activation as a result of exposure to cancer treatments and/or age-associated changes in the brain (see the section on Cancer, Cognition and Aging below).
Animal Studies
Seigers and Fardell (52) recently reviewed the animal studies of chemotherapy-induced cognitive impairment. Studies utilizing common chemotherapeutic agents demonstrated changes in memory and learning which parallel the deficits seen in cancer survivors. Further, animal studies have demonstrated evidence for a variety of potential mechanisms for the effect of chemotherapy on the brain including: 1) inhibition of hippocampal neurogenesis; 2) oxidative damage; 3) white matter damage, including progressive change associated with 5-FU; 4) decreased hypothalamic-pituitary-adrenal axis activity; and 5) reduced brain vascularization and blood flow. Additionally, concentrations of chemotherapy agents which are ineffective in killing tumor cells have been shown to increase cell death and decrease cell division in brain regions including hippocampus suggesting that small amounts of chemotherapy crossing the blood brain barrier can have toxic effects (53).
Emerging evidence from animal studies supports the efficacy of antioxidants in blocking the behavioral and physiological effects in the brain when co-administered with chemotherapy (52). Although this is an interesting proof of principal, antioxidants may not have immediate clinical utility because of concerns that they may decrease the efficacy of chemotherapy. Fluoxetine has been shown to prevent deficits in behavior and hippocampal function when administered before and during administration of 5-FU and may represent a more promising preventative approach (54–55).
Data from imaging and animal studies support the hypothesis that chemotherapy affects brain structure and function and begin to provide evidence for candidate mechanisms of chemotherapy-induced cognitive change. Similar studies examining other aspects of cancer treatments such as endocrine therapy for breast cancer and hormone ablation therapy for prostate cancer are clearly needed.
Cancer, Cognition, and Aging
Given the data described above suggesting an association between age and post-treatment cognitive decline, a potentially useful perspective may be viewing cognitive change within the context of factors that influence the trajectory of normal aging. Cancer and aging are linked, although the molecular mechanisms responsible for the increasing risk of cancer with increasing age are not completely understood. Aging is associated with a variety of biological changes including increased cell senescence, DNA damage, oxidative stress, inflammation, and decreased telomere length (telomerase activity) (56–57). Chemotherapy has been associated with increased DNA damage, oxidative stress, inflammation and shortened telomeres (29, 58). Further, research has suggested that the targets for certain cancer treatments negatively impact biological markers of aging, e.g., increases in tumor suppressor mechanisms through the P53 pathway are associated with increased cell senescence systemically (59). Tamoxifen has also been shown to be genotoxic and other endocrine therapies may be associated with increased DNA damage because of decreased antioxidant capacity (60). Finally, all of the above processes have been implicated in cognitive decline and the development of neurodegenerative diseases (29, 58). This research suggests that biological processes underlying cancer, the impact of cancer treatments, aging, neurodegeneration and cognitive decline are linked, leading to the hypothesis that cancer treatments may accelerate the aging process (58).
In addition to examining specific pathways associated with aging, theoreticians have proposed systems theories of aging which provide interesting insights and hypotheses regarding cognition and cancer treatment. The reliability theory of aging is an example of a model of aging that is not specific to a particular biological process, but is consistent with a systems biology perspective (64). Reliability theory proposes that complex biological systems have developed a high level of redundancy to support survival. In a highly redundant system, failure of one or more components will not result in system failure if other components are available to support a specific pathway. Therefore, aging is determined by the failure rate of systems (loss of redundancy). Loss of redundancy is influenced by the initial extent of system redundancy (primarily genetically determined), the systems repair potential, and factors that increase failure rate such as poor healthcare, lifestyle risk factors, and/or exposure to environmental toxins. Someone with a low failure rate and/or high repair potential will show fewer signs of biological aging as they age chronologically, whereas someone with a high failure rate and/or low repair potential will age more rapidly as evidenced by the development of a disease associated with a specific set of system failures or frailty with a patchwork of failures across multiple systems; hence the difference between chronological and biological aging.
One implication of reliability theory is that vulnerability to post-treatment cognitive change does not necessarily depend upon a given treatment affecting a specific biological pathway. Rather, different patterns of failure rate (redundancy loss) across various biological systems may confer more or less vulnerability to specific treatments for each individual. Therefore, one patient may be vulnerable to the DNA damaging effects of a chemotherapy regimen, whereas another patient may be vulnerable to the impact on the hormonal milieu of endocrine treatments. This vulnerability may be strongly influenced by the pattern of systems failure prior to cancer diagnosis.
Further, investigators have assumed that long-term cognitive problems result from the lack of recovery from the acute effects of treatment, but remain stable after initial recovery (26). However, viewed within the context of models of aging, two additional hypotheses emerge. First, the initial effect of cancer treatment may produce a cascade of biological events which causes continued cognitive decline with aging. Second, a given treatment may not be sufficient to cause enough redundancy loss to immediately effect cognitive function but may produce a delayed effect as aging continues. Support for each of these patterns was reported by Wefel et al (22) who studied patients treated with regimens that included 5-fluorouracil (5-FU): 1) stable cognitive functioning over time after an acute post-treatment decline; 2) Continued cognitive decline over one year; and 3) no acute cognitive decline with new evidence of cognitive decline at one year post-treatment.
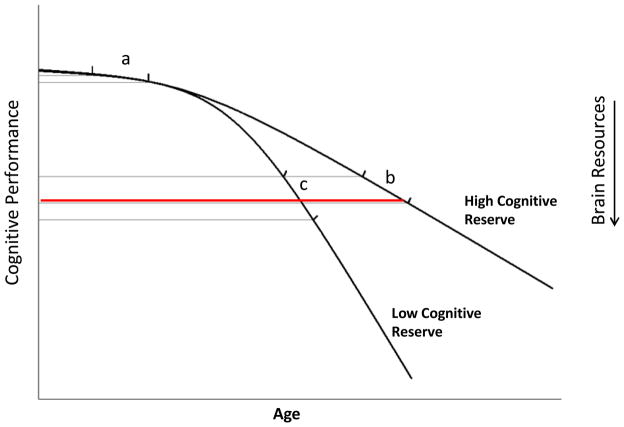
Other investigators have proposed that normal aging is a curvilinear process with relatively little change in young adulthood to middle age and increasing levels of decline in older adults (62). The slope of change in older adults is influenced by a variety of factors including cognitive reserve, diet and exercise, comorbid conditions, and genetic factors (e.g., APOE and COMT). Therefore, even if a given cancer treatment like chemotherapy has the same impact on brain resources across the lifespan, the effect on cognitive performance may differ depending on the age of the individual and the slope of cognitive aging. This point is illustrated in Figure 1 where the same change in brain resources can have a minimal effect on cognitive performance in a young adult (a), a moderate effect in an older adult with high cognitive reserve (b) and a greater effect on an older adult with low cognitive reserve (c). This model may partially explain the emerging pattern of results from imaging studies which seem to indicate group effects of chemotherapy on brain structure and function (suggesting a more consistent effect across individuals) whereas the neuropsychological studies suggest that only a subgroup of patients demonstrate a decline in cognitive performance.
Figure 1.
Impact of Change in Brain Resources on Cognitive Performance by Age
The same change in brain resources can have a minimal effect on cognitive performance in a young adult (a), a moderate effect in an older adult with high cognitive reserve (b) and a greater effect on an older adult with low cognitive reserve (c).
These considerations suggest the need for studying the short and long-term effects of cancer treatments in older cancer patients. Despite the fact that the majority of breast cancer patients are diagnosed at age 65 and older and that the number of older breast cancer survivors is growing dramatically, nearly all of the published research has focused on younger breast cancer patients (mean age <60). Longitudinal studies (9) suggest that older breast cancer patients experience objective cognitive declines shortly after treatment; however, larger scale prospective studies are needed. Additionally, a cross-sectional study of older (>65) long-term breast survivors found lower performance on measures of executive function, working memory, and divided attention as compared to healthy controls (63).
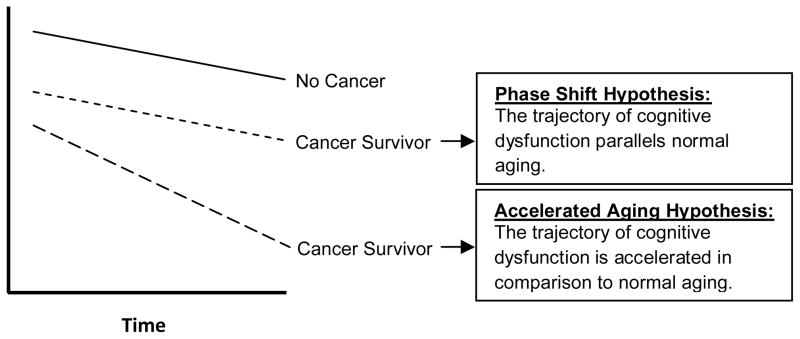
Although the recent focus of research has been on longitudinal studies with pretreatment assessments, data suggesting the possibility of continued or delayed cognitive decline demonstrates the critical need for studies examining the impact of cancer and cancer treatments on the trajectory of age-associated cognitive change, particularly in older long-term survivors. Cross-sectional studies suggest that older, long-term cancer survivors will have lower performance in various areas of neurocognitive functioning as compared to matched older adults without a cancer history (63–65). However, longitudinal assessments are important to define whether age-associated declines parallel those of older adults with no cancer history (Phase Shift Hypothesis) or a steeper slope of decline (Accelerated Aging Hypothesis) (Figure 2). These are not mutually exclusive hypotheses in that one group of survivors may demonstrate the Phase Shift pattern whereas another, vulnerable population may demonstrate the Accelerated Aging pattern. Further, it is critical to define whether the impact on the trajectory of cognitive aging is the same for someone treated as a younger versus older adult.
Figure 2.
Trajectories of Cognitive Change
Although longitudinal studies that include pretreatment assessments continue to be important to answer certain questions, there is an urgent need to study the growing number of older women who are long-term survivors of breast cancer treatment in order to evaluate the trajectory of change of cognitive function with aging. Beginning a large-scale study with pretreatment assessment with the goal of studying the impact of cognitive functioning on long-term survivors is logistically difficult and would delay answering critical questions for 10 or more years. Further, difficulties inherent in the recruitment of patients at diagnosis makes selection on critical factors (e.g., cognitive reserve, age, smoking history) extremely difficult; leaving investigators to conduct post-hoc analyses with samples that may or may not be large enough to allow for sufficient power to test specific hypotheses. An advantage of examining a large survivor cohort is that groups with specific characteristics like age, level of cognitive reserve, smoking history etc can be recruited so that specific hypotheses can be tested.
If cancer treatments accelerate the aging process, some overlap in brain structures affected by cancer treatments and aging would be expected. Imaging studies have demonstrated that total gray matter volume reliably decreases with advancing age (beginning in the mid-40s), with regional changes exhibited mainly in frontal cortex and in regions around the central sulcus (66). Global white matter decreases with advancing age with a trend for anterior white matter integrity decreasing earlier than posterior sites have been found (66–67). As described above, similar areas of the brain are affected by chemotherapy. Therefore, change in brain structure and function may be an interaction between the effects of cancer treatments and changes associated with aging.
Chemotherapy and Increased Risk for Dementia
If cancer treatments accelerate aging, then it is reasonable to assess whether these treatments increase the risk of dementia. Three studies utilized SEER data linked to Medicare claims data to evaluate the increased risk of dementia in breast cancer survivors who were or were not exposed to chemotherapy (68–70). One study (68) found a significantly increased risk for dementia for survivors who had been exposed to chemotherapy (hazard ration-1.20, 95% confidence interval-1.08–1.33), whereas the other two found no association between chemotherapy exposure and risk of dementia (69–70). An earlier twin study reported that the twin who was a cancer survivor was twice as likely to develop dementia as their co-twin, although this difference was not statistically significant (71). The authors of the first three studies recognized the limitations of Medicare claims data for the diagnosis of dementia and recommended further studies with formal neuropsychological assessments. Further, none of these studies evaluated whether exposure to chemotherapy increased risk for dementia in people who had other risk factors for dementia (e.g., APOE4 positive). Therefore, exposure to cancer treatments may not increase risk of dementia generally, but only in those people with existing risk factors for dementia.
Interventions
Few studies designed to evaluate interventions to treat cognitive changes have been reported, although a number of treatment trials are in process. Two studies have found support for the efficacy of modafinil, a psychostimulant, in improving memory and attention and reducing fatigue (72–73). Cognitive rehabilitation approaches are also being developed with initial reports of positive results (74). A recent review of factors associated with prevention of cognitive decline with aging reported evidence for cognitive training, physical exercise and possibly diet as efficacious interventions (75). These data suggest the value of testing exercise and dietary interventions to preserve cognitive function in cancer survivors.
Summary
An impressive body of research supports the hypothesis that a subgroup of breast cancer patients is vulnerable to post-treatment cognitive changes, although not exclusively related to chemotherapy. Further, imaging and animal model studies are providing accumulating evidence for putative mechanisms for chemotherapy-induced cognitive change. Additional research with other cancer groups and treatment modalities (e.g., androgen ablation for prostate cancer) is growing; however, significantly more research is required in order to determine if the findings in breast cancer are generalizable to other cancer types and treatments. Finally, models of aging are reviewed to suggest that a useful perspective is to view cognitive changes associated with cancer and cancer treatments in the context of factors that affect the trajectory of normal aging.
Acknowledgments
Supported by grants (R01 CA87845, R01 CA101318, R01 CA129769, and U54 CA132378) from the National Cancer Institute, Bethesda, MD and from the Starr Foundation.
Footnotes
Based on the presentation given as the Wiley-Blackwell invited speaker for the IPOS World Congress 2011
I have no conflict of interest to report with regard to the preparation of this manuscript.
References
- 1.Oxman TE, Silberfarb PM. Serial cognitive testing in cancer patients receiving chemotherapy. American Journal of Psychiatry. 1980;137:1263–1265. doi: 10.1176/ajp.137.10.1263. [DOI] [PubMed] [Google Scholar]
- 2.Tannock IF, Ahles TA, Ganz PA, van Dam FS. Cognitive impairment associated with chemotherapy for cancer: Report of a workshop. Journal of Clinical Oncology. 2004;22:2233–2239. doi: 10.1200/JCO.2004.08.094. [DOI] [PubMed] [Google Scholar]
- 3.Vardy J, Wefel JS, Ahles TA, et al. Cancer and cancer-therapy related cognitive dysfunction: An international perspective from the Venice Cognitive Workshop. Annals of Oncology. 2008;19:623–629. doi: 10.1093/annonc/mdm500. [DOI] [PubMed] [Google Scholar]
- 4.Wefel JS, Vardy J, Ahles TA, Schagen S. Internation Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in cancer patients. Lancet Oncology. 2011;12:703–708. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- 5.Wefel JS, Lenzi R, Theriault RL. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: Results of a prospective, randomized, longitudinal study. Cancer. 2004;100:2292–2299. doi: 10.1002/cncr.20272. [DOI] [PubMed] [Google Scholar]
- 6.Mar Fan HG, Houede-Tchen, Yi QL, et al. Fatigue, menopausal symptoms, and cognitive function in women after adjuvant chemotherapy for breast cancer: 1- and 2-year follow-up of a prospective study. Journal of Clinical Oncology. 2005;23:8025–8032. doi: 10.1200/JCO.2005.01.6550. [DOI] [PubMed] [Google Scholar]
- 7.Schilling V, Jenkins V, Morris R, et al. The effects of adjuvant chemotherapy on cognition in women with breast cancer-preliminary results of an observational study. Breast. 2005;14:142–150. doi: 10.1016/j.breast.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Bender CM, Sereika SM, Berga SL, et al. Cognitive impairment associated with adjuvant therapy in breast cancer. Psycho-oncology. 2006;15:422–430. doi: 10.1002/pon.964. [DOI] [PubMed] [Google Scholar]
- 9.Hurria A, Rosen C, Hudis C, et al. Cognitive function of older patients receiving adjuvant chemotherapy for breast cancer: a pilot prospective longitudinal study. Journal of the American Geriatric Society. 2006;54:925–931. doi: 10.1111/j.1532-5415.2006.00732.x. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins V, Shilling V, Deutsch G, et al. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. British Journal of Cancer. 2006;94:828–834. doi: 10.1038/sj.bjc.6603029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schagen SB, Muller MJ, Boogerd W, et al. Change in cognitive function after chemotherapy: a prospective longitudinal study in breast cancer patients. Journal of the National Cancer Institute. 2006;98:1742–1745. doi: 10.1093/jnci/djj470. [DOI] [PubMed] [Google Scholar]
- 12.Hermelink K, Untch M, Lux MP, et al. Cognitive function during neoadjuvant chemotherapy for breast cancer: Results of a prospective, multicenter, longitudinal study. Cancer. 2007;109:1905–1913. doi: 10.1002/cncr.22610. [DOI] [PubMed] [Google Scholar]
- 13.Stewart A, Collins B, Mackenzie J, et al. The cognitive effects of adjuvant chemotherapy in early stage breast cancer: A prospective study. Psycho-Oncology. 2008;17:122–130. doi: 10.1002/pon.1210. [DOI] [PubMed] [Google Scholar]
- 14.Collins B, Mackenzie J, Stewart A, et al. Cognitive effects of chemotherapy in post-menopausal breast cancer patients 1 year after treatment. Psycho-oncology. 2009;18:134–143. doi: 10.1002/pon.1379. [DOI] [PubMed] [Google Scholar]
- 15.Hermelink K, Henschel V, Untch M, et al. Short-term effects of treatment-induced hormonal changes on cognitive function in breast cancer patients: Results of a multicenter, prospective, longitudinal study. Cancer. 2008;113:2431–2439. doi: 10.1002/cncr.23853. [DOI] [PubMed] [Google Scholar]
- 16.Mehlsen M, Pedersen AD, Jensen AB, et al. No indications of cognitive side-effects in a prospective study of breast cancer patients receiving adjuvant chemotherapy. Psycho-Oncology. 2009;18:248–257. doi: 10.1002/pon.1398. [DOI] [PubMed] [Google Scholar]
- 17.Quesnel C, Savard J, Ivers H. Cognitive impairments associated with breast cancer treatments: results from a longitudinal study. Breast Cancer Research and Treatment. 2009;116:113–123. doi: 10.1007/s10549-008-0114-2. [DOI] [PubMed] [Google Scholar]
- 18.Vearncombe KJ, Rolfe M, Wright M, et al. Predictors of cognitive decline after chemotherapy in breast cancer patients. Journal of the International Neuropsychological Society. 2009;15:951–962. doi: 10.1017/S1355617709990567. [DOI] [PubMed] [Google Scholar]
- 19.Ahles TA, Saykin AJ, McDonald BC, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: The impact of age and cognitive reserve. Journal of Clinical Oncology. 2010;28:4434–4440. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Debess J, Riis JO, Engebjerg MC, et al. Cognitive function after adjuvant treatment fro early breast cancer: A population-based longitudinal study. Breast Cancer Research and Treatment. 2010;121:91–100. doi: 10.1007/s10549-010-0756-8. [DOI] [PubMed] [Google Scholar]
- 21.Tager FA, McKinley PS, Schnabel FR, et al. The cognitive effets of chemotherapy in post-menopausal breast cancer patients: A controlled longitudinal study. Breast Cancer Research and Treatment. 2010;123:25–34. doi: 10.1007/s10549-009-0606-8. [DOI] [PubMed] [Google Scholar]
- 22.Wefel JS, Saleeba AK, Buzdar AU, et al. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116:3348–3356. doi: 10.1002/cncr.25098. [DOI] [PubMed] [Google Scholar]
- 23.Hedayati E, Alinaghizadeh H, Schedin A, et al. Effects of adjuvant treatment on cognitive function in women with early breast cancer. European Journal of Oncology Nursing. doi: 10.1016/j.ejon.2011.07.006. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Jansen CE, Cooper BA, Dodd MJ, Miaskowski CA. A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients. Supportive Care in Cancer. 2010;19:1647–1656. doi: 10.1007/s00520-010-0997-4. [DOI] [PubMed] [Google Scholar]
- 25.Biglia N, Bounous VE, Malabaila A, et al. Objective and self-reported cognitive dysfunction in breast cancer women treated with chemotherapy: A prospective study. European Journal of Cancer. doi: 10.1111/j.1365-2354.2011.01320.x. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 26.Ahles TA, Root JC, Ryan EL. Cancer and cancer treatment associated cognitive change: An update on the state of the science. Journal of Clinical Oncology. doi: 10.1200/JCO.2012.43.0116. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahles TA, Saykin AJ, McDonald BC, et al. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Research and Treatment. 2008;110:143–152. doi: 10.1007/s10549-007-9686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wefel JS, Lenzi R, Theriault R, et al. Chemobrain in breast carcinoma? A prologue Cancer. 2004;101:466–475. doi: 10.1002/cncr.20393. [DOI] [PubMed] [Google Scholar]
- 29.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nature Reviews Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castellon SA, Ganz PA, Bower JE, et al. Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. Journal of Clinical and Experimental Neuropsychology. 2004;26:955–969. doi: 10.1080/13803390490510905. [DOI] [PubMed] [Google Scholar]
- 31.Schilder C, Seynaeve C, Beex LV, et al. Effects of tamoxifen and exemestane on cognitive function of postmenopausal patients with breast cancer: Results from the neuropsychological side study of the Tamoxifen and Exemestane Adjuvant Multinational Trial. Journal of Clinical Oncology. 2010;28:1294–1300. doi: 10.1200/JCO.2008.21.3553. [DOI] [PubMed] [Google Scholar]
- 32.Hurria A, Somlo G, Ahles T. Renaming “chemobrain”. Cancer Investigation. 2007;25:373–377. doi: 10.1080/07357900701506672. [DOI] [PubMed] [Google Scholar]
- 33.Harris SE, Deary IJ. The genetics of cognitive ability and cognitive ageing in healthy older people. Trends in Cognitive Sciences. 2011;15:388–394. doi: 10.1016/j.tics.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Ahles TA, Saykin AJ, Noll WW, et al. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psycho-Oncology. 2003;12:612–619. doi: 10.1002/pon.742. [DOI] [PubMed] [Google Scholar]
- 35.Small BJ, Sharp Rowson K, Walsh E, et al. Catechol-o-methyltransferase genotype modulates cancer treatment-related cognitive deficits in breast cancer survivors. Cancer. 2011;117:1369–1376. doi: 10.1002/cncr.25685. [DOI] [PubMed] [Google Scholar]
- 36.Inagaki M, Yoshikawa E, Matsuoka Y, et al. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer. 2007;109:146–156. doi: 10.1002/cncr.22368. [DOI] [PubMed] [Google Scholar]
- 37.Abraham J, Haut MW, Moran MT, et al. Adjuvant chemotherapy for breast cancer: effects on cerebral white matter seen in diffusion tensor imaging. Clinical Breast Cancer. 2008;8:88–91. doi: 10.3816/CBC.2008.n.007. [DOI] [PubMed] [Google Scholar]
- 38.Kopplemans V, et al. Global and focal brain volume in long-term breast cancer survivors exposed to adjuvant chemotherapy. Breast Cancer Research and Treatment. doi: 10.1007/s10549-011-1888-1. E-Pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 39.Deprez S, Amant F, Yigit R, et al. Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired functioning in breast cancer patients. Human Brain Mapping. 2011;32:480–493. doi: 10.1002/hbm.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Ruiter MB, et al. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: Converging results from multimodal magnetic resonance imaging. Human Brain Mapping. doi: 10.1002/hbm.21422. E-Pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshikawa E, Matsuoka Y, Inagaki M, et al. No adverse effects of adjuvant chemotherapy on hippocampal volume in Japanese breast cancer survivors. Breast Cancer Research and Treatment. 2005;92:81–84. doi: 10.1007/s10549-005-1412-6. [DOI] [PubMed] [Google Scholar]
- 42.McDonald BC, Conroy SK, Ahles TA, et al. Gray matter reduction associated with systemic chemotherapy for breast cancer: A prospective MRI study. Breast Cancer Research and Treatment. 2010;123:819–828. doi: 10.1007/s10549-010-1088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deprez S, Amant F, Smeets A, et al. Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. Journal of Clinical Oncology. 2012;30:274–281. doi: 10.1200/JCO.2011.36.8571. [DOI] [PubMed] [Google Scholar]
- 44.Ferguson RJ, McDonald BC, Saykin AJ, Ahles TA. Brain structure and function differences in monozygotic twins: Possible effects of breast cancer chemotherapy. Journal of Clinical Oncology. 2007;25:3866–3870. doi: 10.1200/JCO.2007.10.8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kesler SR, Kent JS, O’Hara R. Prefrontal cortex and executive function impairments in primary breast cancer. Archives of Neurology. 2011;68:1447–1453. doi: 10.1001/archneurol.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kesler SR, et al. Regional brain activation during verbal declarative memory in metastatic breast cancer. Clinical Cancer Research. 2009;15:6665–6673. doi: 10.1158/1078-0432.CCR-09-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Ruiter MD, Reneman L, Boogerd W, et al. Cerebral hyperresponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Human Brain Mapping. 2011;38:1206–1219. doi: 10.1002/hbm.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silverman DH, Dy CJ, Castellon SA, et al. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5–10 years after chemotherapy. Breast Cancer Research and Treatment. 2007;103:303–31. doi: 10.1007/s10549-006-9380-z. [DOI] [PubMed] [Google Scholar]
- 49.McDonald BC, Conroy SK, Ahles TA, et al. Alterations in brain activation during working memory processing associated with breast cancer and treatment: A Prospective functional MRI study. Journal of Clinical Oncology. doi: 10.1200/JCO.2011.38.5674. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cimprich B, Reuter-Lorenz P, Nelson J, et al. Prechemotherapy alterations in brain function in women with breast cancer. Journal of Clinical and Experimental Neuropsychology. 2010;32:324–331. doi: 10.1080/13803390903032537. [DOI] [PubMed] [Google Scholar]
- 51.Scherling C, Collins B, MacKenzie J, et al. Pre-chemotherapy differences in visuospatial working memory in breast cancer patients compared to controls: An fMRI study. Frontiers in Human Neuroscience. 2011;5:1–21. doi: 10.3389/fnhum.2011.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seigers R, Fardell JE. Neurobiological basis of chemotherapy-induced cognitive impairment: A review of rodent research. Neuroscience and Biobehavioral Reviews. 2011;35:729–741. doi: 10.1016/j.neubiorev.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 53.Dietrich J, Han R, Yang Y, et al. CNS progenitor cells and oligodendrocytes ae targets of chemotherapeutic agents in vitro and in vivo. Journal of Biology. 2006;5:1–23. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lyons L, ElBeltagy M, Bennett G, Wigmore P. Fluoxetine counteracts the cognitive and cellular effects of 5-fluorouracil in the rat hippocampus by a mechanism of prevention rather than recovery. PLoS One. 2012;1:e30010. doi: 10.1371/journal.pone.0030010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.ElBeltagy M, Mustafa S, Umka J, et al. Fluoxetine improves the memory deficits caused by the chemotherapy agent 5-fluorouracil. Behavioural Brain Research. 2010;208:112–117. doi: 10.1016/j.bbr.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 56.Irminger-Finger I. Science of cancer and aging. Journal of Clinical Oncology. 2007;25:1844–1851. doi: 10.1200/JCO.2007.10.8928. [DOI] [PubMed] [Google Scholar]
- 57.Campisi J, Yaswen P. Aging and cancer cell biology, 2009. Aging Cell. 2009;8:221–225. doi: 10.1111/j.1474-9726.2009.00475.x. [DOI] [PubMed] [Google Scholar]
- 58.Maccormick RE. Possible acceleration of aging by adjuvant chemotherapy: A cause of early onset frailty. Medical Hypothesis. 2006;67:212–215. doi: 10.1016/j.mehy.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 59.Campisi J, d’Adda di Fagagna F. Cellular senescence. When bad things happen to good cells. Nature Reviews Molecular Cell Biology. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 60.Brown K. Is tamoxifen a genotoxic caricinogen in women? Mutagenesis. 2009;24:391–404. doi: 10.1093/mutage/gep022. [DOI] [PubMed] [Google Scholar]
- 61.Gavrilov LA, Gavrilova NS. Reliability theory of aging and longevity. In: Masoro EJ, Sustad ST, editors. Handbook of the Biology of Aging. 6. Academic Press; Burlington, MA: 2006. [Google Scholar]
- 62.Lindenberger U, Nagel IE, Chicherio C, et al. Age-related decline in brain resources modulates genetic effects on cognitive functioning. Frontiers in Neuroscience. 2008;2:234–244. doi: 10.3389/neuro.01.039.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamada TH, Denburg NL, Beglinger LJ, Schultz SK. Neuropsychological outcomes of older breast cancer survivors: Cogntive features ten or more years after chemotherapy. Journal of Neuropsychiatry and Clinical Neurosciences. 2010;22:48–54. doi: 10.1176/appi.neuropsych.22.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahles TA, Saykin AJ, Furstenberg CT, et al. Neuropsychological impact of standard-dose chemotherapy in long-term survivors of breast cancer and lymphoma. Journal of Clinical Oncology. 2002;20:485–493. doi: 10.1200/JCO.2002.20.2.485. [DOI] [PubMed] [Google Scholar]
- 65.Koppelmans V, Breteler MMB, Boogerd W, et al. Neuropsychological performance in breast cancer survivors more than 20 years after adjuvant chemotherapy. Journal of Clinical Oncology. doi: 10.1200/JCO.2011.37.0189. e-Pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 66.Peelle JE, Cusack R, Henson RN. Adjusting for global effects in voxel-based morphometry: Gray matter decline in normal aging. Neuroimage. 2012;60:1503–1516. doi: 10.1016/j.neuroimage.2011.12.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gunning-Dixon FM, et al. Aging of cerebral white matter: A review of MRI findings. International Journal of Geriatric Psychiatry. 2009;24:109–117. doi: 10.1002/gps.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heck JE, Alber SM, Franco R, et al. Patterns of dementia diagnosis in surveillance, epidemiology, and end results breast cancer survivors who use chemotherapy. Journal of the American Geriatrics Society. 2008;56:1687–1692. doi: 10.1111/j.1532-5415.2008.01848.x. [DOI] [PubMed] [Google Scholar]
- 69.Baxter NN, Durham SB, Phillips KA, et al. Risk of dementia in older breast cancer survivors: A population-based cohort study of the association with adjuvant chemotherapy. Journal of the American Geriatrics Society. 2009;57:403–411. doi: 10.1111/j.1532-5415.2008.02130.x. [DOI] [PubMed] [Google Scholar]
- 70.Raji MA, Tamborello LP, Kuo YF, et al. Risk of subsequent dementia diagnoses does not vary by types of adjuvant chemotherapy in older women with breast cancer. Medical Oncology. 2009;26:452–459. doi: 10.1007/s12032-008-9145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heflin LH, Meyerowitz BE, Hall P, et al. Cancer as a risk factor for long-term cognitive deficits and dementia. Journal of the National Caner Institute. 2005;97:854–856. doi: 10.1093/jnci/dji137. [DOI] [PubMed] [Google Scholar]
- 72.Kohli SS, Fisher G, et al. The effect of modafinil on cognitive function in breast cancer survivors. Cancer. 2009;115:2605–2616. doi: 10.1002/cncr.24287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lundorff LE, Jønsson BH, Sjøgren P. Modafinil for attentional and psychomotor dysfunction in advanced cancer: a double-blind, randomised, cross-over trial. Palliative Medicine. 2009;23:731–8. doi: 10.1177/0269216309106872. [DOI] [PubMed] [Google Scholar]
- 74.Ferguson JR, McDonald BC, Rocque MA, et al. Development of CBT for chemotherapy-related cognitive change: Results of a waitlist control trial. Psycho-Oncology. 2012;21:176–186. doi: 10.1002/pon.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Plassman BL, Williams JW, Burke JR, et al. Systematic Review: Factors associated with risk for and possible prevention of cognitive decline in later life. Annals of Internal Medicine. 2010;153:182–193. doi: 10.7326/0003-4819-153-3-201008030-00258. [DOI] [PubMed] [Google Scholar]