Abstract
Background
Mammalian sterile 20-like kinase 1 (Mst1) is a mammalian homolog of Hippo kinase from Drosophila and it is a critical component of the Hippo signaling pathway, which regulates a variety of biological processes ranging from cell contact inhibition, organ size control, apoptosis and tumor suppression in mammals. Mst1 plays essential roles in the heart disease since its activation causes cardiomyocyte apoptosis and dilated cardiomyopathy. However, the mechanism underlying Mst1 activation in the heart is not known.
Methods and Results
To identify novel cardiac proteins that may regulate Mst1 activity in the heart under pathophysiological conditions, a yeast two-hybrid screen of a human heart cDNA library with a dominant-negative Mst1 (K59R) mutant used as bait was performed. As a result, protein-L-isoaspartate (D-aspartate) O-methyltransferase (PCMT1) was identified as an Mst1-interacting protein. The interaction of PCMT1 with Mst1 was confirmed by co-immunoprecipitation in both co-transfected HEK293 cells and native cardiomyocytes, in which PCMT1 interacted with the kinase domain of Mst1, but not with its C-terminal regulatory domain. Overexpression of PCMT1 did not affect the Mst1 expression, but significantly attenuated the Mst1 activation and its apoptotic effects in response to the hypoxia/reoxygenation induced injury in cardiomyocytes. Indeed, upregulation of PCMT1 by CGP3466B, a compound related to the anti-Parkinson’s drug R-(−)-deprenyl with potent antiapoptotic effects, inhibited the hypoxia/reoxygenation induced Mst1 activation and cardiomyocte apoptosis.
Conclusions
These findings implicate PCMT1 as a novel inhibitor of Mst1 activation in cardiomyocytes and suggest that targeting PCMT1 may prevent myocardial apoptosis through inhibition of Mst1.
Keywords: Mst1 kinase, PCMT1, hypoxia/reoxygenation, cardiac myocytes, apoptosis
1. Introduction
Mammalian sterile 20—like kinase 1 (Mst1) is an ubiquitously expressed serine/threonine kinase with a similarity to the Hippo kinase from Drosophila and it is a critical component of the Hippo signaling pathway, which regulates a variety of biological processes ranging from cell contact inhibition, cell growth, organ size control, apoptosis and tumor suppression in mammals [1] [2]. Mst1 contains an N-terminal catalytic domain and an autoinhibitory segment followed by a dimerization domain and a nuclear localization motif in the non-catalytic C-terminal region [3]. In addition, Human Mst1 has two caspase cleavage sites located between the catalytic and regulatory domains, which mediate the cleavage of the autoinhibitory domain [3] [4]. In response to a variety of apoptotic stimuli, Mst1 is cleaved by caspases to produce a 34–36-kDa N-terminal constitutively active fragment and this cleavage markedly increases Mst1 kinase activity and translocates the cleaved Mst1 to the nucleus where it phosphorylates histone H2B on Ser14, resulting in apoptotic cell death [4] [5] [6]. In addition to Histone H2B, several Mst1 substrates, including FOXO [7] [8] [9], LATS1/2 [10] [11], JNK [12] and cardiac troponin I [13], have been recently identified. For instance, MST1 has been shown to phosphorylate FOXO and promote FOXO nuclear translocation, thereby inducing apoptosis in neuronal cells [7] [8].
Regulation of Mst1 appears to occur mainly at posttranslational level. Mst1 autophosphorylation has been proposed to contribute to the kinase activation [14]. Several phosphorylation sites have been identified in Mst1, namely Thr175, Thr177, Thr183, Thr187, Ser327 and Thr387, of which, Thr183 and Thr187 appear to be essential for kinase activation [14] [15] [16]. The effect of phosphorylation at these sites on the activation of Mst1 may be further amplified by dimerization and eventually leads to the caspase cleavage, thereby constituting a powerful amplification loop of apoptotic responses [16]. In addition, protein-protein interactions have also been shown to play critical roles in the regulation of Mst1 activity. Thus far, several proteins including Ras association domain family protein (Rassf) [16] [17] [18], hWW45 [17] [19], PHLPP1 [20], and Heat Shock Protein 70 (Hsp70) [21], have been identified to interact with Mst1 and regulate Mst1 activation. For instance, both Rassf1 and Rassf2 have been shown to interact with and stabilize Mst1, thereby preventing Mst1 from the degradation and inhibiting tumor growth [18] [22]. In contrast, our recent results demonstrated that Hsp70 decreases Mst1 activity through promoting Mst1 degradation via a CHIP dependent pathway, thereby preventing cancer cells from cisplatin induced apoptosis [21]. Further investigation of the mechanisms underlying the regulation of Mst1 activation will provide new insights into the pathogenesis of human diseases such as tumor and heart failure.
Recently, the physiological role of Mst1 in the cardiovascular system has begun to be explored. In cardiomyocytes, Mst1 is activated by pathological stimuli, such as hypoxia/reoxygenation in vitro and ischemia/reperfusion in vivo [23]. Cardiac-specific over-expression of Mst1 has been shown to cause dilated cardiomyopathy in mice [23]. Inhibition of endogenous Mst1 prevents apoptosis of cardiomyocytes and cardiac dysfunction after myocardial infarction without producing cardiac hypertrophy [23] [24]. Recently, we identified Mst1 as a novel kinase that mediates cTnI phosphorylation and plays a critical role in the modulation of myofilament function in the heart [13]. However, despite these important functions, little is relatively known about the mechanisms underlying the regulation of the Mst1 activation in the heart. In the present study, we performed yeast two-hybrid screen of a human heart cDNA library using the dominant negative form of Mst1 (K59R) as bait to identify cardiac proteins that may associate with Mst1 and regulate Mst1 activation in the heart. Subsequently, we identify protein-L-isoaspartate (D-aspartate) O-methyltransferase (PCMT1) as a novel Mst1-interacting protein that negatively regulates Mst1 activation and its function in cardiomyocytes.
2. Materials and Methods
2.1. Yeast Two-Hybrid Library Screening and Interaction Assays
To identify novel proteins that interact with Mst1, we screened a human heart cDNA library using human dominant-negative Mst1 (K59R) as bait and the MATCHMAKER GAL4 yeast 2-hybrid system 3 (Clontech Laboratories Inc) as previously described [21] [13]. The screening was performed in AH109 yeast cells according to the protocol provided by the Clontech Matchmaker Two-Hybrid System. Positive colonies were subject to multiple rounds of additional selection in the appropriate medium and β-galactosidase (β-gal) filter assays to verify specificity.
2.2. Primary Culture of Neonatal Rat Ventricular Myocytes (NRCMs) and Hypoxia/reoxygenation Injury
We obtained ventricles from 1-day-old Sprague-Dawley rats and isolated cardiac myocytes by digestion with trypsin-EDTA and type 2 collagenase as previously described [25]. Neonatal rats were obtained from Charles River Laboratories North Wilmington, Mass. This study was reviewed and approved by the Institutional Animal Care and Use Committee at Thomas Jefferson University. Hypoxia was achieved by placing the cells in a hypoxia chamber filled with 5% CO2 and 95% N2 at 37°C for 12 h. Following hypoxia exposure, cells were reoxygenated by placing cells in the normoxic culture medium for 24 h [26].
2.3. Coimmunoprecipitation of Mst1 and PCMT1 in HEK293 Cells and Cardiomyocytes
HEK293 cells were transiently transfected with Mst1 and PCMT1 cDNAs using FuGENE 6 as described by the manufacturer (Roche Applied Science). Both HEK293 cells and cardiomyocytes were lysed in the buffer containing 1% Nonidet P-40, 150 mmol/L NaCl, 50 mmol/L Tris (pH 8), 100 µmol/L EDTA, and protease inhibitors. Coimmunoprecipitation of Mst1 and PCMT1 was performed essentially as described [27] [25]. The following antibodies were used for detection and immunoprecipitation: rabbit polyclonal c-Myc (Invitrogen), mouse monoclonal FLAG M2 (Sigma), mouse monoclonal PCMT1 (Santa Cruz), rabbit polyclonal PCMT1 (Abcam) and rabbit polyclonal Mst1 (Abgent). Secondary antibodies were peroxidase-conjugated donkey anti-rabbit or anti-mouse (Jackson ImmunoResearch). Detection of the peroxidase was performed with ECL reagents.
2.4. Immunofluorescence Staining
Freshly isolated neonatal rat cardiomyocytes were fixed and sequentially incubated with primary antibodies and appropriate fluorescent-labeled secondary antibodies. Images were visualized using an Olympus IX70 epifluorescence microscope as previously described [25].
2.5. Mst1 Activity Assay
HEK293T cells were transiently transfected with Myc-Mst1, and Flag-PCMT1 cDNAs. Forty-eight hours after transfection, cells were lysed in the buffer containing 50 mmol/L Tris-HCl (pH 7.5), 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L MgCl2, and 0.5% Triton X-100. Cell lysates were subjected to centrifugation at 12,000 × g for 10 min at 4°C. The resulting supernatants were immunoprecipitated with anti-Myc for 2 h at 4°C. Equal amounts of precipitated Mst1 were incubated for 20 min at 30°C with 2 µg Histone H2B (Sigma) in 25 µL kinase buffer 40 mmol/L HEPES-NaOH (pH 7.4), 20 mmol/L MgCl2, 1 mmol/L DTT, and 1 µCi [γ-32P]ATP. Reactions were terminated by the addition of 2 × SDS sample buffer, and then loaded to 15% SDS-PAGE and subjected to autoradiography. For Mst1 kinase assay in cardiomyocytes, cell homogenates (400µg) were immunoprecipitated using anti-Mst1 antibody (BD Transduction Laboratories, San Diego, California, USA), and then incubated with 2 µg Histone H2B (Sigma) in 25 µL kinase assay buffer for 20 min at 30°C. Samples were subjected to SDS-PAGE and phosphorylation of histone H2B was detected by immunoblotting with anti-phospho-Histone H2B (Ser14) antibody (Millipore).
2.6. Small Interfering RNA of PCMT1
PCMT1 small interfering RNA (siRNA) was designed based on sequence specific for rat PCMT1 cDNA. Two pairs of small interfering (si)RNA oligonucleotides for rat PCMT1 (sense strand, 5’-GUG GGA AAG UCA UUG GAA UUG AUC A-3’, and 5’-CAU CAA GAC AGA UAA AGU AUU UGA G-3’) and a pair of control siRNA oligonucleotides (5′-CAG AGA GGA GGA AAG GAG ACG CAG G-3′) were synthesized by Integrated DNA Technologies (Coralville, Iowa). Cardiomyocytes were transfected with Gene Silencer (Gene Therapy System, San Diego, Calif) transfecting reagent with target-specific siRNA (20 nmol/L) and control siRNA (20 nmol/L) in serum-free medium according to the recommendations of the manufacturer.
2.7. Construction of Adenoviruses
Adenoviruses harboring wild-type Mst1 (Ad-Mst1) and dominant-negative Mst1 (Ad-DNMST) were made using AdMax (Microbix) as previously described [21]. Briefly, pBHGloxΔE1,3Cre, including the ΔE1 adenoviral genome, was cotransfected with the pDC shuttle vector containing the gene of interest into Ad293 cells using FuGene 6 Transfection Reagent (Roche, Indianapolis, IN). The viruses were propagated in Ad293 cells and purified using CsCl2 banding, followed by dialysis against 20 mmol/L Tris-buffered saline with 10% glycerol. Titering was performed on Ad293 cells using Adeno-X Rapid Titer kit (Clontech) according to the instructions of the manufacturer. Ad-PCMT1 was a gift from Dr. Takuji Shirasawa (Tokyo Metropolitan Institute of Gerontology, Japan).
2.8. Transverse aortic constriction (TAC)
16 male C57BL/6J mice were randomly divided into 2 groups of 8 mice each as follows: sham 4 wk group and TAC 4 wk group. Essentially, surgical procedures were performed as previously described [28]. Briefly, a partial ligation (constriction) of the aortic arch was introduced between the right innominate artery and the left common carotid artery using a 27-gauge needle as a guide. The sham operation was performed likewise, but the aorta was not ligated. The success of the procedure was confirmed by measuring both left ventricle function and the thickness of the inter-ventricular wall by echocardiography before the animal was sacrificed.
2.9. Quantitative real-time PCR
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) in accordance with the manufacturer’s instructions. Two micrograms of total RNA were reverse transcribed in a total volume of 20 µl containing the SYBR-Green mixture (Bio-Rad Laboratories, Hercules, USA). The specific primers used for 18S rRNA detection were 5'- CGGCTACCACATCCAAGGAA -3' and 5'- CTGGAATTACCGCGGCT -3'. The PCMT1 primers were 5'- GGAAGAATGGGATACGCTGA-3' and 5'- TTCCACCAGGCTTTAACTGG-3'. Reactions were carried out using a MyiQ Single-Color Real-time PCR Detection System (Bio-Rad Laboratories, Hercules, USA) for 40 cycles (95 C for 10 s, 60 C for 30 s) after an initial incubation at 95 C for 3 min. The fold change in expression of each gene was calculated using the 2−ΔΔCT method with 18S rRNA as an internal control.
2.10. Western blotting
Homogenized mouse heart tissues were normalized for protein concentration using the Bradford assay (Bio-Rad Laboratories, Hercules, USA). The proteins (20 µg) were separated using 10% SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, USA). The membranes were then blocked for 1 hr in 5% nonfat milk. Subsequently, the membranes were exposed to primary antibodies against PCMT1 (1:1000 dilution; Proteintech Group, Inc. Chicago, USA). GAPDH (1:1000 dilution; Abcam) was measured as an internal control. Immunodetection was accomplished using a goat anti-rabbit secondary antibody and an enhanced chemiluminescence kit (Millipore, Billerica, USA).
2.11. Assays for Apoptosis
Cardiomyocyte apoptosis measured by using Terminal Transferase-mediated dUTP nick-end labeling (TUNEL) staining was performed using the In Situ Cell Death Detection kit (Roche). Annexin V staining was performed using an annexin V–fluorescein isothiocyanate (FITC) stain detection kit (BioVision). Histone-associated DNA fragments were quantitated by the Cell Death Detection ELISA (Roche) [25]
2.12. Statistical Analyses
Data are expressed as means±SEM. The statistical significance of differences was assessed by Student’s t tests or ANOVA, as appropriate; a value of P<0.05 was considered statistically significant.
3. Results
3.1. Interaction of PCMT1 with Mst1
In an effort to identify novel Mst1 binding proteins, a yeast 2-hybrid screening was performed with human dominant negative Mst1 (DN-Mst1), which lacks kinase activity and does not affect cell apoptosis, as bait in conjunction with a human heart cDNA library. After screening 2.4×106 clones, we identified 3 positive clones bearing cDNA encoding the N-terminal domain (aa122–227) of protein-L-isoaspartate (D-aspartate) O-methyltransferase (PCMT1), suggesting that this region of PCMT1 is sufficient to interact with Mst1 in yeast.
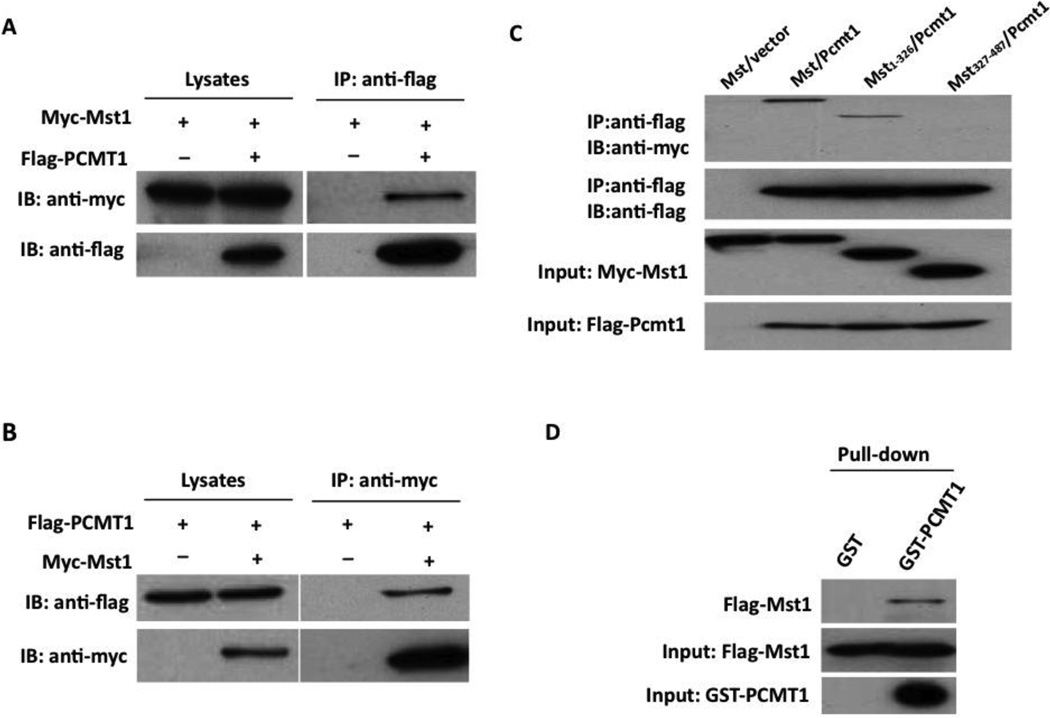
To determine whether the interaction between Mst1 and PCMT1 occurs in mammalian cells, co-immunoprecipitation experiments were performed in HEK293 cells transfected with PCMT1 and Mst1 expression vectors. Immunoprecipitation of FLAG-tagged PCMT1 led to coimmunoprecipitation of Myc-tagged Mst1 when both proteins were cotransfected (Figure 1A). As a control, the anti-FLAG antibody did not immunoprecipitate Myc-tagged Mst1 in the absence of FLAG-PCMT1. Similarly, immunoprecipitation of Myc-tagged Mst1 resulted in coimmunoprecipitation of FLAG-tagged PCMT1, whereas the anti-Myc antibody did not immunoprecipitate FLAG-PCMT1 in the absence of Myc-Mst1 (Figure 1B). Together, these findings indicate that PCMT1 and Mst1 exist in the same complex in mammalian cells.
Figure 1.
Physical interaction between Mst1 and PCMT1 by immunoprecipitation analysis. A, Myc-Mst1 expression vector in combination of either empty vector or pFlag-PCMT1 were co-transfected into HEK293 cells. Extracted proteins were precipitated by anti-FLAG antibody and then separated by 12% SDS-PAGE. The transferred membrane was immunoblotted with either HRP conjugated anti-Myc or HRP conjugated anti-FLAG antibody. B, Flag-PCMT1 expression vector in combination of either empty vector, pMT2-Myc-Mst1 were co-transfected into HEK293 cells. Extracted proteins were precipitated by anti-Myc antibody and then separated by 12% SDS-PAGE. The transferred membrane was immunoblotted with either HRP conjugated anti-Myc or HRP conjugated anti-FLAG antibody. C. FLAG-PCMT1 expression vector in combination with either empty vector or expression vectors of Myc-Mst1 mutants were co-transfected into HEK293 cells. Extracted proteins were precipitated by anti-Flag antibody and then separated by 15% SDS-PAGE. The transferred membrane was immunoblotted with either HRP conjugated anti-FLAG or HRP conjugated anti-Myc antibody. Lysates were also immunoblotted with anti-FLAG antibody to show the expression levels of FLAG-PCMT1 in HEK293 cells. D, Recombinant Flag-Mst1 incubated with either GST or GST-PCMT1 and proteins were precipitated with glutathione-Sepharose 4B beads. The precipitated proteins were separated through SDS-PAGE, and Western blot analysis was done with anti-Mst1 antibody.
Mst1 contains an N-terminal catalytic domain (aa 1–326) and a C-terminal regulatory domain (aa 327–487) [3]. To further map the PCMT1 interaction domain of Mst1, we constructed the kinase domain and regulatory domain of Mst1 in pCS2-6MT vector with a 6×Myc tag and transfected these constructs into HEK293 cells along with the Flag-PCMT1 expression plasmid. Lysates from transfected HEK293 cells were then immunoprecipitated with anti-Flag antibody and analyzed by Western blotting analysis. We found that Flag-PCMT1 bound to the full length and the N-terminal fragment of Mst1, but not to the C-terminal fragment of Mst1 (Figure 1C). As a negative control, the anti-Flag antibody did not immunoprecipitate Myc-Mst1 in cells cotransfected with Myc-Mst1 and empty Flag vector. These results suggest that the N-terminal catalytic domain of Mst1 is required for the binding to PCMT1.
To show whether there is a direct protein interaction between PCMT1 and Mst1, GST Pull-down was performed using bacterially expressed GST fused to full-length PCMT1. Sepharose-conjugated GST-PCMT1 or GST alone was incubated with purified Flag-Mst1, and unbound and bound fractions were analyzed by SDS-PAGE and Western blot. Mst1 bound to GST-PCMT1 but not GST alone, confirming a direct protein interaction between PCMT and Mst1 (Figure 1D).
3.2. Association of Mst1 and PCMT1 in Cardiomyocytes
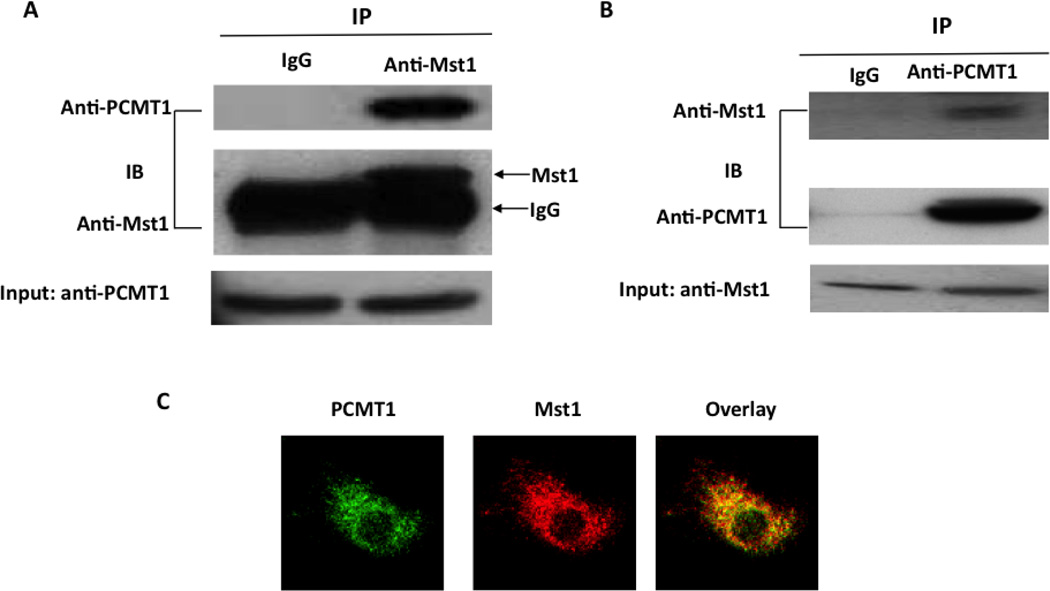
To determine whether there is an endogenous interaction of PCMT1 and Mst1 in cardiomyocytes, we performed immunoprecipitation with anti-Mst1 antibody using lysates obtained from neonatal rat cardiac myocytes. Indeed, PCMT1 coprecipitated with the anti-Mst1 antibody but not with the nonimmune IgG (Figure 2A and 2B). To determine the intracellular localization of this interaction, we performed immunofluorescence staining in neonatal rat cardiac myocytes. Immunofluorescent microscopy showed that PCMT1 and Mst1 are colocalized in the cytoplasm (Figure 2C). The result indicates that PCMT1 interacts with Mst1 in cardiomyocytes under physiological conditions.
Figure 2.
Association of Mst1 with PCMT1 in cardiomyocytes. A. Cell lysates obtained from neonatal rat cardiomyoctes were immunoprecipitated with anti-Mst1 antibody and then separated by 12 % SDS-PAGE. Transferred membrane was immunoblotted with either anti-PCMT1 or Mst1 antibody. B, Cell lysates obtained from neonatal rat cardiomyoctes were immunoprecipitated with anti-PCMT1 antibody and then separated by 12 % SDS-PAGE. Transferred membrane was immunoblotted with either anti-PCMT1 or Mst1 antibody. C, fixed cardiomyocytes were stained with anti-PCMT1 monoclonal antibody and rabbit polyclonal anti-Mst1 antibody and processed for confocal imaging. The merged image shows clear colocalization of these two proteins in cytoplasm of cardiomyocytes.
3.3. Inhibition of Mst1 Activity and Cell Apoptosis by PCMT1
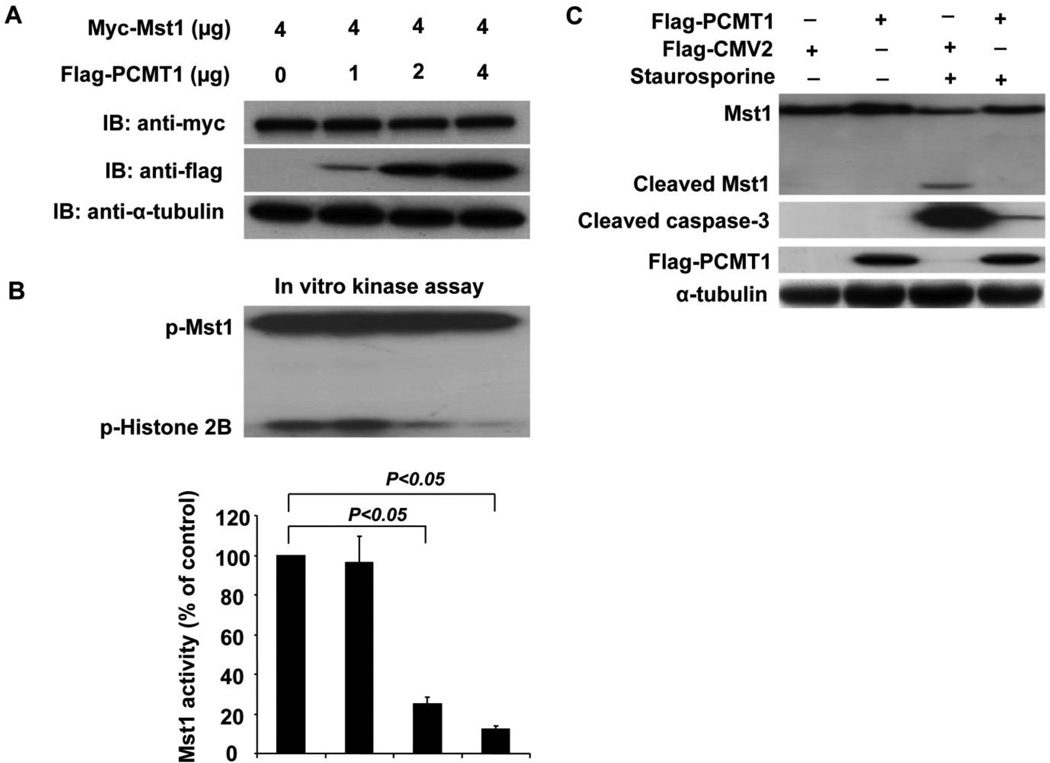
To determine whether the interaction of PCMT1 with Mst1 has functional consequences in terms of affecting Mst1 expression and activity, we cotransfected Mst1 together with increasing concentration of PCMT1 cDNAs into HEK293 cells and then determined the Mst1 expression and its kinase activity. Indeed, transfection of PCMT1 result in an increased expression of PCMT1 in HEK293 cells, as determined by western blotting analysis (Figure 3A). However, the expression of Mst1 was not altered when co-transfected with PCMT1 (Figure 3A). Surprisingly, co-transfection of PCMT1 markedly inhibited Mst1 activity in a dose-dependent manner in HEK293 cells, as determined by an in vitro kinase assay using Histone H2B as a substrate (Figure 3B). Furthermore, PCMT1 was not phosphorylated by Mst1 kinase when GST-PCMT1 was used as a substrate in the kinase assay, indicating that PCMT1 is not a substrate of Mst1 (data not shown). To further examine whether PCMT1 affects Mst1 cleavage and caspase-3 activation, we transfected HEK293 cells with PCMT1. As shown in Figure 3C, overexpression of PCMT1 substantially inhibited the Mst1 cleavage and caspase-3 activation in response to 0.5 µM staurosporine (STS) treatment. Together, these results suggest that PCMT1 is a novel negative regulator of Mst1 in mammalian cells.
Figure 3.
Inhibition of Mst1 activity by PCMT1. A, Myc-Mst1 expression vector together with increasing concentration of Flag-PCMT1 vector were co-transfected into HEK293 cells. Forty-eight hours after transfection, cell lysates were subjected to Western blot analysis of Mst1 and PCMT1 expression. The transferred membrane was immunoblotted with either HRP conjugated anti-Myc or HRP conjugated anti-FLAG antibody. B, HEK293 cells were transfected with Myc-Mst1 expression vector in conjuction with increasing concentration of Flag-PCMT1 expression vector. Forty-eight hours after transfection, equal amount of cell lysates were subjected to immunoprecipitate with anti-Myc antibody, and immunoprecipitates were then subjected to in gel kinase assay using Histone 2B as a substrate. C, HEK293 cells were transfected with either Flag-PCMT1 expression vector or empty vector. Forty-eight hours after transfection, cells were treated with 0.5 µM staurosporine (STS) for 6 hours. Full-length MST1 and a 36-kDa N-terminal fragment (Cleaved MST1), cleaved caspase-3, Flag-PCMT1, and α-tubulin were detected by western blot analysis. The data are representatives of 4 independent experiments.
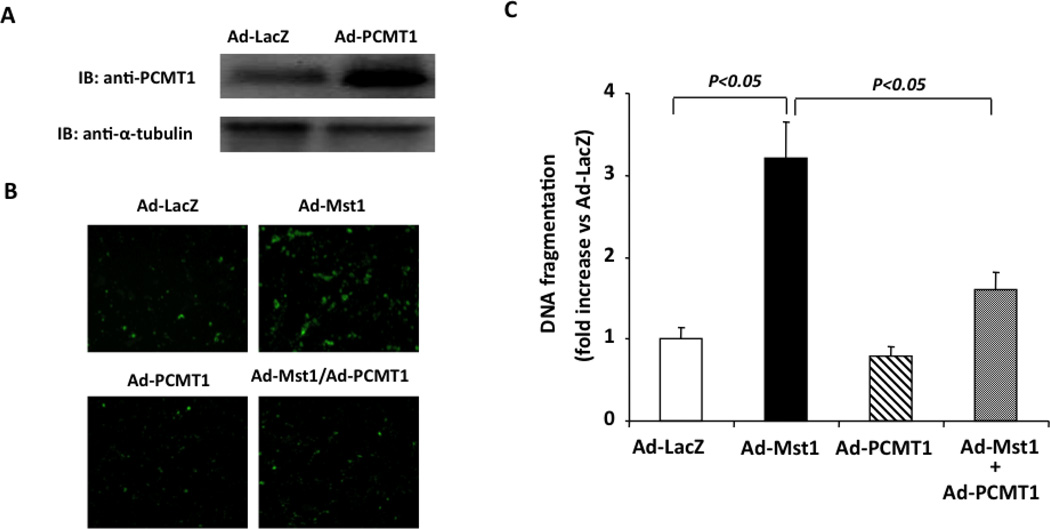
Because Mst1 has been characterized to induce cardiomyocyte apoptosis [23] [24], we investigated whether PCMT1 can affect cell apoptosis via inhibitory effects on Mst1 in cardiac myocytes. To this end, we transduced cardiomyocytes with Ad-PCMT1 (Ad-PCMT1, MOI=50) to increase the expression of PCMT1 (Figure 4A). Transduction of cardiomyocytes with recombinant adenovirus bearing Mst1 (Ad-Mst1, MOI=50) significantly induced apoptosis as compared with cells transduced with Ad-lacZ, as determined by both Annexin V staining and Cell Death Detection ELISA (Roche). Adenovirus mediated overexpression of PCMT1, However, substantially inhibited Mst1 induced cardiomyocyte apoptosis (Figure 4B and 4C). These findings suggest that the interaction of PCMT1 with Mst1 may be functionally important in terms of regulating Mst1-mediated cell apoptosis.
Figure 4.
PCMT1 overexpression Inhibits Mst1 induced cardiomyocyte apoptosis. A, NRCMs were transduced with either Ad-LacZ or Ad-PCMT1 (MOI=50). Forty-eight hours after transduction, PCMT1 expression was analyzed by Western blot analysis. B, NRCMs were transduced with Ad-LacZ, Ad-Mst1, or Ad-PCMT1 with different combinations (total MOI=100). Forty-eight hours after transduction, cell apoptosis was quantitated by Annexin-V (AV)–fluorescein isothiocyanate (FITC) stain detection kit (BioVision). C, Histone-associated DNA fragments were quantitated by the Cell Death Detection ELISA. The data are representatives of 4 independent experiments.
3.4. PCMT1 Attenuates Hypoxia/reoxygenation Induced Mst1 Activation and Apoptosis in Cardiomyocytes
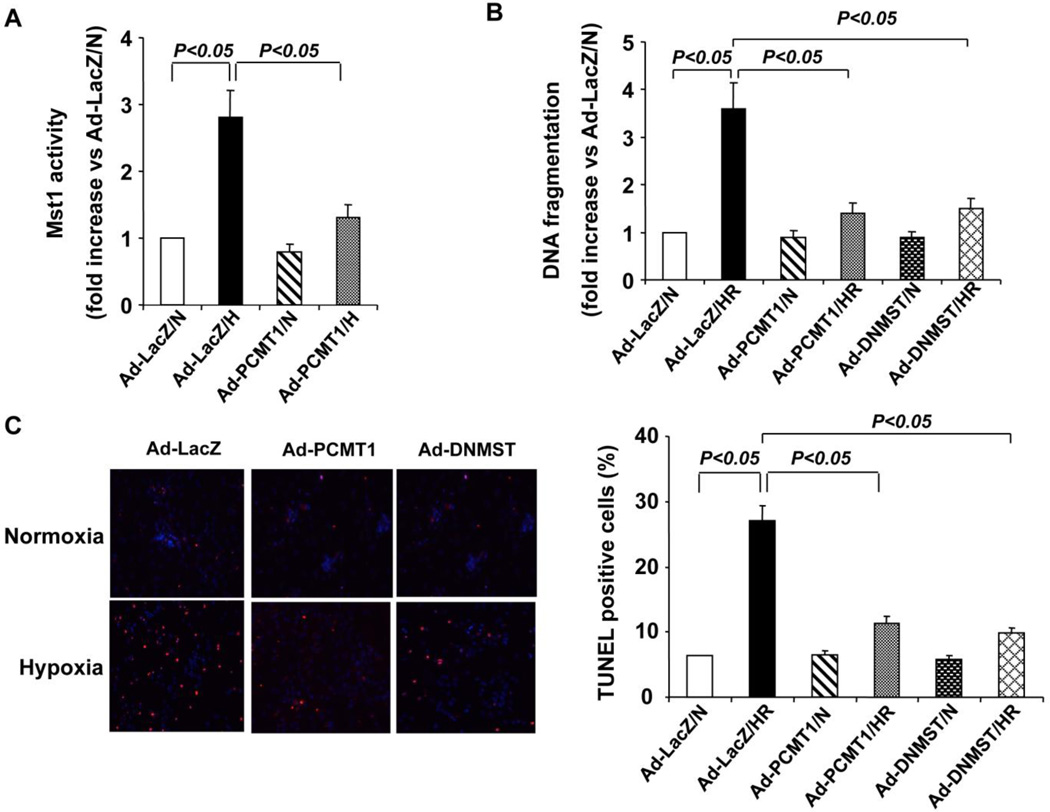
To further substantiate the functional significance of PCMT1-Mst1 interaction, we investigated the role of PCMT1-Mst1 interaction in cardiac cell apoptosis in response to the hypoxia/reoxygenation induced injury. To this end, cardiomyocytes were transduced with either Ad-LacZ or Ad-PCMT1 or Ad-DNMST1. 48 hr after virus transduction, cells were placed in a 37°C airtight box saturated with 95% N2/5% CO2 for 12 hr (hypoxia) and then exposed to normoxic atmosphere for 24 hr (reoxygenation). As shown in Figure 5A, in cardiomyocytes transduced with Ad-LacZ (MOI=50), hypoxia/reoxygenation substantially increased Mst1 activity by ~2.8 fold (Figure 5A), as determined by phosphorylation of histone 2B. Overexpression of PCMT1, however, markedly inhibited the hypoxia/reoxygenation induced Mst1 activation in cardiomyocytes (Figure 5A). Likewise, in Ad-LacZ transduced cardiomyocytes, hypoxia/reoxygenation markedly induced cell apoptosis, which was substantially inhibited by overexpression of either PCMT1 or DN-Mst1, as determined by both TUNEL staining (Figure 5B) and Cell Death ELISA (Figure 5C). Collectively, these findings indicate that PCMT1 may inhibit the hypoxia/reoxygenation induced injury through attenuating the Mst1 activation in cardiomyocytes.
Figure 5.
PCMT1 overexpression inhibits hypoxia/reoxygenation induced Mst1 activation and cell apoptosis in cardiomyoctes. A, NRCMs were transduced with either Ad-LacZ or Ad-PCMT1 (MOI=50). 48 hr after transduction, cells were subjected to hypoxia/reoxygenation injury. Mst1 was immunoprecipitated and subjected to an in vitro kinase assay using Histone 2B as a substrate. B, NRCMs were transduced with either Ad-LacZ or Ad-PCMT1 or Ad-DNMST1 (MOI=50). Forty-eight hours after transduction, cells were subjected to hypoxia/reoxygenation. DNA fragments were quantitated by the Cell Death Detection ELISA. C, NRCMs were transduced with either Ad-LacZ or Ad-PCMT1 or Ad-DNMST (MOI=50). Forty-eight hours after transduction, cells were subjected to hypoxia/reoxygenation injury. Cell apoptosis was quantitated by In Situ Cell Death Detection kit (Roche). N indicates normoxia; HR, hypoxia/reoxygenation. N indicates normoxia; HR, hypoxia/reoxygenation. The data are representatives of 4 independent experiments.
3.5. Knockdown of PCMT1 Augments Hypoxia/reoxygenation Induced Mst1 Activation and Apoptosis
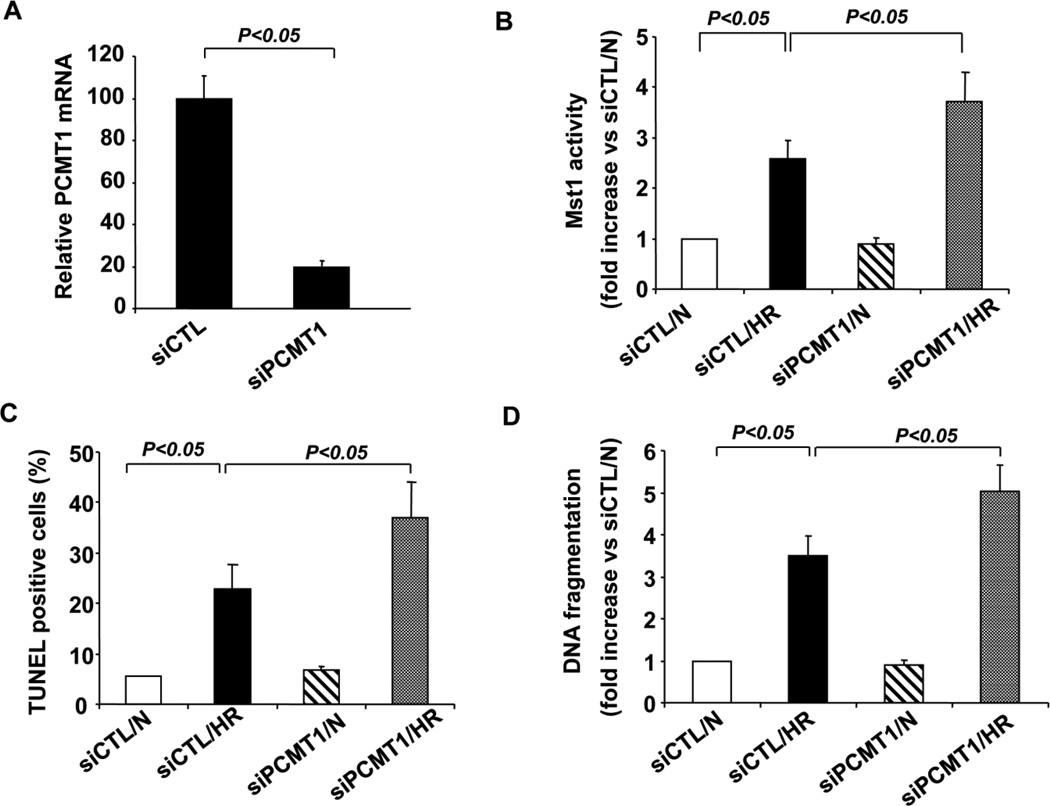
To further investigate the role of PCMT1 in the regulation of Mst1 in cardiomyocytes, we used siRNA to knock down the expression of PCMT1. Transfection of PCMT1 siRNA (siPCMT1) reduced PCMT1 mRNA expression by ~80% in cardiomyocytes, as determined by qRT-PCR (Figure 6A). Knockdown of PCMT1 expression was found to markedly increase Mst1 activity in response to the hypoxia/reoxygenation injury, as compared with that in cells transfected with control siRNA (siCTL) (Figure 6B). Accordingly, knockdown of PCMT1 expression markedly increased the cardiomyocyte apoptosis in response to the hypoxia/reoxygenation injury, as determined by both TUNEL staining (Figure 6C) and Cell Death ELISA (Figure 6D). In contrast, knockdown of PCMT1 expression had no significant effects on the MST1 activity and cell apoptosis under normoxic conditions.
Figure 6.
Knockdown of PCMT1 augments Mst1 activation and cell apoptosis under hypoxic conditions. A, Total RNA extracted from control siRNA (siCTL) and PCMT1 siRNA transfected cells was analyzed for the expression of PCMT1 in NRCMs by qRT-PCR. B, NRCMs were transfected with either control siRNA or PCMT1 siRNA. Forty-eight hours after transfection, cells were subjected to hypoxia/reoxygenation. Cell lysates were immunoprecipitated with anti-Mst1 antibody and Mst1 activity was then determined by an in vitro kinase assay using histone H2B as a substrate. N indicates normoxia; HR, hypoxia/reoxygenation. C, NRCMs were transfected with either control siRNA or PCMT1 siRNA. Forty-eight hours after transfection, cells were subjected to hypoxia/reoxygenation injury. Cell apoptosis was quantitated by In Situ Cell Death Detection kit (Roche). N indicates normoxia; HR, hypoxia/reoxygenation. D, NRCMs were transfected with either control siRNA or PCMT1 siRNA. Forty-eight hours after transfection, cells were subjected to hypoxia/reoxygenation injury. DNA fragments were quantitated by the Cell Death Detection ELISA. The data are representatives of 4 independent experiments. N indicates normoxia; HR, hypoxia/reoxygenation. The data are representatives of 4 independent experiments.
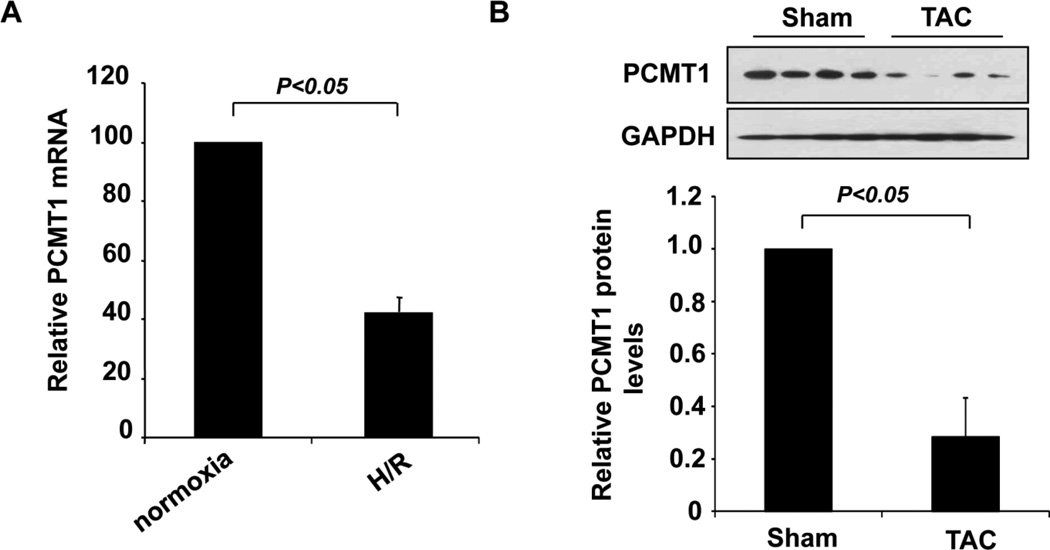
To further substantiate the functional significance of PCMT1 in apoptosis associated heart diseases, we examined the expression of PCMT1 in cardiomyocytes in response to H/R injury as well as in the hearts of mice with heart failure induced by transverse aortic constriction (TAC). The expression of PCMT1 was markedly downregulated under both circumstances as determined by qRT-PCR (Figure 7A) and western blot analysis (Figure 7B). Collectively, these findings further indicate that PCMT1 is a negative regulator of Mst1 activation under hypoxic conditions in cardiomyocytes and its expression is essentially involved in the development of apoptosis associated heart diseases such as heart failure.
Figure 7.
Down-regulation of PCMT1 in apoptotic cardiac cells. A, NRCMs were subjected to hypoxia/reoxygenation injury and the expression of PCMT1 in hypoxic cardiomyocytes was determined by qRT-PCR. B, mice were subjected to transverse aortic constriction (TAC) for 4 weeks and the expression of PCMT1 in the hearts of mice was determined by western blot and quantitated by Densitometric analysis.
3.6. Upregulation of PCMT1 by CGP3466B Attenuates Cardiomyocyte Apoptosis
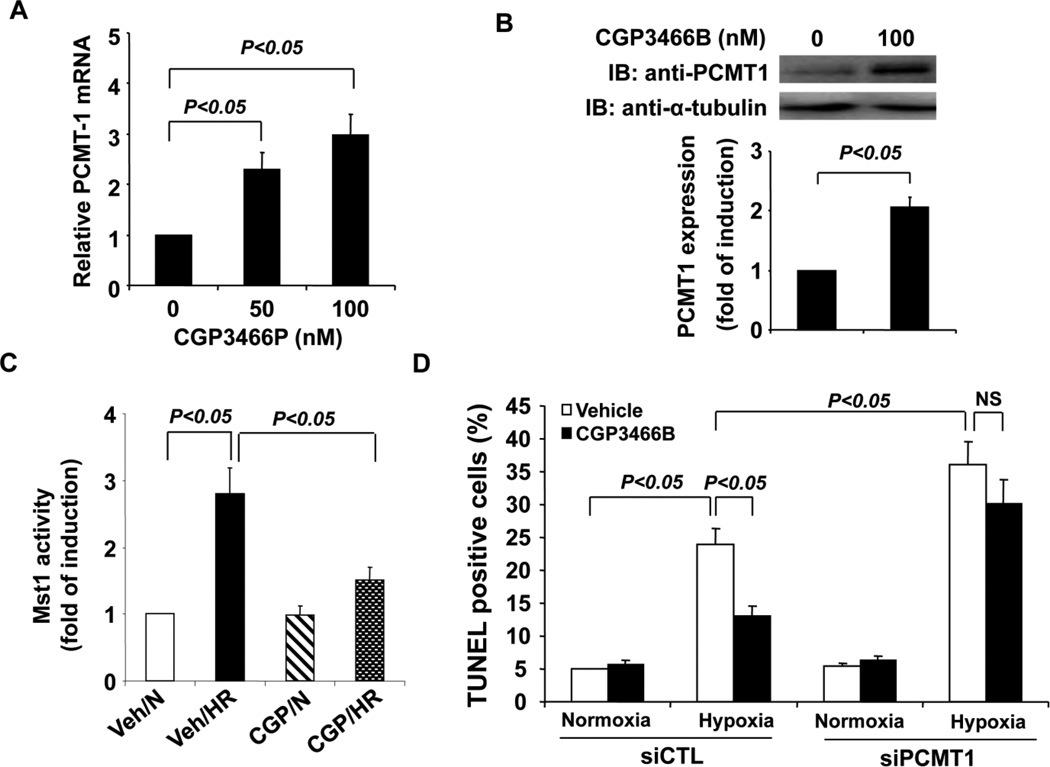
CGP3466B is a compound related to the anti-Parkinson’s drug R-(−)-deprenyl, which has been reported to exert the antiapoptotic effects via upregulating PCMT1 expression in neurons [29]. Interestingly, R-(−)-deprenyl has been recently shown to elicit cardiac protective effects in heart failure via reduction of cardiomyocyte apoptosis and oxidative stress [30]. Based on these observations, we attempted to investigate whether CGP3466B exerts the anti-apoptotic effects in cardiomyocytes via acting on the PCMT1/Mst1 pathway. Indeed, treatment of cardiomycytes with CGP3466B significantly increased both PCMT mRNA and protein levels (Figure 8A and 8B), as determined by qRT-PCR and western blotting analysis. Furthermore, pretreatment of cardiomyocytes with 100 nM CBP3466B for 24 hr substantially inhibited the activation of Mst1 kinase in cardiomyoctes subjected to the hypoxia/reoxygenation injury (Figure 8C). As shown in Figure 8D, in control siRNA transfected cardiomyocytes, pretreatment of cardiac cells with CGP3466B markedly attenuated the hypoxia/reoxygenation induced cell apoptosis as determined by TUNEL staining. However, the anti-apoptotic effects of CGP3466B was markedly attenuated in PCMT1 specific siRNA transfected cells (Figure 8D). Taken together, these findings suggest that some of the cardioprotective effects of CGP3466B may be mediated by upregulation of PCMT1 and subsequent inhibition of Mst1 activation in cardiomyocytes.
Figure 8.
Upregulation of PCMT1 attenuates Mst1 activation and cell apoptosis under hypoxic conditions. A, NRCMs were treated with either vehicle or CGP3466P as indicated for twelve hours. Total RNA was extracted and then subjected to the analysis for the expression of PCMT1 by qRT-PCR. B, Cardiomyoctes were treated with either vehicle or CGP3466P for twenty-four hours. Extracted cell lysates were subjected to the analysis of the expression of PCMT1 by Western blot. C, NRCMs were pretreated with either vehicle or CGP3466P for twenty-four hours and then subjected to hypoxia/reoxygenation. Mst1 was then immunoprecipitated and its activity was determined by an in vitro kinase assay using histone H2B as a substrate. N indicates normoxia; HR, hypoxia/reoxygenation. D, Forty-eight hours after transfection with either control siRNA or PCMT1 siRNA, NRCMs were treated with either vehicle or CGP3466B for twenty-four hours and then subjected to hypoxia/reoxygenation injury as indicated. Cell apoptosis was quantitated by In Situ Cell Death Detection kit (Roche). The data are representatives of 4 independent experiments.
4. Discussion
Using the yeast 2-hybrid system and Mst1 as bait, we isolated several Mst1 cDNAs from a human heart cDNA library. The interaction of PCMT1 with wild-type Mst1 was further supported by coimmunoprecipitation studies showing that in both cotransfected HEK293 cells and cardiomyocytes, PCMT1 specifically interacted with Mst1. The interaction between PCMT1 and Mst1 required the kinase domain of Mst1 and it seems that the C-terminal fragment (aa 122–228) of PCMT1 mediates the binding of Mst1 in PCMT1. The functional consequence of this interaction was demonstrated by the ability of PCMT1 to inhibit Mst1 activity and Mst1-mediated apoptotic effects in cardiomyocytes. Indeed, adenovirus mediated overexpression of PCMT1 promotes cardiomyocyte survival via inhibiting Mst1 activation in response to hypoxia/reoxygenation injury. Importantly, pharmacological upregulation of PCMT1 expression by an anti-apopotic compound CGP3466B also markedly inhibited the hypoxia/reoxygenation induced Mst1 activation and cell apoptosis in cardiomyocytes. These results suggest that the regulation of Mst1 activity by PCMT1 may be an important determinant of cell survival.
Under physiological conditions, L-aspartyl (L-Asp) and L-asparaginyl residues in proteins are spontaneously subjected to nonenzymatic isomerization and/or deamidation leading to the formation of an abnormal isoaspartyl residue via a succinimide intermediate (cyclic imide) [31]. This damage alters the three-dimensional structure of proteins and leads to disruption of cellular function, as it has been demonstrated for calmodulin [32], tubulin [33], eye lens crystallins [33], Alzheimer's β-amyloid [35] and others. PCMT1 is a highly conserved enzyme that recognizes and repairs the abnormal L-isoaspartyl residues in proteins, thus preventing the accumulation of dysfunctional proteins [36] [37]. Recently, accumulating evidence suggests that PCMT1 plays critical roles in the regulation of a wide range of biological processes such as longevity, apoptosis, heat shock response and oxidative stress in different species [38] [39] [40] [29]. Particularly, the protective role of PCMT1 during apoptosis and oxidative stress has been characterized in various cell types [29, 40, 41]. For example, overexpression of PCMT1 has been shown to prevent the Bax induced apoptosis in both neurons and COS-1 cells [28]. Furthermore, in vascular endothelial cells, PCMT1 has been shown to prevent H2O2 induced cell apoptosis possibly through regulating the methylation of various anti-apoptotic proteins such as Hsp90, Hsp70 and Bcl-xL[41]. However, the biological roles of PCMT1 in cardiomyocytes have not been explored thus far. In the present study, we, for the first time, identified PCMT1 as a novel Mst1 binding protein in the heart. Overexpression of PCMT1 confers resistance to the hypoxia/reoxygenation induced apoptosis in cardiac cells. Furthermore, CGP3466B, a R-(−)-deprenyl related compound with potent antiapoptotic properties [42] [43], also significantly upregulated PCMT1 expression in cardiomyocytes and exerted the cardioprotective effects via inhibiting Mst1 activation. In this respect, our finding is in line with previous notion showing that R-(−)-deprenyl could improve the in vivo cardiac function in heart failure via inhibiting cardiomyocyte apoptosis and oxidative stress [30]. Thus, our results unveil a novel function for PCMT1 and suggest its involvement in the regulation of apoptotic processes in the heart.
The mechanism by which PCMT1 inhibits Mst1 is not known, but certainly involves the PCMT1-Mst1 interaction. As PCMT1 is an enzyme with methyltransferase activity, one possibility is that it might regulate Mst1 activity by altering either deamidation or methylation of Mst1. Indeed, oxidative stress conditions, such as hypoxia/reoxygenation, as we used to induce cardiomyocyte apoptosis in the study, have been linked to an increased susceptibility of proteins to undergo deamidation [44]. Moreover, UV irradiation, which activates Mst1 in different cell types [45], has been shown to increase protein deamidation [46]. In this regard, it would be very interesting to investigate whether Mst1 undergoes deamidation and whether PCMT inhibits Mst1 activation through alteration of Mst1 deamidation and methylation under oxidative stress conditions in the heart. Alternatively, the interaction of PCMT1 with Mst1 may cause the conformational change of Mst1, thus affecting the formation of the Mst1/Hippo signaling complex with other proteins, such as Rassf1, hWW45, and Lats, which have been shown to play essential roles in the regulation of cardiomyocyte apoptosis and heart failure [47] [48]. These studies are ongoing.
The molecular mechanism underlying the regulation of PCMT1 expression is largely obscure. In addition to CGP3466B, lithium, which also possesses the anti-apoptotic effects in both neuronal and cardiac cells [49], has been shown to enhance PCMT1 expression by inactivation of GSK-3β in astrocytoma and neuroblastoma cells [50]. Interestingly, the expression of PCMT1 appears to decline during aging, which could partially explain the build up of damaged proteins in old age [38]. As aging is a major risk factor for the cardiovascular diseases such as heat failure, it remains to be investigated whether PCMT1 helps to repair damaged proteins and restore the cardiac function in the aged heart. Recently, PCMT1-deficient mice have been generated, but they exhibit accumulation of L-isoAsp in several tissues, growth retardation, and die from progressive epileptic seizures at a mean age of 42 days [51], making it impossible to investigate the in vivo functional importance of PCMT1 in the development of heart disease. In this regard, further studies using conditional knockout or inducible transgenic mice will be necessary to dissect the functional significance of this protein in the heart in vivo.
In summary, the data reported here represent the first demonstration that Mst1 is an intracellular target of PCMT1 in cardiomyocytes, and that induction of PCMT1 and subsequent inhibition of Mst1 activation may constitute an important mechanism by which PCMT1 confers resistance to the oxidative stress injury in the heart. These findings suggest that PCMT1 may be an important therapeutic target for promoting myocardial survival in ischemia-reperfusion injury and heart failure.
Acknowledgements
The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology [52].
Acknowledgement of grant support: this work was supported by American Heart Association Scientist Development Grant, the Chinese Natural Science Foundation, and the National Institutes of Health R01HL103869 to J.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential conflicts of interest: none
References
- 1.Chan SW, Lim CJ, Chen L, Chong YF, Huang C, Song H, et al. The hippo pathway in biological control and cancer development. J Cell Physiol. 2011;226:928–939. doi: 10.1002/jcp.22435. [DOI] [PubMed] [Google Scholar]
- 2.Avruch J, Zhou D, Fitamant J, Bardeesy N. Mst1/2 signalling to Yap: gatekeeper for liver size and tumour development. Br J Cancer. 2011;104:24–32. doi: 10.1038/sj.bjc.6606011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Creasy CL, Ambrose DM, Chernoff J. The Ste20-like protein kinase, Mst1, dimerizes and contains an inhibitory domain. J Biol Chem. 1996;271:21049–21053. doi: 10.1074/jbc.271.35.21049. [DOI] [PubMed] [Google Scholar]
- 4.Ura S, Masuyama N, Graves JD, Gotoh Y. Caspase cleavage of MST1 promotes nuclear translocation and chromatin condensation. Proc Natl Acad Sci U S A. 2001;98:10148–10153. doi: 10.1073/pnas.181161698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin Y, Khokhlatchev A, Figeys D, Avruch J. Death-associated protein 4 binds MST1 and augments MST1-induced apoptosis. J Biol Chem. 2002;277:47991–48001. doi: 10.1074/jbc.M202630200. [DOI] [PubMed] [Google Scholar]
- 6.Cheung WL, Ajiro K, Samejima K, Kloc M, Cheung P, Mizzen CA, et al. Apoptotic phosphorylation of histone H2B is mediated by mammalian sterile twenty kinase. Cell. 2003;113:507–517. doi: 10.1016/s0092-8674(03)00355-6. [DOI] [PubMed] [Google Scholar]
- 7.Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villen J, Becker EB, et al. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125:987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 8.Yuan Z, Lehtinen MK, Merlo P, Villen J, Gygi S, Bonni A. Regulation of neuronal cell death by MST1-FOXO1 signaling. J Biol Chem. 2009;284:11285–11292. doi: 10.1074/jbc.M900461200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jang SW, Yang SJ, Srinivasan S, Ye K. Akt phosphorylates MstI and prevents its proteolytic activation, blocking FOXO3 phosphorylation and nuclear translocation. J Biol Chem. 2007;282:30836–30844. doi: 10.1074/jbc.M704542200. [DOI] [PubMed] [Google Scholar]
- 10.Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Chan EH, Nousiainen M, Chalamalasetty RB, Schafer A, Nigg EA, Sillje HH. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005;24:2076–2086. doi: 10.1038/sj.onc.1208445. [DOI] [PubMed] [Google Scholar]
- 12.Bi W, Xiao L, Jia Y, Wu J, Xie Q, Ren J, et al. c-Jun N-terminal kinase enhances MST1-mediated pro-apoptotic signaling through phosphorylation at serine 82. J Biol Chem. 2010;285:6259–6264. doi: 10.1074/jbc.M109.038570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.You B, Yan G, Zhang Z, Yan L, Li J, Ge Q, et al. Phosphorylation of cardiac troponin I by mammalian sterile 20-like kinase 1. Biochem J. 2009;418:93–101. doi: 10.1042/BJ20081340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graves JD, Draves KE, Gotoh Y, Krebs EG, Clark EA. Both phosphorylation and caspase-mediated cleavage contribute to regulation of the Ste20-like protein kinase Mst1 during CD95/Fas-induced apoptosis. J Biol Chem. 2001;276:14909–14915. doi: 10.1074/jbc.M010905200. [DOI] [PubMed] [Google Scholar]
- 15.Glantschnig H, Rodan GA, Reszka AA. Mapping of MST1 kinase sites of phosphorylation. Activation and autophosphorylation. J Biol Chem. 2002;277:42987–42996. doi: 10.1074/jbc.M208538200. [DOI] [PubMed] [Google Scholar]
- 16.Praskova M, Khoklatchev A, Ortiz-Vega S, Avruch J. Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras. Biochem J. 2004;381:453–462. doi: 10.1042/BJ20040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo C, Tommasi S, Liu L, Yee JK, Dammann R, Pfeifer GP. RASSF1A is part of a complex similar to the Drosophila Hippo/Salvador/Lats tumor-suppressor network. Curr Biol. 2007;17:700–705. doi: 10.1016/j.cub.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 18.Cooper WN, Hesson LB, Matallanas D, Dallol A, von Kriegsheim A, Ward R, et al. RASSF2 associates with and stabilizes the proapoptotic kinase MST2. Oncogene. 2009;28:2988–2998. doi: 10.1038/onc.2009.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng Q, Hong W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell. 2008;13:188–192. doi: 10.1016/j.ccr.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Qiao M, Wang Y, Xu X, Lu J, Dong Y, Tao W, et al. Mst1 is an interacting protein that mediates PHLPPs' induced apoptosis. Mol Cell. 2010;38:512–523. doi: 10.1016/j.molcel.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 21.Ren A, Yan G, You B, Sun J. Down-regulation of mammalian sterile 20-like kinase 1 by heat shock protein 70 mediates cisplatin resistance in prostate cancer cells. Cancer Res. 2008;68:2266–2274. doi: 10.1158/0008-5472.CAN-07-6248. [DOI] [PubMed] [Google Scholar]
- 22.Guo C, Zhang X, Pfeifer GP. The tumor suppressor RASSF1A prevents dephosphorylation of the mammalian STE20-like kinases MST1 and MST2. J Biol Chem. 2011;286:6253–6261. doi: 10.1074/jbc.M110.178210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto S, Yang G, Zablocki D, Liu J, Hong C, Kim SJ, et al. Activation of Mst1 causes dilated cardiomyopathy by stimulating apoptosis without compensatory ventricular myocyte hypertrophy. J Clin Invest. 2003;111:1463–1474. doi: 10.1172/JCI17459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Odashima M, Usui S, Takagi H, Hong C, Liu J, Yokota M, et al. Inhibition of endogenous Mst1 prevents apoptosis and cardiac dysfunction without affecting cardiac hypertrophy after myocardial infarction. Circ Res. 2007;100:1344–1352. doi: 10.1161/01.RES.0000265846.23485.7a. [DOI] [PubMed] [Google Scholar]
- 25.Sun J, Yan G, Ren A, You B, Liao JK. FHL2/SLIM3 decreases cardiomyocyte survival by inhibitory interaction with sphingosine kinase-1. Circ Res. 2006;99:468–476. doi: 10.1161/01.RES.0000239410.65551.b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caporali A, Sala-Newby GB, Meloni M, Graiani G, Pani E, Cristofaro B, et al. Identification of the prosurvival activity of nerve growth factor on cardiac myocytes. Cell death and differentiation. 2008;15:299–311. doi: 10.1038/sj.cdd.4402263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun J, Liao JK. Functional interaction of endothelial nitric oxide synthase with a voltage-dependent anion channel. Proc Natl Acad Sci U S A. 2002;99:13108–13113. doi: 10.1073/pnas.202260999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao X, Haldar SM, Lu Y, Jeyaraj D, paruchuri K, Nahori M, et al. Krüppel-like factor 4 regulates pressure-induced cardiac hypertrophy. J Mol Cell Cardiol. 2010;49:334–338. doi: 10.1016/j.yjmcc.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huebscher KJ, Lee J, Rovelli G, Ludin B, Matus A, Stauffer D, et al. Protein isoaspartyl methyltransferase protects from Bax-induced apoptosis. Gene. 1999;240:333–341. doi: 10.1016/s0378-1119(99)00443-6. [DOI] [PubMed] [Google Scholar]
- 30.Qin F, Shite J, Liang CS. Antioxidants attenuate myocyte apoptosis and improve cardiac function in CHF: association with changes in MAPK pathways. Am J Physiol Heart Circ Physiol. 2003;285:H822–H832. doi: 10.1152/ajpheart.00015.2003. [DOI] [PubMed] [Google Scholar]
- 31.Furuchi T, Sakurako K, Katane M, Sekine M, Homma H. The role of protein L-isoaspartyl/ D-aspartyl O-methyltransferase (PIMT) in intracellular signal transduction. Chem Biodivers. 2010;7:1337–1348. doi: 10.1002/cbdv.200900273. [DOI] [PubMed] [Google Scholar]
- 32.Johnson BA, Langmack EL, Aswad DW. Partial repair of deamidation-damaged calmodulin by protein carboxyl methyltransferase. J Biol Chem. 1987;262:12283–12287. [PubMed] [Google Scholar]
- 33.Hill CM, Libich DS, Harauz G. Assembly of tubulin by classic myelin basic protein isoforms and regulation by post-translational modification. Biochemistry. 2005;44:16672–16683. doi: 10.1021/bi050646+. [DOI] [PubMed] [Google Scholar]
- 34.Wilmarth PA, Tanner S, Dasari S, Nagalla SR, Riviere MA, Bafna V, et al. Age-related changes in human crystallins determined from comparative analysis of post-translational modifications in young and aged lens: does deamidation contribute to crystallin insolubility? J Proteome Res. 2006;5:2554–2566. doi: 10.1021/pr050473a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lowenson JD, Roher AE, Clarke S. Protein aging Extracellular amyloid formation and intracellular repair. Trends Cardiovasc Med. 1994;4:3–8. doi: 10.1016/1050-1738(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu T, Matsuoka Y, Shirasawa T. Biological significance of isoaspartate and its repair system. Biol Pharm Bull. 2005;28:1590–1596. doi: 10.1248/bpb.28.1590. [DOI] [PubMed] [Google Scholar]
- 37.Reissner KJ, Aswad DW. Deamidation and isoaspartate formation in proteins: unwanted alterations or surreptitious signals? Cell Mol Life Sci. 2003;60:1281–1295. doi: 10.1007/s00018-003-2287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Desrosiers RR, Fanelus I. Damaged proteins bearing L-isoaspartyl residues and aging: a dynamic equilibrium between generation of isomerized forms and repair by PIMT. Curr Aging Sci. 2011;4:8–18. [PubMed] [Google Scholar]
- 39.Oge L, Bourdais G, Bove J, Collet B, Godin B, Granier F, et al. Protein repair L-isoaspartyl methyltransferase 1 is involved in both seed longevity and germination vigor in Arabidopsis. Plant Cell. 2008;20:3022–3037. doi: 10.1105/tpc.108.058479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verma P, Singh A, Kaur H, Majee M. Protein L-isoaspartyl methyltransferase1 (CaPIMT1) from chickpea mitigates oxidative stress-induced growth inhibition of Escherichia coli. Planta. 2010;231:329–336. doi: 10.1007/s00425-009-1050-z. [DOI] [PubMed] [Google Scholar]
- 41.Cimmino A, Capasso R, Muller F, Sambri I, Masella L, Raimo M, et al. Protein isoaspartate methyltransferase prevents apoptosis induced by oxidative stress in endothelial cells: role of Bcl-Xl deamidation and methylation. PLoS One. 2008;3:e3258. doi: 10.1371/journal.pone.0003258. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Yacoubian TA, Standaert DG. Targets for neuroprotection in Parkinson's disease. Biochim Biophys Acta. 2009;1792:676–687. doi: 10.1016/j.bbadis.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sagot Y, Toni N, Perrelet D, Lurot S, King B, Rixner H, et al. An orally active anti-apoptotic molecule (CGP 3466B) preserves mitochondria and enhances survival in an animal model of motoneuron disease. Br J Pharmacol. 2000;131:721–728. doi: 10.1038/sj.bjp.0703633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ingrosso D, D'Angelo S, di Carlo E, Perna AF, Zappia V, Galletti P. Increased methyl esterification of altered aspartyl residues in erythrocyte membrane proteins in response to oxidative stress. Eur J Biochem. 2000;267:4397–4405. doi: 10.1046/j.1432-1327.2000.01485.x. [DOI] [PubMed] [Google Scholar]
- 45.Lu ML, Sato M, Cao B, Richie JP. UV irradiation-induced apoptosis leads to activation of a 36-kDa myelin basic protein kinase in HL-60 cells. Proc Natl Acad Sci U S A. 1996;93:8977–8982. doi: 10.1073/pnas.93.17.8977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mafia K, Gupta R, Kirk M, Wilson L, Srivastava OP, Barnes S. UV-A-induced structural and functional changes in human lens deamidated alphaB-crystallin. Mol Vis. 2008;14:234–248. [PMC free article] [PubMed] [Google Scholar]
- 47.Matsui Y, Nakano N, Shao D, Gao S, Luo W, Hong C, et al. Lats2 is a negative regulator of myocyte size in the heart. Circ Res. 2008;103:1309–1318. doi: 10.1161/CIRCRESAHA.108.180042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Del Re DP, Matsuda T, Zhai P, Gao S, Clark GJ, Van Der Weyden L, et al. Proapoptotic Rassf1A/Mst1 signaling in cardiac fibroblasts is protective against pressure overload in mice. J Clin Invest. 2010;120:3555–3567. doi: 10.1172/JCI43569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bielecka AM, Obuchowicz E. Antiapoptotic action of lithium and valproate. Pharmacol Rep. 2008;60:771–782. [PubMed] [Google Scholar]
- 50.Lamarre M, Desrosiers RR. Up-regulation of protein L-isoaspartyl methyltransferase expression by lithium is mediated by glycogen synthase kinase-3 inactivation and beta-catenin stabilization. Neuropharmacology. 2008;55:669–676. doi: 10.1016/j.neuropharm.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 51.Yamamoto A, Takagi H, Kitamura D, Tatsuoka H, Nakano H, Kawano H, et al. Deficiency in protein L-isoaspartyl methyltransferase results in a fatal progressive epilepsy. J Neurosci. 1998;18:2063–2074. doi: 10.1523/JNEUROSCI.18-06-02063.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shewan LG, Coats AJ. Ethics in the authorship and publishing of scientific articles. Int J Cardiol. 2010;144:1–2. [Google Scholar]