Abstract
While concentric remodeling (CR) and concentric hypertrophy (CH) are common forms of left ventricular (LV) remodeling in heart failure with preserved ejection fraction (HFpEF), eccentric hypertrophy (EH) can also occur in these patients. However, clinical characteristics and outcomes of EH have not been well described in HFpEF. We prospectively studied 402 patients with HFpEF, divided into 4 groups based on LV structure: normal geometry (no LV hypertrophy [LVH] and relative wall thickness [RWT] < 0.42); CR (no LVH and RWT > 0.42); CH (LVH and RWT > 0.42); and EH (LVH and RWT < 0.42). We compared clinical, laboratory, echocardiographic, invasive hemodynamic, and outcome data among groups. Of 402 patients, 48 (12%) had EH. Compared to CH, patients with EH had lower systolic blood pressure and less renal impairment despite similar rates of hypertension. After adjustment for covariates, EH was associated with reduced LV contractility compared to CH (lower LVEF [β-coefficient = −3.2; 95% confidence interval (CI) −5.4, −1.1%] and ratio of systolic blood pressure to end-systolic volume [β-coefficient = −1.0; 95% CI −1.5, −0.5 mmHg/ml]). EH was also associated with increased LV compliance compared to CH (LV end-diastolic volume at an idealized LV end-diastolic pressure of 20 mmHg [EDV20] β-coefficient = 14.2;95% CI 9.4, 19.1 ml). Despite these differences, EH and CH had similarly elevated cardiac filling pressures and equivalent adverse outcomes. In conclusion, the presence of EH denotes a distinct subset of HFpEF that is pathophysiologically similar to HF with reduced EF (HFrEF), and may benefit from HFrEF therapy.
Keywords: heart failure with preserved ejection fraction, left ventricular hypertrophy, cardiac remodeling, echocardiography, outcomes
INTRODUCTION
Left ventricular (LV) structure in heart failure with preserved ejection fraction (HFpEF) has been classically defined as a small, thick ventricle. Contemporary studies show that most patients with HFpEF have a normal sized LV.5,6 Indeed, concentric remodeling (CR) and concentric hypertrophy (CH) have been demonstrated to be the most common LV structural abnormalities observed in HFpEF.7 A smaller subset of HFpEF patients demonstrate eccentric hypertrophy (EH);8however, EH has not been well described in the setting of HFpEF. We sought to define HFpEF based on LV geometry, with a focus on the subset of patients with EH. We hypothesized that this group, while less prevalent, represents a unique pathophysiologic subset of HFpEF patients who may benefit from tailored therapy, which may include medications typically used to treat patients with heart failure and reduced ejection fraction (HFrEF).
METHODS
Between March 2008 and May 2011, consecutive patients were prospectively enrolled from the outpatient clinic of the Northwestern University HFpEF Program as part of a systematic observational study of HFpEF (ClinicalTrials.gov identifier #NCT01030991), as described previously.9 All patients were recruited after hospitalization for HF. Patients were initially identified by an automated daily query of the inpatient electronic medical record at Northwestern Memorial Hospital. The list of patients generated was screened daily, and only those patients with an LV ejection fraction (EF) > 50% and who met Framingham criteria for HF10 were offered post-discharge follow-up in a specialized HFpEF outpatient program. The HF diagnosis was confirmed in the post-hospitalization, outpatient HFpEF clinic. Based on previously published criteria, in addition to the presence of symptomatic HF and EF > 50%, we required evidence of either significant diastolic dysfunction (grade 2 or 3) on echocardiography or evidence of elevated LV filling pressures on invasive hemodynamic testing. Patients with greater than moderate valvular disease, prior cardiac transplantation, prior LVEF < 40%, LV end-diastolic volume (EDV)>97 ml/m2or diagnosis of constrictive pericarditis were excluded. For the present analysis, patients with known sources of extracardiac volume overload or high output HF were also excluded. All study participants gave written, informed consent, and the institutional review board at Northwestern University approved the study.
We collected the following data in all study participants: demographics, race/ethnicity, New York Heart Association (NYHA) functional class, comorbidities (as defined in Table 1), medications, vital signs, body-mass index, and laboratory data, including serum sodium, blood urea nitrogen, creatinine, hemoglobin, and B-type natriuretic peptide (BNP).
Table 1.
Clinical Characteristics by Left Ventricular Geometry Group
| Clinical characteristic | Normal geometry (N=49) |
Concentric remodeling (N=111) |
Concentric hypertrophy (N=194) |
Eccentric hypertrophy (N=48) |
p-value* |
|---|---|---|---|---|---|
| Age (years) | 56±13.2 | 67±12.1 | 66±12.3 | 62±13.9 | <0.001** |
| Female | 29 (59%) | 55 (50%) | 132 (68%) | 35 (73%) | 0.005** |
| Race | |||||
| White | 34 (69%) | 60 (54%) | 87 (45%) | 28 (58%) | 0.01 |
| Black | 10 (20%) | 41 (37%) | 85 (44%) | 18 (38%) | 0.03** |
| Other | 5 (10%) | 10 (9%) | 22 (11%) | 2 (4%) | 0.50 |
| New York Heart Association functional class | |||||
| I | 14 (29%) | 16 (14%) | 16 (8%) | 4 (8%) | 0.001** |
| II | 24 (49%) | 49 (44%) | 68 (35%) | 16 (33%) | 0.16 |
| III | 9 (18%) | 45 (41%) | 103 (53%) | 26 (54%) | <0.001** |
| IV | 2 (4%) | 1 (1%) | 6 (3%) | 1 (2%) | 0.57 |
| Coronary artery disease¶ | 8 (16%) | 36 (32%) | 79 (41%) | 15 (31%) | 0.01 |
| Hypertension‡ | 25 (51%) | 84 (76%) | 163 (84%) | 37 (77%) | <0.001** |
| Hyperlipidemia§ | 18 (37%) | 63 (57%) | 108 (56%) | 28 (58%) | 0.08 |
| Diabetes mellitus | 6 (12%) | 30 (27%) | 73 (38%) | 19 (40%) | 0.003** |
| Chronic kidney disease ‖ | 5 (10%) | 35 (32%) | 75 (39%) | 14 (29%) | 0.002** |
| Smoker | 18 (37%) | 48 (43%) | 78 (40%) | 19 (40%) | 0.88 |
| Atrial fibrillation | 14 (29%) | 27 (24%) | 55 (28%) | 14 (29%) | 0.87 |
| Obesity# | 19 (39%) | 44 (40%) | 114 (59%) | 33 (69%) | <0.001** |
| Chronic obstructive pulmonary disease | 8 (16%) | 29 (26%) | 40 (21%) | 11 (23%) | 0.52 |
| Heart rate (beats per minute) | 71±14.7 | 71±14 | 71±13.6 | 68±13.3 | 0.62 |
| Systolic blood pressure (mmHg) | 118±16 | 121±17 | 129±22 | 121±19† | <0.001** |
| Diastolic blood pressure (mmHg) | 70±10 | 70±10 | 71±13 | 67±13 | 0.23 |
| Pulse pressure (mmHg) | 49±13 | 51±15 | 59±18 | 54±19 | <0.001** |
| Body mass index (kg/m2) | 28±6.5 | 29±6.7 | 34±9.6 | 3 6± 11.1 | <0.001** |
| Serum sodium (mEq/l) | 139±2.1 | 138±3.1 | 138±2.8 | 138±3.2 | 0.75 |
| Blood urea nitrogen (mg/dl) | 17.7±11.2 | 24.9± 18.1 | 26.6±17 | 22±11.5 | 0.006 |
| Serum creatinine (mg/dl) | 1.01±0.4 | 1.41±1.2 | 1.85±1.8 | 1.29±0.9† | <0.001 |
| Estimated glomerular filtration rate (ml/min/1.73m2) | 76±26.8 | 60±26.3 | 52±25.5 | 64±29.2† | <0.001** |
| Fasting glucose (mg/dl) | 99±20 | 125±66 | 121±54 | 121±52 | 0.05 |
| Hemoglobin (g/dl) | 12.0±1.8 | 12.1±1.9 | 11.8±1.8 | 11.6±2.1 | 0.46 |
| B-type natriuretic peptide (pg/ml) | 131 (54–245) | 230 (59–402) | 278 (101–664) | 261 (94–473) | <0.001** |
| Number of antihypertensive medications | 2.0±1.1 | 2.3±1.5 | 3.0±1.4 | 2.7±1.3 | <0.001** |
| Medications | |||||
| Angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker | 27 (55%) | 51 (46%) | 113 (58%) | 30 (63%) | 0.13 |
| β-blocker | 29 (59%) | 69 (62%) | 138 (71%) | 32 (67%) | 0.26 |
| Calcium channel blocker | 6 (12%) | 27 (24%) | 77 (40%) | 15 (31%) | <0.001** |
| Nitrate | 0 (0%) | 13 (12%) | 39 (20%) | 6 (13%) | 0.003** |
| Loop diuretic | 29 (59%) | 54 (49%) | 124 (64%) | 27 (56%) | 0.08 |
| Thiazide diuretic | 6 (12%) | 26 (23%) | 50 (26%) | 12 (25%) | 0.25 |
| Statin | 17 (35%) | 54 (49%) | 98 (51%) | 28 (58%) | 0.12 |
| Aspirin | 17 (35%) | 57 (51%) | 91 (47%) | 21 (44%) | 0.27 |
p value by analysis of variance among groups (or Kruskal-Wallis test for right-skewed variables)
p-value < 0.05 and remains significant after correction using false discovery rate < 5% for comparison of eccentric hypertrophy vs. concentric hypertrophy
p-value < 0.05 and remains significant after correction using false discovery rate < 5% for comparison of left ventricular hypertrophy vs. no left ventricular hypertrophy
Presence of physician-documented history of coronary artery disease, known coronary stenosis >50%, history of myocardial infarction, percutaneous coronary intervention, coronary artery bypass grafting, or abnormal stress test results consistent with myocardial ischemia.
Systolic blood pressure >140 mm Hg or diastolic blood pressure >90 mm Hg, physician-documented history of hypertension, or current use of antihypertensive medications.
Physician-documented history of hyperlipidemia or current use of lipid-lowering medications.
Estimated glomerular filtration rate < 60 ml/min/1.73 m2.
Body-mass index >30 kg/m2
All study participants underwent comprehensive 2-dimensional echocardiography with Doppler and tissue Doppler imaging (TDI) using commercially available ultrasound systems with harmonic imaging (Philips iE33 or 7500, Philips Medical Systems, Andover, MA; or Vivid 7, GE Healthcare, General Electric Corp., Waukesha, WI). Cardiac structure and function were quantified as recommended by the American Society of Echocardiography. 13 Relative wall thickness (RWT) was calculated as 2*(posterior wall thickness)/LV end-diastolic dimension. LV hypertrophy (LVH) was defined as LV mass indexed to height2.7>44g/m2.7 in women and >48g/m2.7 in men. Normal geometry was classified as RWT<0.42 and no LVH; CR was defined as RWT>0.42 and no LV hypertrophy; CH was defined as RWT>0.42 and LVH; and EH was defined as RWT<0.42 and LVH.13 All measurements were made by experienced research sonographer (blinded to clinical data and outcomes) using ProSolv 4.0 software (ProSolv CardioVascular; Indianapolis, IN) and verified by a board-certified echocardiographer.
End-systolic elastance (Ees) was estimated by the single-beat method.14,15 The relationship between end-systolic pressure (Pes) and end-systolic volume (ESV) was related by the equation: [Pes = Ees(ESV – V0)]. Using 0.9*(systolic blood pressure) as an estimate of Pes, we estimated V0, the volume-axis intercept, for each patient. We then used the average V0 and Ees to define the end-systolic pressure volume relationship (ESPVR) in the EH and CH groups. We also generated the estimated ESV at an idealized Pes of 120 mmHg (ESV120) for each patient as a basis for comparison of ESPVR between groups. The effective arterial elastance (Ea) was estimated using the equation:Ea = 0.9*SBP/stroke volume. The end-diastolic pressure-volume relationship (EDPVR) was also characterized by a single-beat method using the equation:LVEDP = α(LVEDV)β. The parameters α and β were calculated for each individual based on their LVEDV and LVEDP (as estimated by [11.96 + (lateral E/e’ ratio)*0.596]).20 These parameters were then used to calculate the LVEDV at an idealized LVEDP of 20 mmHg (EDV20) for each patient as a basis for comparison of EDPVR among groups.
Right-sided heart catheterization was performed from either the right internal jugular or right femoral vein approach using standard Seldinger technique under fluoroscopic guidance. Participants underwent recording of invasive hemodynamics using a fluid-filled, 6F pulmonary artery catheter (Edwards Lifesciences, Irvine, CA) and a properly zeroed pressure transducer. Pressure recordings were analyzed off-line using a WITT Hemodynamic Workstation (Philips Medical Systems, Andover, MA) at a 50 mm/s paper speed with adjustment of pressure (mmHg) scale as needed. All hemodynamic pressure measurements were made at end-expiration and in duplicate using a standardized measurement protocol by a physician blinded to all clinical data.
After enrollment, study participants were evaluated in the Northwestern HFpEF Program as clinically indicated but at least every 6 months. At each visit, inter-current hospitalizations were documented, reviewed, and categorized as due to cardiovascular or non-cardiovascular causes. Every 6 months, participants (or their proxy) were contacted to determine vital status with verification of deaths through query of the Social Security Death Index. Enrollment date was defined as the first visit to the outpatient HFpEF clinic. Date of last follow-up was defined as date of death or last HFpEF clinic visit. Follow-up was complete in all patients. The combined outcome included any hospitalization for HF or any cardiovascular cause, or death.
Study participants were divided into 4 groups based on LV geometry (normal, CR, CH, and EH). Clinical characteristics, laboratory data, and echocardiographic parameters were compared between groups. Categorical variables were compared using Chi-squared tests, and continuous variables were compared using ANOVA (or Kruskal-Wallis test, when appropriate). Pairwise group comparisons were made using t-tests (or Wilcoxon rank-sum test, when appropriate). Variables from the univariate analysis that were significantly different between EH and CH groups were compared using multivariable-adjusted linear regression models. Covariates, chosen on the basis of known associations between the variable of interest and LV geometry, included age, sex, African-American race, hypertension, hyperlipidemia, diabetes mellitus, obesity, heart rate, log BNP, GFR, and wall motion abnormality on echocardiography.
Finally, we used Cox proportional-hazards analyses and the log-rank statistic to evaluate the relationship between LV geometry groups and outcomes. Covariates included in multivariable Cox regression models, chosen based on clinical relevance, included age, sex, African American race, hypertension, DM, obesity, and estimated GFR. To correct for multiple testing, false discovery rate (FDR) methods were applied using all calculated p-values. FDR Q-values were calculated using the Benjamini-Hochberg method.16,21 FDR < 5% corresponded to p-values <0.0172, which was therefore used as the threshold for statistical significance. All analyses were performed using Stata 12 (StataCorp, College Station, TX).
RESULTS
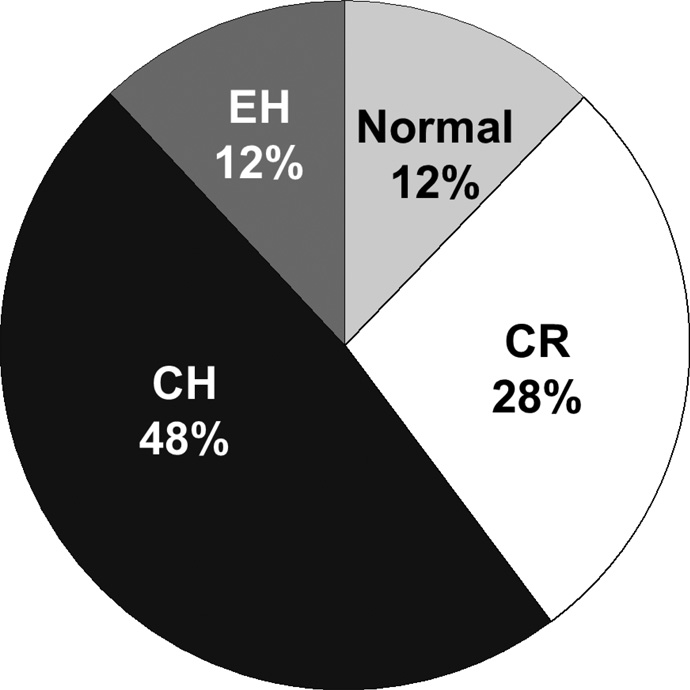
We prospectively enrolled 402 consecutive outpatients with HFpEF after hospitalization for HF. The majority of subjects had CH, followed by CR, and similar frequencies of normal geometry and EH (Figure 1).EH, while less frequent than other forms of LV remodeling, was not rare, occurring in 12% of the study cohort. Table 1 displays the clinical and demographic characteristics by LV geometry. Those with normal geometry were significantly younger than those in any other group. Significant differences among the 4 groups, especially in comorbidities, were largely driven by the differences between those with and without LVH.
Figure 1. Distribution of Left Ventricular Geometries in Heart Failure with Preserved Ejection Fraction.
Left ventricular hypertrophy was defined as left ventricular mass/height2.7>44g/m2.7 in women and >48 g/m2.7 in men. Concentric geometry defined as RWT > 0.42.CR = concentric remodeling; CH = concentric hypertrophy; EH = eccentric hypertrophy.
However, there were significant differences in kidney function and blood pressure between the EH and CH groups. Those with EH had a mean systolic blood pressure (SBP)of 121 mmHg (95% confidence interval [CI]115–127 mmHg), while those with CH had a mean SBP of 129 mmHg (95% CI 127–132 mmHg); P=0.01. Mean diastolic blood pressure was also lower among those with EH compared to CH. Despite the differences in blood pressure, prevalence of hypertension and number of antihypertensive medications were not significantly different between the EH and CH groups. Patients with EH also had lower serum creatinine and higher GFR compared to those with CH, reflecting better renal function in the EH subgroup.
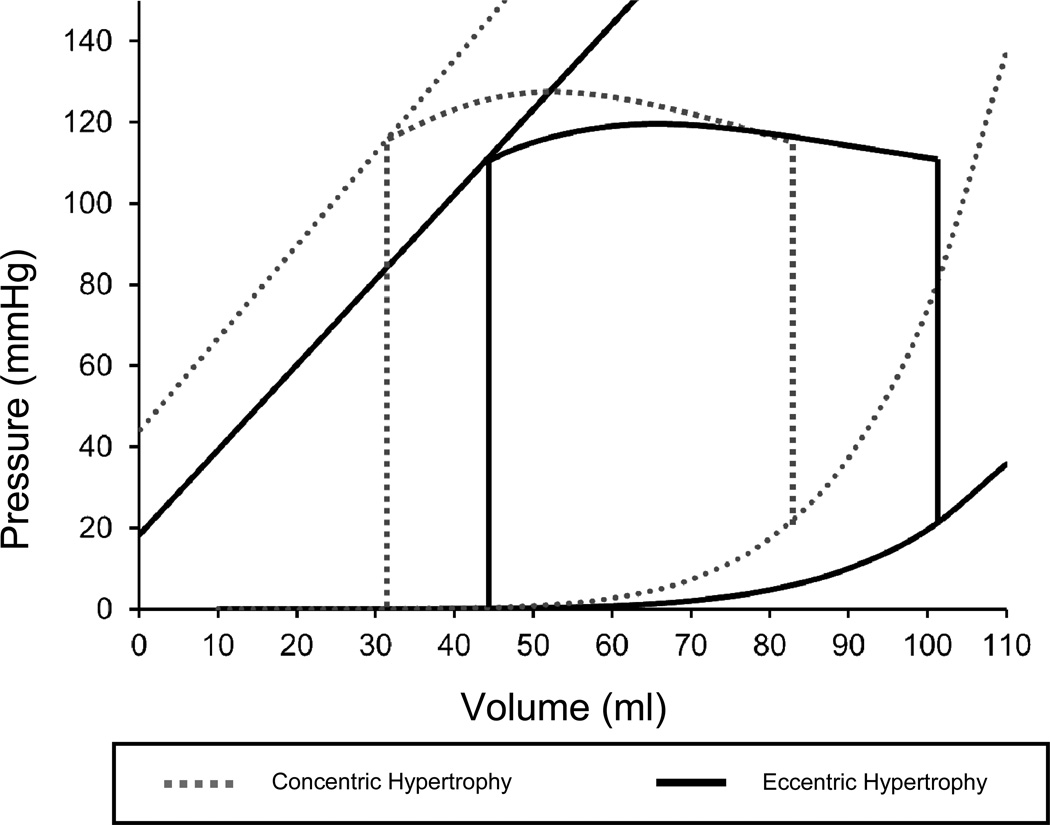
Echocardiographic analysis (Table 2) revealed that subjects with EH had larger LV volumes, lower relative wall thickness, and lower LV mass/volume ratio compared to those with CH. Contractile function was worse in EH compared to CH. Patients with EH had lower LVEF, lower SBP/ESV ratio, and higher ESV120, indicative of worse contractility on LV pressure-volume analysis (Figure 2).Patients with EH also demonstrated less arterial stiffening, as Ea and pulse pressure/stroke volume ratio were significantly lower in EH compared to CH.HFpEF patients with EH also had less LV diastolic stiffness (increased LV compliance) compared to patients with CH as shown in Table 2 and Figure 2 with a rightward- and downward-shifted EDPVR curve, as indicated by a larger EDV20. Diastolic relaxation (i.e., e’ velocity) was also less impaired in EH compared to CH. However, LV filling pressures (E/e’ ratio and PCWP) were similarly elevated in EH and CH (Tables 2 and Table 3). Pulmonary artery systolic pressure was higher in LVH compared to no LVH but was similar in EH and CH groups (Table 3). Rates (and severity) of mitral regurgitation did not vary between groups, and no patient had greater than mild aortic regurgitation.
Table 2.
Echocardiographic Characteristics by Left Ventricular Geometry Group
| Echocardiographic parameter | Normal geometry (N=49) |
Concentric remodeling (N=111) |
Concentric hypertrophy (N=194) |
Eccentric hypertrophy (N=48) |
p-value* |
|---|---|---|---|---|---|
| Left ventricular end-systolic volume index (ml/m2) | 17±5 | 15±4 | 16±6 | 21±9† | <0.001** |
| Left ventricular end-diastolic volume index (ml/m2) | 42±10 | 38±8 | 40±11 | 48±15† | <0.001** |
| Relative wall thickness | 0.38±0.03 | 0.50±0.07 | 0.59±0.17 | 0.38±0.03† | <0.001** |
| Left ventricular mass index (g/m27) | 36±6 | 37±6 | 64±19 | 56±10† | <0.001** |
| Left ventricular mass/volume ratio (g/ml) | 1.9±0.4 | 2.2±0.5 | 3.2±1.2 | 2.3±0.5† | <0.001** |
| Left ventricular ejection fraction (%) | 60±5 | 62±6 | 62±7 | 58±6† | <0.001 |
| Stroke volume (ml) | 50±16 | 46±12 | 50±15 | 56±17† | <0.001** |
| Cardiac index (L/min/m2) | 1.7±0.5 | 1.6±0.5 | 1.7±0.5 | 1.9±0.7 | 0.12 |
| End-systolic volume 120 (ml) | 40±13 | 34±13 | 35±16 | 52±22† | <0.001** |
| Systolic blood pressure/end systolic volume ratio | 3.5±1.3 | 4.3±1.6 | 4.2±1.7 | 2.9±1.4† | <0.001 |
| Effective arterial elastance (mmHg/ml) | 2.3±0.8 | 2.6±0.7 | 2.5±0.8 | 2.1±0.7† | 0.004 |
| Pulse pressure/stroke volume ratio (mmHg/ml) | 1.06±0.41 | 1.18±0.45 | 1.24±0.48 | 1.06±0.53 | 0.02 |
| End-diastolic volume20 (ml) | 86±28 | 75±20 | 82±25 | 101±32† | <0.001** |
| Left atrial volume index (ml/m2) | 33±12 | 32±17 | 35±14 | 35±11 | 0.30 |
| E/A ratio | 1.5±0.6 | 1.3±0.6 | 1.3±0.8 | 1.5±0.8 | 0.23 |
| E deceleration time (ms) | 220±57 | 227±56 | 236±77 | 224±53 | 0.38 |
| Isovolumic relaxation time (ms) | 84±20 | 85±20 | 91±23 | 87±26 | 0.07 |
| Septal e’ tissue velocity (cm/s) | 8.4±3.5 | 7.6±2.9 | 6.4±2.2 | 7.1±2.4 | <0.001** |
| Lateral e’ tissue velocity (cm/s) | 11.2±4.8 | 10.0±4.0 | 8.4±3.2 | 9.7±4.0 | <0.001** |
| Average E/e’ ratio | 12.6±7.1 | 13.4±7.3 | 16.9±9.0 | 14.9±6.7 | <0.001** |
| Tricuspid annular plane systolic excursion (cm) | 2.30±0.71 | 1.89±0.64 | 1.97±0.61 | 2.06±0.54 | 0.002 |
| Mitral Regurgitation | |||||
| None | 26 (53%) | 70 (63%) | 109 (56%) | 23 (48%) | 0.31 |
| Mild | 13 (27%) | 29 (26%) | 55 (28%) | 16 (33%) | 0.82 |
| Moderate | 10 (20%) | 11 (10%) | 29 (15%) | 8 (17%) | 0.32 |
p value by analysis of variance;
p-value < 0.05 and remains significant after correction using false discovery rate < 5% for comparison of eccentric hypertrophy vs. concentric hypertrophy;
p-value < 0.05 for left ventricular hypertrophy compared to no left ventricular hypertrophy (and remains significant after false-discover rate correction)
Figure 2. Pressure-Volume Relationships in Heart Failure with Preserved Ejection Fraction: Eccentric Hypertrophy versus Concentric Hypertrophy.
Patients with eccentric hypertrophy have downward and rightward shifted end-systolic and end-diastolic pressure volume relationships, as indicated by larger ESV120 and EDV20 values.
Table 3.
Invasive Hemodynamics by Left Ventricular Geometry Group (N=217)
| Invasive hemodynamic parameter | Normal geometry (N=27) |
Concentric remodeling (N=62) |
Concentric hypertrophy (N=101) |
Eccentric hypertrophy (N=27) |
p-value* |
|---|---|---|---|---|---|
| Right atrial pressure (mmHg) | 15±7 | 13±6 | 14±7 | 13±6 | 0.31 |
| Pulmonary artery systolic pressure (mmHg) | 48±14 | 45±13 | 56±20 | 54±15 | <0.001** |
| Pulmonary artery diastolic pressure (mmHg) | 26±8 | 22±7 | 27±10 | 27±9 | 0.005 |
| Mean pulmonary artery pressure (mmHg) | 34±10 | 30±8 | 36±12 | 36±10 | 0.006 |
| Pulmonary capillary wedge pressure (mmHg) | 23±8 | 21±7 | 24±10 | 24±9 | 0.06 |
| Cardiac index (L/min/m2) | 3.0±0.8 | 2.7±0.8 | 3.0±1.1 | 3.2±1.4 | 0.06 |
| Pulmonary vascular resistance (Wood units) | 1.7±1.3 | 1.9±1 | 2.0±1.6 | 2.2±2.1 | 0.60 |
p value by analysis of variance between groups;
p-value < 0.05 and remains significant after correction using false discovery rate < 5% for left ventricular hypertrophy compared to no left ventricular hypertrophy. There were no statistically significant differences between the eccentric and concentric hypertrophy groups
Clinical characteristics that were significantly different between EH and CH groups on univariate analysis were analyzed further using multivariable-adjusted linear regression models (Table 4). After adjustment for covariates, SBP, diastolic blood pressure, LVEF, SBP/ESV ratio, and Ea remained significantly lower while GFR, ESV120, EDV20, and stroke volume remained significantly higher in EH compared to CH.
Table 4.
Differences in Clinical Characteristics and Echocardiographic Parameters between Eccentric Hypertrophy and Concentric Hypertrophy Groups after Multivariable-Adjusted Linear Regression Analysis
| Clinical characteristic | β-Coefficient for comparison of EH vs. CH |
p-value |
|---|---|---|
| Systolic blood pressure (mmHg) | −8.3 (−14.9, −1.8) | 0.01 |
| Diastolic blood pressure (mmHg) | −5.3 (−9.1, −1.5) | 0.01 |
| Serum creatinine (mg/dl)* | −0.6 (−1.0, −0.2) | 0.01 |
| Estimated glomerular filtration rate (ml/min/1.73 m2)* | 12.3 (4.7, 20.0) | 0.002 |
| Echocardiographic parameter | ||
| Ejection fraction (%) | −3.2 (−5.4, −1.1) | <0.001 |
| Stroke volume (ml) | 4.9 (0.5, 9.3) | 0.03† |
| End-systolic volume120 (ml) | 15.7 (8.4, 23.0) | <0.001 |
| Systolic blood pressure/end systolic volume ratio (mmHg/ml) | −1.0 (−1.5, −0.5) | <0.001 |
| Ea, arterial elastance (mmHg/ml) | −0.3 (−0.5, −0.1) | 0.01 |
| Pulse pressure/stroke volume ratio (mmHg/ml) | −0.1 (−0.3, 0.0) | 0.10 |
| End-diastolic volume20 (ml) | 14.2 (9.4, 19.1) | <0.001 |
| Lateral e’ velocity (cm/s) | 0.8 (−0.4, 2.0) | 0.19 |
Model adjusted for age, sex, African-American race, hypertension, hyperlipidemia, diabetes mellitus, obesity, heart rate, log B-type natriuretic peptide, presence of wall motion abnormality on echocardiography, and estimated glomerular filtration rate (except as noted)
Estimated glomerular filtration rate omitted from model
No longer significant after adjustment for multiple comparisons using the false discovery rate method
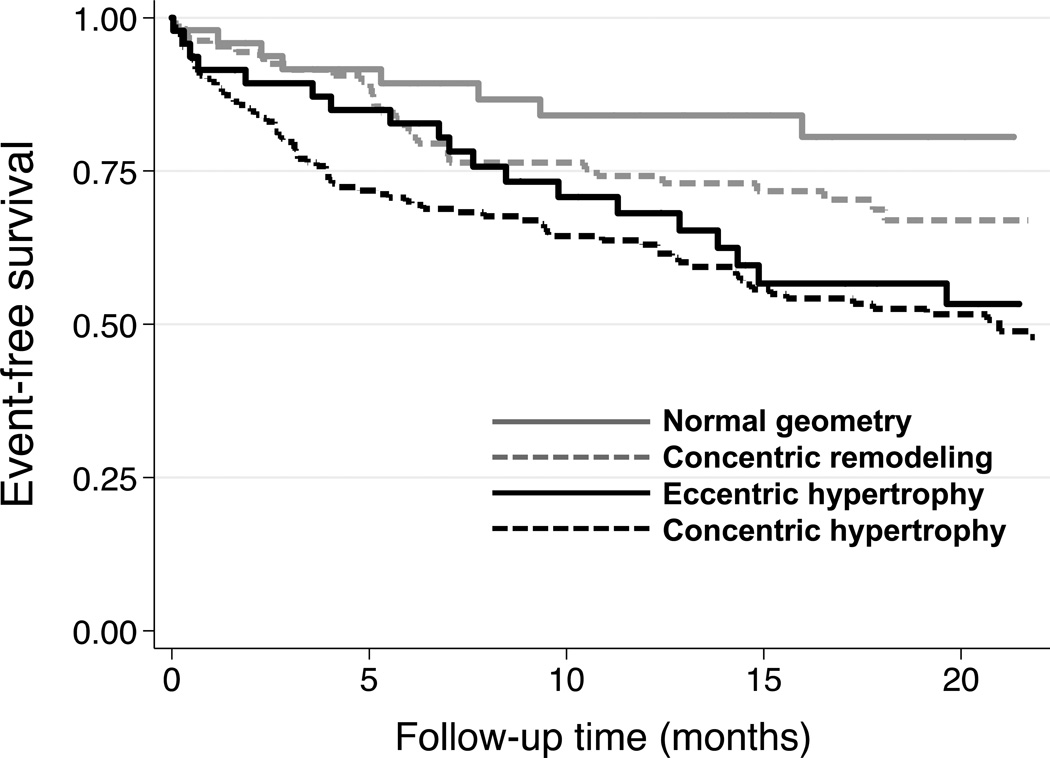
Over a mean follow-up time of 12.2±8.5 months, there was no significant difference in the number of hospitalizations, death, or combined outcomes between subjects with EH and CH(Table 5). There were also no significant differences in the Cox-proportional hazard ratios (HRs) between these groups. The difference between EH and CH groups after multivariable adjustment was not significant (P = 0.24). Figure 3shows the Kaplan-Meier curves for the combined outcome of HF hospitalization, cardiovascular hospitalization, and death. Outcomes did differ by presence or absence of LV hypertrophy; those with LV hypertrophy (EH or CH) had worse outcomes compared to those without LV hypertrophy (Figure 3, log-rank P=0.0005).
Table 5.
Association of Left Ventricular Geometry with Adverse Outcomes on Cox-Proportional Hazards Analysis
| Normal geometry (N=49) |
Concentric remodeling (N=111) |
Concentric hypertrophy (N=194) |
Eccentric hypertrophy (N=48) |
p-value* | |
|---|---|---|---|---|---|
| Outcome | |||||
| Heart failure hospitalization | 3 (6%) | 20 (18%) | 64 (33%) | 15 (31%) | <0.001 |
| Cardiovascular hospitalization | 6 (12%) | 28 (25%) | 86 (44%) | 18 (38%) | <0.001 |
| Death | 3 (8%) | 14 (14%) | 28 (17%) | 3 (8%) | 0.340 |
| Combined endpoint | 8 (16%) | 36 (32%) | 101 (52%) | 20 (42%) | <0.001 |
| Unadjusted HR (95% CI) | |||||
| Heart failure hospitalization | 1.00 | 3.10 (0.92–10.40) | 6.00 (1.88–19.1) | 5.27 (1.51–18.3) | 0.002** |
| Cardiovascular hospitalization | 1.00 | 2.19 (0.91–5.29) | 4.20 (1.83–9.62) | 3.09 (1.22–7.84) | <0.001** |
| Death | 1.00 | 1.78 (0.60–5.29) | 2.12 (0.75–5.98) | 0.73 (0.16–3.28) | 0.192 |
| Combined endpoint | 1.00 | 2.09 (0.97–4.50) | 3.71 (1.80–7.62) | 2.59 (1.14–5.93) | <0.001** |
| Adjusted HR (95% CI)† | |||||
| Heart failure hospitalization | 1.00 | 2.31 (0.67–8.00) | 4.03 (1.22–13.3) | 3.64 (1.01–13.1) | 0.035** |
| Cardiovascular hospitalization | 1.00 | 1.54 (0.62–3.82) | 2.73 (1.15–6.46) | 2.34 (0.90–6.11) | 0.017** |
| Death | 1.00 | 1.28 (0.41–3.99) | 1.76 (0.59–5.19) | 0.85 (0.19–3.93) | 0.452 |
| Combined endpoint | 1.00 | 1.51 (0.68–3.32) | 2.54 (1.20–5.40) | 2.02 (0.86–4.77) | 0.012** |
P-value represents difference across all 4 groups (there were no significant differences between the eccentric and hypertrophy groups);
Adjusted for age, sex, African American race, estimated glomerular filtration rate, hypertension, diabetes mellitus, and obesity;
p-value < 0.05 and remains significant after correction using false discovery rate < 5% for comparison of left ventricular hypertrophy to no left ventricular hypertrophy HR = hazard ratio; CI = confidence interval
Figure 3. Kaplan-Meier Survival Curves for the Combined Outcome of Heart Failure Hospitalization, Cardiovascular Hospitalization, or Death, Stratified by Left Ventricular Geometry Group.
Patients with eccentric hypertrophy had outcomes that were similar to patients with concentric hypertrophy (Log-rank P=0.33).Patients with left ventricular hypertrophy (either eccentric or concentric hypertrophy) had worse outcomes compared to those without left ventricular hypertrophy (Log-rank P=0.0005).
DISCUSSION
In a prospective study of LV geometry in 402 subjects with HFpEF, we found that a non-trivial proportion of these individuals (12%) have EH. Study participants with HFpEF who had EH represent a unique subset with lower blood pressure, better kidney function, and higher LV compliance, but lower contractility, compared to individuals with CH. These differences were not attenuated by multivariable adjustment. LV filling pressures and outcomes were nonetheless similar in patients with EH and CH, underscoring the continued unmet need for therapies for patients with HFpEF who have either type of LVH. To our knowledge this is the most comprehensive analysis of characteristics of EH in HFpEF to date.
The frequency of EH in HFpEF in our study is similar to the16% prevalence found in the Olmsted County HFpEF cohort. Despite having lower BP, those with EH had similar rates of hypertension as those with CR or CH. Only subjects with normal geometry, who were significantly younger, had less prevalent hypertension. Since those with EH were not taking more or significantly different antihypertensive medications compared to CH, our results may suggest that patients with EH have greater responses to anti-hypertensive medication. Results from the Losartan Intervention For Endpoint Reduction in Hypertension (LIFE) study demonstrated that treatment with losartan or atenolol induced conversion from CH to EH in 34% of individuals with hypertension and baseline CH, while only 3% converted from EH to CH. 22 Therefore, the presence of EH in HFpEF may represent a differential response to anti-hypertensive medications compared to those with CH or CR.
Echocardiographic analysis in our study found that when compared to other types of LV geometry in HFpEF, patients with EH have characteristics that are similar to HFrEF, despite a preserved EF. In our study, patients with EH demonstrated lower EF, lower SBP/ESV ratio, and a greater ESV120 compared to those with CH. Taken together, these data suggest worse contractility in the EH group. EH patients also demonstrated better diastolic function compared to CH patients, with increased LV compliance (larger EDV20) and better, but still abnormal, LV relaxation. However, lateral e’ tissue velocity was not different between groups after adjustment for covariates. Figure 2, which summarizes the pressure-volume relationships of the 2 groups, demonstrates that patients with EH have larger ventricles with worse systolic function and better diastolic function. The pressure-volume loop in EH is more like that of a patient with HFrEF compared to the classic model of HFpEF. Additionally, Ea in EH was lower than in CH, suggesting less arterial stiffening, a difference also found when comparing HFpEF with HFrEF.17,2
Despite differences in ventricular and vascular structure and function, patients with EH had similarly elevated cardiac filling pressures and similar adverse outcomes when compared to those with CH. The similarity in left atrial size, LV filling pressures (i.e., E/e’ ratio and PCWP), and outcomes in HFpEF patients with EH and CH underscores the need to develop therapies for HFpEF patients with EH. Although EH is only represented in 12–16% of HFpEF patients, given the high overall prevalence of HFpEF, an estimated 360,000 to 480,000 patients in the United States have both HFpEF and EH.18,19 Given the pathophysiologic profile of patients with HFpEF who have EH, it is possible that these patients may benefit from therapies proven to be effective in HFrEF. These therapies have thus far failed in HFpEF,20–25 but future HFpEF clinical trials may benefit from a priori stratification by type of LV remodeling to determine whether there are differential treatment responses among groups.
From our data we cannot determine what led patients to develop EH rather than other types of LV remodeling. EH is classically thought to result from volume overload states.26,27 We excluded patients with obvious causes of high-output heart failure or extracardiac sources of volume overload. Valvular heart disease (i.e., mitral or aortic regurgitation) is another possible cause of EH, but valvular heart disease greater than moderate in severity was an exclusion criteria for our study, and rates of mitral regurgitation did not differ between the EH and CH groups. Another potential limitation of our study is the recruitment of all patients from a single academic medical center. However, Northwestern Memorial Hospital serves a large, diverse urban environment. While our cohort was younger than epidemiologic and registry studies of HFpEF, rates of comorbidities were similar, and our study sample was more racially diverse; this may better represent the broader population of HFpEF patients compared to other contemporary HFpEF studies. Finally, our study design only included those HFpEF patients who had recently been hospitalized for HF; however, post-hospitalization patients are at the highest risk for adverse outcomes, and have the most urgent need for effective therapies.19
Acknowledgments
Funding Sources: AHA Scientist Development Grant (#0835488N) and National Institutes of Health (R01 HL107557)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: No relationships with industry related to this manuscript.
References
- 1.Komajda M, Carson PE, Hetzel S, McKelvie R, McMurray J, Ptaszynska A, Zile MR, Demets D, Massie BM. Factors associated with outcome in heart failure with preserved ejection fraction: findings from the Irbesartan in Heart Failure with Preserved Ejection Fraction Study (I-PRESERVE) Circ Heart Fail. 2011;4:27–35. doi: 10.1161/CIRCHEARTFAILURE.109.932996. [DOI] [PubMed] [Google Scholar]
- 2.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–1959. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 3.Maurer MS, King DL, El-Khoury Rumbarger L, Packer M, Burkhoff D. Left heart failure with a normal ejection fraction: identification of different pathophysiologic mechanisms. J Card Fail. 2005;11:177–187. doi: 10.1016/j.cardfail.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Shah AM, Pfeffer MA. The many faces of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2012;9:555–556. doi: 10.1038/nrcardio.2012.123. [DOI] [PubMed] [Google Scholar]
- 5.Maurer MS, Burkhoff D, Fried LP, Gottdiener J, King DL, Kitzman DW. Ventricular structure and function in hypertensive participants with heart failure and a normal ejection fraction: the Cardiovascular Health Study. J Am Coll Cardiol. 2007;49:972–981. doi: 10.1016/j.jacc.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 6.Zile MR, Lewinter MM. Left ventricular end-diastolic volume is normal in patients with heart failure and a normal ejection fraction: a renewed consensus in diastolic heart failure. J Am Coll Cardiol. 2007;49:982–985. doi: 10.1016/j.jacc.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Zile M, Gottdiener J, Hetzel S, McMurray J, Komajda M, McKelvie R, Baicu C, Massie B, Carson P, Investigators IP. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124:2491–2501. doi: 10.1161/CIRCULATIONAHA.110.011031. [DOI] [PubMed] [Google Scholar]
- 8.Mohammed SF, Borlaug BA, Roger VL, Mirzoyev SA, Rodeheffer RJ, Chirinos JA, Redfield MM. Comorbidity and ventricular and vascular structure and function in heart failure with preserved ejection fraction: a community-based study. Circ Heart Fail. 2012;5:710–719. doi: 10.1161/CIRCHEARTFAILURE.112.968594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anjan VY, Loftus TM, Burke MA, Akhter N, Fonarow GC, Gheorghiade M, Shah SJ. Prevalence, clinical phenotype, and outcomes associated with normal B-type natriuretic Peptide levels in heart failure with preserved ejection fraction. Am J Cardiol. 2012;110:870–876. doi: 10.1016/j.amjcard.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 11.Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 12.Paulus WJ, van Ballegoij JJ. Treatment of heart failure with normal ejection fraction: an inconvenient truth! J Am Coll Cardiol. 2010;55:526–537. doi: 10.1016/j.jacc.2009.06.067. [DOI] [PubMed] [Google Scholar]
- 13.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA, Kawaguchi M, Kass DA. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol. 2001;38:2028–2034. doi: 10.1016/s0735-1097(01)01651-5. [DOI] [PubMed] [Google Scholar]
- 15.Senzaki H, Chen CH, Kass DA. Single-beat estimation of end-systolic pressure-volume relation in humans. A new method with the potential for noninvasive application. Circulation. 1996;94:2497–2506. doi: 10.1161/01.cir.94.10.2497. [DOI] [PubMed] [Google Scholar]
- 16.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995:289–300. [Google Scholar]
- 17.Schwartzenberg S, Redfield MM, From AM, Sorajja P, Nishimura RA, Borlaug BA. Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol. 2012;59:442–451. doi: 10.1016/j.jacc.2011.09.062. [DOI] [PubMed] [Google Scholar]
- 18.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 20.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A, Investigators IP. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 21.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J, Investigators P-C. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 22.Setaro JF, Zaret BL, Schulman DS, Black HR, Soufer R. Usefulness of verapamil for congestive heart failure associated with abnormal left ventricular diastolic filling and normal left ventricular systolic performance. Am J Cardiol. 1990;66:981–986. doi: 10.1016/0002-9149(90)90937-v. [DOI] [PubMed] [Google Scholar]
- 23.Shah RV, Desai AS, Givertz MM. The effect of renin-angiotensin system inhibitors on mortality and heart failure hospitalization in patients with heart failure and preserved ejection fraction: a systematic review and meta-analysis. J Card Fail. 2010;16:260–267. doi: 10.1016/j.cardfail.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Investigators C Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 25.Aronow WS, Ahn C, Kronzon I. Effect of propranolol versus no propranolol on total mortality plus nonfatal myocardial infarction in older patients with prior myocardial infarction, congestive heart failure, and left ventricular ejection fraction > or = 40% treated with diuretics plus angiotensin-converting enzyme inhibitors. Am J Cardiol. 1997;80:207–209. doi: 10.1016/s0002-9149(97)00320-2. [DOI] [PubMed] [Google Scholar]
- 26.Conrady AO, Rudomanov OG, Zaharov DV, Krutikov AN, Vahrameeva NV, Yakovleva OI, Alexeeva NP, Shlyakhto EV. Prevalence and determinants of left ventricular hypertrophy and remodelling patterns in hypertensive patients: the St. Petersburg study. Blood Press. 2004;13:101–109. doi: 10.1080/08037050410031855. [DOI] [PubMed] [Google Scholar]
- 27.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]