Abstract
While negative emotions and psychiatric morbidity have often been found to increase incident coronary artery disease (CAD) risk, fewer studies have shown positive emotions to be protective against CAD; none have been performed in high-risk healthy populations, taking risk factors into account. Thus, we examined the impact of positive well-being on incident CAD in both a high-risk initially healthy population and in a national probability sample. We screened healthy siblings of probands with documented early-onset CAD from 1985 to 2007 in the GeneSTAR (Genetic Study of Atherosclerosis Risk) population and examined sociodemographics, risk factors, and positive well-being using the General Well Being Schedule (GWBS). We further classified siblings into high, intermediate and low risk strata based on the Framingham Risk Score (FRS) and followed them for 5 to 25 years. Siblings (n=1483) with higher baseline GWBS total scores were significantly less likely to develop CAD (hazard ratio [HR]=0.67, 95% confidence interval [CI] 0.58–0.79), independent of age, sex, race, and traditional risk factors. Protection was strongest in the high FRS stratum (HR=0.52, 95% CI 0.30–0.90). Findings were replicated in the first National Health and Nutrition Examination Survey and Epidemiologic Follow-up Study (n=5992; HR=0.87, 95% CI 0.83–0.93). In conclusion, positive well-being was associated with nearly a third reduction in CAD in a high-risk population with family history, a nearly 50% reduction in incident CAD in the highest risk stratum in those with family history, and a 13% reduction in incident CAD in a national probability sample, independent of traditional CAD risk factors.
Keywords: coronary artery disease, epidemiology, cardiovascular risk factors, psychosocial factors
Introduction
While negative psychological states and psychiatric diagnoses such as depression and anxiety have long been found to predict cardiovascular outcomes,1, 2 positive psychological attributes such as optimism and life satisfaction, have been studied with regard to coronary artery disease (CAD) only more recently.3,4,5,6 Positive well-being is a broad and multi-dimensional construct, encompassing several aspects of psychological health, including affect, outlook, and life satisfaction; positive well-being represents the absence of negative well-being or depression in combination with other positive components.7, 8 Moreover, positive well-being is stable over time9 and functions as a trait.10 Prior studies of positive well-being and CAD have included both healthy and patient populations, but to date, no studies of healthy high-risk populations have examined positive well-being as a trait predicting incident CAD, nor have any studies examined positive well-being in the context of CAD risk factor classification. Thus, our study was designed to determine the extent to which baseline positive well-being measured with the General Well-Being Schedule (GWBS) predicted CAD incidence, in initially healthy individuals with a family history of early-onset CAD, in the context of the Framingham Risk Score (FRS) strata. We further examined the relationship of positive well-being and incident CAD in a national probability sample of the general population in the First National Health and Nutrition Examination Survey (NHANES I) and NHANES I Epidemiologic Follow-up Study (NHEFS).
Methods
The GeneSTAR study was approved by the Johns Hopkins Medicine Institutional Review Board and has been previously described.11 Briefly, given that siblings of people with premature CAD have a more than 2-fold excess risk of CAD, GeneSTAR was designed to explore CAD risk factors in a cohort of high-risk families (www.genestarstudy.com). Probands with documented early-onset (< age 60 years) CAD events including myocardial infarction (42.9%), coronary artery bypass surgery (21.2%), percutaneous coronary intervention (22.6%), or ≥ 50% stenosis in 1 or more vessels confirmed on coronary angiography with or without angina symptoms (13.1%) were identified at the time of hospitalization for the sentinel CAD event. Their apparently healthy siblings < 60 years of age and free of known CAD were recruited and screened from 1985 to 2007. Siblings were excluded from the study for systemic autoimmune disease, chest radiation exposure, any life-threatening disease (AIDS or advanced cancer), or chronic glucocorticosteroid therapy.
All participants gave written informed consent prior to screening. Demographic information (age, sex, race, education, marital status) was obtained from standardized self-administered questionnaires. Siblings who self-reported smoking any cigarettes within the past month or who had an expired carbon monoxide ≥ 8 ppm on 2 successive measurements were considered to be current smokers.
Medical history, including current prescription and over-the-counter medication use, was obtained by a physician and a nurse, and a physical examination was performed by a cardiologist. A history of any psychiatric disorder was considered present if there was any report of a current or past psychiatric diagnosis by a physician, licensed counselor or psychologist according to the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition,12 and psychiatric medication use was considered present if current use of any medications used explicitly for psychiatric or mental problems was recorded.
Blood was obtained after participants had fasted for at least 8 hours overnight. Total and HDL cholesterol, triglycerides, and glucose levels were measured directly using CLIA-approved standardized methods in the Johns Hopkins Analytical Chemistry Laboratory. LDL cholesterol was calculated using the Chen formula.13 Diabetes was defined as a self-reported physician’s diagnosis of current diabetes, current use of insulin or hypoglycemic medication, and/or fasting glucose level > 125 mg/dl (6.9 mmol/L).
Blood pressure was measured 3 times over the course of the 8-hour screening day using standard guidelines,14 and averaged to represent resting blood pressure. Hypertension was defined as resting blood pressure ≥ 140/90 mmHg and/or current antihypertensive medication use. Weight and height were measured; body mass index was calculated as weight(kg) / height(m)2. Maximal graded treadmill tests were performed using a standardized modified Bruce protocol; total metabolic equivalent task levels were calculated as the minutes on the treadmill multiplied by the metabolic equivalent task level associated with the achieved stage of the protocol, expressed as MET-minutes and used to characterize cardiorespiratory fitness.
Ten-year FRS were calculated according to established criteria inclusive of sex, age, total and HDL cholesterol, systolic and diastolic blood pressure, diabetes and smoking.15 Siblings were placed into low (< 10%), intermediate (10–20%), and high risk (>20%) groups.16
The General Well-Being Schedule (GWBS) was self-administered and reviewed for completion. The GWBS questionnaire was originally designed to measure self-report of subjective well-being in normal populations.17 The instrument consists of 18 items, representing 6 psychological domains: relaxed versus tense (anxiety), cheerful versus depressed mood (depression), freedom from health concern (somaticism), energy level (vitality), life satisfaction, and emotional-behavioral control. The domain scores sum to a total score with a maximum of 110 points, where higher scores represent greater “positive well-being”. The GWBS total score has good internal consistency (Cronbach’s alpha 0.90 to 0.94) and test-retest reliability.17 It has been used to characterize healthy populations in several large clinical and epidemiological studies,18,19 including NHANES I,18 and has been validated in European- and African-American populations.20 The instrument and scoring system are available online.18
Siblings completed a standardized health status and cardiovascular disease event questionnaire at approximately 5-year intervals after their baseline visit with trained telephone interviewers between 1992 and 2012. For deceased siblings, proxy interviews were completed with the next of kin. Medical records were then obtained for all reports of a CAD event, any possibly related diagnosis, diagnostic procedure (including exercise tests, thallium imaging, or coronary angiography) or therapeutic procedure. Medical record documentation was reviewed and CAD events were classified independently by 2 cardiologists and 1 epidemiologist, each blinded to the review of the others, according to a standardized Framingham Heart Study15 schema. Whenever there was discordance among the team, an External Adjudication Committee consisting of at least 1 non-study cardiologist from Johns Hopkins and 1 from another institution reviewed and adjudicated the final event classification. For participants with more than 1 documented event, only the first event was used in analysis.
Detailed descriptions of NHANES I and the NHEFS are available elsewhere.18, 21 Briefly, NHANES I was conducted between 1971–1975 in the U.S. by the National Center for Health Statistics, Centers for Disease Control and Prevention, to obtain national estimates on the health and nutritional status of the U.S. population. NHANES I was based on a complex, multistage, stratified, clustered, probability-sample design and comprised a representative sample of the non-institutionalized civilian U.S. population living in households. The NHEFS was a national longitudinal study which comprised studies in 1982, 1987 and 1992 designed to investigate the relationships between factors assessed in NHANES I and subsequent morbidity, mortality, and hospital utilization.21
This study used data from the NHANES I examination sample of adults aged 25–74. Briefly, individuals received standardized interview questionnaires to obtain sociodemographic data. Additional information included smoking, medical history (medication use, diagnosis of diabetes or hypertension, history of psychiatric condition) and physical activity questions. Current smokers were defined as individuals who self-reported that they currently smoked cigarettes. Diabetes, hypertension and psychiatric diseases were defined as a self-reported physician diagnosis and/or medication use. A question asking the level and intensity of recreational physical activity was used to categorize fitness as none-little, moderate or high physical activity. NHANES I also obtained standardized measurements of height and weight; body mass index was calculated by dividing weight (kg) by height (m2). Blood was drawn in mobile examination centers and total serum cholesterol was measured by the Centers for Disease Control and Prevention laboratory using standardized methods; HDL and LDL cholesterol were unavailable. The GWBS was administered in the mobile examination centers by trained interviewers.
CAD events were identified by hospital and/or nursing home discharge reports, and death certificates. CAD was recorded if ICD-9 codes 410–414 were listed. For nonfatal events, the date of the CAD event was the date of admission obtained in the discharge report, or self-reported if not available on the record. The date of a fatal CAD event was the date of death recorded from the death certificate. For participants with more than 1 event, only the earliest event was included.
A total of 6,913 adults 25–74 years of age, were interviewed, received a detailed physical and medical exam, and were eligible for the current analyses. After excluding 921 people with missing covariates and missing outcome information, 5992 (86.7% of those eligible) were included in the analyses.
Data were analyzed using SAS v. 9.2 (SAS Institute Inc, Cary, NC, 2002–2008), STATA 11 (StataCorp LP, College Station, TX, 2009) and R v. 2.12.1 (The R Foundation for Statistical Computing, 2012). For bivariable analyses, frequencies and contingency table arrays and the χ2 statistic were used; for continuous and ordinal variables, student’s t tests and the Wilcoxon rank-sum test were used. Psychometric properties of the GWBS were assessed using measures of internal consistency, Cronbach’s alpha and item-to total correlations. Continuous variables were standardized by dividing by their standard deviations prior to entry in regression models. Hazard ratios (HR) and 95% confidence intervals (CI) of CAD events were estimated using Cox proportional hazards regression modeling incident CAD adjusting for age, sex, race, education, being married, current smoking, diabetes, hypertension, LDL and HDL cholesterol, body mass index, physical fitness, having a psychiatric diagnosis and/or medication use, and GWBS. For GeneSTAR, grouped jackknife estimation, in which the variance of hazard ratios is empirically estimated by a set of regressions leaving one family cluster out at a time, thus implicitly accounting for the non-independence of the time to event within families. This method is appropriate for censored time-to-event analyses because explicit modeling of within-cluster correlation of censored variables, which is required for Generalized Estimating Equations methods, is not possible. Sensitivity analyses examined the same regression analyses with the exclusion of participants with psychological diagnoses and/or medication use; separate regression analyses for each dichotomous group (males and females; European and African Americans; hypertensives and nonhypertensives; diabetics and nondiabetics; smokers and nonsmokers; married and nonmarried; obese and nonobese; high school and less than high school education; psychiatric diagnosis and/or medication use and no diagnosis or medication use; age < 46 and ≥ 46 years; HDL <40 and ≥ 40 mg/dl; LDL <160 and ≥ 160 mg/dl; MET-minutes <median 83 and ≥83); and separate regression analyses with each potentially influential variable expressed as present or absent included with an interaction term with the GWBS total score. FRS analyses were performed separately within each FRS strata with the same adjustments as the primary model.
Results
We screened 1504 siblings at baseline and obtained outcome data on 98.6% or 1483 siblings who comprise the present study sample. Siblings came from 778 families, with a mean 1.9 ± 1.2 siblings per family (range 1 to 9), and a mean 12.1 ± 6 years of follow-up (range 5 to 25). The GWBS was highly internally consistent, with a Cronbach’s alpha of 0.91 for the GWBS total score, and Cronbach’s alphas ranging from 0.61 to 0.83 for the domain scores.
During follow-up, we documented 208 incident CAD events (overall rate 14%), with 71/208 (34.1%) classified as “hard” events (myocardial infarction or sudden death). Unstable angina with > 50% coronary luminal stenoses occurred in 38.5%, 77.5% of whom underwent revascularization. Chronic stable angina with > 50% stenosis in at least one coronary vessel occurred in 27.4%, 68% of whom underwent revascularization. The mean time from baseline to an incident CAD event was 8.2±5.4 years; median 7.1 years. The mean age at the time of the event was 56.2±7.6 years (range 34 to 74 years). Incident events occurred in 68/834 (8.2%) females and in 140/649 (21.6%) males.
Table 1 shows the baseline demographic and risk characteristics for those with and without an incident CAD event. The presence of a psychiatric diagnosis (4.3% depression; 0.68% anxiety) or prescribed psychiatric medications was relatively low. Psychiatric pharmacotherapy included 3.8% benzodiazepines, 4.5% selective serotonin reuptake inhibitors, and 0.9% both agents.
Table 1.
Baseline Characteristics of GeneSTAR by Incident Coronary Artery Disease Event Status (n=1483)
| CAD Event | |||
|---|---|---|---|
|
| |||
| Variable | No (n=1275) | Yes (n=208) | |
|
| |||
| Mean ± SD | Mean ± SD | P value* | |
| Age (years) | 46.4 ± 7.3 | 48.1 ± 6.7 | 0.0017 |
| Education (years) | 13.3 ± 2.8 | 12.7 ± 2.7 | 0.0033 |
| LDL cholesterol (mg/dl) | 133.6 ± 39.2 | 155.8 ± 48.4 | <0.0001 |
| HDL cholesterol (mg/dl) | 54.6 ± 17.1 | 46.5 ± 13.6 | <0.0001 |
| Body mass index (kg/m2) | 29.6 ± 6.3 | 29.4 ± 5.7 | 0.70 |
| Physical fitness (MET-minutes) | 93.6 ± 49.9 | 95.5 ± 52.4 | 0.62 |
| General Well-Being (total score) | 75.0 ± 16.8 | 69.7 ± 17.2 | <0.0001 |
|
| |||
| P value* | |||
|
| |||
| Male sex | 509 (39.9%) | 140 (67.3%) | <0.0001 |
| African American race | 582 (45.7%) | 51 (24.5%) | <0.0001 |
| Currently married | 805 (63.1%) | 145 (69.7%) | 0.0669 |
| Current smoker | 363 (28.5%) | 76 (36.5%) | 0.0181 |
| Diabetes mellitus | 110 (8.63%) | 36 (17.3%) | <0.0001 |
| Hypertension | 607 (47.6%) | 131 (63.0%) | <0.0001 |
| Psychiatric diagnosis | 90 (7.1%) | 8 (3.9%) | 0.0837 |
| Psychiatric prescription | 125 (9.8%) | 19 (9.1%) | 0.7624 |
| Psychiatric diagnosis and/or prescription | 146 (11.5%) | 22 (10.6%) | 0.7123 |
p-values were obtained from student’s t-tests or Wilcoxon tests for continuous variables and chi-square tests for categorical variables
CAD=coronary artery disease
HDL=high density lipoprotein
LDL=low density lipoprotein
MET=metabolic equivalent
SD=standard deviation
GWBS total and domain scores by CAD event status are shown in Table 2. The unadjusted HR for incident CAD related to higher GWBS total scores was 0.77, 95% CI 0.68 to 0.87. Importantly, multivariable survival analysis showed that higher GWBS total scores were associated with a significantly lower risk of incident CAD, independent of all risk factors and salient variables (Table 3). Every 16-point increase in the baseline GWBS total score was associated with a 33% reduction in incident CAD risk even when accounting for important risk factors and covariates.
Table 2.
General Well-Being Characteristics of GeneSTAR by Incident Coronary Artery Disease Event Status (n=1483)
| CAD Event | |||
|---|---|---|---|
|
| |||
| Variable | No (n=1275) | Yes (n=208) | |
|
| |||
| General Well-Being Characteristic | Mean ± SD | Mean ± SD | P value* |
| General Well-Being (total score) | 75.0 ± 16.8 | 69.7 ± 17.2 | <0.0001 |
| Relaxation domain score | 16.5 ± 5.1 | 15.0 ± 5.0 | <0.0001 |
| Cheerful mood domain score | 18.1 ± 4.4 | 16.7 ± 4.9 | <0.0001 |
| Life satisfaction domain score | 6.27 ± 2.1 | 5.77 ± 2.2 | 0.0033 |
| Emotional behavioral control domain score | 12.5 ± 2.5 | 12.0 ± 3.0 | 0.088 |
| Freedom from health concern domain score | 9.61 ± 3.7 | 9.12 ± 3.6 | 0.052 |
| Energy level domain score | 12.0 ± 4.0 | 11.1 ± 3.8 | 0.002 |
p-values were obtained from student’s t-tests or Wilcoxon tests for continuous variables and chi-square tests for categorical variables
CAD=coronary artery disease
SD=standard deviation
Table 3.
Multivariable Cox Proportional Hazards Model Predicting Incident Coronary Artery Disease in GeneSTAR (n=1483)*
| Characteristic | Hazard Ratio (95% CI) | P |
|---|---|---|
| Baseline age (years) † | 1.38 (1.18–1.61) | <0.0001 |
| Male sex | 2.87 (2.03–4.06) | <0.0001 |
| African American race | 0.51 (0.35–0.74) | 0.0004 |
| Hypertension | 1.65 (1.19–2.30) | 0.003 |
| LDL cholesterol (mg/dl) † | 1.31 (1.15–1.49) | <0.0001 |
| HDL cholesterol (mg/dl) † | 0.85 (0.70–1.04) | 0.11 |
| Diabetes mellitus | 1.99 (1.25–3.16) | 0.0036 |
| Current smoker | 1.36 (0.99–1.87) | 0.06 |
| General Well-Being (total score) † | 0.67 (0.58–0.79) | <0.0001 |
Adjusted for education, being married, BMI, physical fitness, and having a psychiatric diagnosis and/or being on psychiatric medications, all non-significant (p>0.2)
Continuous variables were standardized by dividing by their standard deviation: age SD=7.19; LDL SD=41.3; HDL SD=16.9; GWBS SD=16.9.
BMI=body mass index
CI=confidence interval
GWBS=General Well-Being Schedule
HDL=high density lipoprotein
LDL=low density lipoprotein
SD=standard deviation
To assure that siblings with psychiatric disease history and/or use of psychiatric medications were not unduly influencing the results, survival analysis was also run with these siblings (n=168) removed from the dataset; the hazard and significance for GWBS total scores persisted (HR=0.68, 95% CI 0.58–0.80, p=3.5E-6). Additional sensitivity analyses examining potential interactions found no significant changes in the results. Higher GWBS total scores were consistently protective against incident CAD in both whites (HR=0.67, 95% CI 0.56–0.79, p=4.5E-6) and African Americans (HR=0.65, 95% CI=0.46–0.92, p=0.014), and in both men (HR=0.65, 95%CI=0.53–0.78, p=7.06E-6) and women (HR=0.80, 95% CI=0.62–1.03, p=0.088), with essentially the same protection observed in both races and both sexes. While the CI was wider in women, this was most likely due to fewer observed incident events (140 in men, 68 in women). Sensitivity analysis found no interaction between GWBS and FRS.
Because the possibility remained that siblings with higher baseline positive well-being may have had lower CAD incidence merely due to lower levels of baseline risk factors, we further assessed the relationship between well-being and incident CAD by FRS group. Higher GWBS total scores were protective against incident CAD in all FRS strata (Figure 1), with a nearly 50% reduction in incident CAD risk per standardized GWBS unit in the highest FRS group.
Figure 1.
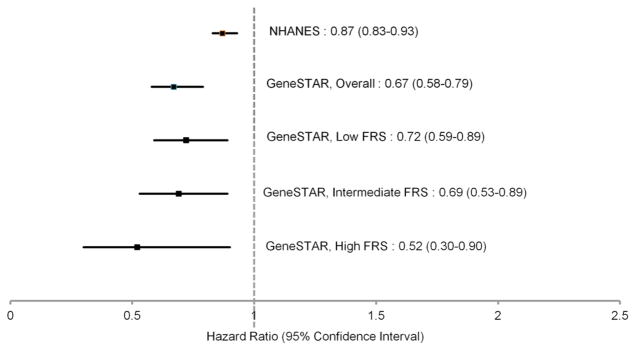
Hazard Ratios and 95% Confidence Intervals for General Well-Being Total Score Predicting Incident Coronary Artery Disease in NHANES and GeneSTAR, Overall and by Framingham Risk Score Group
FRS=Framingham Risk Score
NHANES=National Health and Nutrition Examination Survey
NHANES I baseline characteristics are shown in Table 4. Over an average of 16 ± 6 years of follow-up for 5992 nationally representative participants, 1226 (20.5%) CAD events were observed. Our findings were replicated in NHANES I and the NHEFS, such that higher GWBS total scores were associated with lower risk of incident CAD (Table 5). As we were unable to calculate FRS, we were unable to explore GWBS findings within FRS groups in NHANES I.
Table 4.
Baseline Characteristics of NHANES I by Incident Coronary Artery Disease Event Status (n=5992)
| CAD Event | |||
|---|---|---|---|
|
| |||
| Variable | No (n=4766) | Yes (n=1226) | |
|
| |||
| Mean ± SD | Mean ± SD | P value | |
| Age (years) | 45.9 ± 13.8 | 59 ± 10.6 | <0.0001 |
| Total cholesterol (mmol/L) | 5.7 ± 1.19 | 6.2 ± 1.22 | <0.0001 |
| Body mass index (kg/m2) | 25.3 ± 4.9 | 27.1 ± 5.3 | <0.0001 |
| General Well-Being (total score) | 80.9 ± 17.2 | 78.3 ± 18.6 | <0.0001 |
| Male sex | 2071 (43.5%) | 683 (55.7%) | <0.0001 |
| African American race | 635 (13.3%) | 155 (12.6%) | 0.53 |
| Education, college or higher | 1283 (26.9%) | 197 (16.1%) | <0.0001 |
| Physical activity | <0.001 | ||
| None or little | 1876 (39.4%) | 619 (50.5%) | |
| Moderate | 1979 (41.5%) | 430 (35.1%) | |
| High | 911 (19.1%) | 177 (14.4%) | |
| Currently married | 3688 (77.4%) | 907 (74%) | 0.01 |
| Current smoker | 1806 (37.9%) | 435 (35.5%) | 0.12 |
| Diabetes mellitus | 247 (5.2%) | 149 (12.2%) | <0.0001 |
| Hypertension | 779 (16.3%) | 440 (35.9%) | <0.0001 |
| History of psychiatric disorder | 135 (2.8%) | 50 (4.1%) | 0.03 |
CAD=coronary artery disease
NHANES=National Health and Nutrition Examination Survey
SD=standard deviation
Table 5.
Multivariable Cox Proportional Hazards Model Predicting Incident Coronary Artery Disease in NHANES I (unweighted n= 5992)
| Characteristic | Hazard Ratio (95% CI) | P-value |
|---|---|---|
| Baseline age (years) † | 3.16 (2.91–3.24) | <0.0001 |
| Male sex | 2.02(1.79–2.28) | <0.0001 |
| African American race | 0.75 (0.63–0.89) | 0.001 |
| Hypertension | 1.61 (1.42–1.82) | <0.0001 |
| Total cholesterol (mmol/L) † | 1.15 (1.09–1.20) | <0.0001 |
| Diabetes mellitus | 1.73 (1.45–2.10) | <0.0001 |
| Current smoker | 1.57 (1.39–1.78) | <0.0001 |
| General Well-Being (total score) † | 0.87 (0.83–0.93) | <0.0001 |
| Physical activity | ||
| Moderate vs. none/little | 0.76 (0.67–0.86) | <0.0001 |
| High vs. none/little | 0.77 (0.65–0.91) | 0.004 |
| College or higher education | 0.85 (0.73–0.99) | 0.048 |
| Body mass index (kg/m2) † | 1.04 (1.03–1.05) | <0.0001 |
Adjusted for being married and having a history of psychiatric disease, both non-significant (p>0.25)
Continuous variables were standardized by dividing by their standard deviation: age SD=13.2; total cholesterol SD=1.22; GWBS SD= 17.5; BMI SD=5.1
BMI=body mass index
CI=confidence interval
GWBS=General Well-Being Schedule
SD=standard deviation
Discussion
After an average 12 years of follow-up, positive well-being afforded a large degree of protection against CAD in two markedly different large cohorts, independent of traditional CAD risk factors. Average well-being scores were higher in the general population sample than in the high-risk family cohort, which makes it even more notable to find a protective effect in both groups. Although the extent of protection was smaller in the general population sample than in the high-risk cohort (HR=0.87 vs 0.67), the effect was still statistically significant in both groups.
We observed a consistent protective effect for positive well-being for all levels of Framingham risk: low, intermediate, and high. However, it is notable that an almost 50% reduction in the incidence of CAD, controlling for all major risk factors, occurred in GeneSTAR siblings in the highest FRS category. Also, the intermediate risk sibling group had a 30% reduction in CAD, while the general population had a 13% reduction in CAD risk. This suggests that positive well-being may be particularly important for the highest risk groups. To our knowledge, this is the first study to show that a measure of positive well-being as a trait is so strongly protective against incident CAD in a high-risk healthy population, even in the highest-risk subset with a 10 year CAD risk of > 20%.
Prior studies have assessed different components of positive well-being, including optimism,22 happiness,23 life satisfaction,6 and vitality,4 all of which have been associated with a lower risk of incident cardiovascular disease.5 Only 1 prior study has examined the association of multi-dimensional general well being with incident CAD, and as in our study, a protective effect was reported.3 However, no prior associations with general well-being have been reported in higher risk healthy populations with a family history of disease, where genetic susceptibility as well as exposure to common risk behaviors and risk factors may be particularly potent in the disease causality cascade.
The mechanisms of the protective effect of positive well-being remain unclear. Physiological patterns associated with positive well-being include attenuated stress reactivity,24 better antiinflammatory function,25 and increased vagal activity and parasympathetic control.26 However, many studies of positive traits and biological markers have been cross-sectional, and longitudinal studies have been relatively weak and inconsistent. In general, the relationship between positive well-being and pro-health or cardioprotective biological processes has been less well-studied than negative affective states and their role in the genesis of CAD. It is possible that our observed association between GWBS and CAD is the result of other unmeasured variables; however, we believe that the relationship that we observed demonstrates that the GWB represents those potential unmeasured dimensions well.
The protective effects of positive well-being may be mediated through biology and/or the social environment, and further determined by genetics and gene-environment interactions. Optimism has been associated with physical activity27 and healthy food consumption,28 and life satisfaction has been inversely associated with cigarette smoking,29 suggesting that positive well-being may also be mediated through health behaviors which protect against cardiovascular disease.
Limitations of using two different populations include slightly different measures of smoking, physical activity, education, and lipids between GeneSTAR and NHANES I. However, we would have expected this imprecision to decrease the probability of finding the same effect in both studies, so given our ability to replicate the findings, these differences in measurements are unlikely to have had an impact on the replication. Due to missing blood pressure and HDL data necessary for calculation of FRS in NHANES I, we were unable to show replication for our Framingham risk stratified analysis. Strengths include a well-characterized high-risk population with documented family history of early-onset CAD, well-documented incident CAD events over a long period of follow-up in both populations, and use of a well-validated general well-being instrument.
Acknowledgments
This study was supported in part by grants NR02241, R01 HL49762, and R01 HL59684 from the National Institutes of Health, Bethesda, Maryland, and grant M01 RR00052 from the National Institutes of Health, Bethesda, Maryland, to the Johns Hopkins University School of Medicine General Clinical Research Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferketich AK, Schwartzbaum JA, Frid DJ, Moeschberger ML. Depression as an antecedent to heart disease among women and men in the NHANES I study. National Health and Nutrition Examination Survey. Arch Intern Med. 2000;160:1261–1268. doi: 10.1001/archinte.160.9.1261. [DOI] [PubMed] [Google Scholar]
- 2.Anda R, Williamson D, Jones D, Macera C, Eaker E, Glassman A, Marks J. Depressed affect, hopelessness, and the risk of ischemic heart disease in a cohort of U.S. adults. Epidemiology. 1993;4:285–294. doi: 10.1097/00001648-199307000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Boehm JK, Peterson C, Kivimaki M, Kubzansky L. A prospective study of positive psychological well-being and coronary heart disease. Health Psychol. 2011;30:259–267. doi: 10.1037/a0023124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kubzansky LD, Thurston RC. Emotional vitality and incident coronary heart disease: Benefits of healthy psychological functioning. Arch Gen Psychiatry. 2007;64:1393–1401. doi: 10.1001/archpsyc.64.12.1393. [DOI] [PubMed] [Google Scholar]
- 5.Davidson KW, Mostofsky E, Whang W. Don’t worry, be happy: Positive affect and reduced 10-year incident coronary heart disease: The Canadian Nova Scotia Health Survey. Eur Heart J. 2010;31:1065–1070. doi: 10.1093/eurheartj/ehp603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boehm JK, Peterson C, Kivimaki M, Kubzansky LD. Heart health when life is satisfying: Evidence from the Whitehall II cohort study. Eur Heart J. 2011;32:2672–2677. doi: 10.1093/eurheartj/ehr203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boehm JK, Kubzansky LD. The heart’s content: The association between positive psychological well-being and cardiovascular health. Psychol Bull. 2012;138:655–691. doi: 10.1037/a0027448. [DOI] [PubMed] [Google Scholar]
- 8.Huppert FA, So TT. Flourishing across Europe: Application of a new conceptual framework for defining well-being. Soc Indic Res. 2013;110:837–861. doi: 10.1007/s11205-011-9966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costa PT, Jr, McCrae RR, Zonderman AB. Environmental and dispositional influences on well-being: Longitudinal follow-up of an American national sample. Br J Psychol. 1987;78(Pt 3):299–306. doi: 10.1111/j.2044-8295.1987.tb02248.x. [DOI] [PubMed] [Google Scholar]
- 10.Pressman SD, Cohen S. Does positive affect influence health? Psychol Bull. 2005;131:925–971. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- 11.Vaidya D, Yanek LR, Moy TF, Pearson TA, Becker LC, Becker DM. Incidence of coronary artery disease in siblings of patients with premature coronary artery disease: 10 years of follow-up. Am J Cardiol. 2007;100:1410–1415. doi: 10.1016/j.amjcard.2007.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Arlington, VA: American Psychiatric Association; 2000. [Google Scholar]
- 13.Chen Y, Zhang X, Pan B, Jin X, Yao H, Chen B, Zou Y, Ge J, Chen H. A modified formula for calculating low-density lipoprotein cholesterol values. Lipids Health Dis. 2010;9:52. doi: 10.1186/1476-511X-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The 1980 Report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med. 1980;140:1280–1285. [PubMed] [Google Scholar]
- 15.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 16.National Cholesterol Education Program. Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002;106:3143. [PubMed] [Google Scholar]
- 17.Fazio AF. A concurrent validational study of the NCHS’ General Well-Being Schedule. National Center for Health Statistics; Hyattsville, MD: 1977. Report 78–1347. [PubMed] [Google Scholar]
- 18.National Center for Health Statistics. First National Health and Nutrition Examination Survey (NHANES I) Hyattsville, MD: [Last accessed 5/2/2013]. http://www.cdc.gov/nchs/nhanes/nhanesi.htm. [Google Scholar]
- 19.Wells KB, Manning WG, Jr, Valdez RB. The effects of insurance generosity on the psychological distress and psychological well-being of a general population. Arch Gen Psychiatry. 1989;46:315–320. doi: 10.1001/archpsyc.1989.01810040021004. [DOI] [PubMed] [Google Scholar]
- 20.Taylor JE, Poston WS, 2nd, Haddock CK, Blackburn GL, Heber D, Heymsfield SB, Foreyt JP. Psychometric characteristics of the General Well-Being Schedule (GWB) with African-American women. Qual Life Res. 2003;12:31–39. doi: 10.1023/a:1022052804109. [DOI] [PubMed] [Google Scholar]
- 21.National Center for Health Statistics. NHANES I Epidemiologic Followup Study. Hyatsville, MD: [Last accessed 5/2/2013]. http://www.cdc.gov/nchs/nhanes/nhefs/nhefs.htm. [Google Scholar]
- 22.Tindle HA, Chang YF, Kuller LH, Manson JE, Robinson JG, Rosal MC, Siegle GJ, Matthews KA. Optimism, cynical hostility, and incident coronary heart disease and mortality in the Women’s Health Initiative. Circulation. 2009;120:656–662. doi: 10.1161/CIRCULATIONAHA.108.827642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shirai K, Iso H, Ohira T, Ikeda A, Noda H, Honjo K, Inoue M, Tsugane S. Perceived level of life enjoyment and risks of cardiovascular disease incidence and mortality: The Japan public health center-based study. Circulation. 2009;120:956–963. doi: 10.1161/CIRCULATIONAHA.108.834176. [DOI] [PubMed] [Google Scholar]
- 24.Papousek I, Nauschnegg K, Paechter M, Lackner HK, Goswami N, Schulter G. Trait and state positive affect and cardiovascular recovery from experimental academic stress. Biol Psychol. 2010;83:108–115. doi: 10.1016/j.biopsycho.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda A, Schwartz J, Peters JL, Fang S, Spiro A, 3rd, Sparrow D, Vokonas P, Kubzansky LD. Optimism in relation to inflammation and endothelial dysfunction in older men: The VA Normative Aging Study. Psychosom Med. 2011;73:664–671. doi: 10.1097/PSY.0b013e3182312497. [DOI] [PubMed] [Google Scholar]
- 26.Bhattacharyya MR, Whitehead DL, Rakhit R, Steptoe A. Depressed mood, positive affect, and heart rate variability in patients with suspected coronary artery disease. Psychosom Med. 2008;70:1020–1027. doi: 10.1097/PSY.0b013e318189afcc. [DOI] [PubMed] [Google Scholar]
- 27.Giltay EJ, Geleijnse JM, Zitman FG, Buijsse B, Kromhout D. Lifestyle and dietary correlates of dispositional optimism in men: The Zutphen Elderly Study. J Psychosom Res. 2007;63:483–490. doi: 10.1016/j.jpsychores.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Kelloniemi H, Ek E, Laitinen J. Optimism, dietary habits, body mass index and smoking among young Finnish adults. Appetite. 2005;45:169–176. doi: 10.1016/j.appet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Strine TW, Chapman DP, Balluz LS, Moriarty DG, Mokdad AH. The associations between life satisfaction and health-related quality of life, chronic illness, and health behaviors among U.S. community-dwelling adults. J Community Health. 2008;33:40–50. doi: 10.1007/s10900-007-9066-4. [DOI] [PubMed] [Google Scholar]


