INTRODUCTION
Invasive anal squamous cell cancer (IAC) is a health crisis for gay, bisexual, and other men who have sex with men (MSM), and for male-to-female transgendered women who have had sex with men (TWSM), especially within the context of HIV-coinfection where risk for invasive malignancy is greatest (1-6). Currently, experts recommend using the Dacron swab for anal cytology (Pap test) specimen collection at annual and semi-annual intervals for HIV-infected and -uninfected MSM, respectively (7, 8). Dacron-swab anal cytology poorly correlates with histological evaluation of disease (9) and while cervical-cytology sampling has informed anal-cytology specimen collection, cervical screening tools may not be appropriate for analcancer screening. For example, cytobrushes with and without spatulas, cytopicks; and cotton, Dacron, rayon, and nylon-flocked (NF)-swab have been evaluated in genital sampling for pathogens, including HPV, and for cervical cytology (10-14). Cytobrushes improved cervical sampling over cotton swabs by 58-76% for detecting endocervical cells in a sample of >800 adult females >29 years of age (14) and are shown superior to Ayers spatulas (10). One report suggests the cytobrush may be effective in anal cytology,(15) but may be uncomfortable in blind sampling (16). The NF-swab is evaluated extensively for respiratory-pathogen and -cytology sampling, yielding more cells and pathogens than other collection protocols (17-20). For example, Daley et al. report >2-fold cell yield of respiratory epithelial cells using NF-over Dacron swabs, and Krech et al. report a 5-fold greater yield of Chlamydia trachomatis, as well as greater HPV yield using NF-over rayon-wrapped swabs in cervicovaginal tissues (17, 21). Further, unsatisfactory cervical cytology findings range from 0.3-10.9% and 0.17-2.7% for specimens preserved using PreservCyt® (Hologic, Inc., Marlborough, Massachusetts) and SurePath™ (TriPath Imaging, Inc., Burlington, N.C.) preservatives, respectively (22-25), whereas the prevalence of unsatisfactory anal cytology specimens may range from 1% to upwards of 14% (26-33).
The cervix and the anal canal are distinct anatomical targets that might easily require different sampling tools. The cervix is firm and sampling is more akin to hitting the bulls-eye on a target while the anal canal is soft and folds much like the surface of a deflated balloon. Cervical cytology uses a speculum to visually guide sample collection and the transformation zone (TZ) of the cervix closely approximates the cervical os. The dentate line lies approximately ~5 centimeters proximal to the anal verge, and the anal TZ immediately adjoins it about ~0.5-1 cm, just cephalad (34). Current anal cytology recommendations are to blindly insert a Dacron swab through the anal verge ~5 cm, approximate it to the anal wall, and rotate the swab using lateral pressure to sample the canal circumferentially as it is withdrawn over 10-20 seconds and stored in liquid preservative for laboratory examination (35).
Although experts suggest screening in high-risk populations is important and data show it is cost-effective, there is no current national consensus for screening methods or frequency for anal cancer screening using Papanicolaou staining (Pap test) (8, 36-38). To date, no large studies evaluate the risks or benefits of early detection and treatment for preventing anal cancer and general reluctance among clinicians for anal cytology screening may be due to the imprecision of the test, the rarity of malignancy, and the limited success of available treatments, especially within the context of HIV infection where there is poor control over HPV infections (39-41). Developing a screening strategy with modest-to-high sensitivity and specificity for anal precancers, high-grade anal intraepithelial neoplasia (HG-AIN), is an important public health goal. Thus, to evaluate the sensitivity and specificity for two cytology collection procedures and compare their efficacy for predicting HG-AIN, we evaluated a protocol using an NF-swab (Copan Diagnostics Inc., Murrieta, CA) and the Dacron swab (Thermo Fisher Scientific, Miami, OK), each with preservative, for anal cytology.
MATERIALS AND METHODS
Subjects and Sampling
Fifty-eight adult MSM provided written informed consent for an IRB-approved study (University of California, Los Angeles, Medical IRB2 #11-000668) and all were enrolled in 1 of 4 Multicenter AIDS Cohort Study groups. Prior to examination, examiners collected a self-reported history for HIV infection, AIDS-defining conditions, anal treatments, and presence of recent anal bleeding, discharge, or pain. Men lay in the left lateral position for the exam, with legs flexed, and the anogenital region exposed. External genitalia were examined.
Cytology specimens
were collected in order so that the swab procedures could be compared without biasing cytology findings for specimens collected using Dacron swab (42). Briefly, a Dacron swab was lightly moistened with water, inserted blindly beyond the anal verge ~5 cm, firmly approximated to the anal wall and circularly rotated while being withdrawn over ~30 seconds; thereafter, the swab was deposited into PreservCyt® (Dacron-protocol). Early experience showed the NF swab had a larger diameter that was difficult to pass through the verge unaided. Accordingly, the protocol was amended to use a disposable anoscope (CooperSurgical, Inc., Trumbull, CT) with water-soluble lubricant lightly applied to the leading edge, allowing for comfortable passage of the instrument. Once inserted just beyond the verge, the obturator was removed, and the internal aspect of the anoscope was cleared of residual lubricant using a dry Scopette swab (Owens & Minor, Mechanicsville, VA). Thereafter, an NF-swab passed through the anoscope was approximated to the anal wall ~5 cm beyond the verge close to the anal dentate line, circularly rotated, using lateral pressure during withdrawal, over 20-30 seconds using a standard procedure (35). The NF-swab specimen was placed into SurePath™ preservative and the anoscope was removed (NF-protocol). For both swab protocols, swabs were agitated several minutes in the specimen containers to dislodge the collected material.
Following cytology specimen collection
high-resolution anoscopy (HRA) was performed. First, a 4X4-gauze-padded swab soaked with 3-5% acetic acid was passed through an anoscope, the anoscope was withdrawn, and the gauze was left in place one minute before being withdrawn. The anoscope was reintroduced using water-soluble lubricant, and the anal canal was examined using a colposcope for magnification and bright light. Biopsy was performed where acetowhite lesions showed punctation, friability, or highly vascularized appearances. Where hemorrhoids were significant and obstructed the ability of the examiner to evaluate the tissue, a 2% lidocaine/1:100,000 epinephrine solution was distributed evenly, with <0.5cc in each of four quadrants, using a 25-30 gauge needle. Biopsies were performed using Tischler (Sklar, West Chester, PA) or endoscopic (Pentax Corporation, Montvale, NJ) forceps and hemostasis was achieved using Monsel’s solution (ferric subsulfate; CooperSurgical, Inc., Trumbull, CT) as needed.
Board-certified cyto- and histo-pathologists
in one CLIA-certified laboratory used standard procedures to evaluate cytology and biopsy specimens, blinded to clinical examination findings. The Bethesda Classification System (43, 44) and the International Classification of Diseases for Oncology (45) were used to evaluate cytology and histology specimens, respectively. Cytology specimens were classified as negative for intra-epithelial lesion (NIL); atypical squamous cells, either of unknown significance (ASC-US) or favoring high-grade dysplasia (ASC-H); or low- or high-grade squamous intraepithelial lesions (LSIL and HSIL, respectively). PreservCyt®/Dacron-swab specimens showing fewer than 1-2 nucleated squames/high-power field (hpf) and SurePath™/NF-swab specimens showing fewer than 3-6 nucleated squames/hpf were evaluated as insufficient and reported as unsatisfactory (46). Histology was classified as negative for specimens showing keratosis, and benign hyperplasia, acute inflammation or reactive changes in the absence of dysplasia; low-grade anal intraepithelial neoplasia or mild dysplasia (LG-AIN); and HG-AIN for AIN 2/moderate dysplasia, AIN 2- 3/moderate-to-severe dysplasia, or AIN 3/severe dysplasia. For these analyses, the histology outcome is the most severe of findings where multiple biopsy specimens were evaluated. The sensitivity and specificity for cytology to predict HG-AIN was evaluated first excluding unsatisfactory cytology and then classified.
Sexual, behavioral, and other laboratory data
are part of the Multicenter AIDS Cohort Study repository, and in the case of one subject, by self-report. Demographic, health and illness events, and laboratory data, including HIV infection characteristics, are collected semi-annually using standardized instruments. However, to control for the effects of immune and disease characteristics, adjusted analyses were limited to HIV-infection (versus not) and chronological age at the time of the examination, evaluated as a continuous variable.
Statistical Analysis
Descriptive and tabular analyses evaluated associations between the two protocols and cytology findings to predict risk for HG-AIN. Kappa statistics, sensitivity and specificity were estimated using SAS PROC FREQ (47). To evaluate sensitivity and specificity, cytology specimens showing ASC-US, ASC-H, LSIL or HSIL (≥ASC-US) were compared to findings of NIL, excluding unsatisfactory cytology from the analyses. A series of logistic regression models, using SAS PROC LOGISTIC, (48) examined the crude and adjusted odds of HG-AIN for NF- and Dacron-protocols individually, controlling for the effects of HIV-infection and age. Last, receiver operating characteristic (ROC) curves and the corresponding area under each curve (AUCROC) were estimated for logistic regression analyses to evaluate the accuracy of Dacron- and NF-protocol cytology methods to predict HG-AIN, adjusting for the effect of age and HIV-infection (49). The AUCROC globally evaluates test accuracy, estimating the mean specificity across the range of possible sensitivity estimates for the models (49). Model fits were evaluated using the deviance statistic and were rejected or not using a 0.05 level of significance.
RESULTS
The study sample is best described as white, HIV-infected, and older MSM (Table 1). Most participants were Caucasian (85%, 49/58), 5% (3/58) were Black, and 10% (6/58) reported “other” race groupings; 5% (3/58) reported Hispanic ethnicity. The mean age was 57.9 (+6.6) years, ranging 39.5-72 years, not varying significantly for HIV-infected/-uninfected men. Overall, 36% (21/58) showed HG-AIN by HRA and biopsy, and although estimates did not reach statistical significance, the prevalence of HG-AIN was 62% higher among HIV-infected than uninfected men (Table 1). Older and younger men were equally likely to show HG-AIN (OR=0.9, p=0.2). For 40 of 42 HIV-infected men, infection duration could be estimated, and the average duration was 25.7 (+8.37) years, including estimated values for prevalent positive men (50). Among HIV-infected men, most reported no prior AIDS-defining conditions (79%, 33/40), and for the 36 whose data were complete, CD4+ count showed a downward trend before combined antiretroviral therapy (CART): μ=−340.9 (+/−208.8) cells/mm3/year; however, following CART, CD4+ cell counts trended positively, increasing by 284.7 (+/−171.1) cells/mm3/year.
Table 1.
Sociodemographic (n=58) and HIV-infection Characteristics of Study Participants Evaluating NF- and Dacron-swab Anal Cytology Specimen Procedures
| Seropositives N (%) |
Seronegatives N (%) |
|
|---|---|---|
| Total | 42 (72) | 16 (28) |
| Race a | ||
| White | 36 (86) | 13 (81) |
| Black | 3 ( 7) | 0 |
| Other | 3 ( 7) | 3 (19) |
| Hispanic ethnicity | 3 ( 8) | 0 |
| Biopsy a | ||
| NIL | 18 (42.9) | 7 (43.8) |
| LG-AIN | 7 (16.7) | 5 (31.3) |
| HG-AIN | 17 (40.5) | 4 (25.0) |
| Mean (SD) | Mean (SD) | |
| Age, yearsa | 56.99 (5.7) | 60.41 (8.2) |
| Range | 41.2-66.1 | 39.5-72.0 |
| HIV Infection Characteristics | ||
| Years Duration: HIV infection | 25.7 (8.4) | - |
| Range | 0.5 – 34.0 | - |
| Nadir CD4+ before HAARTb,c | 340.9 (208.8) | - |
| Range | 1.0-819.0 | - |
| Slope of CD4+ Counts before HAART | −30.84 (52.06) | - |
| Nadir CD4+ after HAARTb,c | 284.7 (171.1) | - |
| Range | 10.0-835.0 | - |
| Slope of CD4+ Counts after HAART | 27.66 (64.77) | - |
| HIV-RNA peak after HAARTc | 4.9 (0.7) | - |
| HIV-RNA Set-Point before HAARTc | 3.8 (0.8) | - |
| AIDS-Diagnoses | ||
| None | 33 (79) | 16 (100) |
| Kaposi’s Sarcoma | 1 ( 2) | - |
| Pneumocystis carinii Pneumonia | 2 ( 4) | - |
| Cryptosporidiosis | 1 ( 2) | - |
| Non-Hodgkin’s Lymphoma | 1 ( 2) | - |
| Candida Esophagitis | 1 ( 2) | - |
| Wasting Syndrome | 1 ( 2) | - |
| Pulmonary Tuberculosis | 1 ( 2) | - |
| Multiple AIDS Diagnoses | 1 ( 2) | - |
Non-significant (NS) between HIV groups
T-lymphocytes/mm3
log10 transformation
The prevalence of unsatisfactory cytology specimens
was 15% (9/58), where one or both specimens were independently evaluated as insufficient. Of these, 33% (3/9) showed unsatisfactory results for both specimens. Half as many NF- as Dacron-protocol cytology specimens were evaluated as unsatisfactory by experts: 7% (4/58) versus 14% (8/58).
Agreement between NF- and Dacron protocol cytology
findings was poor to modestly poor (Table 2). Including those evaluated as unsatisfactory, 23 of 58 specimens agreed using either protocol procedure. The simple Kappa statistic, including 3 of 9 unsatisfactory specimens that were so classified by both procedures, was 16% (-1-33%). When values for unsatisfactory specimens were omitted from the analysis, agreement shifted somewhat; the simple and weighted Kappa statistics were 11% (-8-30%) and 24% (3-46%), respectively.
Table 2.
Comparison of Dacron- and NF-Swab Cytology Specimen Cytology Results for 58 MSM
| Dacron-swab Unsatisfactory |
NIL | ASC-US | ASC-H | LG-SIL | HG-SIL | Total | ||
|---|---|---|---|---|---|---|---|---|
| NF- swab |
Unsatisfactory | 3 | 0 | 1 | 0 | 0 | 0 | 4 |
| NIL | 4 | 13 | 7 | 0 | 0 | 1 | 25 | |
| ASC-US | 0 | 8 | 5 | 0 | 1 | 0 | 14 | |
| ASC-H | 0 | 0 | 2 | 0 | 0 | 0 | 2 | |
| LG-SIL | 1 | 4 | 1 | 1 | 1 | 1 | 9 | |
| HG-SIL | 0 | 1 | 0 | 1 | 1 | 1 | 4 | |
| Total | 8 | 26 | 16 | 2 | 3 | 3 | 58 |
Simple Kappa = 0.11 (−0.08, 0.30), and Weighted Kappa = 0.24 (0.03, 0.46). Including unsatisfactory cytology: Kappa = 0.16 (−0.01, 0.33)
Sensitivity and specificity
NF-protocol cytology showed 56% greater sensitivity for detecting HG-AIN and 26% greater specificity than did Dacron-protocol cytology (Table 3). Specifically, sensitivity and specificity for NF-protocol cytology ≥ASC-US was 81% (58-95%) and 73% (50-89%), while Dacron protocol specimens showed 52% (30-74%) and 58% (34-80%) sensitivity and specificity, respectively.
Table 3.
Comparison of Dacron- and NF-Protocol Cytology to Histology for 58 MSM Evaluated Using High-Resolution Anoscopy and Biopsy for Histology
| Histology | Histology | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dacron–Swab Cytology |
Normal (Col %) |
LG-AIN (Col %) |
HG-AIN (Col %) |
Total (Col %) |
NF-Swab Cytology |
Normal (Col %) |
LG-AIN (Col %) |
HG-AIN (Col %) |
Total (Col %) |
| Unsatisfactory | 6(24) | 2(17) | 0 | 8 (14) | Unsatisfactory | 3 (12) | 1 (8) | 0 | 4 (7) |
| NIL | 11(44) | 5(42) | 10(48) | 26 (45) | NIL | 16 (64) | 5(42) | 4 (19) | 25 (43) |
| ASC-US | 7(28) | 5(42) | 4(19) | 16 (28) | ASC-US | 4 (16) | 1 (8) | 9 (43) | 14 (24) |
| ASC-H | 0 | 0 | 2 (10) | 2 ( 3) | ASC-H | 0 | 2 (17) | 0 | 2 (3) |
| LG-SIL | 1(4) | 0 | 2(10) | 3 ( 5) | LG-SIL | 2 (8) | 3 (25) | 4 (19) | 9 (16) |
| HG-SIL | 0 | 0 | 3(14) | 3 ( 5) | HG-SIL | 0 | 0 | 4 (19) | 4 (7) |
| Total (Row %) | 25(43) | 12(21) | 21(36) | 58(100) | Total (Row %) | 25 (43) | 12 (21) | 21 (36) | 58(100) |
Sensitivity: 52% (Exact 95% Confidence Interval (CI): 30-74%). Specificity: 58% (CI: 34-80%)
Sensitivity: 81% (CI: 58-95%). Specificity: 73% (CI: 50-89%)
Cytology using NF-protocol better predicted HG-AIN on histology than did the Dacron protocol cytology
Overall, 81% (17/21) of men with ≥ASC-US cytology on NF-protocol showed histological evidence of HG-AIN, while 52% (11/21) of Dacron-protocol cytology specimens tested similarly positive (Table 3). Put another way, findings from NF-protocol cytology would result in 50% (29/58) of men evaluated being referred for diagnostic follow-up as compared to 41% (24/58) of men showing ≥ASC-US using Dacron protocol. However, among those that would be referred for diagnostic follow-up, a higher proportion of HG-AIN-affected men would be detected using the NF-protocol procedure over Dacron: 59% (17/29) versus 46% (11/24), respectively. Conservatively, Dacron-protocol cytology misclassified men more often as unaffected when compared to NF-protocol findings, i.e., cytology showed NIL and histology showed LG- or HG-AIN: 58% (15/26) vs. 36% (9/25), respectively. HIV-infection and age alone did not predict HG-AIN well in this small sample using either Dacron or NF-protocol (Table 4, Unadjusted Analyses). For example, in unadjusted analyses, HIV-infected men showed 10% greater odds of HG-AIN than did uninfected men, and each additional year in age increased the odds of HG-AIN 10% (OR=1.1 and OR=1.1, respectively, p-values>0.05, Table 4).
Table 4.
Two Multivariate Analyses Evaluating Dacron- and NF-swab Procedures for Predicting HG-AIN on Histology for 58 MSM
| Unadjusted Analyses (95% Confidence Interval) |
Adjusted Analyses a (95% Confidence Interval) |
|||
|---|---|---|---|---|
| Cytology | ||||
| Dacron Swabb | ||||
| NIL | 1 | 1 | ||
| ≥ASCUS | 2.0 | (1.0, 4.0) | 2.0 | (0.9, 4.2) |
|
| ||||
| Nylon-Flocked Swabb | ||||
| NIL | 1 | 1 | ||
| ≥ASCUS | 2.7 | (1.4, 5.3)b | 3.0 | (1.5, 6.2)c |
Adjusting for the effect of age and HIV infection (infected vs. not).
8 unsatisfactory Dacron-protocol cytology, n=50; and 4 unsatisfactory NF-protocol cytology, n=54.
p<0.05
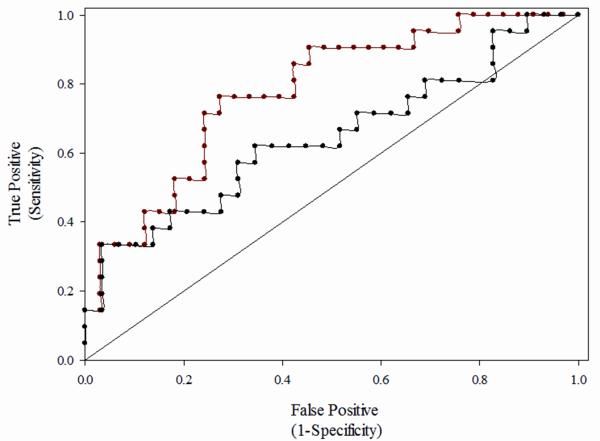
The multivariate analyses suggests that after controlling for the effects of age and HIV infection, men who show ≥ASC-US cytology using the NF-protocol have 3-fold greater odds for HG-AIN over men showing NIL (adjusted OR=3.0 (1.5, 6.2), Table 4). However, men showing ≥ASC-US using the Dacron-procedure showed no statistically significantly greater odds of HG-AIN than men showing NIL on (Dacron) cytology (adjusted OR=2.0 (1.0, 4.0), Table 4). In both adjusted analyses, age and HIV-infection did not independently predict HG-AIN. Last, the AUCROC analysis suggested the accuracy of NF-cytology showing ≥ASC-US to predict HG-AIN was 21% greater than was Dacron cytology showing similar findings: C-statistics 0.776 vs. 0.643, respectively (Fig. 1).
Figure 1.
Comparison of Receiver Operating Characteristic (ROC) Curves for Anal Cytology Showing > Atypical Squamous Cells of Unknown Significance (ASC-US) Using Dacron- (black) and NF-swab (red) Protocols to Predict High Grade Anal Intraepithelial Neoplasia, Adjusted for the Effect of Age and HIV-infection Among 58 MSM (C=0.643 and C= 0.776, respectively).
DISCUSSION
Analyses showed HG-AIN was commonly detected among HIV-infected and –uninfected MSM screened using two anal cancer screening protocols. Currently, a blindly-passed Dacron swab is customary for anal cytology specimen collection (35, 51). However, sensitivity and specificity for detecting HG-AIN were higher for NF- than Dacron-protocol specimens: 81% vs. 52%, albeit confidence intervals overlapped. Sensitivity and specificity of Dacron cytology were within published ranges: 42-98% and 32-96%, respectively (9, 28, 44, 52-56). Comparatively, sensitivity and specificity of cervical cytology for detecting high-grade cervical intraepithelial neoplasia ranges 11-99% and 14-97%, respectively (57). Experts currently recommend HRA and biopsy for anal cytology > ASC-US (27, 58). Poor specificity, even in high-risk populations, makes the predictive value of positive cytology poor and escalates healthcare costs associated with unnecessary diagnostic follow-up. Nonetheless, even relatively costly strategies can improve screening-test specificity enough to provide significant overall cost-savings. For example, the addition of molecular HPV testing or repeat cytology improves the test performance of cervical cytology two-fold when compared to immediate colposcopy referral for ASC-US cytology (59). Future efforts are best directed toward developing adjunctive or alternative screening strategies that improve specificity for anal cytology, so as to assure that additional diagnostic follow-up and healthcare costs are offset by improved performance of screening tests.
This study compares two instruments and procedures for cytology collection. The NF-procedure described herein uses a small plastic anoscope to open the verge for comfortable passage of the NF-swab with larger surface area. We found no statistically significantly greater odds of HG-AIN for men showing ≥ASC-US on Dacron cytology, but found a 3-fold greater odds of HG-AIN for NF-cytology ≥ASC-US when compared to otherwise similar men showing NIL. Nonetheless, our sample population is small, the Dacron-procedure was systematically collected first, and some findings vary from published works. For example, Vajdic et al. report first-collected and blindly passed Dacron anal cytology was superior to anoscope-guided and second-collected Dacron specimens in a sample of 151 MSM, 63% of whom were HIV-infected (29). Conversely, Gage et al. reported no significant difference in the mean number of detectable anal epithelial cells using an endogenous retrovirus biomarker, when first-collected NF- and Dacron anal cytology specimens were compared (60). However, authors showed higher (log) cell counts for second-drawn NF- (over Dacron-swab) specimens and in the overall comparison: μ=8.1 vs. 7.1 (p=0.03) and 8.3 vs. 7.8 (p=0.03), respectively (60). Herein, the NF-protocol more precisely predicted HG-AIN and yielded half as many unsatisfactory cytology specimens despite that Dacron cytology sampling was performed first.
Few head-to-head trials have been performed and a large number of published anal cytology studies employ Dacron protocol, with few using cytobrush or other specimen collection instruments (9, 53, 55, 61-63). Since ~1950, comparative studies informed cervical cytology specimen collection and improved care, showing cervical cytobrush to be superior to swab and spatula specimen collection methods (64-70). Our experience and that of experts suggests cervical cytobrushes cause discomfort, making them ill-advised for care (16, 46). NF-swabs are designed to collect more cells and pathogens over the conventional Dacron swabs; however, PreservCyt® and SurePath™ preservatives may have differentially affected the number of insufficient cytology specimens. One large cervical cytology study showed fewer unsatisfactory cytology results attributable to SurePath™ over PreservCyt®, and that by reprocessing using SurePath™ converted 60% of insufficient specimens to satisfactory for interpretation, suggesting some differences herein may be due to specimen processing alone (22). Additionally, data show water-based lubricants are associated with higher rates of unsatisfactory cytology specimens and, in one randomized dose-response controlled trial, showed an inverse association for one water-soluble lubricant (0.1-0.5g/vial) and cell counts/field using PreservCyt® specimens and an overall lower number of cells per field using a second (42). However, one trial showed no association between cytology findings and (0.5 mL) water-based lubricant added randomly to 1 of 2 paired PreservCyt® specimens (n=200); for ~8% discordant specimens, 8/15 showed cytology less severe in the lubricant-contaminated specimens, 5/15 showed vice versa, and 2/15 showed unsatisfactory results in one (71).
Additional, albeit modest, costs of NF-protocol sampling increase individual cytology costs. The NF-swab and anoscope add ~$3.00 to the examination expense. However, some data show NF-swabs significantly improve the cell yield, while collecting comparable levels of nucleic acids, when compared to rayon swabs for respiratory sampling (17, 18). While an anoscope may be less comfortable than blind sampling with Dacron swab, men in this study voiced no complaints about the procedure. Thus, balancing cost, comfort, and yield of HG-AIN is an important public health goal. Herein, the NF-procedure netted a 31% greater yield of HG-AIN than Dacron-protocol but would result in 22% higher referral for diagnostic follow-up using current guidelines of ≥ASCUS (30).
Few studies have evaluated anal cytology in older men. Our analyses showed age had little effect on risk for HG-AIN. Nonetheless, population data show men over 60 are at higher risk than younger men for invasive anal cancer,(3, 72) and that invasive malignancy may show slightly earlier onset among HIV-infected MSM than -uninfected men. For example, among the 219 anal cancer cases reported to the HIV/AIDS Cancer Match Study (N=263,254), the median age at diagnosis was ~3 years younger than expected: 42 versus 45 years (73).
A small sample size may limit these analyses and self-report data may contribute to misclassification of some characteristics. Systematic sampling may have introduced some bias; however, the ordered collection and choice of preservatives was based on randomized control trial findings that show water-soluble lubricants produce unpredictable artifacts and lower cell counts per field in PreservCyt®-preserved cytology specimens that are not seen with SurePath™(42, 71, 74). However, Dacron swab collection into PreservCyt® is the current standard for anal Pap test against which the NF-swab collection and SurePath™ were compared (35). Also, Scopette swabs removed lubricant from the internal wall of the anoscope, were not used to swab anal tissues, and facilitated unimpeded passage of the NF-swab. Albeit infrequent, some studies show wiping increases drying and crush artifact and more frequently noted limited cellularity by pathologist in cervical sampling (75, 76). Also, there may be residual confounding that is not controlled for solely by adjusting for age and HIV-infection in the multivariate analyses (77). Differences in unsatisfactory cytology specimens we measured cannot be attributed solely to the swab collection device. One meta-analysis of >2-million cervical cytology specimens analyzed in 42 studies shows the prevalence of unsatisfactory cytology was 0.3% and 1.3% for SurePath™ and PreservCyt® specimens, respectively, and three head-to-head comparisons show SurePath™ yields fewer unsatisfactory test results than does PreservCyt® (25). Last, samples were collected systematically, not randomly, as even small amounts of water-soluble lubricant might have irreparably disadvantaged the Dacron-collection procedure. Reports show first-collected Dacron cytology specimens more often detect HG-AIN over second-collected swabs, where specimens are discordant (p<0.001) (29). Thus, if biased, our findings may conservatively estimate of the sensitivity and specificity of the NF-swab procedure.
Future research should focus on improving anal cancer screening specificity to lower overall costs and improve the predictive value of positive screening tests. For example, molecular HPV testing to triage ASC-US cervical cytology for diagnostic colposcopy has significantly reduced the overall cost of mass cervical cancer screening and improved detection of HG-disease (78). Finding similar effective adjunctive tests for anal cytology holds promise of improving care for individuals and minimizing healthcare expenditures.
Acknowledgements
We thank all Multicenter AIDS Cohort Study participants and investigators. We also thank Zoe Masongsong and Tristan Grogan for assistance in the preparation of this manuscript.
Sources of Funding:
Grant support for this project was provided by the UCLA Center for AIDS Research, 5P30AI028697, and the National Institutes of Health, National Cancer Institute, Cancer Center Support Grant, UCLA Jonsson Comprehensive Cancer Center, 5P30CA016042. Support for this study and demographic data in this manuscript were collected by the Multicenter AIDS Cohort Study with centers (Principal Investigators) located at: The Johns Hopkins Bloomberg School of Public Health (Joseph Margolick); Howard Brown Health Center and Northwestern University Medical School (John Phair, Steven Wolinsky); University of California, Los Angeles (Roger Detels, Otoniel Martinez-Maza); University of Pittsburgh (Charles Rinaldo); and Data Analysis Center (Lisa Jacobson). The Multicenter AIDS Cohort Study is funded by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute; and the National Heart, Lung, and Blood Institute: UO1-AI-35042, UM1-AI-35043, UO1-AI-35039, UO1-AI-35040, and UO1-AI-35041.
Nylon-flocked swab materials supporting for this project were received from Copan Diagnostics Inc. (Murrieta, CA) a branch of Copan Italia S.p.a. (Via Perotti, 10; 25125 Brescia Italy).
Footnotes
Conflicts of Interest:
Dr. Wiley has received grants and payment for lectures including service on speakers bureaus from Merck & Co., Inc. Dr. Young is as an Advisory Board Member for Roche Molecular Systems and Quidel Corporation. None were declared for the remaining authors.
Portions of the analysis were presented at: (1) 13th International Conference on Malignancies in AIDS and other Acquired Immunodeficiencies (ICMAOI). Bethesda, MD USA. 7-8 November 2011. P49: “Nylon-flocked swab collection method better predicts high-grade AIN than does Dacron swab method” (2) 2012 Western Institute of Nursing (WIN) Annual Communicating Nursing Research Conference, Portland, OR, April 18–21, 2012. “Directed anal Pap using flocked swab better predicts high-grade AIN than Dacron swab.”
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frisch M, Smith E, Grulich A, Johansen C. Cancer in a population-based cohort of men and women in registered homosexual partnerships. Am J Epidemiol. 2003 Jun 1;157(11):966–72. doi: 10.1093/aje/kwg067. [DOI] [PubMed] [Google Scholar]
- 2.Frisch M, Glimelius B, van den Brule AJ, Wohlfahrt J, Meijer CJ, Walboomers JM, et al. Sexually transmitted infection as a cause of anal cancer. N Engl J Med. 1997;337(19):1350–8. doi: 10.1056/NEJM199711063371904. [DOI] [PubMed] [Google Scholar]
- 3.Frisch M, Melbye M, Moller H. Trends in incidence of anal cancer in Denmark. BMJ. 1993;306(6875):419–22. doi: 10.1136/bmj.306.6875.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clifford GM, Polesel J, Rickenbach M, Dal Maso L, Keiser O, Kofler A, et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst. 2005 Mar 16;97(6):425–32. doi: 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 5.Seaberg EC, Wiley D, Martinez-Maza O, Chmiel JS, Kingsley L, Tang Y, et al. Cancer incidence in the Multicenter AIDS Cohort Study before and during the HAART era: 1984 to 2007. Cancer. 2010 Dec 1;116(23):5507–16. doi: 10.1002/cncr.25530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simard EP, Pfeiffer RM, Engels EA. Spectrum of cancer risk late after AIDS onset in the United States. Arch Intern Med. 2010 Aug 9;170(15):1337–45. doi: 10.1001/archinternmed.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darragh TM, Winkler B. Anal cancer and cervical cancer screening: key differences. Cancer Cytopathol. 2011 Feb 25;119(1):5–19. doi: 10.1002/cncy.20126. [DOI] [PubMed] [Google Scholar]
- 8.Goldie SJ, Kuntz KM, Weinstein MC, Freedberg KA, Welton ML, Palefsky JM. The clinical effectiveness and cost-effectiveness of screening for anal squamous intraepithelial lesions in homosexual and bisexual HIV-positive men. JAMA. 1999;281(19):1822–9. doi: 10.1001/jama.281.19.1822. [DOI] [PubMed] [Google Scholar]
- 9.Panther LA, Wagner K, Proper J, Fugelso DK, Chatis PA, Weeden W, et al. High resolution anoscopy findings for men who have sex with men: inaccuracy of anal cytology as a predictor of histologic high-grade anal intraepithelial neoplasia and the impact of HIV serostatus. Clin Infect Dis. 2004 May 15;38(10):1490–2. doi: 10.1086/383574. [DOI] [PubMed] [Google Scholar]
- 10.Boon ME, de Graaff Guilloud JC, Rietveld WJ. Analysis of five sampling methods for the preparation of cervical smears. Acta Cytol. 1989 Nov-Dec;33(6):843–8. [PubMed] [Google Scholar]
- 11.Gao L, Zhou F, Li X, Yang Y, Ruan Y, Jin Q. Anal HPV infection in HIV-positive men who have sex with men from China. PLoS One. 2010;5(12):e15256. doi: 10.1371/journal.pone.0015256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benschop CC, Wiebosch DC, Kloosterman AD, Sijen T. Post-coital vaginal sampling with nylon flocked swabs improves DNA typing. Forensic Sci Int Genet. 2010 Feb;4(2):115–21. doi: 10.1016/j.fsigen.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 13.El Aila NA, Tency I, Claeys G, Saerens B, De Backer E, Temmerman M, et al. Genotyping of Streptococcus agalactiae (group B streptococci) isolated from vaginal and rectal swabs of women at 35-37 weeks of pregnancy. BMC Infect Dis. 2009;9:153. doi: 10.1186/1471-2334-9-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kristensen GB, Holund B, Grinsted P. Efficacy of the cytobrush versus the cotton swab in the collection of endocervical cells. Acta Cytol. 1989 Nov-Dec;33(6):849–51. [PubMed] [Google Scholar]
- 15.Calore EE, Nadal SR, Manzione CR, Horta SC, Santos RR, Nadal LM. Anal cytology in patients with AIDS. Diagn Cytopathol. 2010 Apr;38(4):260–3. doi: 10.1002/dc.21201. [DOI] [PubMed] [Google Scholar]
- 16.Darragh T, Birdsong G, Luff R, Davey D. Anal-Rectal Cytology. In: Solomon D, Nayar R, editors. The Bethesda system for reporting cervical cytology: definitions, criteria, and explanatory notes. Springer Verlag; New York: 2004. pp. 169–76. [Google Scholar]
- 17.Daley P, Castriciano S, Chernesky M, Smieja M. Comparison of flocked and rayon swabs for collection of respiratory epithelial cells from uninfected volunteers and symptomatic patients. J Clin Microbiol. 2006 Jun;44(6):2265–7. doi: 10.1128/JCM.02055-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esposito S, Molteni CG, Daleno C, Valzano A, Cesati L, Gualtieri L, et al. Comparison of nasopharyngeal nylon flocked swabs with universal transport medium and rayon-bud swabs with a sponge reservoir of viral transport medium in the diagnosis of paediatric influenza. J Med Microbiol. 2010 Jan;59(Pt 1):96–9. doi: 10.1099/jmm.0.015305-0. [DOI] [PubMed] [Google Scholar]
- 19.Scansen KA, Bonsu BK, Stoner E, Mack K, Salamon D, Leber A, et al. Comparison of polyurethane foam to nylon flocked swabs for collection of secretions from the anterior nares in performance of a rapid influenza virus antigen test in a pediatric emergency department. J Clin Microbiol. 2010 Mar;48(3):852–6. doi: 10.1128/JCM.01897-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walsh P, Overmyer CL, Pham K, Michaelson S, Gofman L, DeSalvia L, et al. Comparison of respiratory virus detection rates for infants and toddlers by use of flocked swabs, saline aspirates, and saline aspirates mixed in universal transport medium for room temperature storage and shipping. J Clin Microbiol. 2008 Jul;46(7):2374–6. doi: 10.1128/JCM.00714-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krech T, Castriciano S, Jang D, Smieja M, Enders G, Chernesky M. Detection of high risk HPV and Chlamydia trachomatis in vaginal and cervical samples collected with flocked nylon and wrapped rayon dual swabs transported in dry tubes. J Virol Methods. 2009 Dec;162(1-2):291–3. doi: 10.1016/j.jviromet.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Nance KV. Evolution of Pap testing at a community hospital: a ten year experience. Diagn Cytopathol. 2007 Mar;35(3):148–53. doi: 10.1002/dc.20607. [DOI] [PubMed] [Google Scholar]
- 23.Kirschner B, Simonsen K, Junge J. Comparison of conventional Papanicolaou smear and SurePath liquid-based cytology in the Copenhagen population screening programme for cervical cancer. Cytopathology. 2006 Aug;17(4):187–94. doi: 10.1111/j.1365-2303.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 24.Ferris DG, Heidemann NL, Litaker MS, Crosby JH, Macfee MS. The efficacy of liquid-based cervical cytology using direct-to-vial sample collection. J Fam Pract. 2000 Nov;49(11):1005–11. [PubMed] [Google Scholar]
- 25.Fontaine D, Narine N, Naugler C. Unsatisfactory rates vary between cervical cytology samples prepared using ThinPrep and SurePath platforms: a review and meta-analysis. BMJ Open. 2012;2(2):e000847. doi: 10.1136/bmjopen-2012-000847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao C, Domfeh AB, Austin RM. Histopathologic outcomes and clinical correlations for high-risk patients screened with anal cytology. Acta Cytol. 2012;56(1):62–7. doi: 10.1159/000331431. [DOI] [PubMed] [Google Scholar]
- 27.Berry JM, Palefsky JM, Jay N, Cheng SC, Darragh TM, Chin-Hong PV. Performance characteristics of anal cytology and human papillomavirus testing in patients with high-resolution anoscopy-guided biopsy of high-grade anal intraepithelial neoplasia. Dis Colon Rectum. 2009 Feb;52(2):239–47. doi: 10.1007/DCR.0b013e31819793d9. [DOI] [PubMed] [Google Scholar]
- 28.Mathews WC, Sitapati A, Caperna JC, Barber RE, Tugend A, Go U. Measurement characteristics of anal cytology, histopathology, and high-resolution anoscopic visual impression in an anal dysplasia screening program. J Acquir Immune Defic Syndr. 2004 Dec 15;37(5):1610–5. doi: 10.1097/00126334-200412150-00014. [DOI] [PubMed] [Google Scholar]
- 29.Vajdic CM, Anderson JS, Hillman RJ, Medley G, Grulich AE. Blind sampling is superior to anoscope guided sampling for screening for anal intraepithelial neoplasia. Sex Transm Infect. 2005 Oct;81(5):415–8. doi: 10.1136/sti.2004.014407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bean SM, Chhieng DC, Roberson J, Raper JL, Broker TR, Hoesley CJ, et al. Anal-rectal cytology: correlation with human papillomavirus status and biopsy diagnoses in a population of HIV-positive patients. J Low Genit Tract Dis. 2010 Apr;14(2):90–6. doi: 10.1097/LGT.0b013e3181ba9bcd. [DOI] [PubMed] [Google Scholar]
- 31.Cranston RD, Hart SD, Gornbein JA, Hirschowitz SL, Cortina G, Moe AA. The prevalence, and predictive value, of abnormal anal cytology to diagnose anal dysplasia in a population of HIV-positive men who have sex with men. Int J STD AIDS. 2007 Feb;18(2):77–80. doi: 10.1258/095646207779949772. [DOI] [PubMed] [Google Scholar]
- 32.Conley L, Bush T, Darragh TM, Palefsky JM, Unger ER, Patel P, et al. Factors associated with prevalent abnormal anal cytology in a large cohort of HIV-infected adults in the United States. J Infect Dis. 2010 Nov 15;202(10):1567–76. doi: 10.1086/656775. [DOI] [PubMed] [Google Scholar]
- 33.Kojic EM, Cu-Uvin S, Conley L, Bush T, Onyekwuluje J, Swan DC, et al. Human Papillomavirus Infection and Cytologic Abnormalities of the Anus and Cervix Among HIV-Infected Women in the Study to Understand the Natural History of HIV/AIDS in the Era of Effective Therapy (The SUN Study) Sex Transm Dis. 2011 Apr;38(4):253–9. doi: 10.1097/OLQ.0b013e3181f70253. [DOI] [PubMed] [Google Scholar]
- 34.Jorge JMN, Habr-Gama A. Anatomy and Embryology. In: Beck DE, Roberts PL, Saclarides TJ, Senagore AJ, Stamos MJ, Wesner SD, editors. The ASCRS Textbook of Colon and Rectal Surgery. 2nd ed Springer Science & Businesss Media, LLC; New York: 2011. [Google Scholar]
- 35.Panther LA, Schlecht HP, Dezube BJ. Spectrum of human papillomavirus-related dysplasia and carcinoma of the anus in HIV-infected patients. AIDS Read. 2005 Feb;15(2):79–82. 85-6, 88, 91. [PubMed] [Google Scholar]
- 36.Goldie S, Palefsky JM, Workowsi K. Anal cancer in HIV infection: to screen or not to screen? AIDS Clin Care. 2004 Jul;16(7):53–5. 57. [PubMed] [Google Scholar]
- 37.Goldie SJ, Kuntz KM, Weinstein MC, Freedberg KA, Palefsky JM. Cost-effectiveness of screening for anal squamous intraepithelial lesions and anal cancer in human immunodeficiency virus-negative homosexual and bisexual men. Am J Med. 2000;108(8):634–41. doi: 10.1016/s0002-9343(00)00349-1. [DOI] [PubMed] [Google Scholar]
- 38.Division of STD Prevention . CDC Fact Sheet: HPV and Men. U.S. Center for Disease Control and Prevention; Atlanta, GA: [updated December 2007; cited 2009 April 25]. 2007. Available from: http://www.cdc.gov/std/hpv/HPV&Men-Fact-Sheet-press.pdf. [Google Scholar]
- 39.Goldstone SE, Hundert JS, Huyett JW. Infrared coagulator ablation of high-grade anal squamous intraepithelial lesions in HIV-negative males who have sex with males. Dis Colon Rectum. 2007 May;50(5):565–75. doi: 10.1007/s10350-006-0874-x. [DOI] [PubMed] [Google Scholar]
- 40.Wexler A, Berson AM, Goldstone SE, Waltzman R, Penzer J, Maisonet OG, et al. Invasive anal squamous-cell carcinoma in the HIV-positive patient: outcome in the era of highly active antiretroviral therapy. Dis Colon Rectum. 2008 Jan;51(1):73–81. doi: 10.1007/s10350-007-9154-7. [DOI] [PubMed] [Google Scholar]
- 41.Devaraj B, Cosman BC. Expectant management of anal squamous dysplasia in patients with HIV. Dis Colon Rectum. 2006 Jan;49(1):36–40. doi: 10.1007/s10350-005-0229-z. [DOI] [PubMed] [Google Scholar]
- 42.Holton T, Smith D, Terry M, Madgwick A, Levine T. The effect of lubricant contamination on ThinPrep (Cytyc) cervical cytology liquid-based preparations. Cytopathology. 2008 Aug;19(4):236–43. doi: 10.1111/j.1365-2303.2007.00525.x. [DOI] [PubMed] [Google Scholar]
- 43.Solomon D, Davey D, Kurman R, Moriarty A, O’Connor D, Prey M, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002 Apr 24;287(16):2114–9. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 44.Arain S, Walts AE, Thomas P, Bose S. The Anal Pap Smear: Cytomorphology of squamous intraepithelial lesions. Cytojournal. 2005 Feb 16;2(1):4. doi: 10.1186/1742-6413-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fritz AG. International classification of diseases for oncology: ICD-O. 3rd ed World Health Organization; Geneva: 2000. [Google Scholar]
- 46.Darragh TM, Winkler B. Anal cancer and cervical cancer screening: Key differences. Cancer Cytopathol. 2011;119(1):5–19. doi: 10.1002/cncy.20126. [DOI] [PubMed] [Google Scholar]
- 47.SAS Institute Inc . BASE SAS 92 Procedures Guide, Statistical Procedures. 3rd ed SAS Institute Inc.; Cary: 2010. The FREQ Procedure; pp. 63–216. [Google Scholar]
- 48.SAS Institute Inc . SAS/STAT 922 User’s Guide. SAS Institute Inc.; Cary, NC: 2010. The Logistic Procedure; pp. 3861–4100. [Google Scholar]
- 49.Obuchowski NA, Lieber ML, Wians FH., Jr. ROC curves in clinical chemistry: uses, misuses, and possible solutions. Clin Chem. 2004 Jul;50(7):1118–25. doi: 10.1373/clinchem.2004.031823. [DOI] [PubMed] [Google Scholar]
- 50.Munoz A, Carey V, Taylor JM, Chmiel JS, Kingsley L, Van Raden M, et al. Estimation of time since exposure for a prevalent cohort. Stat Med. 1992 May;11(7):939–52. doi: 10.1002/sim.4780110711. [DOI] [PubMed] [Google Scholar]
- 51.Palefsky J, Handley J. What your doctor may not tell you about HPV and abnormal pap smears. Warner Books; New York, NY: 2002. [Google Scholar]
- 52.Nathan M, Singh N, Garrett N, Hickey N, Prevost T, Sheaff M. Performance of anal cytology in a clinical setting when measured against histology and high-resolution anoscopy findings. AIDS. 2010 Jan 28;24(3):373–9. doi: 10.1097/QAD.0b013e328333ab8e. [DOI] [PubMed] [Google Scholar]
- 53.Fox PA, Seet JE, Stebbing J, Francis N, Barton SE, Strauss S, et al. The value of anal cytology and human papillomavirus typing in the detection of anal intraepithelial neoplasia: a review of cases from an anoscopy clinic. Sex Transm Infect. 2005 Apr;81(2):142–6. doi: 10.1136/sti.2003.008318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Papaconstantinou HT, Lee AJ, Simmang CL, Ashfaq R, Gokaslan ST, Sokol S, et al. Screening methods for high-grade dysplasia in patients with anal condyloma. J Surg Res. 2005 Jul 1;127(1):8–13. doi: 10.1016/j.jss.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 55.Cranston RD, Darragh TM, Holly EA, Jay N, Berry JM, Da Costa M, et al. Self-collected versus clinician-collected anal cytology specimens to diagnose anal intraepithelial neoplasia in HIV-positive men. J Acquir Immune Defic Syndr. 2004 Aug 1;36(4):915–20. doi: 10.1097/00126334-200408010-00004. [DOI] [PubMed] [Google Scholar]
- 56.Chiao EY, Giordano TP, Palefsky JM, Tyring S, El Serag H. Screening HIV-infected individuals for anal cancer precursor lesions: a systematic review. Clin Infect Dis. 2006 Jul 15;43(2):223–33. doi: 10.1086/505219. [DOI] [PubMed] [Google Scholar]
- 57.Fahey MT, Irwig L, Macaskill P. Meta-analysis of Pap test accuracy. Am J Epidemiol. 1995 Apr 1;141(7):680–9. doi: 10.1093/oxfordjournals.aje.a117485. [DOI] [PubMed] [Google Scholar]
- 58.Darragh TM. Anal cytology for anal cancer screening: is it time yet? Diagn Cytopathol. 2004 Jun;30(6):371–4. doi: 10.1002/dc.20110. [DOI] [PubMed] [Google Scholar]
- 59.Manos MM, Kinney WK, Hurley LB, Sherman ME, Shieh-Ngai J, Kurman RJ, et al. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA. 1999;281(17):1605–10. doi: 10.1001/jama.281.17.1605. [DOI] [PubMed] [Google Scholar]
- 60.Gage JC, Ghosh A, Borgonovo S, Follansbee S, Wentzensen N, Gravitt PE, et al. A comparison of dacron versus Flocked nylon swabs for anal cytology specimen collection. Acta Cytol. 2011;55(4):364–7. doi: 10.1159/000329488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chin-Hong PV, Berry JM, Cheng SC, Catania JA, Da Costa M, Darragh TM, et al. Comparison of patient- and clinician-collected anal cytology samples to screen for human papillomavirus-associated anal intraepithelial neoplasia in men who have sex with men. Ann Intern Med. 2008 Sep 2;149(5):300–6. doi: 10.7326/0003-4819-149-5-200809020-00004. [DOI] [PubMed] [Google Scholar]
- 62.Chin-Hong PV, Vittinghoff E, Cranston RD, Browne L, Buchbinder S, Colfax G, et al. Age-related prevalence of anal cancer precursors in homosexual men: the EXPLORE study. J Natl Cancer Inst. 2005 Jun 15;97(12):896–905. doi: 10.1093/jnci/dji163. [DOI] [PubMed] [Google Scholar]
- 63.Palefsky JM. Anal squamous intraepithelial lesions in human immunodeficiency virus-positive men and women. Semin Oncol. 2000;27(4):471–9. [PubMed] [Google Scholar]
- 64.Brink AL, du Toit JP, Deale CJ. In search of more representative cervical cytology. A preliminary prospective study. S Afr Med J. 1989 Jul 15;76(2):55–7. [PubMed] [Google Scholar]
- 65.Dotters DJ, Carney CN, Droegemueller W. Nylon brush improves collection of cervical cytologic specimens. Am J Obstet Gynecol. 1988 Oct;159(4):814–9. doi: 10.1016/s0002-9378(88)80143-1. [DOI] [PubMed] [Google Scholar]
- 66.Rubio CA, Berglund K, Kock Y. Studies on the distribution of abnormal cells in cytologic preparations. IV. Importance of the topographical position of the cells in material collected by wooden spatulas. Gynecol Oncol. 1980 Oct;10(2):146–51. doi: 10.1016/0090-8258(80)90076-1. [DOI] [PubMed] [Google Scholar]
- 67.Rubio CA, Berglund K, Kock Y, Zetterberg A. Studies on the distribution of abnormal cells in cytologic preparations. III. Making the smear with a plastic spatula. Am J Obstet Gynecol. 1980 Aug 1;137(7):843–6. doi: 10.1016/0002-9378(80)90897-2. [DOI] [PubMed] [Google Scholar]
- 68.Rubio CA, Kock Y, Berglund K. Studies of the distribution of abnormal cells in cytologic preparations. I. Making the smear with a wooden spatula. Acta Cytol. 1980 Jan-Feb;24(1):49–53. [PubMed] [Google Scholar]
- 69.Rubio CA, Kock Y, Berglund K, Thomassen P. Studies on the distribution of abnormal cells in cytological preparations II. Making the smear with the cotton swab applicator. Gynecol Oncol. 1980 Apr;9(2):127–34. doi: 10.1016/0090-8258(80)90020-7. [DOI] [PubMed] [Google Scholar]
- 70.Trimbos JB, Arentz NP. The efficiency of the Cytobrush versus the cotton swab in the collection of endocervical cells in cervical smears. Acta Cytol. 1986 May-Jun;30(3):261–3. [PubMed] [Google Scholar]
- 71.Hathaway JK, Pathak PK, Maney R. Is liquid-based pap testing affected by water-based lubricant? Obstet Gynecol. 2006 Jan;107(1):66–70. doi: 10.1097/01.AOG.0000192512.03576.da. [DOI] [PubMed] [Google Scholar]
- 72.Nielsen A, Munk C, Kjaer SK. Trends in incidence of anal cancer and high-grade anal intraepithelial neoplasia in Denmark, 1978-2008. Int J Cancer. 2012 Mar 1;130(5):1168–73. doi: 10.1002/ijc.26115. [DOI] [PubMed] [Google Scholar]
- 73.Shiels MS, Pfeiffer RM, Engels EA. Age at cancer diagnosis among persons with AIDS in the United States. Ann Intern Med. 2010 Oct 5;153(7):452–60. doi: 10.1059/0003-4819-153-7-201010050-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alsharif M, McKeon DM, Gulbahce HE, Savik K, Pambuccian SE. Unsatisfactory SurePath liquid-based Papanicolaou tests: causes and significance. Cancer. 2009 Feb 25;117(1):15–26. doi: 10.1002/cncy.20009. [DOI] [PubMed] [Google Scholar]
- 75.AbdullGaffar B, Kamal MO, Khalid M, Samuel R, AlGhufli R. Lubricant, mucus, and other contaminant materials as a potential source of interpretation errors in ThinPrep cervical cytology. J Low Genit Tract Dis. 2010 Jan;14(1):22–8. doi: 10.1097/LGT.0b013e3181ab4584. [DOI] [PubMed] [Google Scholar]
- 76.Hild-Mosley KA, Lindblade JA, Julian TM. The Management of Cervical Mucus in Obtaining a Papanicolaou Smear. Journal of Lower Genital Tract Disease. 1997 Jan;1(1):1–4. doi: 10.1097/00128360-199701000-00001. [DOI] [PubMed] [Google Scholar]
- 77.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR., Jr. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. American Journal of Epidemiology. 1987;126(2):310–8. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]