Abstract
Signals from the T cell Ig- and mucin-domain-containing molecules (TIMs) have been demonstrated to be actively involved in regulating the progression of carcinomas. However, the expression and distribution of these molecules in osteosarcoma, the most common primary bone malignancy with poor prognosis, have not been investigated. In this study, the expression of TIMs was examined in nine invasive human osteosarcomas using immunohistochemistry, and the phenotypes were detected by dual immunofluorescence staining. Using immunohistochemistry, it was observed that only TIM-3, rather than TIM-1 or TIM-4, was expressed in these tumor specimens, where it was localized in the cytoplasm and plasma membrane of tumor cells. Dual immunofluorescence staining revealed that the expression of TIM-3 was observed in all cell types investigated, including CD68+ macrophages, CD31+ endothelial cells, CK-18+ epithelial cells and PCNA+ tumor cells. Notably, in sarcoma cells, TIM-3 was co-expressed with certain biomarkers of epithelial-mesenchymal transition (EMT), including vimentin, Slug, Snail and Smad. These combined results suggest that TIM-3 triggers tumor cells to acquire features of aggressive EMT and may be involved in the pathogenesis of this malignancy.
Keywords: osteosarcoma, T cell Ig- and mucin-domain-containing molecules, immunohistochemistry, tumor pathogenesis
Introduction
Osteosarcoma is the most common type of non-hematopoietic primary malignant bone tumor. Following the initial diagnosis, patients are usually treated with multi-agent preoperative chemotherapy and surgical resection, followed by postoperative chemotherapy. Despite significant progress in chemotherapy, patients who have metastases at diagnosis continue to have poor prognoses (1). Therefore, it is essential to identify additional biomarkers for use in diagnosis and novel therapeutic strategies.
T cell Ig- and mucin-domain-containing molecules (TIMs) are a recently described family. Three members of which, TIM-1, TIM-3 and TIM-4, have been identified in humans (2). TIM-1 was demonstrated to be preferentially expressed on Th2 cells and to support T-cell activation, promoting the pathogenesis of asthma and allergies (3–5). TIM-4 was observed on antigen-presenting cells (APCs) and mediates the phagocytosis of apoptotic cells (6). By contrast, TIM-3 was originally identified as a surface molecule expressed on CD4+ Th1 cells. The interaction of TIM-3 with its potential ligand, galectin-9, induces Th1 cells to undergo apoptosis and inhibits their production of IFN-γ (7–9).
In addition to activated T cells, ectopic expression of TIMs has been observed on tumor cells, where they were also described as being actively involved in the pathogenesis of tumor development. For example, non-small cell lung cancer patients whose tumor tissues were positive for TIM-3 had significantly shorter survival times compared with those with TIM-3- tumor tissues (10). In patients with hepatitis B virus-associated hepatocellular carcinoma, the number of TIM-3+ infiltrating tumor cells was negatively associated with patient survival (11). Moreover, TIMs were also shown to function as specific markers for the diagnosis of Langerhans cell sarcoma, head and neck cancer and follicular B cell non-Hodgkin lymphoma (12,13), while the combined blockade of TIM-3 and TIM-4 augments the efficacy of cancer vaccines against established melanomas (14).
Epithelial-mesenchymal transition (EMT) is an essential process for normal development and is also crucial in cancer dissemination, endowing cells with metastatic and cancer stem cell properties (15). EMT is characterized by the down-regulation of epithelial markers (such as E-cadherin) and the upregulation of vimentin, as well as other mesenchymal markers, resulting in numerous phenotypic changes, including the loss of cell-cell adhesion and cell polarity and the acquisition of migratory and invasive properties (16). TWIST1, SNAIL and SLUG are transcription factors that govern EMT and are regulated by TGF-β and microRNA (17,18). The increased expression of EMT biomarkers has been associated with poor prognostic clinicopathological features of various types of cancer, including osteosarcoma (19–21).
In the present study, the expression of TIMs in osteosarcoma samples was analyzed by immunohistochemistry, and the associations between TIMs and EMT biomarkers were further analyzed by dual immunofluorescence staining.
Materials and methods
Patients
Samples from nine cases of osteosarcoma were collected at the Department of Orthopedics, Affiliated Hospital of Chifeng University (Chifeng, China). The histopathology and pathological characteristics of the patients were analyzed by CT examination and H&E staining, with the results demonstrating that all patients had osteosarcoma (Fig. 1). All osteosarcoma samples were obtained from the legs, and the tissues were fixed in 10% neutral buffered formalin, and then embedded in paraffin. This study protocol was approved by the review board of the ethics committee of Chifeng University. Written informed consent was obtained from all patients.
Figure 1.
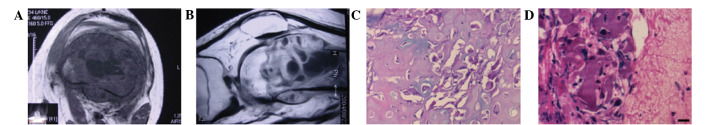
Histopathology and pathological characteristics of osteosarcoma were analyzed by CT examination and H&E staining. (A) Axial and (B) sagittal CT images demonstrating that tumor development arose from the bone. (C) Histopathology and (D) pathological characteristics of osteosarcoma from two patients were detected by H&E staining. Scale bar, 20 μm.
Immunohistochemistry
The immunohistochemistry was performed as published previously, but with slight modifications (22). Briefly, paraffin-embedded tissue blocks were cut into 2–3-μm sections and mounted on poly-L-lysine-charged glass slides (Sigma, St. Louis, MO, USA). After the sections were dewaxed and rehydrated, antigen retrieval was performed by microwaving in 10 mM citrate buffer (pH 6.0). The sections were cooled to room temperature (RT) and endogenous peroxidase activity was blocked by incubation with a solution of 0.5% hydrogen peroxide in 50% methanol for 1 h. The sections were then incubated in 3% BSA (Sigma) with 0.1% Nonidet P-40 (Beyotime, Haimen, China) in PBS (Wuhan Boster Biological Technology Ltd., Wuhan, China) for 1 h at RT to block nonspecific binding. Subsequently, the sections were incubated overnight at 4°C with primary antibodies (Table I), including anti-TIM-1, anti-TIM-3 or anti-TIM-4, diluted in 1% BSA. After washing, the sections were incubated with the corresponding secondary antibodies for 1 h at RT. The Vectastain ABC kit (Vector Laboratories, San Diego, CA, USA) was used for the avidin-biotin complex method according the manufacturer’s instructions. Sections incubated with isotype-matched, concentration-matched Ig without primary antibodies were used as isotype controls. Peroxidase activity was visualized with the DAB Elite kit (K3465; Dako, Copenhagen, Denmark) and brown coloration of tissues represented positive staining. The sections were lightly counterstained with hematoxylin, dehydrated through an ethanol series, cleared in xylene and mounted. Subsequently, the sample sections were viewed using a light microscope (Axioplan 2; Zeiss, Berlin, Germany).
Table I.
Immunohistochemical study: Antibodies, source and dilution.
| Ab | Dilution | Clone | Source |
|---|---|---|---|
| TIM-1 | 1:100 | Polyclonal Goat IgG | R&D System |
| TIM-3 | 1:100 | Polyclonal Goat IgG | R&D System |
| TIM-4 | 1:100 | Polyclonal Goat IgG | R&D System |
| CD3 | 1:50 | Monoclonal mouse IgG (F7.2.38) | Dako |
| CK-18 | 1:400 | Monoclonal mouse IgG (C-04) | Santa Cruz |
| CD68 | 1:50 | Monoclonal mouse IgG (3F103) | Santa Cruz |
| CD31 | 1:50 | Monoclonal mouse IgG (10G9) | Santa Cruz |
| CD1a | 1:200 | Monoclonal mouse IgG (7A7) | Abcam |
| PCNA | 1:200 | Monoclonal mouse IgG (F-2) | Santa Cruz |
| Bcl-2 | 1:100 | Monoclonal mouse IgG (C-2) | Santa Cruz |
| Slug | 1:100 | Monoclonal mouse IgG | Abcam |
| Snail | 1:100 | Polyclonal Goat IgG | Abcam |
| Smad2 | 1:100 | Polyclonal Goat IgG | Santa Cruz |
| Smad3 | 1:100 | Polyclonal Rabbit IgG | Santa Cruz |
| Vimentin | 1:50 | Monoclonal mouse IgG (RV202) | Santa Cruz |
| E-cad | 1:200 | Polyclonal Rabbit IgG | Santa Cruz |
Dual immunofluorescence staining
For dual immunofluorescence staining, the sections were incubated with primary anti-TIM-3 antibodies at 4°C overnight. After washing with PBS (three washes, 5 min per wash), the sections were incubated with Alexa Fluor® 555-conjugated goat anti-mouse/rabbit IgG antibodies (Invitrogen, Carlsbad, CA, USA) for 1 h. The sections were further incubated with anti-CD68, anti-CD31, anti-PCNA, anti-Bcl-2, anti-Snail, anti-Slug, anti-Smad, anti-pSmad2/3 or anti-CK-18 antibodies at 4°C overnight, and incubated with Alexa Fluor® 488-conjugated goat anti-mouse/rabbit IgG1 antibodies (Invitrogen) for an additional hour. Subsequently, the sections were incubated with 1 μg/ml DAPI (Sigma) for 10 min to stain the nuclei. Sections incubated with the appropriate isotype control primary antibodies and fluorescently labeled secondary antibodies were used as negative controls. The results were analyzed by fluorescence microscopy (Axioplan 2; Zeiss).
Results
Expression and anatomical distribution of TIMs in sections from osteosarcoma
Axial and sagittal CT images demonstrated that tumor development originated from the bone (Fig. 1A and B). The specimens from all nine cases of osteosarcoma were highly cellular tumors consisting of enlarged round cells. The neoplastic cells showed the presence of cytological atypia, with multiple hyperchromatic and prominent nucleoli (Fig. 1C and D). Immunohistochemistry showed that TIM-3+ and TIM-4+, but not TIM-1+, cells were observed in all cases. These molecules were located on cell membranes and in the cytoplasm. However, the distributions of TIM-3 and TIM-4 were noticeably different. TIM-3 was identified on macrophages (Fig. 2A), infiltrated inflammatory cells (Fig. 2B) and tumor cells (Fig. 2C and D), and TIM-3+ cells were distributed throughout the tissue sections. TIM-4, however, was expressed on macrophage-like cells (Fig. 2E) and was absent from tumor cells (Fig. 2F). No positive results were observed in sections incubated with only secondary antibodies (goat IgG1), which were used as the negative controls (data not shown).
Figure 2.

Expression of TIM-1, TIM-3 and TIM-4 in osteosarcoma sections as detected by immunohistochemistry. (A) Goat IgG isotype control antibodies showed no positive staining; (B) TIM-1 was absent in sections from osteosarcoma patients. TIM-3 was expressed on (C) infiltrating lymphocytes, (D) macrophages and (E and F) tumor cells in sections from osteosarcoma patients. TIM-4 was expressed on (G) macrophage-like cells while it was absent from (H) tumor cells. Arrows indicate the positive cells. Scale bar, 20 μm. TIM, T cell Ig- and mucin-domain-containing molecule.
Phenotypes of TIM-3 in sections from osteosarcoma
As TIM-3 was observed in tumor cells from osteosarcomas, the phenotypes of the TIM-3+ cells were further examined by dual immunofluorescence staining. As shown in Fig. 3, TIM-3 was expressed on CD31+ endothelial cells, CK-18+ epithelial cells and CD68+ macrophages. In addition, TIM-3 was co-expressed with Bcl-2 and PCNA, indicating that TIM-3 may regulate tumor cell apoptosis and proliferation (Fig. 3).
Figure 3.
Morphology of TIM-3+ cells in osteosarcoma sample sections as detected by dual immunofluorescence staining. Dual immunofluorescence staining showed that TIM-3 was expressed on CD68+ macrophages, CD31+ endothelial cells, CK-18+ epithelial cells, Bcl-2+ tumor cells and PCNA+ tumor cells. Arrows indicate positive cells. Nuclei were stained with DAPI. Scale bar, 20 μm. TIM, T cell Ig- and mucin-domain-containing molecule.
Associations between TIM-3 and EMT biomarkers in sections from osteosarcoma
Previous studies demonstrated that the expression of EMT biomarkers, including Slug, Snail and Smad, could be detected in samples from patients with osteosarcoma, suggesting that EMT may be involved in the pathogenesis of osteosarcoma (23,24). In the present study, the correlations between TIM-3 and these EMT biomarkers were also investigated. The results showed that TIM-3 was co-expressed with Slug, Snail and Smad in the same sarcoma cells (Fig. 4).
Figure 4.
Association between the expression of TIM-3 and certain bio-markers of EMT in osteosarcoma sections detected by immunofluorescent dual staining. Dual immunofluorescence staining showed that the expression of certain biomarkers of EMT, including Slug, Snail and Smad, were co-expressed with TIM-3 in the same carcinoma cells. Nuclei were stained with DAPI. Scale bar, 20 μm. EMT, epithelial-mesenchymal transition. TIM, T cell Ig- and mucin-domain-containing molecule.
Discussion
Osteosarcoma, derived from primitive mesenchymal cells and originating from bone, is the most common type of primary bone tumor in children and adolescents. Characteristically, this sarcoma occurs frequently in the metaphyseal regions of long bones and metastasizes preferentially to the lung (1,25). Although it is a relatively uncommon type of cancer, the incidence is increasing (26,27). With advances in multimodal treatments consisting of adjuvant chemotherapy and surgical resection, the prognosis and quality of life of patients with non-metastatic osteosarcoma of the extremities are greatly improved. Nevertheless, the five-year progression-free survival of high-grade osteosarcoma is only ∼50% due to the failure of rescue chemotherapy (28). Therefore, it is necessary to establish new therapeutic strategies to improve the overall rate of survival.
TIMs are proteins that are actively involved in tumor development, in addition to the pathogenesis of rheumatoid arthritis, asthma, systemic lupus erythematosus, multiple sclerosis and diabetes (2). TIMs have been reported to be aberrantly expressed in carcinoma tissues, and the presence of TIM-3 modulates tumor development and carcinoma traits (3). In the present study, the expression of TIMs was analyzed in nine cases of osteosarcoma and the results demonstrated that only TIM-3, rather than TIM-1 or TIM-4, was detected on the tumor cells of osteosarcoma patients. Notably, the morphological analysis demonstrated that TIM-3 was also present on CD68+ macrophages, CD31+ endothelial cells, CK-18+ epithelial cells, as well as on Bcl-2+ and PCNA+ tumor cells. These results suggested that TIM-3 is involved in the progression of osteosarcoma via the promotion of tumor cell proliferation, as well as the inhibition of apoptosis.
In patients with advanced cancer, widespread manifestation of distant metastases is a major cause of cancer-associated mortality. Despite this important clinical problem, little is known about the mediators that promote tumor outgrowth in the metastatic organ. At present, the transdifferentiation of polarized epithelial cells to mesenchymal cells (EMT), which occurs during tumor invasion and metastasis, is recognized as a key developmental process (29). The acquisition of invasiveness by cancer cells through the invasion and destruction of the basement membrane is considered to represent the onset of a multistep process that eventually leads to metastatic dissemination with life-threatening consequences. In addition to promoting tumor cell invasion and metastasis, EMT generates cancer cells with stem cell-like characteristics, including increased self-renewal and tumor-initiating capabilities, as well as increased resistance to apoptosis and chemotherapy (30). Previous studies have examined the expression of EMT biomarkers, such as Slug, Snail and Smad, in osteosarcoma sections, suggesting that EMT may be involved in the pathogenesis of osteosarcoma (23,24). The present study also detected correlations between TIM-3 expression and the expression of these EMT biomarkers. The results showed that TIM-3 was co-expressed with Slug, Snail and Smad in the same sarcoma cells, suggesting that TIM-3 may trigger the process of EMT to promote tumor development. However, the exact mechanism of this requires further investigation.
In summary, the present study investigated the expression of TIMs in sections from osteosarcoma patients and an understanding of the functional roles of TIM-3 may aid in the development of novel strategies for disease diagnosis or immunotherapy.
Acknowledgments
The present study was supported by grants from the National Natural Science Foundation of China (grant no. 81171585).
References
- 1.Szuhai K, Cleton-Jansen AM, Hogendoorn PC, Bovée JV. Molecular pathology and its diagnostic use in bone tumors. Cancer Genet. 2012;205:193–204. doi: 10.1016/j.cancergen.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Kuchroo VK, Dardalhon V, Xiao S, Anderson AC. New roles for TIM family members in immune regulation. Nat Rev Immunol. 2008;8:577–580. doi: 10.1038/nri2366. [DOI] [PubMed] [Google Scholar]
- 3.Kuchroo VK, Umetsu DT, DeKruyff RH, Freeman GJ. The TIM gene family: emerging roles in immunity and disease. Nat Rev Immunol. 2003;3:454–462. doi: 10.1038/nri1111. [DOI] [PubMed] [Google Scholar]
- 4.Sonar SS, Hsu YM, Conrad ML, et al. Antagonism of TIM-1 blocks the development of disease in a humanized mouse model of allergic asthma. J Clin Invest. 2010;120:2767–2781. doi: 10.1172/JCI39543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Souza AJ, Oriss TB, O’malley KJ, Ray A, Kane LP. T cell Ig and mucin 1 (TIM-1) is expressed on in vivo-activated T cells and provides a costimulatory signal for T cell activation. Proc Natl Acad Sci USA. 2005;102:17113–17118. doi: 10.1073/pnas.0508643102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez-Manzanet R, Sanjuan MA, Wu HY, et al. T and B cell hyperactivity and autoimmunity associated with niche-specific defects in apoptotic body clearance in TIM-4-deficient mice. Proc Natl Acad Sci USA. 2010;107:8706–8711. doi: 10.1073/pnas.0910359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monney L, Sabatos CA, Gaglia JL, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 8.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev. 2010;235:172–189. doi: 10.1111/j.0105-2896.2010.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhuang X, Zhang X, Xia X, et al. Ectopic expression of TIM-3 in lung cancers: a potential independent prognostic factor for patients with NSCLC. Am J Clin Pathol. 2012;137:978–985. doi: 10.1309/AJCP9Q6OVLVSHTMY. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Wu K, Tao K, et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2012;56:1342–1351. doi: 10.1002/hep.25777. [DOI] [PubMed] [Google Scholar]
- 12.Dorfman DM, Hornick JL, Shahsafaei A, Freeman GJ. The phosphatidylserine receptors, T cell immunoglobulin mucin proteins 3 and 4, are markers of histiocytic sarcoma and other histiocytic and dendritic cell neoplasms. Hum Pathol. 2010;41:1486–1494. doi: 10.1016/j.humpath.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang ZZ, Grote DM, Ziesmer SC, et al. IL-12 upregulates TIM-3 expression and induces T cell exhaustion in patients with follicular B cell non-Hodgkin lymphoma. J Clin Invest. 2012;122:1271–1282. doi: 10.1172/JCI59806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baghdadi M, Nagao H, Yoshiyama H, Akiba H, Yagita H, Dosaka-Akita H, Jinushi M. Combined blockade of TIM-3 and TIM-4 augments cancer vaccine efficacy against established melanomas. Cancer Immunol Immunother. 2013;62:629–637. doi: 10.1007/s00262-012-1371-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim J, Thiery JP. Epithelial-mesenchymal transitions: insights from development. Development. 2012;139:3471–3486. doi: 10.1242/dev.071209. [DOI] [PubMed] [Google Scholar]
- 16.Wu CY, Tsai YP, Wu MZ, Teng SC, Wu KJ. Epigenetic reprogramming and post-transcriptional regulation during the epithelial-mesenchymal transition. Trends Genet. 2012;28:454–463. doi: 10.1016/j.tig.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez IE, Eickelberg O. The impact of TGF-β on lung fibrosis: from targeting to biomarkers. Proc Am Thorac Soc. 2012;9:111–116. doi: 10.1513/pats.201203-023AW. [DOI] [PubMed] [Google Scholar]
- 18.Hermeking H. MicroRNAs in the p53 network: micromanagement of tumour suppression. Nat Rev Cancer. 2012;12:613–626. doi: 10.1038/nrc3318. [DOI] [PubMed] [Google Scholar]
- 19.Gao D, Vahdat LT, Wong S, Chang JC, Mittal V. Microenvironmental regulation of epithelial-mesenchymal transitions in cancer. Cancer Res. 2012;72:4883–4889. doi: 10.1158/0008-5472.CAN-12-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rangel MC, Karasawa H, Castro NP, Nagaoka T, Salomon DS, Bianco C. Role of Cripto-1 during epithelial-to-mesenchymal transition in development and cancer. Am J Pathol. 2012;180:2188–2200. doi: 10.1016/j.ajpath.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton DH, Litzinger MT, Fernando RI, Huang B, Palena C. Cancer vaccines targeting the epithelial-mesenchymal transition: tissue distribution of brachyury and other drivers of the mesenchymal-like phenotype of carcinomas. Semin Oncol. 2012;39:358–366. doi: 10.1053/j.seminoncol.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Wang C, Guo G, Gao C, Wu Y, Chen Y. The characteristic expression of B7-associated proteins in Langerhans cell sarcoma. Acta Histochem. 2012;114:733–743. doi: 10.1016/j.acthis.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Guo Y, Zi X, Koontz Z, Kim A, Xie J, Gorlick R, Holcombe RF, Hoang BH. Blocking Wnt/LRP5 signaling by a soluble receptor modulates the epithelial to mesenchymal transition and suppresses met and metalloproteinases in osteosarcoma Saos-2 cells. J Orthop Res. 2007;25:964–971. doi: 10.1002/jor.20356. [DOI] [PubMed] [Google Scholar]
- 24.Niinaka Y, Harada K, Fujimuro M, et al. Silencing of autocrine motility factor induces mesenchymal-to-epithelial transition and suppression of osteosarcoma pulmonary metastasis. Cancer Res. 2010;70:9483–9493. doi: 10.1158/0008-5472.CAN-09-3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarsilmaz A, Argin M, Sezak M, Altay C, Erdogan N. Primary osteosarcoma arising from subcutaneous tissue: 5-year follow-up. Clin Imaging. 2012;36:402–405. doi: 10.1016/j.clinimag.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Eyre R, Feltbower RG, James PW, Blakey K, Mubwandarikwa E, Forman D, McKinney PA, Pearce MS, McNally RJ. The epidemiology of bone cancer in 0–39 year olds in northern England, 1981–2002. BMC Cancer. 2010;10:357. doi: 10.1186/1471-2407-10-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrews EB, Gilsenan AW, Midkiff K, Sherrill B, Wu Y, Mann BH, Masica D. The US postmarketing surveillance study of adult osteosarcoma and teriparatide: Study design and findings from the first 7 years. J Bone Miner Res. 2012;27:2429–2437. doi: 10.1002/jbmr.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mavrogenis AF, Rossi G, Palmerini E, et al. Palliative treatments for advanced osteosarcoma. J BUON. 2012;17:436–445. [PubMed] [Google Scholar]
- 29.McConkey DJ, Choi W, Marquis L, et al. Role of epithelial-to-mesenchymal transition (EMT) in drug sensitivity and metastasis in bladder cancer. Cancer Metastasis Rev. 2009;28:335–344. doi: 10.1007/s10555-009-9194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunning NL, Laversin SA, Miles AK, Rees RC. Immunotherapy of prostate cancer: should we be targeting stem cells and EMT? Cancer Immunol Immunother. 2011;60:1181–1193. doi: 10.1007/s00262-011-1065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]




