Abstract
Telomerase activation is a critical step in human carcinogenesis through the maintenance of telomeres. Telomerase activity is primarily regulated by the human telomerase reverse transcriptase gene (hTERT), thus, an improved understanding of the transcriptional control of hTERT may provide potential therapeutic targets for the treatment of leukemia and other forms of cancer. Epigenetic modulation, a significant regulatory process in cell biology, has recently been shown to be involved in the regulation of the hTERT gene. Moreover, several epigenetic modifiers, including DNA methyltransferase (DNMT) and histone deacetylase (HDAC) inhibitors, are now in pre- and early clinical trials of leukemia as monotherapies or in combination with other drugs, and have achieved significant clinical success. In the present review, the epigenetic mechanisms associated with telomerase activity in leukemia, and the therapeutic potential of an antitelomerase strategy that combines epigenetic modifiers with telomerase hTR subunit small molecule inhibitors are discussed.
Keywords: human telomerase reverse transcriptase, epigenetic, leukemia
Contents
Introduction
Epigenetic regulation of hTERT and telomere length
Targeting telomerase (hTERT) in leukemia cells through epigenetic modifiers presents new anticancer therapeutic approaches for leukemia
Future perspectives
1. Introduction
Telomeres serve as essential structures that protect the ends of linear chromosomes from DNA repair and degradation, and their maintenance is critical for long-term cell proliferation and survival (1,2). Mammalian telomeres consist of tandem TTAGGG repeats that are bound by a specialized six-protein complex known as shelterin and may be replenished by telomerase (3). Telomerase is composed of two essential components, a catalytic subunit with reverse transcriptase activity, telomerase reverse transcriptase (TERT), and a telomerase RNA component (TERC), that acts as a template for DNA synthesis (4–6). Telomerase activity is overexpressed in the majority of cancer cells but is barely detectable in the predominance of normal somatic cells (7).
Among the various aspects of gene control, epigenetic alterations have gained attention as critical determinants for tumor initiation and subsequent cancer progression (8,9). The forms of epigenetic control of gene expression include DNA methylation and histone modification. DNA methylation involves a covalent modification at the fifth carbon position of cytosine residues within CpG dinucleotides, resulting in the transcriptional silencing of the affiliated gene (10). Promoter hypermethylation of tumor suppressor genes has been increasingly considered as a fundamental mechanism for the silencing of these genes in cancer cells, resulting in tumor initiation and progression (11,12). In addition to DNA methylation, another key element in the epigenetic control of gene expression is histone modification, including acetylation, methylation, phosphorylation and ubiquitination. Aberrant patterns of histone modifications have been associated with a large number of human malignancies (13,14). DNA methylation and histone modifications have been extensively recognized as epigenetic mechanisms that regulate gene transcription in carcinogenesis.
Human (h)TERT, a catalytic subunit of telomerase, is a key determinant for the control of telomerase activity (15). The hTERT promoter contains two E-box regions and five GC boxes (16). Similar to numerous human genes, hTERT also contains a CpG island in its promoter region, indicating a role for methylation in the regulation of hTERT expression (17). Accumulating evidence indicates that hTERT contains an increased level of DNA methylation in its promoter region in numerous cancers. Moreover, hTERT hypermethylation has been associated with the stable silencing of hTERT promoter expression (18,19). Histone deacetylation/methylation has also been reported to be responsible for the repressive status of the hTERT promoter (20). In the present review, the contribution of the epigenetic dysregulation of hTERT expression to leukemogenesis, and the prospect of this regulation as a basis for developing new anticancer therapies for leukemia are discussed.
2. Epigenetic regulation of hTERT and telomere length
Telomere length, maintained by telomerase, is a prominent mechanism for long-term cell proliferation and survival, and is strongly involved in cancer, cell senescence and aging (21–23). It has been demonstrated that the epigenetic plasticity of the hTERT gene promoter is a determinant for the control of telomerase activity. Therefore, inhibiting the expression of the hTERT gene through epigenetic mechanisms usually results in telomeric attrition. The epigenetic changes associated with the inhibition of telomerase activity include hypermethylation and histone modifications of the hTERT promoter.
The proximal core promoter region of the hTERT gene harbors a high GC content and therefore, may be partly regulated by DNA methylation. Currently, there are three major DNA methyltransferases (DNMTs) identified to be responsible for the establishment of DNA methylation in the hTERT promoter (24). In the majority of cases, the aberrant methylation of CpG islands in promoter regions results in the heritable silencing of genes without a change in their coding sequence (25). Recent studies have shown that telomerase activity is repressed through the epigenetic silencing of hTERT, which is accompanied by telomere shortening (26,27). Shin et al reported that hypermethylation of the hTERT promoter played a critical role in the negative regulation of telomerase activity in normal human oral cells (27). Zinn et al also showed that the DNA methylation patterns of the hTERT promoter decreased hTERT transcription and telomerase activity, which was consistent with the normal paradigm of methylation-induced gene silencing (28). Paradoxically, there are conflicting studies with regard to the correlation between hypermethylation of the hTERT promoter, hTERT gene expression and telomerase activity. It is increasingly apparent that the hTERT promoter is partially or completely hyper-methylated in telomerase-positive tumors, but unmethylated or hypomethylated in telomerase-negative normal tissues (16,29). Treatment using 5-azacytidine (azacitidine) and its deoxy analogue 5-aza-2′-deoxycytidine (decitabine; DAC), two common demethylating agents, is able to cause a reduction in hTERT gene expression and consequently, telomerase activity (Fig. 1) (30–32). This correlation was in contrast with the general model of gene regulation by promoter methylation. Taken together, these studies indicate that hTERT may have an effect on telomerase activity through epigenetic regulation. However, the exact mechanism by which DNA methylation affects hTERT gene expression and telomerase activity remains to be elucidated (Fig. 2).
Figure 1.
Chemical structures of selected (A) DNMT inhibitors and (B) HDAC inhibitors. DNMT, DNA methyltransferase; HDAC, histone deactylase.
Figure 2.
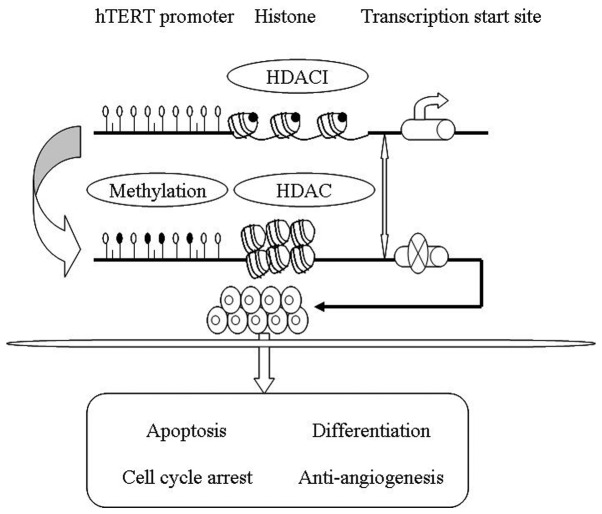
Complex molecular mechanisms and biological effects of hTERT. Epigenetic modification may affect hTERT expression and will form a permissive or inhibitive condition for hTERT transcription, depending on the specific cellular context. The suppression of hTERT promotes growth inhibition, differentiation, apoptosis and anti-angiogenesis. hTERT, human telomerase reverse transcriptase; HDAC, histone deacetylase; HDACI, HDAC inhibitor.
In addition to DNA methylation, another prevalent epigenetic mechanism that affects hTERT transcription is histone modification, including histone acetylation, methylation, phosphorylation and ubiquitinization. Histone tails carry basic charges and are associated with DNA molecules by electrostatic attraction. The acetylation of the histone proteins neutralizes the charge status of the histone tails, which decreases the attraction force between DNA and the histone tails, thus conferring an opened chromatin structure, allowing transcription factors, including c-MYC, MAD1 and CTCF, to bind to the DNA. Conversely, the deacetylation of histones results in the transcription factors having less access to the DNA (33,34). It has been demonstrated that Trichostatin A (TSA), a histone deacetylase (HDAC) inhibitor, is able to induce hTERT transcription and telomerase activity in normal cells and telomerase-negative immortal cell lines through the inhibition of histone deacetylation (Fig. 1) (35,36). Furthermore, FR901288, a novel cyclic peptide inhibitor of HDAC, has also been shown to activate hTERT mRNA expression in oral cancer cell lines (37). However, there are conflicting studies with regard to hTERT transcription and telomerase activity in cancer cells induced by HDAC inhibitors. Zhu et al reported that HDAC inhibitors prevented cell proliferation and induced apoptosis, but had no effect on the expression of hTERC and hTERT mRNA, or on telomerase activity (38). In prostate and brain cancer cells, the hTERT gene expression and telomerase activity were inhibited by HDAC inhibitors (30,40). Therefore, the HDAC inhibitors may exhibit various effects on hTERT transcription and telomerase activity in cancer cells. In addition to histone acetylation, hTERT transcription was also reported to be associated with histone methylation, of which three varying forms, including mono-, di- and trimethylation, may emerge in methylation modifications of the histone lysine residues. It has been demonstrated that mono- and dimethylated histone3-lysine9 (H3-K9) are localized to distinct domains of silent chromatin, where they are associated with inactive genes, whereas trimethylated H3-K9 is enriched in pericentric heterochromatin (41). Further studies have shown that a lack of hTERT expression in telomerase-negative cell lines is associated with histone H3 and H4 hypoacetylation and the methylation of H3-K9. However, hTERT transcription in telomerase-positive cell lines is associated with the hyperacetylation of H3 and H4 and the methylation of Lys4-H3 (H3-K4) (42). Histone methyltransferase (HMTase) is considered to be responsible for histone methylation at the hTERT promoter. Liu et al reported that SET and MYND domain-containing protein 3 (SMYD3), a HMTase, may directly transactivate hTERT transcription and telomerase activity in normal human fibroblasts and cancer cell lines through histone H3-K4 trimethylation (43). These results suggest that the epigenetic regulation of histones may contribute to hTERT gene expression and telomerase activity (Fig. 2).
3. Targeting telomerase (hTERT) in leukemia cells through epigenetic modifiers presents new anticancer therapeutic approaches for leukemia
Telomerase activity is a hallmark of the immortal cell phenotype and several mechanisms have been reported to be involved in its regulation, including transcriptional factors, DNA methylation and histone deacetylation. Furthermore, it has been shown that cells in numerous types of leukemia are able to maintain their telomere length and prevent replicative senescence or apoptosis by the epigenetic regulation of hTERT (44–46). Therefore, telomerase suppression using epigenetic modifications should be a promising target for the treatment of leukemia.
Studies have linked differentiation therapy to the epigenetic regulation of hTERT, and a large number of demethylating agents and HDAC inhibitors have achieved significant clinical successes in inducing the differentiation of human leukemia cells (Table I). Low methylation levels of the hTERT promoter core domain have been shown to correlate with high telomerase activity in patients with B-cell chronic lymphocytic leukemia (B-CLL), whereas a high degree of methylation indicates low enzyme activity. Moreover, patients with a high level of telomerase activity show a worse prognosis (47,48). Azacitidine and its deoxy analogue, decitabine, which are two DNMT inhibitors, have been approved as single agents to treat patients with leukemia through the induction of cell differentiation (Fig. 1) (49–52). HDAC inhibitors are agents that have attracted interest due to their ability to induce the differentiation of leukemic cells, and are now in pre- and early clinical trials as monotherapies and in combination with other drugs (53,54). Previous studies have shown that the transcriptional suppression of the hTERT gene during all-trans retinoic acid (ATRA) treatment is associated with the differentiation of leukemia cells, partly due to DNA methylation and histone deacetylation in the hTERT promoter region (47,55–56). Recently, it has been revealed that hTERT is downregulated 5-fold through epigenetic and protein acetylation mechanisms using a combined treatment of aurora kinase inhibitors (AKi) and HDAC inhibitors (57,58). Azouz et al identified two distinct functional domains of the hTERT promoter, the proximal and distal domains, and identified that the epigenetic modifications of the distal region determined the retinoid capacity to repress telomerase in maturation-resistant acute promyelocytic leukemia cells during cellular differentiation (59). Love et al showed that epigenetic regulation stabilized hTERT inhibition and thus maintained telomerase activity in a silenced state during the ATRA-induced differentiation of HL60 human leukemia cells (60). Altogether, these data indicate that epigenetic mechanisms may represent a target for maintaining the differentiated phenotype of human leukemia cells.
Table I.
Selected drugs with epigenetic targets in the preclinical and clinical development of leukemia.
| Drug target | Drug | Chemical class | Study in leukemia | Clinical trials |
|---|---|---|---|---|
| DNMT inhibitor | Azacitidine | Nucleoside analog | ALL, AML, CML | + |
| DNMT inhibitor | Decitabine (DAC) | Nucleoside analog | ALL, AML, CML | + |
| HDAC inhibitor | Valproic acid (VPA) | Short-chain fatty acid | AML, CLL, CML | + |
| HDAC inhibitor | Trichostatin A (TSA) | Hydroxamic acid | Preclinical trials | N/A |
| HDAC inhibitor | Panobinostat (LBH589) | Hydroxamic acid | ALL, AML | + |
| HDAC inhibitor | Depsipeptide (FR901228/FK228) | Cyclic tetrapeptide | AML | + |
| HDAC inhibitor | Entinostat (MS275/SNDX-275) | Benzamide | ALL, AML | + |
| HDAC inhibitor | MGCD0103 | Benzamide | AML, CLL | + |
AML, acute myelogenous leukemia; ALL, acute lymphocytic leukemia; CLL, chronic lymphocytic leukemia; CML, chronic myelogenous leukemia. DNMT, DNA methyltransferase; HDAC, histone deactylase.
In addition to inducing cell differentiation, telomerase inhibition through epigenetic mechanisms has been reported to promote growth arrest, apoptosis and sensitivity to certain chemotherapeutic reagents in human acute leukemia cells. Woo et al demonstrated that TSA had an antiproliferative and apoptosis-inducing effect on the human leukemic cell line U937, and that these growth-inhibitory effects were associated with the inhibition of hTERT expression and telomerase activity. Therefore, a loss of telomerase activity may be a good surrogate biomarker to assess the antitumor activity of TSA in human leukemic cells (61). The resistance to imatinib is a major problem in chronic myelogenous leukemia (CML) treatment, and recent studies have shown that by targeting telomerase expression using a dominant-negative form of the catalytic protein subunit of hTERT, or by the treatment with HDAC inhibitors, the risk of imatinib resistance may be reduced and the imatinib-induced apoptosis in leukemia cells may be enhanced, suggesting that antitelomerase strategies may be able to prevent, or at least delay the onset of such resistance (62,63).
4. Future perspectives
The hTERT gene is usually transcriptionally inactivated in differentiated cells, but is reactivated in the majority of leukemia cells. As previously discussed, accumulating evidence suggests that epigenetic changes in the hTERT promoter may be a prominent mechanism of telomerase activity control. Therefore, antitelomerase strategies using epigenetic mechanisms may represent a promising target for the treatment of leukemia. There are two major approaches in advanced clinical trials to target telomerase-positive leukemia cells. Firstly, the use of direct telomerase hTR subunit small molecule inhibitors, such as telomestatin (SOT-095), several of which are currently in preclinical trials for acute leukemia (64,65). The second approach involves using epigenetic modification drugs against the hTERT protein; these drugs are currently being used for, or have completed trials for the treatment of leukemia. At present, there is an increasing interest in using epigenetic modifiers as candidate chemotherapeutic agents in human leukemia.
Epigenetic modifiers that are currently available in preclinical and early clinical trials of leukemia target DNMTs through DNMT inhibitors, or alter the status of the histones using HDAC inhibitors, in order to modulate gene transcription. It has been noted that epigenetic modifications contribute to hTERT gene expression and telomerase activity, resulting in a positive effect in the treatment of leukemia (59,61). In addition to epigenetic modifiers, the use of several telomerase hTR subunit small molecule inhibitors has resulted in the specific inhibition of telomerase activity. Therefore, an antitelomerase strategy involving a combination of epigenetic modifiers and telomerase hTR subunit small molecule inhibitors may exert a more potent effect for the treatment of human leukemia (Fig. 3).
Figure 3.
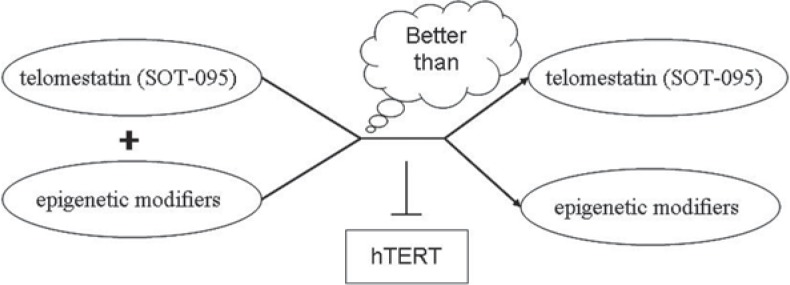
A hypothesis is associated with antitelomerase strategy. The antitelomerase strategy, created by combining epigenetic modifiers with telomerase hTR subunit small molecule inhibitors (such as SOT-095), may exert a more potent effect for the treatment of human leukemia, since each approach is able to individually inhibit telomerase activity. hTERT, human telomerase reverse transcriptase.
Although epigenetic modifiers have shown promise as therapies for human leukemia in early clinical trials, certain limitations prevent their widespread clinical application. Firstly, the exact molecular mechanisms underlying the epigenetic regulation and hTERT expression remain to be elucidated, as do numerous details with regard to telomerase regulation. An improved understanding of the linkage will facilitate the identification of more specific and selective epigenetic modifiers for leukemia cells (66). Secondly, a broad spectrum of biological and potentially adverse effects have been identified following treatment using epigenetic modifiers. Further investigation with regard to these effects is required in large-scale and multicentric populations of treated patients (67). Thirdly, further studies will be required to identify whether the inhibition of hTERT gene expression is causal or consequential to the anticancer effects of epigenetic modifiers, and whether the hTERT gene or telomerase activity may be an appropriate predictive biomarker for assessing the antitumor activity of these agents in human leukemia cells (68). Finally, it should be taken into account whether the antitelomerase approach using epigenetic modifiers with telomerase hTR subunit small molecule inhibitors may be a better combinatorial strategy when compared with methods that are already used in prospective clinical trials.
Despite the unanswered biological questions, an increased understanding of the role of epigenetic regulation in hTERT gene expression and the treatment of leukemia may provide a prospective anticancer therapeutic approach in the form of the antitelomerase strategy.
Acknowledgments
This study is supported by Zhejiang University and grants from the National Natural Science Foundation of China (grant no. 81272593) and the Zhejiang Provincial Natural Science Foundation of China (grant no. LQ13H160008).
References
- 1.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 2.Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 3.Morin GB. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989;59:521–529. doi: 10.1016/0092-8674(89)90035-4. [DOI] [PubMed] [Google Scholar]
- 4.Cohen SB, Graham ME, Lovrecz GO, Bache N, Robinson PJ, Reddel RR. Protein composition of catalytically active human telomerase from immortal cells. Science. 2007;315:1850–1853. doi: 10.1126/science.1138596. [DOI] [PubMed] [Google Scholar]
- 5.Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661–673. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- 6.Pinto AR, Li H, Nicholls C, Liu JP. Telomere protein complexes and interactions with telomerase in telomere maintenance. Front Biosci. 2011;16:187–207. doi: 10.2741/3683. [DOI] [PubMed] [Google Scholar]
- 7.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 8.Agrelo R, Wutz A. Cancer progenitors and epigenetic contexts: an Xisting connection. Epigenetics. 2009;4:568–570. doi: 10.4161/epi.4.8.10186. [DOI] [PubMed] [Google Scholar]
- 9.Vineis P, Chuang SC, Vaissière T, Cuenin C, Ricceri F, Genair-EPIC Collaborators. Johansson M, Ueland P, Brennan P, Herceg Z. DNA methylation changes associated with cancer risk factors and blood levels of vitamin metabolites in a prospective study. Epigenetics. 2011;6:195–201. doi: 10.4161/epi.6.2.13573. [DOI] [PubMed] [Google Scholar]
- 10.Laird PW. The power and the promise of DNA methylation markers. Nat Rev Cancer. 2003;3:253–266. doi: 10.1038/nrc1045. [DOI] [PubMed] [Google Scholar]
- 11.Baylin SB, Ohm JE. Epigenetic gene silencing in cancer - a mechanism for early oncogenic pathway addiction? Nat Rev Cancer. 2006;6:107–116. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- 12.Shao C, Lacey M, Dubeau L, Ehrlich M. Hemimethylation footprints of DNA demethylation in cancer. Epigenetics. 2009;4:165–175. doi: 10.4161/epi.4.3.8277. [DOI] [PubMed] [Google Scholar]
- 13.Sawan C, Herceg Z. Histone modifications and cancer. Adv Genet. 2010;70:57–85. doi: 10.1016/B978-0-12-380866-0.60003-4. [DOI] [PubMed] [Google Scholar]
- 14.Rivière G, Lienhard D, Andrieu T, Vieau D, Frey BM, Frey FJ. Epigenetic regulation of somatic angiotensin-converting enzyme by DNA methylation and histone acetylation. Epigenetics. 2011;6:478–489. doi: 10.4161/epi.6.4.14961. [DOI] [PubMed] [Google Scholar]
- 15.Cairney CJ, Keith WN. Telomerase redefined: integrated regulation of hTR and hTERT for telomere maintenance and telomerase activity. Biochimie. 2008;90:13–23. doi: 10.1016/j.biochi.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 16.Dessain SK, Yu H, Reddel RR, Beijersbergen RL, Weinberg RA. Methylation of the human telomerase gene CpG island. Cancer Res. 2000;60:537–541. [PubMed] [Google Scholar]
- 17.Zhu J, Zhao Y, Wang S. Chromatin and epigenetic regulation of the telomerase reverse transcriptase gene. Protein Cell. 2010;1:22–32. doi: 10.1007/s13238-010-0014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iliopoulos D, Oikonomou P, Messinis I, Tsezou A. Correlation of promoter hypermethylation in hTERT, DAPK and MGMT genes with cervical oncogenesis progression. Oncol Rep. 2009;22:199–204. doi: 10.3892/or_00000425. [DOI] [PubMed] [Google Scholar]
- 19.Gigek CO, Leal MF, Silva PN, Lisboa LC, Lima EM, Calcagno DQ, Assumpção PP, Burbano RR, Smith Mde A. hTERT methylation and expression in gastric cancer. Biomarkers. 2009;14:630–636. doi: 10.3109/13547500903225912. [DOI] [PubMed] [Google Scholar]
- 20.Wang S, Hu C, Zhu J. Distinct and temporal roles of nucleosomal remodeling and histone deacetylation in the repression of the hTERT gene. Mol Biol Cell. 2010;21:821–832. doi: 10.1091/mbc.E09-06-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jia W, Wang S, Horner JW, Wang N, Wang H, Gunther EJ, DePinho RA, Zhu J. A BAC transgenic reporter recapitulates in vivo regulation of human telomerase reverse transcriptase in development and tumorigenesis. FASEB J. 2011;25:979–989. doi: 10.1096/fj.10-173989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baird DM, Rowson J, Wynford-Thomas D, Kipling D. Extensive allelic variation and ultrashort telomeres in senescent human cells. Nat Genet. 2003;33:203–207. doi: 10.1038/ng1084. [DOI] [PubMed] [Google Scholar]
- 23.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Liu L, Andrews LG, Tollefsbol TO. Genistein depletes telomerase activity through cross-talk between genetic and epigenetic mechanisms. Int J Cancer. 2009;125:286–296. doi: 10.1002/ijc.24398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457–463. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- 26.Lopatina NG, Poole JC, Saldanha SN, Hansen NJ, Key JS, Pita MA, Andrews LG, Tollefsbol TO. Control mechanisms in the regulation of telomerase reverse transcriptase expression in differentiating human teratocarcinoma cells. Biochem Biophys Res Commun. 2003;306:650–659. doi: 10.1016/s0006-291x(03)01033-7. [DOI] [PubMed] [Google Scholar]
- 27.Shin KH, Kang MK, Dicterow E, Park NH. Hypermethylation of the hTERT promoter inhibits the expression of telomerase activity in normal oral fibroblasts and senescent normal oral keratinocytes. Br J Cancer. 2003;89:1473–1478. doi: 10.1038/sj.bjc.6601291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zinn RL, Pruitt K, Eguchi S, Baylin SB, Herman JG. hTERT is expressed in cancer cell lines despite promoter DNA methylation by preservation of unmethylated DNA and active chromatin around the transcription start site. Cancer Res. 2007;67:194–201. doi: 10.1158/0008-5472.CAN-06-3396. [DOI] [PubMed] [Google Scholar]
- 29.Devereux TR, Horikawa I, Anna CH, Annab LA, Afshari CA, Barrett JC. DNA methylation analysis of the promoter region of the human telomerase reverse transcriptase (hTERT) gene. Cancer Res. 1999;59:6087–6090. [PubMed] [Google Scholar]
- 30.Kumari A, Srinivasan R, Vasishta RK, Wig JD. Positive regulation of human telomerase reverse transcriptase gene expression and telomerase activity by DNA methylation in pancreatic cancer. Ann Surg Oncol. 2009;16:1051–1059. doi: 10.1245/s10434-009-0333-8. [DOI] [PubMed] [Google Scholar]
- 31.Kumari A, Srinivasan R, Wig JD. Effect of c-MYC and E2F1 gene silencing and of 5-azacytidine treatment on telomerase activity in pancreatic cancer-derived cell lines. Pancreatology. 2009;9:360–368. doi: 10.1159/000212094. [DOI] [PubMed] [Google Scholar]
- 32.Guilleret I, Yan P, Grange F, Braunschweig R, Bosman FT, Benhattar J. Hypermethylation of the human telomerase catalytic subunit (hTERT) gene correlates with telomerase activity. Int J Cancer. 2002;101:335–341. doi: 10.1002/ijc.10593. [DOI] [PubMed] [Google Scholar]
- 33.Krajewski WA. Histone acetylation status and DNA sequence modulate ATP-dependent nucleosome repositioning. J Biol Chem. 2002;277:14509–14513. doi: 10.1074/jbc.M107510200. [DOI] [PubMed] [Google Scholar]
- 34.Lai SR, Phipps SM, Liu L, Andrews LG, Tollefsbol TO. Epigenetic control of telomerase and modes of telomere maintenance in aging and abnormal systems. Front Biosci. 2005;10:1779–1796. doi: 10.2741/1661. [DOI] [PubMed] [Google Scholar]
- 35.Takakura M, Kyo S, Sowa Y, Wang Z, Yatabe N, Maida Y, Tanaka M, Inoue M. Telomerase activation by histone deacetylase inhibitor in normal cells. Nucleic Acids Res. 2001;29:3006–3011. doi: 10.1093/nar/29.14.3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hou M, Wang X, Popov N, Zhang A, Zhao X, Zhou R, Zetterberg A, Björkholm M, Henriksson M, Gruber A, Xu D. The histone deacetylase inhibitor trichostatin A derepresses the telomerase reverse transcriptase (hTERT) gene in human cells. Exp Cell Res. 2002;274:25–34. doi: 10.1006/excr.2001.5462. [DOI] [PubMed] [Google Scholar]
- 37.Murakami J, Asaumi J, Kawai N, Tsujigiwa H, Yanagi Y, Nagatsuka H, Inoue T, Kokeguchi S, Kawasaki S, Kuroda M, Tanaka N, Matsubara N, Kishi K. Effects of histone deacetylase inhibitor FR901228 on the expression level of telomerase reverse transcriptase in oral cancer. Cancer Chemother Pharmacol. 2005;56:22–28. doi: 10.1007/s00280-004-0976-x. [DOI] [PubMed] [Google Scholar]
- 38.Zhu K, Qu D, Sakamoto T, Fukasawa I, Hayashi M, Inaba N. Telomerase expression and cell proliferation in ovarian cancer cells induced by histone deacetylase inhibitors. Arch Gynecol Obstet. 2008;277:15–19. doi: 10.1007/s00404-007-0423-4. [DOI] [PubMed] [Google Scholar]
- 39.Suenaga M, Soda H, Oka M, Yamaguchi A, Nakatomi K, Shiozawa K, Kawabata S, Kasai T, Yamada Y, Kamihira S, Tei C, Kohno S. Histone deacetylase inhibitors suppress telomerase reverse transcriptase mRNA expression in prostate cancer cells. Int J Cancer. 2002;97:621–625. doi: 10.1002/ijc.10082. [DOI] [PubMed] [Google Scholar]
- 40.Khaw AK, Silasudjana M, Banerjee B, Suzuki M, Baskar R, Hande MP. Inhibition of telomerase activity and human telomerase reverse transcriptase gene expression by histone deacetylase inhibitor in human brain cancer cells. Mutat Res. 2007;625:134–144. doi: 10.1016/j.mrfmmm.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 41.Rice JC, Briggs SD, Ueberheide B, Barber CM, Shabanowitz J, Hunt DF, Shinkai Y, Allis CD. Histone methyltransferases direct different degrees of methylation to define distinct chromatin domains. Mol Cell. 2003;12:1591–1598. doi: 10.1016/s1097-2765(03)00479-9. [DOI] [PubMed] [Google Scholar]
- 42.Atkinson SP, Hoare SF, Glasspool RM, Keith WN. Lack of telomerase gene expression in alternative lengthening of telomere cells is associated with chromatin remodeling of the hTR and hTERT gene promoters. Cancer Res. 2005;65:7585–7590. doi: 10.1158/0008-5472.CAN-05-1715. [DOI] [PubMed] [Google Scholar]
- 43.Liu C, Fang X, Ge Z, Jalink M, Kyo S, Björkholm M, Gruber A, Sjöberg J, Xu D. The telomerase reverse transcriptase (hTERT) gene is a direct target of the histone methyltransferase SMYD3. Cancer Res. 2007;67:2626–26. doi: 10.1158/0008-5472.CAN-06-4126. [DOI] [PubMed] [Google Scholar]
- 44.Röth A, Vercauteren S, Sutherland HJ, Lansdorp PM. Telomerase is limiting the growth of acute myeloid leukemia cells. Leukemia. 2003;17:2410–2417. doi: 10.1038/sj.leu.2403177. [DOI] [PubMed] [Google Scholar]
- 45.Annaloro C, Onida F, Saporiti G, Lambertenghi Deliliers G. Cancer stem cells in hematological disorders: current and possible new therapeutic approaches. Curr Pharm Biotechnol. 2011;12:217–225. doi: 10.2174/138920111794295747. [DOI] [PubMed] [Google Scholar]
- 46.Liu L, Saldanha SN, Pate MS, Andrews LG, Tollefsbol TO. Epigenetic regulation of human telomerase reverse transcriptase promoter activity during cellular differentiation. Genes Chromosomes Cancer. 2004;41:26–37. doi: 10.1002/gcc.20058. [DOI] [PubMed] [Google Scholar]
- 47.Bechter OE, Eisterer W, Dlaska M, Kühr T, Thaler J. CpG island methylation of the hTERT promoter is associated with lower telomerase activity in B-cell lymphocytic leukemia. Exp Hematol. 2002;30:26–33. doi: 10.1016/s0301-472x(01)00760-3. [DOI] [PubMed] [Google Scholar]
- 48.Terrin L, Trentin L, Degan M, Corradini I, Bertorelle R, Carli P, Maschio N, Bo MD, Noventa F, Gattei V, Semenzato G, De Rossi A. Telomerase expression in B-cell chronic lymphocytic leukemia predicts survival and delineates subgroups of patients with the same igVH mutation status and different outcome. Leukemia. 2007;21:965–972. doi: 10.1038/sj.leu.2404607. [DOI] [PubMed] [Google Scholar]
- 49.Lübbert M, Suciu S, Baila L, Rüter BH, Platzbecker U, Giagounidis A, Selleslag D, Labar B, Germing U, Salih HR, Beeldens F, Muus P, Pflüger KH, Coens C, Hagemeijer A, Eckart Schaefer H, Ganser A, Aul C, de Witte T, Wijermans PW. Low-dose decitabine versus best supportive care in elderly patients with intermediate- or high-risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy: final results of the randomized phase III study of the European Organisation for Research and Treatment of Cancer Leukemia Group and the German MDS Study Group. J Clin Oncol. 2011;29:1987–1996. doi: 10.1200/JCO.2010.30.9245. [DOI] [PubMed] [Google Scholar]
- 50.Kantarjian HM, Giles FJ, Greenberg PL, Paquette RL, Wang ES, Gabrilove JL, Garcia-Manero G, Hu K, Franklin JL, Berger DP. Phase 2 study of romiplostim in patients with low- or intermediate-risk myelodysplastic syndrome receiving azacitidine therapy. Blood. 2010;116:3163–3170. doi: 10.1182/blood-2010-03-274753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baer MR, Gojo I. Novel agents for the treatment of acute myeloid leukemia in the older patient. J Natl Compr Canc Netw. 2011;9:331–335. doi: 10.6004/jnccn.2011.0029. [DOI] [PubMed] [Google Scholar]
- 52.Garcia-Manero G, Gore SD, Cogle C, Ward R, Shi T, Macbeth KJ, Laille E, Giordano H, Sakoian S, Jabbour E, Kantarjian H, Skikne B. Phase I study of oral azacitidine in myelodysplastic syndromes, chronic myelomonocytic leukemia, and acute myeloid leukemia. J Clin Oncol. 2011;29:2521–2527. doi: 10.1200/JCO.2010.34.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Claus R, Lübbert M. Epigenetic targets in hematopoietic malignancies. Oncogene. 2003;22:6489–6496. doi: 10.1038/sj.onc.1206814. [DOI] [PubMed] [Google Scholar]
- 54.Lane AA, Chabner BA. Histone deacetylase inhibitors in cancer therapy. J Clin Oncol. 2009;27:5459–5468. doi: 10.1200/JCO.2009.22.1291. [DOI] [PubMed] [Google Scholar]
- 55.Pendino F, Sahraoui T, Lanotte M, Ségal-Bendirdjian E. A novel mechanism of retinoic acid resistance in acute promyelocytic leukemia cells through a defective pathway in telomerase regulation. Leukemia. 2002;16:826–832. doi: 10.1038/sj.leu.2402470. [DOI] [PubMed] [Google Scholar]
- 56.Pendino F, Flexor M, Delhommeau F, Buet D, Lanotte M, Segal-Bendirdjian E. Retinoids down-regulate telomerase and telomere length in a pathway distinct from leukemia cell differentiation. Proc Natl Acad Sci USA. 2001;98:6662–6667. doi: 10.1073/pnas.111464998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kretzner L, Scuto A, Dino PM, Kowolik CM, Wu J, Ventura P, Jove R, Forman SJ, Yen Y, Kirschbaum MH. Combining histone deacetylase inhibitor vorinostat with aurora kinase inhibitors enhances lymphoma cell killing with repression of c-Myc, hTERT, and microRNA levels. Cancer Res. 2011;71:3912–3920. doi: 10.1158/0008-5472.CAN-10-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quintás-Cardama A, Santos FP, Garcia-Manero G. Histone deacetylase inhibitors for the treatment of myelodysplastic syndrome and acute myeloid leukemia. Leukemia. 2011;25:226–235. doi: 10.1038/leu.2010.276. [DOI] [PubMed] [Google Scholar]
- 59.Azouz A, Wu YL, Hillion J, Tarkanyi I, Karniguian A, Aradi J, Lanotte M, Chen GQ, Chehna M, Ségal-Bendirdjian E. Epigenetic plasticity of hTERT gene promoter determines retinoid capacity to repress telomerase in maturation-resistant acute promyelocytic leukemia cells. Leukemia. 2010;24:613–622. doi: 10.1038/leu.2009.283. [DOI] [PubMed] [Google Scholar]
- 60.Love WK, Berletch JB, Andrews LG, Tollefsbol TO. Epigenetic regulation of telomerase in retinoid-induced differentiation of human leukemia cells. Int J Oncol. 2008;32:625–631. [PMC free article] [PubMed] [Google Scholar]
- 61.Woo HJ, Lee SJ, Choi BT, Park YM, Choi YH. Induction of apoptosis and inhibition of telomerase activity by trichostatin A, a histone deacetylase inhibitor, in human leukemic U937 cells. Exp Mol Pathol. 2007;82:77–84. doi: 10.1016/j.yexmp.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 62.Okabe S, Tauchi T, Nakajima A, Sashida G, Gotoh A, Broxmeyer HE, Ohyashiki JH, Ohyashiki K. Depsipeptide (FK228) preferentially induces apoptosis in BCR/ABL-expressing cell lines and cells from patients with chronic myelogenous leukemia in blast crisis. Stem Cells Dev. 2007;16:503–514. doi: 10.1089/scd.2007.9994. [DOI] [PubMed] [Google Scholar]
- 63.Deville L, Hillion J, Pendino F, Samy M, Nguyen E, Ségal-Bendirdjian E. hTERT promotes imatinib resistance in chronic myeloid leukemia cells: therapeutic implications. Mol Cancer Ther. 2011;10:711–719. doi: 10.1158/1535-7163.MCT-10-0979. [DOI] [PubMed] [Google Scholar]
- 64.Nakajima A, Tauchi T, Sashida G, Sumi M, Abe K, Yamamoto K, Ohyashiki JH, Ohyashiki K. Telomerase inhibition enhances apoptosis in human acute leukemia cells: possibility of anti-telomerase therapy. Leukemia. 2003;17:560–567. doi: 10.1038/sj.leu.2402825. [DOI] [PubMed] [Google Scholar]
- 65.Tauchi T, Shin-ya K, Sashida G, Sumi M, Okabe S, Ohyashiki JH, Ohyashiki K. Telomerase inhibition with a novel G-quadruplex-interactive agent, telomestatin: in vitro and in vivo studies in acute leukemia. Oncogene. 2006;25:5719–5725. doi: 10.1038/sj.onc.1209577. [DOI] [PubMed] [Google Scholar]
- 66.Altucci L, Clarke N, Nebbioso A, Scognamiglio A, Gronemeyer H. Acute myeloid leukemia: therapeutic impact of epigenetic drugs. Int J Biochem Cell Biol. 2005;37:1752–1762. doi: 10.1016/j.biocel.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 67.Bhalla KN. Epigenetic and chromatin modifiers as targeted therapy of hematologic malignancies. J Clin Oncol. 2005;23:3971–3993. doi: 10.1200/JCO.2005.16.600. [DOI] [PubMed] [Google Scholar]
- 68.Piekarz RL, Bates SE. Epigenetic modifiers: basic understanding and clinical development. Clin Cancer Res. 2009;15:3918–3926. doi: 10.1158/1078-0432.CCR-08-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]