Abstract
The changes in testosterone and gonadotropin levels in patients who have undergone radical prostatectomy (RP) for clinically localized prostate cancer (PCa) remain unclear. The aim of the present study was to prospectively evaluate the changes in serum testosterone (Te), luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels in the early months after RP for PCa and the correlation between these hormones at various follow-up times. A total of 100 male patients with clinically localized PCa were consecutively included in the study. The serum levels of Te, LH and FSH were measured prior to RP (baseline) and at 1 and 3 months post-operatively. Changes in the levels of Te, LH and FSH between the baseline and at 1 and 3 months after RP were analyzed with paired sample t-tests. The correlations between LH and Te levels at the various follow-up times were evaluated with a Spearman’s rank correlation coefficient. At 1 month subsequent to RP, the Te levels were significantly decreased (baseline vs. 1 month, P=0.021) and subsequently recovered to the pre-operative value at 3 months (baseline vs. 3 months, P=0.372). The mean Te level at baseline was 15.3 nmol/l, while at 1 and 3 months it was 13.8 and 14.4 nmol/l, respectively. By contrast, the levels of LH and FSH were significantly increased at 1 and 3 months post-surgery, compared with the baseline value (baseline vs. 1 or 3 months, P<0.0001). The pre-operative correlation between LH and Te was lost 1 month after RP and recovered after 3 months. Notably, the LH level at 1 month was markedly correlated with the Te levels recorded after 3 months. In the present study, patients developed compensated hypergonadotropic hypogonadism 3 months after RP.
Keywords: prostate neoplasms, radical prostatectomy, hypogonadism, serum testosterone, luteinizing hormone, follicle-stimulating hormone
Introduction
Several studies have demonstrated that erectile dysfunction and lower urinary tract symptoms due to prostatic disorders may share a common pathophysiological pathway (1,2). The serum level of testosterone (Te) may affect sexual function in males treated by radical prostatectomy (RP) for clinically localized prostate cancer (PCa) (3). It has been suggested that Te may also affect urinary continence and increase bladder compliance following RP, with a relaxing effect in smooth muscle bladder cells through the nitric oxide synthase/nitric oxide pathway (4,5). Moreover, certain authors have reported a significant correlation between Te levels and adverse pathological features and biochemical recurrence, even though the prognostic value of pre-operative Te remains unclear (6–7). Few preliminary studies have evaluated the changes in the levels of Te and gonadotropins following RP, and these have produced conflicting results (9–12). In addition, little is known concerning the possible effect of PCa cells on the hypothalamic-pituitary hormone axis, although certain authors have suggested that patients with PCa showed lower gonadotropin levels compared with similarly aged males without PCa (12). Furthermore, there is no data with regard to changes in sex hormones in the early post-operative period following RP. The aim of the present study was to prospectively evaluate the changes in the serum levels of Te, luteinizing hormone (LH) and follicle-stimulating hormone (FSH) within the first 3 months after RP for clinically localized PCa and to analyze the correlation between LH and Te at various follow-up times.
Materials and methods
Study design
A total of 100 male patients with clinically localized PCa were consecutively included in the study, from February to July 2010. The levels of Te, LH and FSH were assessed pre-operatively and at 1 and 3 months post-operatively. This study was approved by the ethics committee of the University of Florence. Informed consent was obtained from all patients. This study was planned as a longitudinal cohort study.
Patients
A total of 100 male patients with newly diagnosed, biopsy proven, clinically localized PCa [prostate-specific antigen (PSA) <10 mg/ml, clinical stage <T3, negative computed tomography (CT) scan or bone scintigraphy] prior to RP were included in the study. Subjects with a known history of endocrinological diseases, including hypogonadism, previous Te substitutive therapy and/or hormonal manipulation (including 5α-reductase inhibitors, androgen receptor blockers and LH-releasing hormone analogues), were excluded from the study. The adjunctive exclusion criteria were a classification of Eastern Cooperative Oncology Group (ECOG) grade >1, previous radiation therapy and chronic ingestion of alcohol or other drugs, including steroids, barbiturates, spironolactone and cimetidine, that may have interfered with the serum hormone levels.
Clinical and serological data collection
The clinical variables registered for each patient included age at surgery, corporal features [body mass index(BMI), weight and height] and sex hormone profiles, including total Te, LH and FSH. Serum samples were collected by venipuncture between 8:00 and 11:00 a.m., 7 to 14 days prior to RP and at the 1- and 3-month follow-ups. The serum levels of Te, LH and FSH were quantified using an electrochemoluminescence method (Modular Roche, Milan, Italy) at the laboratories of the Sexual Medicine Outpatient Clinic, University of Florence, Italy.
Surgical procedures
The surgical indications were in accordance with the European Association of Urology guidelines for clinically localized PCa (13). RP was performed with an open retropubic antegrade approach, according to our previously described surgical procedures (14). All surgical procedures were performed at the Department of Urology, University of Florence, Florence, Italy, by the same 3 surgeons (M.G., M.C. and S.S.). Each patient provided written informed consent prior to their surgical procedure.
Statistical analysis
The Kolmogorov-Smirnov test was used to test the distribution of each parameter. Data are expressed as the median (quartiles) when not normally distributed and as the mean ± SD when normally distributed. The distributions of LH and FSH were normalized through logarithmic transformations: logarithmically transformed LH and FSH were expressed as logLH and logFSH, respectively.
Comparisons between the serum hormone levels at baseline and the levels at 1 and 3 months after RP were performed with paired t-tests for normally distributed parameters. The same test was applied to the logarithmically transformed data for LH and FSH. The correlation between the logarithmically transformed LH and Te values at each visit was calculated using Pearson’s correlation coefficient. All statistical tests were performed using SPSS version 17.0 statistical software (SPSS, Inc., Chicago, IL, USA).
Results
Pre-operative data
The baseline patient characteristics are shown in Table I. Subsequent to 3 months, complete hormonal data (baseline, 1 and 3 months) were available for 92/100 patients. In total, 4 patients voluntarily withdrew from the study and were excluded from the results, while 4 were not analyzed due to adjuvant radiation and/or hormonal treatment for persistent PCa (R+, N+) or as they were lost to follow-up.
Table I.
Pre-operative clinical and hormonal characteristics of 100 male patients with PCa.
| Characteristic | Value |
|---|---|
| Pre-operative clinical data | |
| Age | |
| Mean ± SD (years) | 64.8±6.5 |
| Range (years) | 48–75 |
| <65 (n) | 52 |
| ≥65 (n) | 48 |
| BMI (kg/m2) | |
| Mean ± SD | 25.8±3.1 |
| Range | 19.5–34.6 |
| PSA (ng/ml) | |
| Mean ± SD | 7.3±4.0 |
| 0–2.4 | 6 |
| 2.5–3.9 | 8 |
| 4–10 | 69 |
| 10.1–19.9 | 17 |
| Pathological outcome | |
| Pathological Stage (n) | |
| pT2 | 54 |
| pT3 | 46 |
| pT4 | 0 |
| Gleason Score of surgical specimens (n) | |
| <6 | 0 |
| 6 | 55 |
| 7 | 29 |
| 8–10 | 16 |
| Lymph nodes status (n) | |
| N0 | 98 |
| N+ | 2 |
| Surgical margins (n) | |
| Positive | 2 |
| Negative | 98 |
| Hormone levels | |
| Testosterone (nmol/ml) | |
| Mean ± SD | 15.3±5.9 |
| Range | 5.7–37.8 |
| LH (mIU/ml) | |
| Median | 3.5 |
| Quartiles | 2.5–3.5–5.1 |
| FSH (mIU/ml) | |
| Median | 5.0 |
| Quartiles | 3.3–5.0–8.1 |
Values are expressed as the mean ± SD when normally distributed and as median (quartiles) when not normally distributed. LH, luteinizing hormone; FSH, follicle-stimulating hormone; PSA, prostate-specific antigen; BMI, body mass index; PCa, prostate cancer.
Changes in sex hormone levels following RP
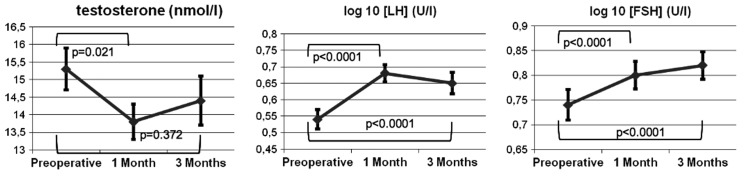
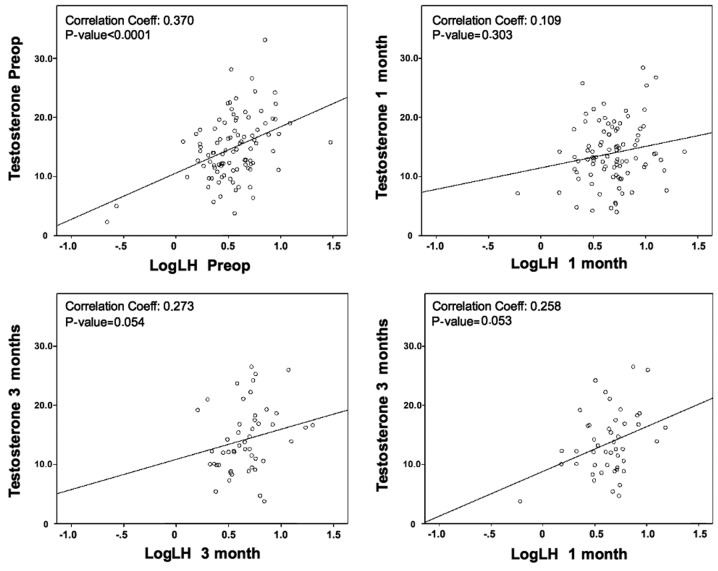
The results of the laboratory analyses of Te, logLH and logFSH are shown in Fig. 1. A significant reduction in Te levels was observed 1 month after RP, as compared with the baseline (baseline vs. 1 month, 15.3 vs. 13.8 nmol/l; P=0.021), with an increase at 3 months that nearly reached the pre-operative baseline value (baseline vs. 3 months, 15.3 vs. 14.4 nmol/l; P=0.372). In contrast, the logLH level was significantly increased at 1 and 3 months post-surgery, compared with the baseline (baseline vs. 1 month logLH, 0.54 vs. 0.68 mIU/ml; P<0.0001; and baseline vs. 3 months logLH, 0.54 vs. 0.65 mIU/ml; P<0.0001). Furthermore, the logFSH level was significantly increased at 1 month after RP (baseline vs. 1 month logFSH, 0.74 vs. 0.80 mIU/ml; P<0.0001) and reached the highest value at the 3-month follow-up (baseline vs. 3 months logFSH, 0.74 vs. 0.82 mIU/ml; P<0.0001). The correlation between the LH and Te serum levels was then evaluated at various follow-up times (Fig. 2). A marked positive correlation was observed between Te and LH pre-operatively (pre-operative logLH-Te, r=0.370; P<0.0001). This correlation was not apparent 1 month after RP (one month logLH-Te, r=0.109; P=0.303). However, the LH level measured at 1 month was markedly correlated with the Te level at 3 months (one month logLH-3 month Te, r=0.258; P=0,053). At 3 months post-surgery, the correlation between Te and LH was completely recovered (three month logLH-Te, r=0.273; P=0.054).
Figure 1.
Comparisons of the serum hormone levels at baseline with those at 1 and 3 months from 92 PCa patients with complete data. Paired t-tests were used for Te (normally distributed, expressed as nmol/l) and logarithmically transformed LH and FSH (not normally distributed, expressed as U/l). Data are expressed as the mean ± standard error of the mean. LH, luteinizing hormone; FSH, follicle-stimulating hormone; Te, testosterone; PCa, prostate cancer.
Figure 2.
Correlation analysis (Pearson’s correlation coefficient) between testosterone and logarithmically transformed LH values at each visit of the study, of the 92 PCa patients with complete data. LH, luteinizing hormone; Pre-op, pre-operative; PCa, prostate cancer.
Discussion
In the early months after RP, the present study observed a significant decline and subsequent recovery of Te, associated with increased LH and FSH, which persisted at higher levels at 3 months compared with the pre-operative value. Therefore, the removal of the whole prostate gland was followed by the development of compensated hypergonadotropic hypogonadism. To date, only a few studies have evaluated the changes in sex hormone levels following prostatic surgery, and little is known with regard to the effect of healthy prostatic tissue or PCa on the hypothalamic-pituitary hormone axis (15).
Certain studies have shown that there are no significant changes in these hormones following surgical treatment for benign prostatic hyperplasia (BPH) (12,16,17). Further investigations are required, but on the basis of these studies it appears that BPH does not significantly affect the hypothalamic-pituitary axis. In contrast, other studies have previously presented changes in the levels of sex hormones after RP for PCa, but there is no consensus with regard to the existence and degree of these changes. Olsson et al reported a significant increase in LH and FSH levels in 55 males following RP in a pilot study (10). These findings have been supported by Madersbacher et al, who observed that LH and FSH were increased by 71 and 63%, respectively, in 49 patients 12 months after RP, without any evident changes to the Te level (12). In a slightly different manner, Miller et al showed a significant increase in Te, as well as LH and FSH, in 63 patients 1 year after RP, when compared with the pre-operative value (9). There are two main differences between the present and previous studies. First, the first post-operative evaluation was subsequent to only 1 month, whereas in the previous studies, it was at 3 or 6 months. This enabled us to make the new observation, not reported by previous studies, that Te was transiently decreased 1 month after RP. The second difference was the correlation analysis with LH. The decrease in Te levels at 1 month was unassociated with LH, while at 3 months, the Te level recovered the correlation with LH, as well as its baseline value. A possible explanation for these data is that the surgical intervention caused a reduction in the testicular production of Te, which stimulated the increased production of gonadotropins by negative feedback, thus normalizing the Te level after a few weeks. The reduction in Te levels that was identified at the 1-month follow-up may be a significant etiological factor of the increased gonadotropins, which have previously been observed even at 12 months after RP (9,10,12). In the present study, we were not able to dismiss other potential explanations, nor identify the cause of the reduced testicular production of Te following RP. However, we suggest that there may be numerous factors, including psychological and organic considerations. The psychological stress of facing a cancer diagnosis or the fear of surgery have already been considered as potential causes of the suppression of LH/FSH prior to RP (12). This may also cause a temporary reduction in testicular endocrine function. Organic factors, e.g., the ligature of Santorini’s venous plexus or of prostatic vascular pedicles and the Trendelenburg position, may theoretically cause transient ischemic or hypoxic damage to the testicles. Moreover, damage to the cavernous nerves may alter testicular function in humans, as has been reported in animal models (18). Following a bilateral cavernous neurotomy in rats, Vignozzi et al observed the onset of overt hypogonadism, characterized by reduced Te levels and testis function, including testis weight and number of Leydig cells, with an inadequate compensatory increase of LH (19). More detailed studies are required to completely investigate whether and how RP has an effect on the testicular production of Te, and if this is similar to the effect that is described after external beam radiation therapy (20).
Previous studies have presented various interpretations of the increased gonadotropin levels following RP, alternately attributing them to the removal of the PCa or healthy prostatic tissue, or to the surgical event itself. In the study by Madersbacher et al, the authors suggested that the hypothalamic-pituitary axis is inhibited in patients with PCa and that this inhibition is removed following RP. The authors presented evidence for a possible direct inhibitory effect of tumor cells, as there was a correlation between higher Gleason scores and lower pre-operative Te, and significantly lower LH and FSH levels were observed in the PCa group compared with the BPH group (12). However, Miller et al suggested that it was the healthy prostatic tissue, producing dihydrotestosterone (DHT) and inhibin-B, that caused the pre-operative inhibition of the hypothalamic-pituitary axis (9). Furthermore, DHT induces negative feedback for LH and FSH, and decreases in its levels in serum and urine following RP have also been observed in other studies (10,12). By contrast, Olsson et al did not report any changes to the serum inhibin-B levels in 44 patients following RP, nor any difference between the PCa and BPH patient groups (10). The present study does not disagree with any previous justifications, although it does provide a new observation (the early reduction of Te) which may present a new interpretation. Further studies are required to confirm whether RP affects testicular production of Te. In the present study, this premise is suggested by the levels of LH and FSH, which remained high at 3 months compared with the baseline value, despite a normal Te value. This premise is also supported by the correlation analysis between Te and LH, which showed that the correlation was lost at 1 month and recovered at 3 months. This explanation would also justify the increased gonadotropin levels observed with the normal Te levels in previous studies at 6 and 12 months following RP (9,12). In addition to possessing significance in endocrinological terms, the fluctuation of the Te levels in the first 3 months following RP may be important from a clinical perspective, since this period of time has critical relevance in the recovery of urinary continence and potency (21), and the resultant Te levels are significantly involved in the recovery of these functions (4,5). Although the present study did not record data on the functional recovery of these patients, we suggest that future investigations into the correlation between functional recovery and sex hormones should focus on the first months after prostatectomy. In our opinion, the strengths of the present study are inherent in its prospective nature and the high significance of the statistical results; conversely, the limits include the lack of sex hormone-binding globulin (SHBG) and estrogens results in the analysis, which were excluded due to their cost. However, not all previous studies on this issue have included SHBG and estrogens in the evaluation, and no remarkable adjunctive outcomes have resulted from those that did (9–12). In conclusion, in the present series, RP induced an early significant decline in the Te serum levels and a significant increase in the LH and FSH levels. At 3 months after RP, the full recovery of the Te level, with concomitant high levels of gonadotropins, appears to delineate the features of compensated hypergonadotropic hypogonadism. Studies evaluating the effect of these hormonal changes on continence and potency are currently in progress.
References
- 1.Gacci M, Eardley I, Giuliano F, Hatzichristou D, Kaplan SA, Maggi M, McVary KT, Mirone V, Porst H, Roehrborn CG. Critical analysis of the relationship between sexual dysfunctions and lower urinary tract symptoms due to benign prostatic hyperplasia. Eur Urol. 2011;60:809–825. doi: 10.1016/j.eururo.2011.06.037. [DOI] [PubMed] [Google Scholar]
- 2.Lowe FC. Treatment of lower urinary tract symptoms suggestive of benign prostatic hyperplasia: sexual function. BJU Int. 2005;95(Suppl 4):12–18. doi: 10.1111/j.1464-410X.2005.05486.x. [DOI] [PubMed] [Google Scholar]
- 3.Khera M. Androgens and erectile function: a case for early androgen use in postprostatectomy hypogonadal men. J Sex Med. 2009;6(Suppl 3):234–238. doi: 10.1111/j.1743-6109.2008.01159.x. [DOI] [PubMed] [Google Scholar]
- 4.Gacci M, Corona G, Apolone G, Lanciotti M, Tosi N, Giancane S, Masieri L, Serni S, Maggi M, Carini M. Influence of serum testosterone on urinary continence and sexual activity in patients undergoing radical prostatectomy for clinically localized prostate cancer. Prostate Cancer Prostatic Dis. 2010;13:168–172. doi: 10.1038/pcan.2010.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gacci M, Ierardi A, Rose AD, Tazzioli S, Scapaticci E, Filippi S, Maggi M, Nicita G, Carini M, Montorsi F. Vardenafil can improve continence recovery after bilateral nerve sparing prostatectomy: results of a randomized, double blind, placebo-controlled pilot study. J Sex Med. 2010;7:234–243. doi: 10.1111/j.1743-6109.2009.01471.x. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto S, Yonese J, Kawakami S, Ohkubo Y, Tatokoro M, Komai Y, Takeshita H, Ishikawa Y, Fukui I. Preoperative serum testosterone level as an independent predictor of treatment failure following radical prostatectomy. Eur Urol. 2007;52:696–701. doi: 10.1016/j.eururo.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 7.Isom-Batz G, Bianco FJ, Jr, Kattan MW, Mulhall JP, Lilja H, Eastham JA. Testosterone as a predictor of pathological stage in clinically localized prostate cancer. J Urol. 2005;173:1935–1937. doi: 10.1097/01.ju.0000158040.33531.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lane BR, Stephenson AJ, Magi-Galluzzi C, Lakin MM, Klein EA. Low testosterone and risk of biochemical recurrence and poorly differentiated prostate cancer at radical prostatectomy. Urology. 2008;72:1240–1245. doi: 10.1016/j.urology.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Miller LR, Partin AW, Chan DW, Bruzek DJ, Dobs AS, Epstein JI, Walsh PC. Influence of radical prostatectomy on serum hormone levels. J Urol. 1998;160:449–453. [PubMed] [Google Scholar]
- 10.Olsson M, Ekström L, Schulze J, Kjellman A, Akre O, Rane A, Gustafsson O. Radical prostatectomy: influence on serum and urinary androgen levels. Prostate. 2010;70:200–205. doi: 10.1002/pros.21053. [DOI] [PubMed] [Google Scholar]
- 11.Lackner JE, Maerk I, Koller A, Bieglmayer C, Marberger M, Kratzik C, Schatzl G. Serum inhibin - not a cause of low testosterone levels in hypogonadal prostate cancer? Urology. 2008;72:1121–1124. doi: 10.1016/j.urology.2008.01.066. [DOI] [PubMed] [Google Scholar]
- 12.Madersbacher S, Schatzl G, Bieglmayer C, Reiter WJ, Gassner C, Berger P, Zidek T, Marberger M. Impact of radical prostatectomy and TURP on the hypothalamic-pituitary-gonadal hormone axis. Urology. 2002;60:869–874. doi: 10.1016/s0090-4295(02)01893-9. [DOI] [PubMed] [Google Scholar]
- 13.Heidenreich A, Aus G, Bolla M, Joniau S, Matveev VB, Schmid HP, Zattoni F. European Association of Urology: EAU guidelines on prostate cancer. Eur Urol. 2008;53:68–80. doi: 10.1016/j.eururo.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Carini M, Masieri L, Minervini A, Lapini A, Serni S. Oncological and functional results of antegrade radical retropubic prostatectomy for the treatment of clinically localised prostate cancer. Eur Urol. 2008;53:554–561. doi: 10.1016/j.eururo.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Corona G, Boddi V, Lotti F, Gacci M, Carini M, De Vita G, Sforza A, Forti G, Mannucci E, Maggi M. The relationship of testosterone to prostate-specific antigen in men with sexual dysfunction. J Sex Med. 2010;7:284–292. doi: 10.1111/j.1743-6109.2009.01549.x. [DOI] [PubMed] [Google Scholar]
- 16.Sasagawa I, Nakada T, Suzuki H, Adachi Y, Adachi M. Effect of transurethral resection of prostate on plasma hormone levels in benign prostatic hyperplasia. Br J Urol. 1993;72:611–614. doi: 10.1111/j.1464-410x.1993.tb16219.x. [DOI] [PubMed] [Google Scholar]
- 17.Türkölmez K, Bozlu M, Sarica K, Gemalmaz H, Ozdiler E, Gögüş O. Effects of transurethral prostate resection and transurethral laser prostatectomy on plasma hormone levels. Urol Int. 1998;61:162–167. doi: 10.1159/000030314. [DOI] [PubMed] [Google Scholar]
- 18.Reyes JG, Farias JG, Henríquez-Olavarrieta S, Madrid E, Parraga M, Zepeda AB, Moreno RD. The hypoxic testicle: physiology and pathophysiology. Oxid Med Cell Longev. 2012;2012:929285. doi: 10.1155/2012/929285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vignozzi L, Filippi S, Morelli A, Marini M, Chavalmane A, Fibbi B, Silvestrini E, Mancina R, Carini M, Vannelli GB, Forti G, Maggi M. Cavernous neurotomy in the rat is associated with the onset of an overt condition of hypogonadism. J Sex Med. 2009;6:1270–1283. doi: 10.1111/j.1743-6109.2008.01208.x. [DOI] [PubMed] [Google Scholar]
- 20.Daniell HW, Clark JC, Pereira SE, Niazi ZA, Ferguson DW, Dunn SR, Figueroa ML, Stratte PT. Hypogonadism following prostate-bed radiation therapy for prostate carcinoma. Cancer. 2001;91:1889–1895. doi: 10.1002/1097-0142(20010515)91:10<1889::aid-cncr1211>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 21.Gacci M, Simonato A, Masieri L, Gore JL, Lanciotti M, Mantella A, Rossetti MA, Serni S, Varca V, Romagnoli A, Ambruosi C, Venzano F, Esposito M, Montanaro T, Carmignani G, Carini M. Urinary and sexual outcomes in long-term (5+ years) prostate cancer disease free survivors after radical prostatectomy. Health Qual Life Outcomes. 2009;7:94. doi: 10.1186/1477-7525-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]