Abstract
The acoustic and thermal properties as well as the temperature change within a tissue volume during high-intensity focused ultrasound ablation are critically important for treatment planning and monitoring. Described in this article is a tomographic reconstruction method used to determine the tissue properties and increase in temperature in a 3-D volume. On the basis of the iterative finite-element solution to the bioheat equation coupled with Tikhonov regularization techniques, our reconstruction algorithm solves the inverse problem of bioheat transfer and uses the time-dependent temperature measured on a tissue surface to obtain the acoustic absorption coefficient, thermal diffusivity and temperature increase within the subsurface volume. Numerical simulations were performed to validate the reconstruction algorithm. The method was initially conducted in ex vivo experiments in which time-dependent temperature on a tissue surface was measured using high-resolution, non-invasive infrared thermography.
Keywords: Image reconstruction, High-intensity focused ultrasound, Bioheat equation, Infrared thermography
INTRODUCTION
High-intensity focused ultrasound (HIFU) is a unique ablation technology (Kennedy 2005) used in a number of applications, including control of hemorrhage from vessel puncture and trauma (Vaezy et al. 2007); treatment of tumors in the liver (Illing et al. 2005), prostate (Crouzet et al. 2010; Uchida et al. 2009) and uterus (Ter Haar 2008); and cardiac ablation (Groh et al. 2007; Natale et al. 2000; Strickberger et al. 1999). With the ability to readily focus energy to deep locations non-invasively without insertion of a device into the bulk tissue, HIFU ablation uses concentrated ultrasound intensities (e.g., 500–5000 W/cm2) to destroy a targeted volume of tissue with no or minimal damage to the intervening and surrounding tissue. HIFU produces a localized increase in temperature, which can result in lesion formation (e.g., protein denaturation and necrosis) depending on the thermal dose associated with the HIFU exposure (Damianou and Hynynen 1994; Sapareto and Dewey 1984). Online monitoring of lesion production and the temperature increase during HIFU application provides important feedback for controlling and tailoring therapy to individual patients (Ries et al. 2010; Vanne and Hynynen 2003).
As the “gold” standard currently used clinically in HIFU treatment of breast carcinoma and uterine fibroids (Hynynen 2010), magnetic resonance imaging (MRI) provides millimeter spatial resolution in detecting HIFU lesions and temperature increases, but the acquisition duration required by MRI limits its use in treatments using short HIFU pulses with rapid tissue heating (Rivens et al. 2007). In addition, the high cost of MRI scanners, the claustrophobia and discomfort of some patients in closed-bore systems and the lack of portability of MRI systems are disadvantages that may restrict the widespread use of this technology for HIFU therapy (Kennedy 2005).
Except for MRI, no method is currently available that reliably and non-invasively measures temperature at locations within a 3-D tissue volume. Thermocouples can be used to obtain temperature measurements at discrete locations, but invasive insertion of thermocouples into tissue is difficult to implement in clinical settings and is inadequate for 3-D temperature measurement. Ultrasound imaging is inexpensive and portable, but has insufficient image contrast for visualization of HIFU lesions without the presence of gas bodies, which are sometimes produced during HIFU application. It has been proposed that ultrasound imaging methods be used to detect HIFU lesions on the basis of changes in attenuation (Zhong et al. 2007) and tissue elasticity (Lizzi et al. 2003), but lesion characterization via these methods is often subject to degradation in the presence of gas bodies. Non-invasive temperature sensing based on temperature-induced changes in acoustic propagation speed (Abolhassani et al. 2007; Liu et al. 2010; Seip et al. 1996) has also been proposed, but can be affected by artifacts from tissue movement and the large range of temperature increase in HIFU ablation. Ultrasound phase contrast thermal imaging with reflex transmission imaging has also been proposed as method for 3-D temperature interrogation and has been validated in phantoms (Farny and Clement 2009), but requires a 2-D array or long imaging times to acquire the necessary data.
Infrared (IR) thermography provides high-sensitivity (e.g., 0.025°C) and high-spatial-resolution (~0.1 mm) temperature measurement in real time (e.g., rate of 100 frames/s) in a non-contact fashion. Because IR imaging can measure temperatures only on a surface, its use in monitoring therapy has been limited (Campbell and Thomas 2008; Ogan et al. 2003), and its potential has not been fully exploited. IR thermography has been used to characterize the sub-surface structure of porcine skin burns in a discrete multi-layer model (Nowakowski 2008; Ruminski et al. 2007). However, continuous 3-D imaging of temperature profiles or thermal properties has not been achieved.
The temperature increase induced in a tissue exposed to HIFU as a function of space and time is described by the bioheat equation and is affected by tissue properties (e.g., acoustic absorption coefficient and thermal diffusivity), the HIFU beam profile and exposure parameters (e.g., duration and intensity). In tissue of finite size, thermal diffusion may result in measurable temperature change on the tissue surface, even if the HIFU focus is located within the tissue volume. Liu et al. (2000) proposed an algorithm based on the dual reciprocity boundary element method (DRBEM) to solve the integral inverse or direct bioheat transfer problems for predicting the thermal states of biological bodies. However, as Liu et al. (2000) mentioned in their article, the inverse problem is generally ill posed. Thus, regularization techniques like the Tikhonov method must be used to solve the inverse problem appropriately. In Liu and colleagues’ work, no regularization method was applied. In addition, for a 3-D problem, Liu and colleagues’ method requires information on temperature distribution over the skin surface where more complex boundary conditions (BCs) are encountered, which may be a disadvantage of the boundary element method. In the work described in this article, we formulated a model-based strategy to solve the inverse problem of heat transfer and reconstruct the tissue properties and HIFU-induced temperature within the tissue volume using the time-dependent, 2-D temperature distribution on the tissue surface. Because the thermal properties of lesions differ from those of normal tissue, the method can also be used to detect lesion formation. Moreover, this model-based strategy can be employed for monitoring other thermal therapies such as radiofrequency ablation. We developed a tomographic reconstruction method to obtain tissue properties and temperature within a tissue volume based on the surface temperatures measured using IR thermography. The inverse tomographic reconstruction algorithm is based on the iterative finite-element (FE) solution to the bioheat equation using the Newton method coupled with the Tikhonov regularization method.
RECONSTRUCTION ALGORITHM
Finite-element method to solve the bioheat transfer equation
High-intensity focused ultrasound-induced temperature change, T(x,y,z,t), is described by the bioheat transfer equation (Nyborg 1988; Pennes 1948)
| (1) |
where κ is the thermal diffusivity, and Qm is the heat generated by metabolic process (Qm was assumed to be zero in this study). The perfusion time constant is defined as τ = ρbcv/wcvb, where ρb is the density of blood, w is the blood flow rate specified in terms of mass/volume-time, and cvb is the volume specific heat of blood. The volume specific heat and density of blood used in the work described here were 4.2 J cm−3 C−1 and 1 g mL−1, respectively; the thermal diffusivity was 0.0014 cm2 s−1; a moderate blood flow was considered for a perfusion length of 10 mm. Under these conditions, the corresponding τ = 714 s. For the ex vivo specimens used, perfusion was not a concern. Hence, we neglected this effect in the image reconstructions. The heat source associated with a HIFU exposure, Q(x,y,z,t), is, as reported in Lizzi et al. (1992)
| (2) |
where α(x,y,z,t) is the acoustic absorption coefficient per unit frequency, f is the center frequency of the HIFU, I(x,y,z,t) is the in situ intensity of the HIFU exposure and cv is the volume-specific heat of the tissue. The boundary conditions for the bioheat equation are , where is the unit normal vector at the tissue surface, h is the convection coefficient, ρ is the density of the tissue and Tc is the background temperature before HIFU application.
The finite-element discretization of equation (1) in the space domain is
| (3) |
where {T}and are the vectors of temperature in the space domain and the rate of temperature change, respectively; [K] is the diffusivity matrix, with its elements expressed as
where ⟨⟩ indicates integration over the reconstruction region, and ∮ expresses integration over the convection boundary surface. T and κ have been expanded as the sum of coefficients multiplied by a set of locally spatially varying Lagrangian basis functions Nl, Ni, and (l, i, j = 1, …, NN). The three dimensional FE mesh has a total of NN = 2479 nodes in the mesh with M = 4 nodes in each element. [C] is the specific heat matrix, and its elements are written as Cij = ⟨NiNj⟩. {p} is the source matrix with elements Pi = ⟨(Q+Qm)Ni⟩.
Next, equation (3) is discretized in the time domain where the temperature is expanded as the sum of coefficients multiplied by a set of basis functions in the time domain, allowing calculation of the temperature at a future time at each node,
| (4) |
where (0≤θ≤1) is a weighting factor (θ = 0.8 was chosen empirically, which often yields stable and quick convergence in our calculations); Δt is the time step; Tn and Tn+1 are the temperature at time steps n and n+1, respectively; and N is the total number of time steps. Thus, in the forward problem of bioheat transfer, temperature is calculated directly from equation (4) with known values for the absorption coefficient and thermal diffusivity, assuming the initial temperature change is zero.
Inverse reconstruction of the 3-D absorption coefficient, thermal diffusivity, and temperature
The inverse problem of bioheat transfer is formulated as the task of obtaining or reconstructing the parameters in the bioheat equation (the absorption coefficient α and thermal diffusivity κ at each FE node) from the known time-dependent 2-D temperature on a tissue surface or boundary. Our reconstruction algorithm is based on regularization-based non-linear methods, because these can offer highly accurate reconstructions (Jiang et al. 1996, 2000; Sun et al. 2011; Xi et al. 2012).
Tikhonov is a commonly used regularization method for ill-posed problems, and the objective function is given as (Tikhonov 1977)
| (5) |
The inverse solution is obtained through iterative solution to the matrix equation
| (6) |
where χ expresses α and/or, and λ is a scalar. A very effective method for determining λ is to set it equal to the trace of the Hessian matrix (trace[JTJ]) multiplied by an empirically determined factor α and the relative least-squares error ((Po – Pc)2), so the final λ was formulated as λ = α(Po – Pc) × trace[JTJ]; I, the identity matrix, is used to realize an invertible system of equations (i.e., equation [6]); T(o) is the temperature at M boundary locations (M = 247) over N time steps (N = 500), which can be measured by IR thermography; T(c) is the temperature calculated at each iteration step using equation (4) with updated κ and α. J = [J1, J2, …, Jt,…, JN]T is the time-dependent Jacobian matrix formed by ∂T/∂χ at the boundary measurement sites;Jt is written as
| (7) |
From the known time-dependent temperature on the boundary of tissue exposed to HIFU, κ and α within the target volume are determined. The objective is to obtain the estimates of κ and α distribution that can sustain the measured boundary quantities under the bioheat equation. The 3-D temperature distribution at each time step is calculated from equation (4) with the converged values of κ and α. A 3-D FE mesh with 2497 nodes and 12,000 tetrahedron elements was used.
METHODS
Simulation: reconstruction of thermal diffusivity and absorption coefficient
Simulation was conducted to test the reconstruction algorithm. The test geometry (Fig. 1a) was a volume of cylindrical shape (radius = 1.5 cm, height = 1 cm) with a uniform absorption coefficient of 0.5 dB/cm-MHz and thermal diffusivity of 0.14 mm2/s, except in a small cylinder (radius = 2 mm, height = 3 mm) located at (0, −5 mm, 3.5 mm), which has a thermal diffusivity of 0.56 mm2/s and an absorption coefficient the same as the background. These values were chosen based on reported values for porcine cardiac tissue and HIFU lesions (Bhavaraju and Valvano 1999; Zderic et al. 2004).
Fig. 1.
(a) Test geometry and volume for simulation. (b) Normalized spatial distribution of the high-intensity focused ultrasound intensity in the XZ plane when Y = 0.
To generate “measured” temperature data, we first calculated the ultrasound intensity field and the associated heat source produced by a HIFU transducer (Sumi and Yanagimura 2007) (radius = 21 mm, focal distance = 57 mm, center frequency = 3.8 MHz).Figure 1b gives the spatial distribution of the HIFU intensity in the XZ (Y = 0) plane (intensity = 1000 W/cm2, duration = 3 s). By solving the bioheat equation with the assumed absorption coefficient and thermal diffusivity for the lesion and surrounding tissue, we calculated the HIFU-induced spatial temporal change in temperature in the tissue, including the temperature on the tissue surface. The calculated time-dependent temperature on the top surface (Z = 1 cm) was then used as “measured” data to reconstruct the absorption coefficient and thermal diffusivity in the subsurface tissue volume using the algorithm described under Reconstruction Algorithm. The FE mesh resolution was chosen to be ~1 mm in the XY plane and only ~2 mm in the XZ and YZ planes given the shape of the HIFU beam profile. The recovered tissue absorption and thermal diffusivity were compared with the assumed values and then used to compute the time-dependent temperature at each FE node using equation (4). In this simulation case, we also present the temperature profile in the center of the lesion considering the effect of noise.
Experiments
Fresh porcine cardiac tissue specimens obtained from a local abattoir and cut into blocks (the volume of the tissue is about 20 × 20 × 14 mm2) were used. An HIFU transducer (3.98 MHz, radius = 20.5 mm, focal distance = 41 mm) was submerged in water (22°C) to deliver HIFU to the tissue; the focus was slightly below the top tissue surface, which was above the water level to allow IR temperature measurement. The tissue specimen was protected on the bottom by a membrane wrap that is acoustically transparent to allow unobstructed HIFU transmission while preventing water from permeating the tissue. An IR camera (Silver SC5600, FLIR, Boston, MA, USA) was mounted on top facing downward to record temperature on the top tissue surface. All devices were synchronized through a data acquisition system (OMB-DAQ-3000, Omega, Stamford, CT, USA).
Thermal probing to detect lesion
The tissue was exposed to tone-burst HIFU at high intensity (e.g., 2000 W/cm2, 5–10 s) to generate a lesion in the tissue and allowed to cool to room temperature. Another exposure to tone-burst HIFU of sub-ablative intensity (283 W/cm2 and duration 10 s) was used to increase temperature in the tissue without generating tissue necrosis. The time-dependent, 2-D temperature on the top tissue surface was measured with the IR camera (resolution = 0.025°) and used for reconstruction of the thermal diffusivity and absorption coefficient in the tissue volume. Because the absorption coefficient and thermal diffusivity can differ between a HIFU lesion and non-ablated tissue, reconstruction of the subsurface tissue properties permits non-invasive probing of the tissue that can reveal the presence of a HIFU lesion.
Validation of HIFU-induced temperature increase
Tone-burst HIFU exposure (duration = 20 s, 125 W/cm2) was used to induce a temperature increase in the tissue without generating tissue necrosis. Temperature on the top surface was measured with IR imaging. Two thermocouples were placed within the tissue at (0.8 mm, 7.3 mm, 5.1 mm) and (0.3 mm, 2.9 mm, 4.5 mm) relative to the focal point (0, 0, 0), determined non-invasively using an ultrasound imaging system (Vevo 770, Visualsonics, Toronto, ON, Canada; the spatial resolution of Vevo 770 is 0.1 mm). The temperature measured on the tissue surface was used to reconstruct the absorption coefficient, thermal diffusivity and temperature in three dimensions. The reconstructed temperatures were compared with the thermocouple measurements.
RESULTS
Simulation: reconstruction of thermal diffusivity and absorption coefficient
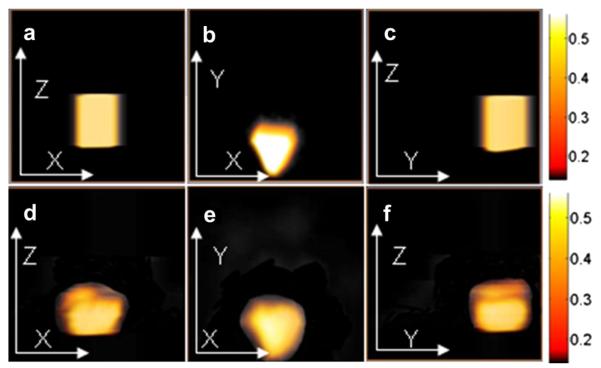
Figure 2 provides the assumed (Fig. 2a–c) and recovered (Fig. 2d–f) thermal diffusivity distributions in different planes in the test volume. The computation time using a 1.2-GHz CPU computer was about 1 h. The lesion is revealed in terms of the thermal diffusivity with respect to magnitude, spatial position and shape, compared with the assumed values. Because of the higher resolution in the XY plane than in the XZ and YZ planes in the finite-element mesh, the image recovered in the XY plane is better than that recovered in the YZ and XZ planes. Because this is an ill-posed inverse problem, it is not surprising to see artifacts near the lesion boundary. These artifacts arise primarily from noise caused by the relatively coarse finite-element mesh and by the value of the initial guess. Figure 3 compares the exact temperature profile in the center of the lesion with reconstructed profiles with various levels of noise, revealing that the reconstruction is still accurate with up to 5% noise. In addition, Figure 3 indicates that if a homogeneous distribution of thermal diffusivity is used instead of an exact distribution, a large deviation will occur. We have also done some simulations to test the ability of our method to detect targets located at different depths. Table 1 provides the temperature difference at the surface versus target depth.
Fig. 2.
Images of the assumed and recovered thermal diffusivity (mm2/s) for the first simulation case in the XZ plane (Y = −5 mm) (a, d); XY plane (Z = 3.5 mm) (b, e); and YZ plane (X = 0 mm) (c, f).
Fig. 3.

Temperature profiles in the center of the lesion with different noise levels.
Table 1.
Temperature difference at the surface versus target depth
| Depth of target | 5 mm | 10 mm | 13 mm | 15 mm | 20 mm |
| Temperature difference | 0.075°C | 0.023°C | 0.014°C | 0.013°C | 0.012°C |
Experiments
Thermal probing to detect lesion
Two tissue specimens, each with a pre-existing HIFU lesion (Fig. 4), were used to illustrate lesion detection using reconstruction of tissue properties for tissue thermal probing. The computation time is proportional to the square of the mesh density used. For the experiment, the reconstruction region was doubled compared with that used for the simulation case, which means the mesh density is two times larger than that for the experimental case, resulting in four times more computation time. Figure 4a illustrates the geometry of the setup and one plane across the lesion.Figure 4b is the gross image of the lesion viewed from the top surface, and Figure 4c is the cross-sectional image. The lesion was 2 mm wide and 7 mm long.Figure 4d, e are photographs of the lesion in the second specimen; this lesion was 5 mm wide and 14 mm long.
Fig. 4.
(a) Schematic drawing illustrating the XY and XZ planes across the high-intensity focused ultrasound lesion in tissue. (b–e) Images of the lesion in the first tissue specimen in the (b) XY and (c) XZ planes and in the second specimen in the (d) XY and (e) XZ planes.
Figure 5 are images of the reconstructed absorption coefficient (Fig. 5a, b) and thermal diffusivity (Fig. 5c, d) for the first tissue specimen. The reconstructed absorption coefficient and thermal diffusivity in the lesion are 0.85 dB/cm-MHz and 0.16 mm2/s, respectively. The absorption coefficient in the lesion is about 1.7 times that in of the non-ablated tissue, whereas the thermal diffusivity is similar to that in non-ablated tissue. The reconstructed absorption coefficient and the thermal diffusivity show that this lesion is about 2 mm in diameter and 3 mm in depth. Figure 6 are the recovered absorption coefficient (Fig. 6a, b) and thermal diffusivity (Fig. 6c, d) for the second specimen. The reconstructed absorption coefficient in the lesion is 1.5 dB/cm-MHz, and the thermal diffusivity is 0.22 mm2/s; structurally, the lesion is about 5 mm in diameter and 8 mm in length. In both cases, the reconstructed absorption coefficient and thermal diffusivity are in agreement with reported values and revealed the lesion in terms of location and diameter, with the reconstructed lesion length about 50% of the length measured from the gross image. The lesion in specimen 1 has a lower-contrast thermal diffusivity and absorption coefficient than the lesion in specimen 2 because of its lower thermal dose, so the recovered results are somewhat worse in specimen 1 than in specimen 2.
Fig. 5.

Images of the recovered absorption coefficient (dB/cm-MHz) (a, b) and thermal diffusivity (mm2/s) (c, d) for the first experimental case in the XY plane (a, c) and XZ plane (b, d).
Fig. 6.

Images of the recovered absorption coefficient (dB/cm-MHz) (a, b) and thermal diffusivity (mm2/s) (c, d) for the second experimental case in the XY (a, c) and the XZ (b, d) planes.
Validation of temperature distribution during HIFU application
In these experiments, tone-burst HIFU induced a temperature increase in the tissue without generating tissue necrosis. Because no lesion was present before or generated during the sub-ablative HIFU exposure, the reconstructed tissue absorption coefficient (0.5 dB/cm-MHz) and thermal diffusivity (0.14 mm2/s) were expected and confirmed to be uniform in the tissue volume. The HIFU-induced temperature increase in the tissue volume was computed with the recovered tissue absorption coefficient and thermal diffusivity. Figure 7(a–c) are the surface temperatures measured with IR thermography at selected time points (13, 20 and 40 s) after HIFU application (intensity = 125 W/cm2, and duration = 20 s), and Figure 7(d, e) are the corresponding calculated temperatures on the top tissue surface. The reconstructed temperatures are in good agreement with the measured temperatures, except at early time points during HIFU, likely because of the small temperature increases.
Fig. 7.
Measured and recovered temperatures (°C) on the top surface 13 s (a, d), 20 s (b, e) and 40 s (c, = f) after the start of high-intensity focused ultrasound ablation (t = 0) at an intensity of 125 W/cm2.
Figure 8 compares the calculated/reconstructed temperature with the measured temperature at the thermocouple locations. Good agreement was reached between temperatures at the thermocouple 2 location, at (0.32 cm, 2.9 cm, 4.45 cm) relative to the HIFU focus (0, 0, 0), and temperatures at the thermocouple 1 location at (0.74 cm, 7.3 cm, 5.05 cm), where the change in temperature is small because it is farther away from the focus than thermocouple 2. Some of the observed discrepancy can be attributed to the fact that thermocouple 1 is not on a finite-element mesh node. Figure 9 is the temperature rise profile along the acoustic axis at different time points (10, 20, 30 and 40 s) and an intensity of 125 W/cm2. The line extends from the HIFU focus to the end of the tissue; we can see that the temperature is highest at 20 s, and also, HIFU has almost no effect on the area surrounding the lesion. When HIFU is turned off, the temperature decreased quickly to normal.
Fig. 8.

Measured and recovered temperature changes as a function of time at the thermocouple locations (TC1 = thermocouple 1, TC2 = thermocouple 2) for a high-intensity focused ultrasound intensity of 125 W/cm2.
Fig. 9.

Temperature rise along the acoustic axis at different time points.
DISCUSSION
We have developed a reconstruction algorithm that uses the time-dependent 2-D temperature on a tissue surface to obtain the tissue absorption coefficient and thermal diffusivity, as well as temperature changes within the subsurface tissue volume, during exposure to HIFU. Our technique provides a unique method of thermal probing to detect lesions in the subsurface volume noninvasively. It can also be used to obtain the thermal properties within a tissue volume before the actual HIFU ablation therapy, which is useful in formulating treatment planning models to predict spatiotemporal temperature evolution during HIFU. Below we discuss the implications of the results, specific conditions used in reconstruction and limitations of the method.
Thermal probing via reconstruction of the absorption coefficient and thermal diffusivity
The acoustic absorption coefficient and thermal diffusivity are significant issue for thermal therapy such as HIFU ablation; however, there has been limited studies of the measurement of these subsurface thermal properties in situ.
Thermal diffusivity
Magnetic resonance imaging thermometry has been used for the measurement of thermal conductivity ex vivo (tofu, turkey muscle, liver) and in vivo (rabbit muscle) (Cheng et al. 2002), under the assumption of homogeneous thermal properties, but the results were limited somewhat by the temperature resolution of the 1.5-T scanner employed. A method to determine thermal conductivity, heat capacity, and thermal diffusivity using volumetric temperature measurements of reference material has also been proposed and tested in phantoms using temperatures measured via ultrasound strain (Sumi and Yanagimura 2007). Local subsurface thermal diffusivity has been non-invasively measured using backscattered ultrasound and focused ultrasound heating (Anand and Kaczkowski 2008) in phantoms. A framework has recently been proposed and computationally evaluated to determine subsurface thermal conductivity, blood perfusion and metabolic heat generation properties from IR thermography of the breast based on iterative optimization with FE solutions of the forward problem, although no experimental data have been reported (Jiang et al. 2010). This method can determine only the thermal property contrast (a ratio of the thermal property with respect to their population average), not the absolute value.
Acoustic absorption
Limited work has been reported regarding the local measurement of acoustic absorption. MRI calorimetry with focused ultrasound heating in ex vivo liver has been employed to make direct tissue ultrasound absorption measurements (Wang et al. 1999). However, this method does not appear to lend itself easily to imaging or 3-D measurement of acoustic absorption.
Reconstruction of tissue properties based on surface temperature in thermal probing
In our experiments, HIFU exposure of sub-ablative intensity was used to induce an increase in temperature (without generating tissue necrosis). On the basis of the HIFU-induced temperature on the tissue surface, reconstruction of the acoustic absorption coefficient and thermal diffusivity in the tissue volume revealed the presence of a preexisting lesion because its different thermal properties differed from those of the untreated surrounding tissue. The reconstructed absorption coefficient (0.85 dB/cm-MHz) and thermal diffusivity (0.16 mm2/s) for the first lesion are well in line with reported values (Sumi and Yanagimura 2007) for HIFU lesions, whereas the reconstructed values (1.5 dB/cm-MHz and 0.22 mm2/s) are higher for the second lesion. This may be due to the fact that the second lesion has a core that appears to have been over-treated (cf. Fig. 4e with 3c), resulting in the different properties of this lesion. For both cases, lesion width was well recovered; the recovered length was under-estimated compared with the actual size observed in the gross image. The far end of the lesion from the surface may be less represented in the surface temperature measurement. Even though theoretically we can detect any target anywhere, our simulations for the minimum detectable temperature on the surface, based on our current camera resolution (0.025°) and our computation accuracy, which is related to the mesh size, indicate that that if the target is seated deeper than 10 mm, the temperature difference in the surface is smaller than 0.025°, so it is undetectable. That is the reason we could recover only a part of the lesion in the experiments. Another reason may be the lesion shape (large aspect ratio with small width relative to length) and orientation with respect to the tissue surface where the temperature was measured and used for reconstruction.
Reconstruction of time-dependent tissue properties during HIFU ablation
In this pilot study, we focused on illustrating the principle of our reconstruction algorithm for thermal probing applications where tone-burst HIFU exposures of sub-ablative intensity were used to induce temperature increases without generating tissue necrosis. In this way, the acoustic absorption coefficient and thermal diffusivity can be assumed to be independent of time in the reconstruction to simplify and speed up the reconstruction.
For HIFU ablation that generates tissue necrosis during HIFU application, however, reconstruction of the tissue properties must take into consideration the temperature or time dependence of tissue properties. Our general reconstruction formulation has already included this consideration. Future study is planned to reconstruct time-dependent tissue properties and temperatures within the subsurface tissue volume for HIFU ablation. More rigorous validation of reconstruction results can be conducted using MRI for temperature and lesion imaging.
Enhanced heating by gas body activities
In this study, the heat source due to absorption of HIFU energy by tissue itself was used; this does not include heating caused by other factors such as cavitation and bubble activity. Although cavitation and gas bubbles are not necessarily desired for controlled generation of lesions of predictable size and extent, these are frequent effects of HIFU ablation. These gaseous bodies strongly scatter the HIFU beam, affect HIFU energy distribution and can enhance heating from the viscous absorption by the bubbles (Leighton 1994). Because of the intrinsic stochastic nature of cavitation inception and dynamic activity, it is generally difficult to adequately account for these effects. It may be possible to include the enhanced heating effect if a suitable model can be constructed to relate the enhanced heating effect of bubbles to modified acoustic absorption (Chavrier et al. 2000;Farny et al. 2010).
Reconstruction coupled with acoustic wave propagation and nonlinear effect
We assumed the HIFU intensity a priori in the reconstruction; thus, the absorption coefficient in the heat source (equation [2]) can be recovered more easily when I(x,y,z,t) is known. This assumption can be removed by incorporating an acoustic wave propagation model into the reconstruction algorithm, allowing simultaneous estimation of the HIFU intensity distribution while iteratively updating the acoustic absorption coefficient and thermal diffusivity. However, the computing time added by incorporating the wave propagation model is negligible compared with the overall reconstruction time. The separation of terms in the heat source (product of the acoustic absorption coefficient and HIFU intensity profile) is analogous to that in quantitative photoacoustic tomography where we have successfully separated the product of the optical absorption coefficient and optical fluence by incorporating a light transport model into the reconstruction algorithm for the recovery of absorbed energy density (Yuan and Jiang 2006, 2009).
A non-linear acoustic propagation model that accounts for non-linear acoustical effects, for example, harmonic generation, can further improve the formulation.
Computation speed
Online implementation of the reconstruction method requires fast reconstruction speed, which depends on the size of the mesh used in an FE mesh-based method to solve differential equations. The computational memory size required to store the matrices in equation (7) can be a limiting factor in high-accuracy reconstruction. Implementation of a GPU-based algorithm can reduce the computation time dramatically and make possible real-time detection of tissue changes during HIFU therapy.
Limitations of the method
Our reconstruction method is based on the ability to obtain the temperature on a tissue surface. Because IR emission is highly attenuated in water, the method involving IR thermography is limited to HIFU applications in which IR thermography is feasible. Zderic et al. (2008) and Ogan et al. (2003) have described HIFU experiments on muscle and liver treatment in animals in which an IR camera was used to measure the temperature rise during HIFU exposure. In their experiments, the IR camera could obtain the surface temperature while the HIFU focus was 1–2 cm below the surface. Although so far no specific clinical applications have been reported, this method is still promising for future treatment of breast and other cancers.
CONCLUSIONS
A tomographic inverse computational approach was developed to reconstruct tissue properties and temperature in a tissue volume based on the time-dependent temperature on a tissue surface. With the use of sub-ablative HIFU, the method can be used as a thermal probe to obtain sub-surface tissue properties and detect sub-surface lesions. Although this technique can also be used to detect thermal lesions in heterogenous tissue, in the current study we used it in homogenous tissue. The non-invasive reconstruction technique can be extended to reconstruction of time-dependent tissue properties and, hence, can improve non-invasive volumetric temperature imaging during HIFU ablation.
Acknowledgments
This research was supported in part by NIH (R01 EB008999). The authors also thank J. Beeney for assistance with implementation of the infrared system.
REFERENCES
- Abolhassani MD, Norouzy A, Takavar A, Ghanaati H. Noninvasive temperature estimation using sonographic digital images. J. Ultrasound Med. 2007;26:215–222. doi: 10.7863/jum.2007.26.2.215. [DOI] [PubMed] [Google Scholar]
- Anand A, Kaczkowski PJ. Noninvasive measurement of local thermal diffusivity using backscattered ultrasound and focused ultrasound heating. Ultrasound Med Biol. 2008;34:1449–1464. doi: 10.1016/j.ultrasmedbio.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhavaraju NC, Valvano JW. Thermophysical properties of swine myocardium. Int J Thermophys. 1999;20:665–676. [Google Scholar]
- Campbell P, Thomas R. Thermal imaging in surgery. In: Diakides NA, Bronzino JD, editors. Medical infrared imaging. CRC Press; Boca Raton, FL: 2008. [Google Scholar]
- Chavrier F, Chapelon JY, Gelet A, Cathignol D. Modeling of high-intensity focused ultrasound-induced lesions in the presence of cavitation bubbles. J Acoust Soc Am. 2000;108:432–440. doi: 10.1121/1.429476. [DOI] [PubMed] [Google Scholar]
- Cheng HLM, Plewes DB. Tissue thermal conductivity by magnetic resonance thermometry and focused ultrasound heating. J Magn Reson Imaging. 2002;16:598–609. doi: 10.1002/jmri.10199. [DOI] [PubMed] [Google Scholar]
- Crouzet S, Murat FJ, Pasticier G, Cassier P, Chapelon JY, Gelet A. High intensity focused ultrasound (HIFU) for prostate cancer: Current clinical status, outcomes and future perspectives. Int J Hypertherm. 2010;26:796–803. doi: 10.3109/02656736.2010.498803. [DOI] [PubMed] [Google Scholar]
- Damianou C, Hynynen K. The effect of various physical parameters on the size and shape of necrosed tissue volume during ultrasound surgery. J Acoust Soc Am. 1994;95:1641–1649. doi: 10.1121/1.408550. [DOI] [PubMed] [Google Scholar]
- Farny CH, Clement GT. Ultrasound phase contrast thermal imaging with reflex transmission imaging methods in tissue phantoms. Ultrasound Med Biol. 2009;35:1995–2006. doi: 10.1016/j.ultrasmedbio.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farny CH, Holt RG, Roy RA. The correlation between bubble-enhanced HIFU heating and cavitation power. IEEE Trans Biomed Eng. 2010;57:175–184. doi: 10.1109/TBME.2009.2028133. [DOI] [PubMed] [Google Scholar]
- Groh MA, Binns OA, Burton HG, III, Champsar GL, Ely SW, Johnson AM. Ultrasonic cardiac ablation for atrial fibrillation during concomitant cardiac surgery: Long-term clinical outcomes. Ann Thorac Surg. 2007;84:1978–1983. doi: 10.1016/j.athoracsur.2007.06.081. [DOI] [PubMed] [Google Scholar]
- Hynynen K. MRI-guided focused ultrasound treatments. Ultrasonics. 2010;50:221–229. doi: 10.1016/j.ultras.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Illing RO, Kennedy JE, Wu F, Haar GT, Protheroe AS, Friend PJ, Gleeson SV, Cranston DW, Philips RR. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population. Br J Cancer. 2005;93:890–895. doi: 10.1038/sj.bjc.6602803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Paulsen KD, Osterberg UL. Optical image reconstruction using DC data: Simulations and experiments. Phys Med Biol. 1996;41:1483–1498. doi: 10.1088/0031-9155/41/8/015. [DOI] [PubMed] [Google Scholar]
- Jiang H, Xu Y, Iftimia N. Experimental three-dimensional optical image reconstruction of heterogeneous turbid media from continuous-wave data. Opt Express. 2000;7:204–209. doi: 10.1364/oe.7.000204. [DOI] [PubMed] [Google Scholar]
- Jiang L, Zhan W, Loew MH. A numerical study of the inverse problem of breast infrared thermography modeling. In: Molthen RC, Weaver JB, editors. Medical Imaging 2010: Biomedical Applications in Molecular, Structural, and Functional Imaging, 7626; Proc SPIE.2010. pp. 1–8. [Google Scholar]
- Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer. 2005;5:321–327. doi: 10.1038/nrc1591. [DOI] [PubMed] [Google Scholar]
- Leighton TG. The acoustic bubble. Academic Press; San Diego: 1994. [Google Scholar]
- Liu D, Ebbini ES. Real-time 2-D temperature imaging using ultrasound. IEEE Trans Biomed Eng. 2010;57:12–16. doi: 10.1109/TBME.2009.2035103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Xu LX. Boundary information based on the thermal states of biological bodies. Int J Heat Mass Transfer. 2000;43:2827–2839. [Google Scholar]
- Lizzi FL, Driller J, Lunzer B, Kalisz A, Coleman DJ. Computer model of ultrasonic hyperthermia and ablation for ocular tumors using B-mode data. Ultrasound Med Biol. 1992;18:59–73. doi: 10.1016/0301-5629(92)90009-y. [DOI] [PubMed] [Google Scholar]
- Lizzi FL, Muratore R, Deng CX, Muratore R, Ketterling JA, Alam SK, Mikaelian S. Radiation-force technique to monitor lesions during ultrasonic therapy. Ultrasound Med Biol. 2003;29:1593–1605. doi: 10.1016/s0301-5629(03)01052-4. [DOI] [PubMed] [Google Scholar]
- Natale A, Pisano E, Shewchik J, Bash D, Fanelli R, Potenza D. First human experience with pulmonary vein isolation using a through-the-balloon circumferential ultrasound ablation system for recurrent atrial fibrillation. Circulation. 2000;102:1879–1882. doi: 10.1161/01.cir.102.16.1879. [DOI] [PubMed] [Google Scholar]
- Nowakowski A. Quantitative active dynamic thermal IR-imaging and thermal tomography in medical diagnostics. In: Diakides NA, Bronzino JD, editors. Medical infrared imaging. CRC Press; Boca Raton, FL: 2008. [Google Scholar]
- Nyborg WL. Solutions of the bio-heat transfer equation. Phys Med Biol. 1988;33:785–792. doi: 10.1088/0031-9155/33/7/002. [DOI] [PubMed] [Google Scholar]
- Ogan K, Roberts WW, Wilhelm DM, Bonnell L, Leiner D, Lindberg G, Kavoussi LR, Cadeddu JA. Infrared thermography and thermocouple mapping of radiofrequency renal ablation to assess treatment adequacy and ablation margins. Urology. 2003;62:146–151. doi: 10.1016/s0090-4295(03)00040-2. [DOI] [PubMed] [Google Scholar]
- Pennes HH. Analysis of tissue and arterial blood temperatures in the resting human forearm. J Appl Physiol. 1948;1:93–122. doi: 10.1152/jappl.1948.1.2.93. [DOI] [PubMed] [Google Scholar]
- Ries M, Senneville BD, Roujol S, Berber Y, Quesson B, Moonen C. Real-time 3D target tracking in MRI guided focused ultrasound ablations in moving tissues. Magn Reson Med. 2010;64:1704–1712. doi: 10.1002/mrm.22548. [DOI] [PubMed] [Google Scholar]
- Rivens I, Shaw A, Civale J, Morris H. Treatment monitoring and thermometry for therapeutic focused ultrasound. Int J Hypertherm. 2007;23:121–139. doi: 10.1080/02656730701207842. [DOI] [PubMed] [Google Scholar]
- Ruminski J, Kaczmarek M, Renkielska A, Nowakowski A. Thermal parametric imaging in the evaluation of skin burn depth. IEEE Trans Biomed Eng. 2007;54:303–312. doi: 10.1109/TBME.2006.886607. [DOI] [PubMed] [Google Scholar]
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys. 1984;10:787–800. doi: 10.1016/0360-3016(84)90379-1. [DOI] [PubMed] [Google Scholar]
- Seip R, VanBaren P, Cain C, Ebbini E. Noninvasive real-time multipoint temperature control for ultrasound phased array treatments. IEEE Trans Ultrason Ferroelectr Freq Control. 1996;43:1063–1073. [Google Scholar]
- Strickberger SA, Tokano T, Kluiwstra JU, Morady F, Cain C. Extracardiac ablation of the canine atrioventricular junction by use of high-intensity focused ultrasound. Circulation. 1999;100:203–208. doi: 10.1161/01.cir.100.2.203. [DOI] [PubMed] [Google Scholar]
- Sumi C, Yanagimura H. Reconstruction of thermal property distributions of tissue phantoms from temperature measurements—Thermal conductivity, thermal capacity and thermal diffusivity. Phys Med Biol. 2007;52:2845–2863. doi: 10.1088/0031-9155/52/10/014. [DOI] [PubMed] [Google Scholar]
- Sun Y, Sobel E, Jiang H. First assessment of three-dimensional quantitative photoacoustic tomography for in vivo detection of osteoarthritis in the finger joints. Med Phys. 2011;38:4009–4017. doi: 10.1118/1.3598113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter Haar G. Harnessing the interaction of ultrasound with tissue for therapeutic benefit: High-intensity focused ultrasound. Ultrasound Obstet Gynecol. 2008;32:601–604. doi: 10.1002/uog.6228. [DOI] [PubMed] [Google Scholar]
- Tikhonov A. Scripta series in mathematics. Winston; New York: 1977. Solutions of ill-posed problems. [Google Scholar]
- Uchida T, Shoji S, Nakano M, Hongo S, Nitta M, Usui Y, Nagata Y. Transrectal high-intensity focused ultrasound for the treatment of localized prostate cancer: Eight-year experience. Int J Urol. 2009;16:881–886. doi: 10.1111/j.1442-2042.2009.02389.x. [DOI] [PubMed] [Google Scholar]
- Vaezy S, Zderic V. Hemorrhage control using high intensity focused ultrasound. Int J Hypertherm. 2007;23:203–211. doi: 10.1080/02656730601169779. [DOI] [PubMed] [Google Scholar]
- Vanne A, Hynynen K. MRI feedback temperature control for focused ultrasound surgery. Phys Med Biol. 2003;48:31–43. doi: 10.1088/0031-9155/48/1/303. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hunt JW, Foster FS, Plewes DB. Tissue ultrasound absorption measurement with MRI calorimetry. IEEE Trans Utrason Ferroelectr Freq Control. 1999;46:1192–1200. doi: 10.1109/58.796125. [DOI] [PubMed] [Google Scholar]
- Xi L, Li X, Yao L, Grobmyer S, Jiang H. Design and evaluation of a hybrid photoacoustic tomography and diffuse optical tomography system for breast cancer detection. Med Phys. 2012;39:2584–2594. doi: 10.1118/1.3703598. [DOI] [PubMed] [Google Scholar]
- Yuan Z, Jiang H. Quantitative photoacoustic tomography: Recovery of optical absorption coefficient maps of heterogeneous media. Appl Phys Lett. 2006;88:23. Art. 231101. [Google Scholar]
- Yuan Z, Jiang H. Quantitative photoacoustic tomography. Philos Trans A Math Phys Eng Sci. 2009;367:3043–3054. doi: 10.1098/rsta.2009.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zderic V, Foley J, Luo W, Vaezy S. Prevention of post-focal thermal damage by formation of bubbles at the focus during high intensity focused ultrasound therapy. Med Phys. 2008;35:4292–4299. doi: 10.1118/1.2975149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zderic V, Keshavarzi A, Andrew MA, Vaezy S, Martin RW. Attenuation of porcine tissues in vivo after high-intensity ultrasound treatment. Ultrasound Med Biol. 2004;30:61–66. doi: 10.1016/j.ultrasmedbio.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Zhong H, Wan MX, Jiang YF, Wang SP. Monitoring imaging of lesions induced by high intensity focused ultrasound based on differential ultrasonic attenuation and integrated backscatter estimation. Ultrasound Med Biol. 2007;33:82–94. doi: 10.1016/j.ultrasmedbio.2006.07.034. [DOI] [PubMed] [Google Scholar]