Abstract
Development of hypertension is influenced by genes, environmental effects and their interactions, and the human metabolome is a measurable manifestation of gene-environment interaction. We explored the metabolomic antecedents of developing incident hypertension in a sample of African Americans, a population with a high prevalence of hypertension and its comorbidities. We examined 896 (565 females, aged 45–64 years) African American normotensives from the Atherosclerosis Risk in Communities (ARIC) Study, whose metabolome was measured in serum collected at the baseline examination and analyzed by high throughput methods. The analyses presented here focus on 204 stably measured metabolites over a period of 4–6 weeks. Weibull parametric models considering interval censored data were used to assess the hazard ratio for incident hypertension. We used a modified Bonferroni correction accounting for the correlations among metabolites to define a threshold for statistical significance (p<3.9×10−4). During 10-years of follow-up, 38% of baseline normotensives developed hypertension (N=344). With adjustment for traditional risk factors and estimated glomerular filtration rate, each +1-standard deviation difference in baseline 4-hydroxyhippurate, a product of gut microbial fermentation, was associated with 17% higher risk of hypertension (p=2.5×10−4), which remained significant after adjusting for both baseline systolic and diastolic blood pressure (p=3.8×10−4). After principal component analyses, a sex steroids pattern was significantly associated with risk of incident hypertension (highest versus lowest quintile hazard ratio, 1.72; 95% CI, 1.05 to 2.82; p for trend=0.03), and stratified analyses suggested that this association was consistent in both genders. Metabolomic analyses identify novel pathways in the etiology of hypertension.
Keywords: metabolomics, hypertension, African Americans, risk factor
Introduction
Hypertension is a leading risk factor for cardiovascular disease mortality, causing more than 7 million deaths every year worldwide.1 African Americans have greater prevalence and severity of hypertension than European-Americans,2 and are, thus, prime candidates for primary prevention efforts. Hypertension and its underlying pathophysiology may be present for years before clinical diagnosis, when irreversible pathology has already occurred. Current clinical evaluation of hypertension risk, such as blood pressure measurement, provides limited insight into relevant abnormal mechanisms for a particular patient. Because blood pressure is regulated and hypertension is controlled by a multiple physiologic and anatomic systems,3 it has been proposed that a “systems-biology” approach to hypertension risk management and control would be beneficial.4 The metabolome represents the outcome of multiple physiologic and metabolic processes and the ultimate downstream expression of the interaction between gene action and environmental exposure.5, 6 Therefore, the metabolome may provide a high-resolution, multifactorial phenotypic signature of the etiology, manifestation, or pathophysiology7 of hypertension.
To date, no study has explored the metabolomic antecedents of incident hypertension in African-Americans, who have the highest prevalence and rate of incident hypertension among all races.2 Therefore, we measured the metabolome in a well-characterized sample of African-Americans from the Atherosclerosis Risk in Communities (ARIC) Study and identified individual metabolites and metabolite patterns that are significantly associated with incident hypertension during approximately 10-years of follow-up.
Methods
Study Sample
The ARIC Study consists of a prospective cohort designed to identify the causes and outcomes of cardiovascular diseases. The ARIC study population was selected as a probability sample of 15,792 men and women aged 45 to 64 years from 4 communities (Forsyth County, NC; Jackson, MS; suburbs of Minneapolis, MN; and Washington County, MD). Detailed descriptions of its study design, objectives and procedures have been published elsewhere.8 Eligible participants were interviewed at home and then invited to a baseline clinical examination between 1987 and 1989. Participants returned for multiple follow-up clinical examinations. The ARIC Study was approved by the institutional review boards at each site. Metabolomic profiles were measured in a subsample of ARIC African American participants (N=1,977) randomly selected from the Jackson, MS field center from all of those who provided informed consented for genetic research, provided quality dietary data, and fasted ≥8 hours before the baseline exam.
Assessment of Baseline Covariates and Metabolites
The baseline examinations included standardized medical history, physical examination with anthropometric measures, and laboratory testing. Sitting blood pressure was measured by trained technicians on the left arm of the participants with an appropriately sized cuff three times with a random-zero mercury sphygmomanometer after a 5-minute rest, and the average of the last two readings was used. Hypertension was defined as systolic blood pressure (SBP) ≥140 mmHg or diastolic blood pressure (DBP) ≥90 mmHg or currently taking anti-hypertensive medication. Incident hypertension was defined as new occurrences of hypertension during the follow-up examinations among the baseline normotensives. Leisure-time physical activity was measured using a modification of the Baecke Physical Activity questionnaire.9 Alcohol intake was ascertained from a standardized questionnaire and the alcohol amount in grams per week was used in these analyses. Cigarette smoking status was self-reported and categorized as current and noncurrent smoker. Diabetes was defined as a fasting glucose level ≥7.0 mmol/L, a nonfasting level ≥11.1 mmol/L, a self-reported physician diagnosis, or pharmacologic hypoglycemic treatment. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.10
Among all normotensive participants (N=927) at the baseline examination, 31 were excluded from the analysis because of either prevalent coronary heart disease (by history or ECG criteria, N=23), prevalent heart failure (by Gothenburg criteria or heart failure medication use,11 N=12), or prevalent stage 4 or 5 chronic kidney disease (eGFR <30 mL/min/1.73 m2,12 N=1) leaving 896 normotensive participants (males, N=331) for the analyses presented here.
Detection and quantification of metabolites in fasting serum samples continuously stored at −80° was completed by Metabolon Inc. (Durham, USA) using an untargeted, gas chromatography-mass spectrometry and liquid chromatography-mass spectrometry (GC-MS and LC-MS)-based metabolomic quantification protocol. Metabolites were identified by comparison to library entries of purified standards or recurrent unknown entities.11 This untargeted approach identified and quantified 602 metabolites, including named compounds whose chemical identity is known (N=361; 90 in amino acid metabolism pathways, 16 in carbohydrate, 12 in cofactors and vitamins, 8 in energy, 147 in lipid, 14 in nucleotide, 29 in peptide and 45 in xenobiotics), as well as unnamed compounds that do not currently have a chemical standard (N=241).13 The unknown chemical identities are tagged beginning with “X” and followed by numbers, such as “X-12345”. Measurement methods of these metabolites and rigorous laboratory quality control process were described in detail elsewhere.11 A repeatability study was carried out to evaluate the biologic stability of metabolites in fasting serum collected 4–6 week apart. Based on these analyses, 204 out of total 602 metabolites were selected based on having a reliability coefficient ≥0.6 as well as having fewer than 80% of the values below the detection limit or missing (BDL/missing),11 and these values were assigned the lowest detected value for that metabolite in all samples. These 204 metabolites consisted of 187 metabolites treated as continuous variables in the analyses (<50% BDL/missing observations; 108 named and 79 unnamed compounds), and 17 metabolites treated as ordinal variables in the analyses (50–80% BDL/missing observations; 1= BDL/missing values, 2= values below the median, and 3= values equal or above the median; 10 named and 7 unnamed compounds) (supplement table S1).
For descriptive analyses across groups, Chi-square tests were used for categorical variables, and two-sample t-tests or Wilcoxon’s rank-sum tests were used for continuous traits. The Weibull parametric model for interval censored data, which is an accelerated-failure time proportional-hazards model,14 was used to estimate the hazard ratio (HR) of developing incident hypertension. Two multivariable models were used to assess the relation between metabolites (either an individual metabolite or metabolomic pattern) and incident hypertension. Covariates were selected based on published reports.15–18 The basic model (Model 1) adjusted for traditional risk factors, i.e., age, gender, leisure-time physical activity, alcohol intake, current cigarette smoking status, body mass index (BMI) and diabetes status. Model 2 included the covariates in Model 1 with the additional of a measure of kidney function, i.e., eGFR. For the identified individual metabolomic biomarker candidates and the potential hypertension-related principal-components, we also investigated whether the observed association was robust to further adjustment for baseline SBP and DBP.
In analyses of association between an individual metabolite and hypertension, all HRs were calculated and reported per +1-SD for the continuous variables or per +1-category change in the categorical variables. A modified stepwise Bonferroni procedure, the Dubey/Armitage-Parmar algorithm11, 19 was used to correct for multiple comparisons and a significance level of 3.9×10−4 (2 tailed) was considered for each individual test. This adjustment takes into account the full correlation matrix of metabolites and uses the mean correlation among the metabolites in the formula, where the new α-level for the kth hypothesis for k=1,2,…, K is readjusted for each individual metabolites according to: αk = 1 − (1 − α)1/mk, where mk = K1−r.k, , and rjk is the correlation coefficient between the jth and kth metabolites. When the average of the correlation coefficients is zero, this adjustment is according to the Bonferroni procedure and when it is one, the adjusted and the unadjusted p-values are the same.
Metabolites are expected to be correlated in complex ways. Thus, a principal-components analysis (PCA) was used to group the 187 metabolites into metabolomic patterns. The three patterns retained were selected based on three criteria: 1) the Kaiser criterion (eigenvalues >1), 2) inflection point of the scree plot, and 3) the interpretability of the patterns. 20, 21 A factor score for each study participant was calculated from the sum of the levels from all the 187 metabolites, multiplied by their respective factor loadings. Metabolomic patterns were named according to the metabolite groups loading highest on each of the three factor patterns: sex steroids, alpha amino acids and branch-chain amino acids (supplement table S2). The scores for each PCA-derived pattern were entered separately into the Weibull parametric models. HR of developing incident hypertension for the highest versus lowest quintile of these three patterns, as well as the corresponding p value for trend across quintiles were calculated in all analyses. For the PCA analyses, a significance level of 0.05 (2 tailed) was used.
All statistical analyses were performed in SAS version 9.2 (SAS Institute, Cary, NC).
Results
During the approximate 10-year follow-up period, 38.4% of the 896 normotensives at baseline (N=344; 39.3% of the female and 36.9% of the male normotensives) developed hypertension (incidence rate: 63.94 per 1000 person-years; median 7.67 years to first diagnosis of hypertension), and 209 reported having begun antihypertensive medications. Participants who developed hypertension were more likely to weigh more, be diabetic, and have higher SBP and DBP at the baseline examination (Table 1). The median and interquartile range for the percentage of BDL/missing values for the 204 metabolites included in our analysis were 2.6% and 20.4%, respectively.
Table 1.
Distribution of baseline risk factors by incident hypertension status among normotensives at baseline
| Characteristics | Normotensive at baseline (N=896) | Non-incident hypertension (N=552) | Incident hypertension (N=344) |
|---|---|---|---|
| Age, y | 51.47 ± 5.4 | 51.40 ± 5.5 | 51.59 ± 5.3 |
| Male, n (%) | 331 (36.9) | 209 (37.9) | 122 (35.5) |
| Leisure-time physical activity* | 2.13 ± 0.6 | 2.13 ± 0.6 | 2.13 ± 0.6 |
| Current cigarette smoking, n (%) | 258 (28.8) | 165 (29.9) | 93 (27.0) |
| Ethanol intake in grams/week, median (IQR) | 0.0 (0.0–13.2) | 0.0 (0.0–13.2) | 0.0 (0.0–13.2) |
| BMI, kg/m2† | 28.65 ± 5.5 | 28.09 ± 5.3 | 29.55 ± 5.8 |
| Prevalent diabetes, n (%)‡ | 97 (10.8) | 47 (8.5) | 50 (14.5) |
| eGFR, mL/min/1.73 m2 | 107.35 ± 15.6 | 107.46 ± 15.4 | 107.18 ± 15.9 |
| SBP, mmHg† | 116.70 ± 11.1 | 114.01 ± 10.8 | 121.02 ± 10.2 |
| DBP, mmHg† | 74.70 ± 7.8 | 73.38 ± 7.8 | 76.83 ± 7.2 |
BMI indicates body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; IQR, interquartile range; SBP, systolic blood pressure.
For continuous variables except “ethanol intake in grams/week”, mean values ± standard errors are shown. Percentages for categorical variables are shown in parentheses.
2.13 was interpreted as “moderate active”42 based on the tertile distribution of the leisure-time physical activity score (low active: <2.00, moderate active: 2.00–2.49, high active: ≥2.50) among the current study participants.
Test for difference in each risk factor between non-incident hypertension group and incident hypertension group:
.001<P<.01,
P<.001.
The metabolite 4-hydroxyhippurate was consistently associated with incident hypertension across models (HR per SD=1.17 and 1.18 respectively in Model 1 and 2, p<3.9×10−4, Table 2), suggesting its association with hypertension may be independent of kidney function. The adjusted HR per SD for 4-hydroxyhippurate also remained significant after adjusting for baseline blood pressure (HR per SD, 1.18; 95% CI, 1.08 to 1.29; p=3.8×10−4; Table 2). No other metabolite was significantly associated with incident hypertension (supplement table S1).
Table 2.
Association of Individual Metabolites with Incident Hypertension
| Metabolite | Loading in sex steroids pattern | Model 1 | Model 2 | Model 2+ baseline BP | |||
|---|---|---|---|---|---|---|---|
| HR per SD (95% CI) | P value | HR per SD (95% CI) | P value | HR per SD (95% CI) | P value | ||
| Individual metabolite | |||||||
| 4-hydroxyhippurate | - | 1.17 (1.08, 1.28) | 3.1× 10−4 | 1.18 (1.08, 1.28) | 2.5× 10−4 | 1.18 (1.08, 1.29) | 3.8× 10−4 |
| Metabolites in sex steroids pattern | |||||||
| 5α-androstan-3β,17β-diol disulfate | 0.70 | 1.12 (1.04, 1.20) | 0.003 | 1.17 (1.05, 1.30) | 0.003 | 1.12 (1.01, 1.25) | 0.03 |
| androsterone sulfate | 0.43 | 1.10 (1.02, 1.19) | 0.02 | 1.15 (1.03, 1.30) | 0.01 | 1.12 (1.00, 1.26) | 0.04 |
| epiandrosterone sulfate | 0.50 | 1.09 (1.00, 1.09) | 0.04 | 1.14 (1.01, 1.30) | 0.03 | 1.12 (0.99, 1.27) | 0.06 |
CI indicates confidence interval; HR, hazard ratio; SD, standard deviation.
Weibull parametric models adjusted for traditional risk factors (age, gender, leisure-time physical activity, alcohol intake, current cigarette smoking status, prevalent diabetes and body mass index) in Model 1, traditional risk factors plus estimated glomerular filtration rate in Model 2.
Principal component analyses yielded three biologically plausible metabolomic patterns: sex steroids, alpha amino acids and branch-chain amino acids (Table 3). The first pattern, sex steroids, was statistically significantly associated with incident hypertension in both models and the other two patterns were uniformly not significant (Table 3). Stratified analysis suggested a consistent association of the sex steroids pattern with incident hypertension in both males and females (in males, highest versus lowest quintile HR=1.93; in females, highest versus lowest quintile HR=1.50). With further adjustment for baseline SBP and DBP, the significance of the sex steroids pattern was slightly attenuated (highest versus lowest quintile HR, 1.40; 95% CI, 0.84 to 2.32; p for trend in quintile number =0.10; Figure 1). To assess whether the association ascribed to sex steroids pattern was driven by one or a few metabolites, individual metabolites composing the sex steroids pattern were assessed. Three metabolites, epiandrosterone sulfate, 5alpha-androstan-3beta 17beta-diol disulfate and androsterone sulfate were nominal significant predictors of incident hypertension (Table 2). It is of note that the variance of the metabolites explained by the three PCA-derived patterns was relatively low (17%), which is believed to be a result of the pathway diversity of the human metabolome.
Table 3.
Principal component patterns
| Pattern (consisting metabolites) | Individual Named Components | Eigen- Value | Variance explained | Model 1 HR (p for trend in quintile number) | Model 2 HR (p for trend in quintile number) | |
|---|---|---|---|---|---|---|
| Pattern1 (sex steroids, branched-chain amino acids, and other AAs) | 4-androsten-3β,17β-diol disulfate 2 pregnen-diol disulfate 5α-androstan-3β,17β-diol disulfate DHEA-S 4-androsten-3β,17β-diol disulfate 1 pregn steroid monosulfate 21- hydroxypregnenolone disulfate androsterone sulfate epiandrosterone sulfate | 3-(4- hydroxyphenyl)lactate isoleucine pyroglutamine α-hydroxyisocaproate leucine α-hydroxyisovalerate 2- methylbutyroylcarnitine valine deoxycarnitine | 15.88 | 0.08 | 1.58 (p=0.05) | 1.72 (p=0.03) |
| Pattern2 (Alpha Amino acids and their derivatives) | N-acetylalanine glycine 3-methoxytyrosine erythronate cholesterol | erythritol γ-glutamylleucine 5-oxoproline phosphate threonate | 8.81 | 0.05 | 0.79 (p=0.19) | 0.80 (p=0.24) |
| Pattern3 (Branched- chain Amino acid, long chain fatty acid, other lipids, peptide) | leucine valine leucylleucine isoleucine glycerol phosphate HXGXA cholesterol dihomo-linolenate (20:3n3 or n6) | pyroglutamylglycine tyrosine glycylvaline docosapentaenoate (n3 DPA; 22:5n3) docosahexaenoate (DHA; 22:6n3) mannose glycylleucine | 7.09 | 0.04 | 1.39 (p=0.23) | 1.39 (p=0.24) |
HR indicates hazard ratio for highest versus lowest quintile.
Weibull parametric models adjusted for traditional risk factors (age, gender, leisure-time physical activity, alcohol intake, current cigarette smoking status, prevalent diabetes and body mass index) in Model 1, traditional risk factors plus estimated glomerular filtration rate in Model 2.
Figure 1.
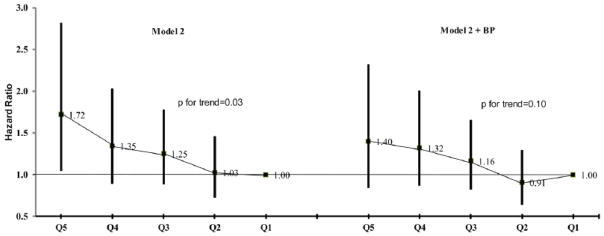
Hazard ratios and 95% CIs of incident hypertension by quintiles of the sex steroids pattern at baseline from Weibull parametric models. Model 2 was adjusted for age, gender, leisure-time physical activity, current cigarette smoking status, alcohol intake, prevalent diabetes, body mass index, and estimated glomerular filtration rate. Compared to the quintile of the lowest scores for the sex steroids pattern, only the quintile of the highest scores had a significant higher risk of incident hypertension, but the trend of associations across quintiles was statistically significant (p<0.05). In the “Model 2+BP” model, baseline systolic blood pressure and diastolic blood pressure were added, and the effects of the sex steroid pattern were attenuated.
Discussion
We prospectively examined a sample of middle-aged African American normotensives having serum metabolomic data. After adjustment for traditional risk factors and kidney function, each SD increment of baseline 4-hydroxyhippurate was associated with an 18% higher risk of hypertension, which remained significant after adjusting for baseline blood pressure. In addition, a sex steroids pattern derived from principal components analysis was also associated with elevated risk of incident hypertension (highest versus lowest quintile HR=1.72; p for trend in quintile number =0.03). To our knowledge, our study is among the first to study human serum metabolomic antecedents of hypertension in a well-defined prospective cohort setting. Although there is limitation in our study such as a limited sample size and lack of availability of an independent replication sample, our findings provide potential novel biomarkers associated with incident disease independently of traditional risk factors, and hold promise for better defining the underlying pathophysiology of hypertension.
4-Hydroxyhippurate is an end product of benzoate metabolism from microbial fermentation of polyphenols.22 It may also originate in the oxidative break-down of many exogenous benzenoid substances by detoxifying enzymes in the endoplasmic reticulum or microsomes.23, 24 In the cardiovascular system, oxidative stress plays a critical physiological role in controlling endothelial function, vascular tone, and cardiac function in hypertension.3 Therefore, we speculate that its mechanism of action on blood pressure regulation may be through a multitude of pathways such as gut microbial fermentation and/or oxidative stress.23–27
A sex steroids pattern was positively and independently associated with incident hypertension after adjustment for traditional risk factors. This pattern may reflect the catabolism of pregnenolone, and the subsequent metabolism of its estrogen and androgen derivatives. It is not prudent to promote firm conclusions regarding the role of progesterone in hypertension,28 though it may lead to hypertension via the body’s response to stress.29 Of note, stress may have a more important effect on hypertension in African Americans than other groups.30, 31 For estrogens and androgens, a balance between activation mechanisms of vasoconstriction32–35 and vasorelaxation36, 37 determines the net effect on vascular tone and blood pressure.38 The mechanisms by which sex steroids affect blood pressure involve direct effects on vascular, renal and heart cells, indirect effects mediated by humoral factors,28 as well as modifying aldosterone, renin and aldosterone to renin ratio.39 Our findings provide further insight into important questions regarding the role of sex hormones in hypertension, though further research is required.
Enhancing the ability to identify high-risk individuals for developing hypertension is particularly important, because proven, preventive therapies exist, end-organ complications accrue over time and the whole process can be delayed. Our prospective cohort study detected biomarkers of hypertension well before the onset of apparent clinical condition. The strength of our study includes a population-based prospective cohort with detailed clinical characterization, and the strict quality standards11 to ensure valid and reliable inference with a single measurement.
The candidate metabolites of interest from this study should be measured in an independent replication sample of African Americans before further application. The present study is among the largest to date and the first prospective cohort to explore serum metabolite profiles in hypertension. High-throughput metabolomics, like other –omic technologies, brings a danger of generating false-positive associations due to multiple comparisons and over-fitting. Application of traditional statistical approaches (e.g., Bonferroni correction) without taking into account the correlations among metabolites may levy an insurmountable statistical penalty that can obscure biologically relevant associations (i.e. false negative results).40 The modified Bonferroni procedure used in this study considers the full correlation matrix among metabolites, which should preserve statistical power and minimize false negative results. Clearly, the sources of variation underlying the human metabolome are varied, and the ability to predict incident hypertension after years of follow-up is influenced by many factors including biologic characteristics of the metabolites, study design, and laboratory factors.
Supplementary Material
Perspectives.
Previous studies suggested that targeting high-risk, normotensive individuals for treatment may delay hypertension onset, allow earlier implementation of intervention measures, thereby possibly mitigating vascular complications.41 The extra information from metabolomic studies may help target such individuals, and potentially improve the sensitivity and specificity of the final algorithm for prediction of hypertension. The present study identified potential single metabolomic biomarker (i.e. 4-hydroxyhippurate) as well as a metabolomic pattern (i.e. sex steroids) independently pointing to dysregulated metabolic pathways underlying hypertension. The findings in this study may impact clinical care by allowing scarce resources to be concentrated on those at greatest risk of hypertension. In addition, an early indicator of hypertension may indicate earlier therapeutic interventions that could minimize the likelihood of serious complications.
Novelty and Significance: 1) What Is New, 2) What Is Relevant?
1. What Is New? (the novelty)
One metabolite, 4-hydroxyhippurate and a sex steroids pattern (consisting of pregnenolone and its estrogen and androgen derivatives) were identified as independent predictors of incident hypertension among African Americans by untargeted high-through metabolomic profiling protocol.
2. What Is Relevant? (how the study relates to hypertension)
Metabolomic studies may help target individuals of high risk in hypertension, and indicate earlier therapeutic interventions to them when necessary.
3. Summary (the conclusions of the study)
The present work is the first studying metabolomic antecedents of hypertension in African Americans, who have the highest rates of hypertension among all races. It shows that metabolomic biomarkers of hypertension can be detected well before the onset of the clinical condition.
Acknowledgments
The authors thank the staff and participants of the Atherosclerosis Risk in Communities (ARIC) Study for their important contributions.
Funding Sources
The ARIC Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The metabolomics measurements were sponsored by National Human Genome Research Institute (3U01HG004402-02S1). Dr. Jennifer A. Nettleton is supported by a K01 from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (5K01DK082729-04). Drs. Yan Zheng and Bing Yu are supported in part by a training fellowship from Burroughs Wellcome Fund – The Houston Laboratory and Population Science Training Program in Gene-Environment Interaction (BWF Grant No. 1008200).
Footnotes
Disclosures
None.
References
- 1.Danaei G, Finucane MM, Lin JK, Singh GM, Paciorek CJ, Cowan MJ, Farzadfar F, Stevens GA, Lim SS, Riley LM, Ezzati M. National, regional, and global trends in systolic blood pressure since 1980: Systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5.4 million participants. Lancet. 2011;377:568–577. doi: 10.1016/S0140-6736(10)62036-3. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2011 update: A report from the american heart association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montezano AC, Touyz RM. Molecular mechanisms of hypertension--reactive oxygen species and antioxidants: A basic science update for the clinician. Can J Cardiol. 2012;28:288–295. doi: 10.1016/j.cjca.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Thongboonkerd V. Genomics, proteomics and integrative “omics” in hypertension research. Curr Opin Nephrol Hypertens. 2005;14:133–139. doi: 10.1097/00041552-200503000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O’Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayr M. Metabolomics: Ready for the prime time? Circ Cardiovasc Genet. 2008;1:58–65. doi: 10.1161/CIRCGENETICS.108.808329. [DOI] [PubMed] [Google Scholar]
- 7.Kenny LC, Broadhurst DI, Dunn W, Brown M, North RA, McCowan L, Roberts C, Cooper GJ, Kell DB, Baker PN. Robust early pregnancy prediction of later preeclampsia using metabolomic biomarkers. Hypertension. 2010;56:741–749. doi: 10.1161/HYPERTENSIONAHA.110.157297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Investigators TA. The atherosclerosis risk in communities (aric) study: Design and objectives. The aric investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 9.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng Y, Yu B, Alexander D, Manolio TA, Aguilar D, Coresh J, Heiss G, Boerwinkle E, Nettleton JA. Associations between metabolomic compounds and incident heart failure among african americans: The atherosclerosis risk in communities (aric) study. Am J Epidemiol. 2013 doi: 10.1093/aje/kwt004. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National kidney foundation. K/doqi clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 13.Suhre K, Meisinger C, Doring A, Altmaier E, Belcredi P, Gieger C, Chang D, Milburn MV, Gall WE, Weinberger KM, Mewes HW, Hrabe de Angelis M, Wichmann HE, Kronenberg F, Adamski J, Illig T. Metabolic footprint of diabetes: A multiplatform metabolomics study in an epidemiological setting. PLoS One. 2010;5:e13953. doi: 10.1371/journal.pone.0013953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collett D. Modelling survival data in medical research. New York, NY: Chapman & Hall; 1994. [Google Scholar]
- 15.Schroeder EB, Liao D, Chambless LE, Prineas RJ, Evans GW, Heiss G. Hypertension, blood pressure, and heart rate variability: The atherosclerosis risk in communities (aric) study. Hypertension. 2003;42:1106–1111. doi: 10.1161/01.HYP.0000100444.71069.73. [DOI] [PubMed] [Google Scholar]
- 16.Alonso A, Nettleton JA, Ix JH, de Boer IH, Folsom AR, Bidulescu A, Kestenbaum BR, Chambless LE, Jacobs DR., Jr Dietary phosphorus, blood pressure, and incidence of hypertension in the atherosclerosis risk in communities study and the multi-ethnic study of atherosclerosis. Hypertension. 2010;55:776–784. doi: 10.1161/HYPERTENSIONAHA.109.143461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mellen PB, Bleyer AJ, Erlinger TP, Evans GW, Nieto FJ, Wagenknecht LE, Wofford MR, Herrington DM. Serum uric acid predicts incident hypertension in a biethnic cohort: The atherosclerosis risk in communities study. Hypertension. 2006;48:1037–1042. doi: 10.1161/01.HYP.0000249768.26560.66. [DOI] [PubMed] [Google Scholar]
- 18.Zheng ZJ, Folsom AR, Ma J, Arnett DK, McGovern PG, Eckfeldt JH. Plasma fatty acid composition and 6-year incidence of hypertension in middle-aged adults: The atherosclerosis risk in communities (aric) study. Am J Epidemiol. 1999;150:492–500. doi: 10.1093/oxfordjournals.aje.a010038. [DOI] [PubMed] [Google Scholar]
- 19.Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat Med. 1997;16:2529–2542. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 20.Pett MA, Lackey NR, Sullivan JJ. Making sense of factor analysis: The use of factor analysis for instrument development in health care research. 2003. [Google Scholar]
- 21.Conway JM, Huffcutt AI. A review and evaluation of exploratory factor analysis practices in organizational research. Org Res Methods. 2003;6:147–168. [Google Scholar]
- 22.Heinzmann SS, Merrifield CA, Rezzi S, Kochhar S, Lindon JC, Holmes E, Nicholson JK. Stability and robustness of human metabolic phenotypes in response to sequential food challenges. J Proteome Res. 2012;11:643–655. doi: 10.1021/pr2005764. [DOI] [PubMed] [Google Scholar]
- 23.Lis AW, McLaughlin I, Mpclaughlin RK, Lis EW, Stubbs EG. Profiles of ultraviolet-absorbing components of urine from autistic children, as obtained by high-resolution ion-exchange chromatography. Clin Chem. 1976;22:1528–1532. [PubMed] [Google Scholar]
- 24.Gelboin HV, Wiebel FJ, Kinoshita N. Microsomal aryl hydrocarbon hydroxylases: On their role in polycyclic hydrocarbon carcinogenesis and toxicity and the mechanism of enzyme induction. New York, N.Y: Academic Press; 1972. [PubMed] [Google Scholar]
- 25.Rechner AR, Smith MA, Kuhnle G, Gibson GR, Debnam ES, Srai SK, Moore KP, Rice-Evans CA. Colonic metabolism of dietary polyphenols: Influence of structure on microbial fermentation products. Free Radic Biol Med. 2004;36:212–225. doi: 10.1016/j.freeradbiomed.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 26.van Duynhoven J, Vaughan EE, Jacobs DM, Kemperman RA, van Velzen EJ, Gross G, Roger LC, Possemiers S, Smilde AK, Dore J, Westerhuis JA, Van de Wiele T. Metabolic fate of polyphenols in the human superorganism. Proc Natl Acad Sci U S A. 2011;108 (Suppl 1):4531–4538. doi: 10.1073/pnas.1000098107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Motojima M, Hosokawa A, Yamato H, Muraki T, Yoshioka T. Uraemic toxins induce proximal tubular injury via organic anion transporter 1-mediated uptake. Br J Pharmacol. 2002;135:555–563. doi: 10.1038/sj.bjp.0704482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubey RK, Oparil S, Imthurn B, Jackson EK. Sex hormones and hypertension. Cardiovasc Res. 2002;53:688–708. doi: 10.1016/s0008-6363(01)00527-2. [DOI] [PubMed] [Google Scholar]
- 29.Roberts E. Pregneolone--from selye to alzheimer and a model of the pregnenolone sulfate binding site on the gabaa receptor. Biochem Pharmacol. 1995;49:1–16. doi: 10.1016/0006-2952(94)00258-n. [DOI] [PubMed] [Google Scholar]
- 30.Anderson NB, McNeilly M, Myers H. Autonomic reactivity and hypertension in blacks: A review and proposed model. Ethn Dis. 1991;1:154–170. [PubMed] [Google Scholar]
- 31.Barnes V, Schneider R, Alexander C, Staggers F. Stress, stress reduction, and hypertension in african americans: An updated review. J Natl Med Assoc. 1997;89:464–476. [PMC free article] [PubMed] [Google Scholar]
- 32.Brooks-Asplund EM, Tupper CE, Daun JM, Kenney WL, Cannon JG. Hormonal modulation of interleukin-6, tumor necrosis factor and associated receptor secretion in postmenopausal women. Cytokine. 2002;19:193–200. doi: 10.1006/cyto.2002.1963. [DOI] [PubMed] [Google Scholar]
- 33.Livingstone C, Collison M. Sex steroids and insulin resistance. Clin Sci (Lond) 2002;102:151–166. doi: 10.1042/cs1020151. [DOI] [PubMed] [Google Scholar]
- 34.Schror K, Morinelli TA, Masuda A, Matsuda K, Mathur RS, Halushka PV. Testosterone treatment enhances thromboxane a2 mimetic induced coronary artery vasoconstriction in guinea pigs. Eur J Clin Invest. 1994;24 (Suppl 1):50–52. doi: 10.1111/j.1365-2362.1994.tb02428.x. [DOI] [PubMed] [Google Scholar]
- 35.Lahita RG. Sex hormones and systemic lupus erythematosus. Rheum Dis Clin North Am. 2000;26:951–968. doi: 10.1016/s0889-857x(05)70178-2. [DOI] [PubMed] [Google Scholar]
- 36.Caulin-Glaser T, Garcia-Cardena G, Sarrel P, Sessa WC, Bender JR. 17 beta-estradiol regulation of human endothelial cell basal nitric oxide release, independent of cytosolic ca2+ mobilization. Circ Res. 1997;81:885–892. doi: 10.1161/01.res.81.5.885. [DOI] [PubMed] [Google Scholar]
- 37.Chou TM, Sudhir K, Hutchison SJ, Ko E, Amidon TM, Collins P, Chatterjee K. Testosterone induces dilation of canine coronary conductance and resistance arteries in vivo. Circulation. 1996;94:2614–2619. doi: 10.1161/01.cir.94.10.2614. [DOI] [PubMed] [Google Scholar]
- 38.Wang L, Szklo M, Folsom AR, Cook NR, Gapstur SM, Ouyang P. Endogenous sex hormones, blood pressure change, and risk of hypertension in postmenopausal women: The multi-ethnic study of atherosclerosis. Atherosclerosis. 2012;224:228–234. doi: 10.1016/j.atherosclerosis.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmed AH, Gordon RD, Taylor PJ, Ward G, Pimenta E, Stowasser M. Are women more at risk of false-positive primary aldosteronism screening and unnecessary suppression testing than men? J Clin Endocrinol Metab. 2011;96:E340–346. doi: 10.1210/jc.2010-1355. [DOI] [PubMed] [Google Scholar]
- 40.Lewis GD, Asnani A, Gerszten RE. Application of metabolomics to cardiovascular biomarker and pathway discovery. J Am Coll Cardiol. 2008;52:117–123. doi: 10.1016/j.jacc.2008.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parikh NI, Pencina MJ, Wang TJ, Benjamin EJ, Lanier KJ, Levy D, D’Agostino RB, Sr, Kannel WB, Vasan RS. A risk score for predicting near-term incidence of hypertension: The framingham heart study. Ann Intern Med. 2008;148:102–110. doi: 10.7326/0003-4819-148-2-200801150-00005. [DOI] [PubMed] [Google Scholar]
- 42.Hertogh EM, Monninkhof EM, Schouten EG, Peeters PH, Schuit AJ. Validity of the modified baecke questionnaire: Comparison with energy expenditure according to the doubly labeled water method. Int J Behav Nutr Phys Act. 2008;5:30. doi: 10.1186/1479-5868-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


