Abstract
We report principles for active, user-defined control over the locations and timing with which DNA is expressed in cells. Our approach exploits unique properties of a ferrocenyl cationic lipid that is inactive when oxidized, but active when chemically reduced. We show that methods that exert spatial control over the administration of reducing agents can lead to local activation of lipoplexes and spatial control over gene expression. The versatility of this approach is demonstrated using both soluble and solid-phase reducing agents. These methods provide control over cell transfection, including methods for remote activation and the patterning of expression using solid-phase redox agents, that are difficult to achieve using conventional lipoplexes.
Keywords: Amphiphiles, Chemical Activation, DNA, Ferrocene, Redox Chemistry
Introduction
Cationic lipids are used widely to deliver DNA to cells.1-3 This class of materials binds cooperatively to DNA to form compact, nanostructured ‘lipoplexes’ with sizes and properties that facilitate transport of DNA across cell membranes. The overall efficiency with which lipoplexes promote transgene expression also depends on their ability to overcome several intracellular barriers to delivery.4 Those challenges are often addressed by designing multifunctional lipids that release DNA or perform other functions – after internalization by cells – in response to intracellular stimuli (e.g., changes in pH, redox potential, or the presence of enzymes) or other external stimuli (e.g., heat, light, etc.).5-11 A common property of most lipoplexes formed from cationic lipids, however, is that they are ‘active’ from the time at which they are first formed and will, thus, start to be internalized immediately upon presentation to cells. The design of ‘inactive’ or dormant lipoplexes that can be transformed and activated on-demand in extracellular environments – at points in time or space selected by the investigator – has received little past attention.
Lipoplexes that can be activated by external stimuli in extracellular environments offer the potential to control the times at which otherwise quiescent lipoplexes will be internalized by cells (to achieve temporal control over gene delivery) or dictate with new degrees of spatial control which specific sub-populations of cells receive a transgene (by spatial control over the ‘activation’ of selected sub-populations of lipoplexes) – without requiring that the lipoplexes themselves be administered in a spatially or temporally resolved manner. The development of principles that permit such control would constitute a significant fundamental advance and would also be useful in a broad range of applications, including (i) generation of transfected cell arrays to screen for protein-protein and protein-drug interactions,12 (ii) provision of new tools for tissue engineering13 and developmental biology14 studies, and (iii) new approaches to the remote activation of lipoplexes and the directed delivery of DNA to sub-populations of cells in ways that are difficult (or, in some cases, impossible) using conventional ‘active’ lipoplexes. We note that other approaches to spatiotemporal control over the delivery of lipoplexes have been reported.12,15-17 As mentioned above, however, those methods are based on the use of lipoplexes that are introduced into a system in an already active state and, thus, require the use of additional (and sometimes complex) methods to distribute or physically pattern them to exert control over transgene expression.
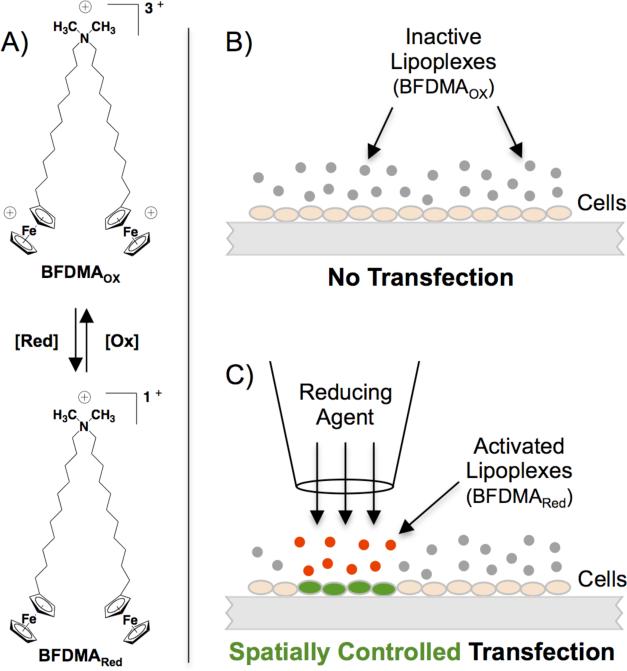
Here, we report principles for spatial and temporal control over transgene expression in cells using lipoplexes prepared from a cationic lipid containing two redox-active ferrocene groups (bis-11-ferrocenylundecyldimethylammonium bromide; BFDMA, Scheme 1A).18 The ferrocene groups in BFDMA can be cycled reversibly between reduced (no net charge) and oxidized (a charge of +1 per ferrocenium ion) states. Past studies demonstrate that the oxidation state of BFDMA strongly influences the efficiency with which lipoplexes of BFDMA and DNA are internalized by cells.19,20 Specifically, lipoplexes of oxidized BFDMA (referred to from here on as ‘inactive lipoplexes’) are not internalized significantly by cells and, thus, promote negligible levels of transfection.21,22 These same lipoplexes, when transformed to contain reduced BFDMA are internalized readily by cells and promote high levels of transfection.19,20 These differences in activity correlate to substantial differences in both the nanostructures and the ζ-potentials of these lipoplexes as a function of the oxidation state of ferrocene;23,24 the addition of biologically compatible oxidizing25 and reducing agents19,20 can be used to affect these changes.
Scheme 1.
(A) Structure of BFDMA, a redox-active ferrocenyl lipid. (B-C) Schematic illustration demonstrating core principles: (B) Lipoplexes of oxidized BFDMA (gray) are inactive and can be distributed uniformly over cells without promoting transfection. (C) Controlled administration of soluble or solid-phase reducing agents results in localized activation of lipoplexes (red) and spatially controlled transfection (green); adjacent cells are unaffected.
In this work, we demonstrate that the unique redox properties of BFDMA can be exploited to exert active, external, and user-defined control over the locations and the timing with which DNA is internalized by and expressed in cultures of cells. These methods are based on the controlled administration of chemical activating agents to sub-populations of uniformly dispersed ‘inactive’ lipoplexes (Scheme 1B-C). This approach thus provides methods for control over transgene expression in cells – including new methods for remote chemical activation and the patterning of expression using solid-phase redox agents – that cannot be achieved using methods that require the delivery or patterning of active lipoplexes.
Results and Discussion
We performed a series of initial experiments to demonstrate that the spatially controlled introduction of chemical reducing agents in a system containing cells and uniformly dispersed, inactive lipoplexes of oxidized BFDMA could lead to localized activation and spatial control over patterns of transgene expression in cells. For these experiments, we used the non-toxic small-molecule reducing agent ascorbic acid (AA) to activate20 and pattern expression in a small, circular sub-population of cells in a larger 2-D culture. In all studies described below, we used lipoplexes of BFDMA and plasmid DNA encoding enhanced green fluorescent protein (EGFP) prepared at oxidized BFDMA/DNA charge ratios of 4.2:120 and a total lipid concentration of 30 μM. BFDMA can be used to control transfection in a range of cell types;20 COS-7 cells were used here to demonstrate proof of concept.
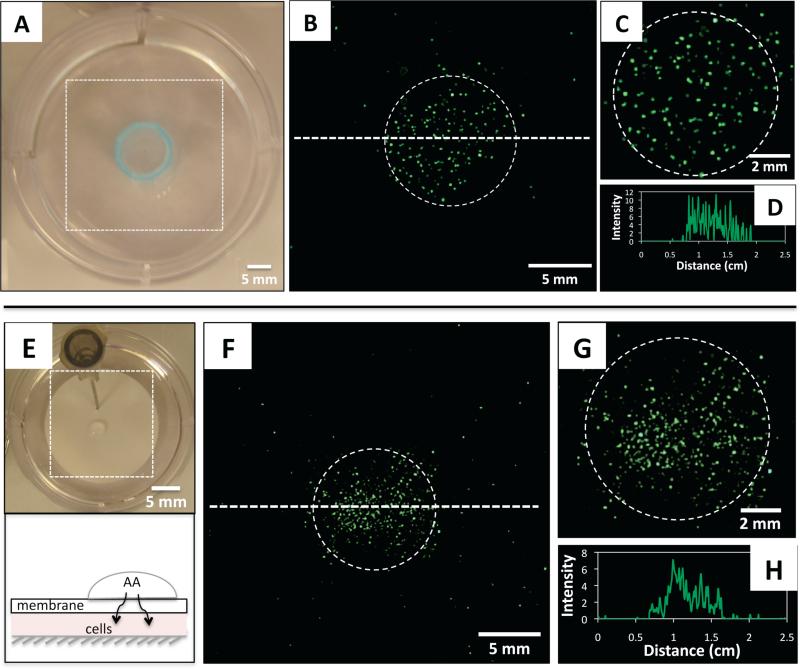
In a first series of experiments, lipoplexes of oxidized BFDMA were introduced as a uniform dispersion above cells growing on the bottom of a culture dish. A hollow cylinder (Fig. 1A, blue circle) was subsequently placed in the middle of the well and positioned against the bottom to isolate a small, circular sub-population of cells (Fig. 1A). A solution of AA was then added to the lipoplex-containing medium confined within the cylinder; the cylinder was removed after 1 h, and cells were then washed and covered with fresh medium. Fig. 1B is a composite of fluorescence micrographs, acquired 48 h after the initial addition of AA, showing an area of the culture well corresponding to the dotted square in Fig. 1A. The dotted circle shows the location of the cylinder used to confine administration of AA, and reveals EGFP to be localized in the sub-population of cells contained in that region. Fig. 1D shows a line intensity profile of EGFP fluorescence in cells distributed along the dotted line in Fig. 1B.
Fig. 1.
(A) Top-down view of a cell culture well containing inactive lipoplexes of oxidized BFDMA and a hollow cylinder (blue) used to localize delivery of AA (see text). (B) Composite of fluorescence micrographs showing the area of the culture well delineated by the dotted square in (A); the dotted circle shows the location of the cylinder shown in (A). (C) Magnified view of the circled area in (B). (D) Line intensity profile of EGFP fluorescence measured along the dotted line shown in (B). (E) Top: picture of a culture well with a porous membrane fixed in place (using a syringe needle) above lipoplex-containing culture medium; also shown is a small droplet of AA (center) added to the surface of the membrane (see text). Bottom: schematic showing a side view of the setup in the top image. (F) Fluorescence micrographs showing the area of the culture well delineated by the dotted square in (E). (G) Magnified view of the circled area in (F). (H) Line intensity profile of EGFP fluorescence measured along the dotted line shown in (F).
The results of this experiment demonstrate that localized treatment with AA leads to localized activation of lipoplexes and – more importantly – that cells in other regions of the system (which were exposed to a uniform dispersion of lipoplexes of oxidized BFDMA during this experiment) do not exhibit high levels of EGFP expression. We note that the essential principle demonstrated here – localized delivery of chemical activating agents to otherwise quiescent and broadly dispersed lipoplexes – provides means of achieving spatial control over transgene expression that do not require methods for controlled placement of lipoplexes. In addition, because lipoplexes of oxidized BFDMA can be incubated with cells without promoting internalization or transgene expression, manipulation of the time at which AA is added can be used to exert control over the timing with which transfection is initiated (see Fig. S1 of the SI).
This small-molecule approach to chemical activation can be used to promote and control transgene expression in other ways that are difficult or impossible to achieve using methods that require the local or patterned delivery of lipoplexes. The large sizes and charges of lipoplexes and other nanoparticles, for example, can place limits on the efficiency with which they are transported through complex media to desired locations (e.g., due to hindered diffusion through nanoscopic pores of extracellular matrices, mucus layers, or gels and materials used in many biotechnology applications).26,27 In contrast, small molecules such as AA can diffuse freely or be transported under active control through environments that lipoplexes cannot pass. We hypothesized that these differences in transport properties could be exploited to develop approaches to remote activation and patterning of gene expression in cells.
To develop these principles and evaluate the potential of this approach, we used a simple system to isolate cells beneath nanoporous membranes with pores (~25 nm) small enough to prevent the passive diffusion of lipoplexes. Placement of solutions containing lipoplexes formed using either reduced BFDMA or a commercially available cationic lipid (Lipofectamine) on top of these membranes did not result in observable expression of EGFP in cells located beneath the membranes (see Fig. S2 of the SI). We then placed small droplets of solutions of AA (radius = 2 mm) at defined positions on top of membranes positioned above cells bathed in uniform dispersions of lipoplexes of oxidized BFDMA (Fig. 1E). This resulted in localized expression of EGFP in small sub-populations of cells residing in locations beneath those of the droplets (Fig. 1F-H), indicating that these chemical methods can be used to activate transfection remotely via passive diffusion of AA through the membrane. We used a commercial membrane in these experiments to demonstrate proof of concept; however, the principles developed are applicable to the use of gel overlays and other chemically and physically complex media used to contain, immobilize, or sequester cells and lipoplexes. As noted above, these methods can also be used, in combination with strategies for time-controlled addition of AA, to achieve spatio-temporal control over the remote activation of transfection (see Fig. S1 of the SI).
In a separate series of studies, we performed experiments using chemical reducing agents immobilized on solid supports (e.g., on polymer microparticles). Methods for the solid-phase chemical activation of DNA lipoplexes are, to our knowledge, without precedent, and offer several practical advantages because polymer beads can be added, removed, clustered, and manipulated in cell culture environments using a variety of methods (e.g., physical placement, sedimentation, electromagnetic focusing, etc.) that cannot be used to localize or sequester soluble reducing agents. While past studies have used immobilization of lipoplexes on solid particles to enhance transfection,15,28 we are not aware of methods that exploit immobilized agents to activate transfection in systems that contain uniform dispersions of lipoplexes.
Whereas past studies provide a framework from which to understand the chemical reduction of oxidized BFDMA lipoplexes (and its influence on properties that influence transfection) using soluble agents,19,20 it was not obvious at the outset of this work that reducing agents immobilized on solid phase beads would be able to reduce oxidized BFDMA or affect the types of physicochemical transformations of lipoplexes (e.g., changes in nanostructure or ζ-potential) that lead to changes in biological behavior. We therefore performed a series of experiments using commercial agarose beads (~50-170 μm) containing covalently immobilized tris(2-carboxyethyl)phosphine (TCEP). TCEP is widely used to reduce disulfide bonds in biological applications,29 and characterization using UV/vis spectrophotometry demonstrated that a 10-fold molar excess of immobilized TCEP could reduce oxidized BFDMA lipoplexes in cell culture media rapidly and completely (in ~5 min; see Fig. S3 of the SI). In addition, biophysical characterization revealed the ζ-potentials of oxidized BFDMA lipoplexes (–25 ± 1 mV) to become significantly less negative (–14 ± 2 mV) after treatment (Table 1). These results are consistent with those obtained after reduction of BFDMA in lipoplexes using soluble AA (Table 1) and, more broadly, with the results of other past studies demonstrating that lipoplexes of reduced BFDMA exhibit zeta potentials that are more positive than those containing oxidized BFDMA (an outcome we attribute to large differences in the amphiphilicity of these two species; interactions of DNA with oxidized BFDMA likely lead to more loosely-structured complexes with an excess of negative charge arising from the presence of DNA in the outer regions of the aggregate).20,23 As discussed above, past studies demonstrate that increases in the ζ-potentials of these BFDMA lipoplexes after chemical reduction result in lipoplexes that are readily internalized by cells.19,20
Table 1.
Zeta Potentials of Lipoplexes Before and After Reduction.[a]
| Lipoplex Sample | Zeta Potential (mV) |
|---|---|
| DNA/BFDMAOX | –25 ± 1 |
| DNA/BFDMAOX + AA | –8 ± 1 |
| DNA/BFDMAOX + TCEP beads | –14 ± 2 |
Lipoplexes were prepared in aqueous Li2SO4 and diluted in culture medium to a BFDMA concentration of 30 μM. Oxidized BFDMA/DNA charge ratios were fixed at 4.2:1. Lipoplexes were then treated with a 10-fold molar excess of AA or TCEP-immobilized polymer beads.
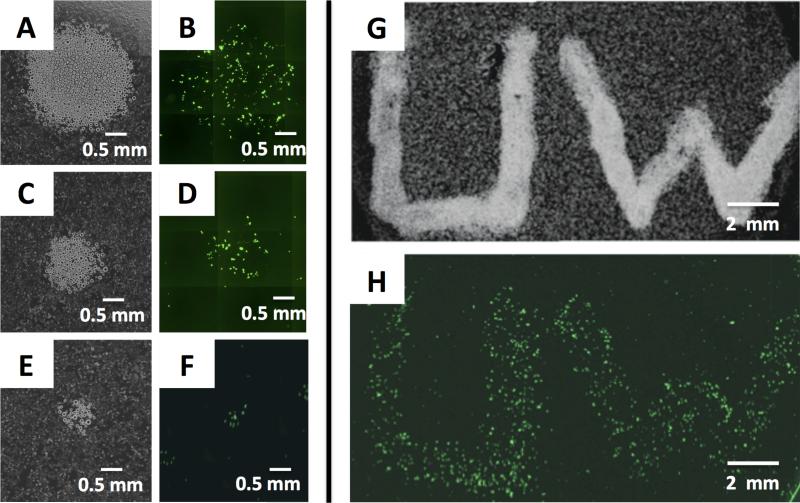
The beads used in our studies were sufficiently dense that they sedimented readily in culture media and could thus be used as liquid suspensions (or ‘inks’) to deposit clusters of beads on cells without substantially influencing viability. Fig. 2A-F shows the results of an experiment in which defined clusters of TCEP-immobilized beads were deposited manually in circular patterns via pipette in a culture dish containing cells bathed in a uniform dispersion of oxidized BFDMA lipoplexes. These clusters of beads were allowed to incubate for 1 h and were then removed by washing; the cells were then incubated for an additional 48 h (in the absence of beads or lipoplexes) before imaging by fluorescence microscopy.
Fig. 2.
Spatial control over the activation of lipoplexes using immobilized TCEP. (A-F) Phase contrast (A,C,E) and fluorescence (B,D,F) micrographs showing different-sized clusters of TCEP-immobilized beads deposited in circular patterns in a culture dish containing cells and a uniform dispersion of oxidized BFDMA lipoplexes. Beads were allowed to sit for 1 h before removal; cells were then incubated for 48 h before imaging by fluorescence microscopy. (G-H) Phase contrast (G) and fluorescence (H) micrographs showing patterns of EGFP expression in cells created by manual writing with a suspension of TCEP-immobilized beads.
The results in Fig. 2A-F reveal that the placement of TCEP-immobilized solid-phase beads can be used to activate localized transgene expression in cells. We observed significant levels of EGFP expression in cells located in areas in which beads were deposited, and very low/negligible levels of expression in cells located in other lipoplex-treated regions. The images in Fig. 2A-F (and additional experiments shown in Fig. S4) reveal the majority of EGFP expression to occur in cells located underneath or immediately adjacent to the beads. Control experiments using otherwise identical beads that did not contain TCEP did not result in significant levels of transfection (see Fig. S4). This result suggests that the localized expression shown in Fig. 2B, D, and F was the result of localized reduction of BFDMA by immobilized TCEP, and that it did not result from the presence of the beads themselves (the average size of these beads reduces the likelihood of internalization of physically-adsorbed lipoplexes by the fibroblast cells used here).
This solid-phase approach permitted the transfer or direct ‘writing’ of other user-defined patterns of beads to produce patterns of transfected cells with other arbitrarily designed (but well-defined) shapes. Fig. 2H shows patterns of EGFP expression in confluent monolayers of cells induced by manual writing (via pipette) with a culture media suspension of TCEP-immobilized beads (the location of the pattern of beads initially placed in this experiment is shown in Fig. 2G). The widths of the patterns of transfected cells in Fig. 2H is ~2 mm, a resolution dictated in part by the manual nature of the bead deposition methods used here. However, the images in Fig. 2E-F show that sub-populations of transfected cells with feature sizes of ~500 μm can be patterned by the deposition of smaller numbers of beads (e.g., using clusters of as few as ~40 beads). When combined with routine methods for the macro/microscale manipulation of polymer beads (including methods for the remote positioning of magnetic beads, etc.) we anticipate that it will be possible to fabricate higher-fidelity and smaller-scale sub-populations of transfected features using this approach. Because solid-phase beads can be easily and quantitatively removed when they are no longer needed, these methods could also form the basis of iterative activation processes to create more complex patterns of expression (e.g., to create spatially adjacent sup-populations of cells expressing different transgenes, etc.) that are difficult to achieve with spatiotemporal control using conventional methods for the patterning of active lipoplexes.
In conclusion, we have demonstrated that the localized and extracellular activation of lipoplexes of oxidized BFDMA can provide a practical approach to exert spatial and temporal control over transgene expression in cultures of cells. In contrast to lipoplex-based delivery systems that are already active when first introduced into a system (and that must therefore be placed or moved into specific locations to achieve spatial control of delivery), lipoplexes of oxidized BFDMA can be administered uniformly using a variety of methods without promoting internalization by cells. Localized introduction of chemical reducing agents thus triggers localized activation of lipoplexes and, subsequently, localized expression in defined sub-populations of cells at points in time and space that can be dictated by the investigator. The versatility of this approach was demonstrated by the use of both soluble (ascorbic acid) and solid-phase (TCEP) reducing agents to exert control of gene delivery in contexts that are difficult or impossible to achieve using conventional lipoplexes. These approaches address practical issues associated with the patterning or delivery of lipoplexes and introduce new principles for chemical transformation, remote activation, and patterning using solid-phase methods that could be useful in a range of biotechnical contexts.
Experimental Section
A detailed description of experimental procedures can be found in the Supporting Information.
Supplementary Material
Acknowledgments
Financial support was provided by the NIH (EB006168 and EB006820) and the NSF through CBET 0754921 and a grant to the MRSEC at the UW-Madison (DMR 1121288). We gratefully acknowledge S. Hata and H. Takahashi for assistance with the synthesis of BFDMA.
Footnotes
Supporting Information: Details of experimental procedures and additional characterization and control experiments. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, Northrop JP, Ringold GM, Danielsen M. Proc. Natl. Acad. Sci. U. S. A. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zabner J. Adv. Drug Deliv. Rev. 1997;27:17–28. doi: 10.1016/s0169-409x(97)00019-7. [DOI] [PubMed] [Google Scholar]
- 3.Kabanov AV, Felgner PL, Seymour LW. Self-Assembling Complexes for Gene Delivery: From Laboratory to Clinical Trial. John Wiley and Sons; New York: 1998. [Google Scholar]
- 4.Bally MB, Harvie P, Wong FMP, Kong S, Wasan EK, Reimer DL. Adv. Drug Deliv. Rev. 1999;38:291–315. doi: 10.1016/s0169-409x(99)00034-4. [DOI] [PubMed] [Google Scholar]
- 5.Gerasimov OV, Boomer JA, Qualls MM, Thompson DH. Adv. Drug Deliv. Rev. 1999;38:317–338. doi: 10.1016/s0169-409x(99)00035-6. [DOI] [PubMed] [Google Scholar]
- 6.Meers P. Adv. Drug Deliv. Rev. 2001;53:265–272. doi: 10.1016/s0169-409x(01)00205-8. [DOI] [PubMed] [Google Scholar]
- 7.Guo X, Szoka FC. Acc. Chem. Res. 2003;36:335–341. doi: 10.1021/ar9703241. [DOI] [PubMed] [Google Scholar]
- 8.Zhang XX, McIntosh TJ, Grinstaff MW. Biochimie. 2012;94:42–58. doi: 10.1016/j.biochi.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagasaki T, Taniguchi A, Tamagaki S. Bioconjugate Chem. 2003;14:513–516. doi: 10.1021/bc0256603. [DOI] [PubMed] [Google Scholar]
- 10.Wolff JA, Rozema DB. Mol. Ther. 2008;16:8–15. doi: 10.1038/sj.mt.6300326. [DOI] [PubMed] [Google Scholar]
- 11.Liu YC, Le Ny ALM, Schmidt J, Talmon Y, Chmelka BF, Lee CT. Langmuir. 2009;25:5713–5724. doi: 10.1021/la803588d. [DOI] [PubMed] [Google Scholar]
- 12.Ziauddin J, Sabatini DM. Nature. 2001;411:107–110. doi: 10.1038/35075114. [DOI] [PubMed] [Google Scholar]
- 13.Saltzman WM, Olbricht WL. Nat. Rev. Drug Discov. 2002;1:177–186. doi: 10.1038/nrd744. [DOI] [PubMed] [Google Scholar]
- 14.Cambridge SB, Geissler D, Calegari F, Anastassiadis K, Hasan MT, Stewart AF, Huttner WB, Hagen V, Bonhoeffer T. Nat. Methods. 2009;6:527–U586. doi: 10.1038/nmeth.1340. [DOI] [PubMed] [Google Scholar]
- 15.Isalan M, Santori MI, Gonzalez C, Serrano L. Nat. Methods. 2005;2:113–118. doi: 10.1038/nmeth732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houchin-Ray T, Whittlesey KJ, Shea LD. Mol. Ther. 2007;15:705–712. doi: 10.1038/mt.sj.6300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tavana H, Jovic A, Mosadegh B, Lee QY, Liu X, Luker KE, Luker GD, Weiss SJ, Takayama S. Nat. Materials. 2009;8:736–741. doi: 10.1038/nmat2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshino N, Shoji H, Kondo Y, Kakizawa Y, Sakai H, Abe M. J. Jpn. Oil Chem. Soc. 1996;45:769–775. [Google Scholar]
- 19.Jewell CM, Hays ME, Kondo Y, Abbott NL, Lynn DM. Bioconjugate Chem. 2008;19:2120–2128. doi: 10.1021/bc8002138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aytar BS, Muller JPE, Golan S, Hata S, Takahashi H, Kondo Y, Talmon Y, Abbott NL, Lynn DM. J. Control. Release. 2012;157:249–259. doi: 10.1016/j.jconrel.2011.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbott NL, Jewell CM, Hays ME, Kondo Y, Lynn DM. J. Am. Chem. Soc. 2005;127:11576–11577. doi: 10.1021/ja054038t. [DOI] [PubMed] [Google Scholar]
- 22.Jewell CM, Hays ME, Kondo Y, Abbott NL, Lynn DM. J. Control. Release. 2006;112:129–138. doi: 10.1016/j.jconrel.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Hays ME, Jewell CM, Kondo Y, Lynn DM, Abbott NL. Biophys. J. 2007;93:4414–4424. doi: 10.1529/biophysj.107.107094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pizzey CL, Jewell CM, Hays ME, Lynn DM, Abbott NL, Kondo Y, Golan S, Tahnon Y. J. Phys. Chem. B. 2008;112:5849–5857. doi: 10.1021/jp7103903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aytar BS, Muller JPE, Golan S, Kondo Y, Talmon Y, Abbott NL, Lynn DM. J. Colloid Interface Sci. 2012;387:56–64. doi: 10.1016/j.jcis.2012.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cu Y, Saltzman WM. Mol. Pharmaceut. 2009;6:173–181. doi: 10.1021/mp8001254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suh J, Dawson M, Hanes J. Adv. Drug Deliv. Rev. 2005;57:63–78. doi: 10.1016/j.addr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Luo D, Saltzman WM. Nat. Biotechnol. 2000;18:893–895. doi: 10.1038/78523. [DOI] [PubMed] [Google Scholar]
- 29.Cline DJ, Redding SE, Brohawn SG, Psathas JN, Schneider JP, Thorpe C. Biochemistry. 2004;43:15195–15203. doi: 10.1021/bi048329a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.