Abstract
Plasma concentrations of antimicrobial drugs have long been used to correlate exposure with effect, yet one cannot always assume that unbound plasma and tissue concentrations are similar. Knowledge about unbound tissue concentrations is important in the development of antimicrobial drugs, since most infections are localised in tissues. Therefore, a clinical microdialysis study was conducted to evaluate the distribution of tedizolid (TR-700), the active moiety of the antimicrobial prodrug tedizolid phosphate (TR-701), into interstitial fluid (ISF) of subcutaneous adipose and skeletal muscle tissues following a single oral 600 mg dose of tedizolid phosphate in fasting conditions. Twelve healthy adult subjects were enrolled. Two microdialysis probes were implanted into the thigh of each subject, one into the vastus medialis muscle and one into subcutaneous adipose tissue. Probes were calibrated using retrodialysis. Dialysate samples were collected every 20 min for 12 h following a single oral dose of 600 mg tedizolid phosphate, and blood samples were drawn over 24 h. Unbound tedizolid levels in plasma were similar to those in muscle and adipose tissue. The ratios of unbound (free) AUC in tissues over unbound AUC in plasma (fAUCtissue/fAUCplasma) were 1.1 ± 0.2 and 1.2 ± 0.2 for adipose and muscle tissue, respectively. The median half-life was 8.1, 9.2 and 9.6 h for plasma, adipose tissue and muscle tissue, respectively. Mean protein binding was 87.2 ± 1.8%. The study drug was very well tolerated. The results of this study show that tedizolid distributes well into ISF of adipose and muscle tissues. Unbound levels of tedizolid in plasma, adipose tissue and muscle tissue were well correlated. Free plasma levels are indicative of unbound levels in the ISF of muscle and adipose tissues.
Keywords: Microdialysis, Tissue distribution, Tedizolid, Pharmacokinetics
1. Introduction
There has been a steady increase in the number of infections caused by meticillin-resistant Staphylococcus aureus (MRSA), meticillin-resistant coagulase-negative staphylococci and vancomycin-resistant enterococci [1]. Although there appears to be a trend towards decreasing numbers of hospital-onset and hospital-associated MRSA infections with community onset [2], there is still a need for novel treatments with an optimised efficacy, safety and pharmacodynamic profile to bolster the armamentarium against potentially severe or fatal S. aureus and Enterococcus spp. infections.
The first oxazolidinone drug to enter the market was linezolid in 2000. Linezolid has good in vitro and in vivo properties against staphylococci, enterococci and streptococci [3]. Linezolid also shows good pharmacokinetic properties with an oral bioavailability of ca. 100%, and tissue penetration following multiple and single doses that are close to the free concentration in plasma [4,5]. One disadvantage of linezolid is that it has to be administered twice daily [6]. Furthermore, linezolid pharmacokinetics have been shown to have considerable interindividual variability and there are safety concerns due to monoamine oxidase interactions and potential myelosuppression [5,6].
Tedizolid is a novel oxazolidinone compound with four to eight times improved antibacterial potency compared with linezolid [7].
The rationale behind studying tissue concentrations is the understanding that for most antibiotics it is the free drug available at the site of action, the biophase, that is responsible for the antibacterial effect [8]. Moreover, most bacteria cause infection not in the bloodstream but in the tissue itself, therefore measuring concentrations in tissue should give greater clarity on the amount of drug available for action [9,10]. One method that can easily be used for measuring drug concentrations in tissue is microdialysis [11]. It has been widely used to measure tissue concentrations, for example, in lungs, soft tissues, and skin and soft-tissue infections [12–16]. Measurement of biophase concentrations is also recommended by regulatory authorities [17,18].
The purpose of this study was to assess the tissue distribution of tedizolid, the microbiologically active moiety, following a single oral dose of tedizolid phosphate prodrug.
2. Materials and methods
This clinical study was conducted according to the Declaration of Helsinki and Good Clinical Practices. Approval for the study was obtained from the institutional review board of Shands Hospital at the University of Florida (Gainesville, FL) before any volunteers were recruited for the study.
2.1. Healthy volunteers
Fifteen healthy volunteers (ten female and five male) participated in the study. To confirm eligibility of the subjects, a physical examination and electrocardiography were performed and urinalysis, haematology and blood chemistry laboratory samples were evaluated. Females also had to have a negative serum β-human chorionic gonadotropin pregnancy test at screening and a negative urine pregnancy test on Day 1. Eligible subjects had to be between 18 years and 50 years of age, healthy and not receiving any other medication; hormonal contraception was allowed for females. The body mass index had to range from 20 kg/m2 to 29 kg/m2.
2.2. Study design
This study was an open-label, single-dose, single-centre study in 15 healthy volunteers; 3 volunteers were enrolled into a pilot study to confirm the feasibility of the microdialysis method in vivo, and 12 volunteers were enrolled into the main part of the study that included a single oral dose of tedizolid. For subjects in the pilot study, one microdialysis membrane each was inserted into the muscle and subcutaneous adipose tissue of the upper thigh. After feasibility was confirmed, subjects were enrolled and screened for the main part of the study. Once eligibility was confirmed, subjects were admitted to the General Clinical Research Unit at Shands Hospital. On the first study day, microdialysis probes were placed and perfused with lactated Ringer’s solution for 30 min, before probe calibration by retrodialysis was performed. For this, the probe was connected to a syringe containing tedizolid at a concentration of 2 μg/mL, which was perfused for 30 min before a sample was collected for another 30 min. Thereafter, the probe was perfused again with lactated Ringer’s solution with a washout period of 4 h. Microdialysis samples were collected every 20 min for 12 h after study drug administration, and blood samples were collected for 24 h post dose. Subjects had to remain on bed rest for the duration of active microdialysis and had to remain fasted for 5 h before dose administration followed by a fast from food for ≥3 h post dose.
2.3. Study drug
Study drug was supplied in 200 mg capsules (Trius Therapeutics, Inc., San Diego, CA). Subjects were administered a single oral dose of 600 mg tedizolid phosphate (the prodrug) and a hand and mouth check was performed for verification. A 600 mg dose was selected as early dosing estimates projected that it could be in the range of the therapeutic dose for the treatment of skin infections. However, results of a recent Phase 2 study showed a high degree of efficacy at lower doses [19].
2.4. Clinical microdialysis
The microdialysis method has been described in detail and has been used in many clinical studies [5,11,12,20]. Briefly, to assess interstitial fluid (ISF) concentrations in the tissues of interest, two microdialysis probes are placed, one into the subcutaneous adipose tissue and one into the muscle. The probes were perfused with lactated Ringer’s solution at a steady rate of 1.5 μL/min. This constant perfusion results in an incomplete equilibrium between the ISF of the tissue and the inside of the microdialysis probe. The factor that correlates the drug measured in the dialysate to the actual concentration in the ISF is the recovery factor. One method to assess this in vivo is the retrodialysis method, which was first described by Ståhle et al. [20]. For this method, tedizolid was perfused through the probe at a concentration of 2 μg/mL and the recovered amount of tedizolid in the dialysate was analysed. The recovery is then calculated as the recovered amount divided by the nominal amount. The recovery factor is used to adjust the measured tissue concentrations.
2.5. Analysis
2.5.1. Microdialysis sample analysis
Microdialysis samples were collected every 20 min for a total of 12 h post dose and were placed on ice and frozen at −80 °C within 2 h of collection. For analysis, samples were thawed at room temperature and were analysed using high-performance liquid chromatography with ultraviolet light detection. The method was validated according to US Food and Drug Administration (FDA) and Good Laboratory Practice guidelines. The limit of quantitation was 50 ng/mL. The accuracy and precision of the microdialysis samples ranged from −5.4% to 4.2% and 1.1% to 7.3%, respectively.
2.6. Plasma and protein binding samples
Blood samples were collected pre dose and at 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, 18 and 24 h post dose. For protein binding, additional blood was drawn at 0, 2 and 12 h post dose. Samples were centrifuged and frozen immediately at −80 °C.
2.7. Data analysis
A non-compartmental analysis of the data from the three sampling sites was conducted using WinNonlin® v.5.2 software (Pharsight, St Louis, MO). Pharmacokinetic parameters included the area under the concentration–time curve (AUC), calculated by linear trapezoidal rule, the maximum concentration (Cmax), the time to Cmax and the terminal half-life (T1/2). Apparent oral clearance (CL/F) was calculated as dose/AUC, where CL is drug clearance and F is the fraction absorbed. The apparent volume of distribution (Vz/F) was calculated as CL×T1/2/ln2. To derive free plasma concentrations, each subject’s total values were adjusted by their individual protein-binding results by multiplying the total tissue concentrations by the subject’s individual fraction of unbound drug in plasma (fu = 1–fraction bound). Adipose and muscle tissue concentrations were derived from the measured concentrations in tissue and were adjusted by the measured recovery value. The calculation was done as follows: concentration in tissue = 100 × (sample concentration/in vivo recovery [%]).
3. Results
The results of the pilot study indicated that tedizolid recovery from the microdialysis probe was very high at 87.0% and 94.7% for adipose and muscle tissue, respectively. The pilot phase also revealed that a washout period of 4 h was sufficient to ensure that there was no leftover drug from probe calibration in the tissue.
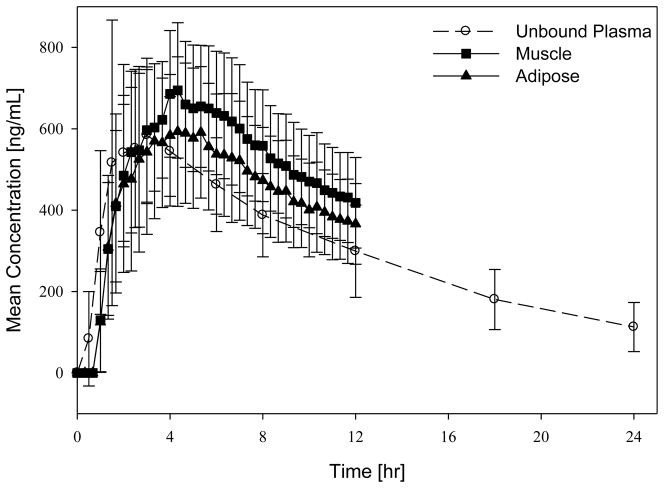
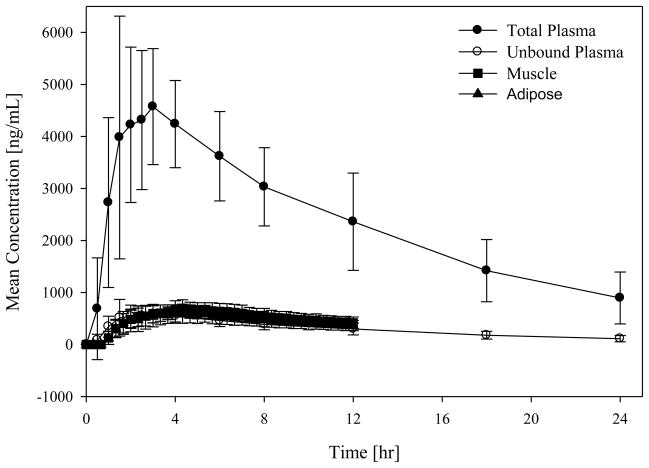
Mean recovery values (± standard deviation) for the 12 volunteers in the main study were 95.3 ± 2.8% for adipose tissue and 96.3 ± 1.9% for muscle tissue. For one subject the probe in the adipose tissue malfunctioned, therefore calculations for adipose tissue are based on 11 subjects. The mean protein binding of tedizolid was measured at 87.3 ± 1.3%. The pharmacokinetic results of the study are in good agreement with a previous study of the same dose [21]. Mean concentration–time profiles for total and free plasma and free tissue concentrations are given in Figs 1 and 2. Table 2 shows a summary of estimated pharmacokinetic parameters. The ratios of unbound AUC in tissues over unbound (free) AUC in plasma (fAUCtissue/fAUCplasma) were 1.1 ± 0.2 and 1.2 ± 0.2 for adipose and muscle tissue, respectively, indicating slightly higher tedizolid tissue distribution (at least for the two tissues measured) relative to plasma. Wilcoxon matched pairs tests showed that the AUC0–12h (AUC over 0–12 h) for muscle tissue was statistically significantly (P < 0.05) higher than the AUC0–12h in free plasma and the AUC0–12h in adipose tissue. The AUC0–12h values of adipose tissue and free plasma were not statistically significantly different from each other.
Fig. 1.
Mean concentration–time profiles for free (unbound) plasma and tissue concentrations.
Fig. 2.
Mean concentration–time profiles for total plasma concentrations and free (unbound) plasma and tissue concentrations.
Table 2.
Estimated pharmacokinetic parameters
| Parameter | Mean ± standard deviation
|
|||
|---|---|---|---|---|
| Total plasma | Free plasma | Adipose tissue | Muscle | |
| Cmax (mg/L) | 5.4 ± 1.5 | 0.69 ± 0.20 | 0.66 ± 0.16 | 0.74 ± 0.15 |
| Tmax(h) | 2.4 ± 1.1 | N/D | 4.3 ± 2.4 | 3.7 ± 1.5 |
| T1/2 (h)a | 8.1 (5.9–12.8) | N/D | 9.2 (5.9–85.9) | 9.6 (6.2–48.2) |
| AUC0–12h (mg h/L) | 38.8 ± 7.5 | 4.9 ± 1.1 | 5.3 ± 1.3 | 5.9 ± 1.1 |
| AUC0–24h (mg h/L) | 57.1 ± 14.7 | 7.3 ± 1.9 | N/A | N/A |
| CL/F (L/hr) | 9.5 ± 2.9 | N/D | N/D | N/D |
| Vz/F (L) | 113.3 ± 19.3 | N/D | N/D | N/D |
| fAUCtissue/fAUCplasma | 1.1 ± 0.2 | 1.2 ± 0.2 | ||
Cmax, maximum concentration; Tmax, time to Cmax; T1/2, terminal half-life; AUC, area under the concentration–time curve over the specified time interval; CL/F, apparent oral clearance; Vz/F, apparent volume of distribution; fAUCtissue/fAUCplasma, ratio of AUC0–12h between specified matrices; N/D, not determined; N/A, not available.
T1/2 is shown as median (range).
4. Discussion
This study shows that the concentrations of tedizolid in adipose and muscle tissue following a single oral dose are similar to the free plasma concentrations, indicating that tedizolid can freely distribute into the tissues. In addition to having similar concentrations over time, the T1/2 values obtained for adipose and muscle tissue were similar to the T1/2 in plasma, suggesting that plasma is a good surrogate for tissue concentrations and that these single-dose results would be predictive of multiple dosing. The distribution of tedizolid was found to be similar to that of the currently marketed oxazolidinone linezolid, which shows ratios of fAUCtissue/fAUCplasma of 0.9 and 1.0 for adipose and muscle tissue, respectively [5]. The bioavailability of tedizolid was recently reported to be 91.7% [22], which is comparable with the reported value for linezolid [23].
One limiting factor of the microdialysis method is that it cannot measure intracellular concentrations of the drug. Yet intracellular colonisation of neutrophils has been associated with recurrent disease [24]. In a study by Lemaire et al. [25], at pH 7.4 intracellular concentrations of tedizolid were ca. 10–15 times those of extracellular concentrations and accumulation was rapid, whereas linezolid concentrations in the cell equilibrated slowly to those measured extracellularly. This could be an explanation as to why tedizolid showed an excellent effect in a clinical study in patients with drug-resistant skin and soft-tissue infections [26] at a dose of 200 mg once daily, yet when examining the pharmacokinetic/pharmacodynamic (PK/PD) index (fAUCtissue/minimum inhibitory concentraiton) for both linezolid and tedizolid (data not shown) for clinically relevant strains one would not expect this high effect. Indeed, PK/PD studies and clinical studies performed subsequent to the present study support the selection of a 200 mg once-daily therapeutic dose for the treatment of skin infections [19,27]. However, there is no reason to speculate that the results of the present study using a 600 mg single dose cannot be extrapolated to what would be expected at the 200 mg dose selected for therapeutic use.
Table 1.
Study demographics (main study)
| Parameter | |
|---|---|
| No. of subjects | 12 |
| Age (years) [mean (± S.D.)] | 24 ± 4 |
| Height (cm) [mean (± S.D.)] | 172 ± 11 |
| Weight (kg) [mean (± S.D.)] | 72 ± 16 |
| Race/ethnicity [n (%)] | |
| Caucasian | 9 (75) |
| African-American | 2 (17) |
| Hispanic | 1 (8) |
| Sex [n (%)] | |
| Female | 7 (58) |
| Male | 5 (42) |
S.D., standard deviation.
Acknowledgments
The authors would like to thank the General Clinical Research Center of Shands Hospital at the University of Florida (Gainesville, FL) for their continued support and excellence in clinical trial conduct, without whose support this study would not have been possible.
Funding
This research was supported in part by the Clinical Research Center at the University of Florida (Gainesville, FL) (grant M01 RR0000082 NCRR/NIH). Trius Therapeutics, Inc. (San Diego, CA) are also thanked for funding this study.
Footnotes
Competing interests
CdA and PP are employees of Trius Therapeutics, Inc. (San Diego, CA). All other authors declare no competing interests.
Ethical approval
The study was approved by the University of Florida Institutional Review Board (IRB protocol #680-2007).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–85. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 2.Kallen AJ, Mu Y, Bulens S, Reingold A, Petit S, Gershman K, et al. Active Bacterial Core surveillance (ABCs) MRSA Investigators of the Emerging Infections Program. Health care-associated invasive MRSA Infections, 2005–2008. JAMA. 2010;304:641–7. doi: 10.1001/jama.2010.1115. [DOI] [PubMed] [Google Scholar]
- 3.Zurenko GE, Yagi BH, Schaadt RD, Allison JW, Kilburn JO, Glickman SE, et al. In vitro activities of U-100592 and U-100766, novel oxazolidinone antibacterial agents. Antimicrob Agents Chemother. 1996;40:839–45. doi: 10.1128/aac.40.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacGowan AP. Pharmacokinetic and pharmacodynamic profile of linezolid in healthy volunteers and patients with Gram-positive infections. J Antimicrob Chemother. 2003;51(Suppl 2):ii17–25. doi: 10.1093/jac/dkg248. [DOI] [PubMed] [Google Scholar]
- 5.Dehghanyar P, Burger C, Zeitlinger M, Islinger F, Kovar F, Muller M, et al. Penetration of linezolid into soft tissues of healthy volunteers after single and multiple doses. Antimicrob Agents Chemother. 2005;49:2367–71. doi: 10.1128/AAC.49.6.2367-2371.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfizer. Zyvox® prescribing information. New York, NY: Pfizer; 2010. [accessed 3 April 2012]. http://www.pfizerpro.com/hcp/zyvox. [Google Scholar]
- 7.Yum JH, Choi SH, Yong D, Chong Y, Im WB, Rhee DK, et al. Comparative in vitro activities of torezolid (DA-7157) against clinical isolates of aerobic and anaerobic bacteria in South Korea. Antimicrob Agents Chemother. 2010;54:5381–6. doi: 10.1128/AAC.00728-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merrikin DJ, Briant J, Rolinson GN. Effect of protein binding on antibiotic activity in vivo. J Antimicrob Chemother. 1983;11:233–8. doi: 10.1093/jac/11.3.233. [DOI] [PubMed] [Google Scholar]
- 9.Chisholm GD, Waterworth PM, Calnan JS, Garrod LP. Concentration of antibacterial agents in interstitial tissue fluid. Br Med J. 1973;1:569–73. doi: 10.1136/bmj.1.5853.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryan DM. Pharmacokinetics of antibiotics in natural and experimental superficial compartments in animals and humans. J Antimicrob Chemother. 1993;31(Suppl D):1–16. doi: 10.1093/jac/31.suppl_d.1. [DOI] [PubMed] [Google Scholar]
- 11.Chaurasia CS, Muller M, Bashaw ED, Benfeldt E, Bolinder J, Bullock R, et al. AAPS-FDA workshop white paper: microdialysis principles, application and regulatory perspectives. Pharm Res. 2007;24:1014–25. doi: 10.1007/s11095-006-9206-z. [DOI] [PubMed] [Google Scholar]
- 12.Barbour A, Schmidt S, Sabarinath SN, Grant M, Seubert C, Skee D, et al. Soft-tissue penetration of ceftobiprole in healthy volunteers determined by in vivo microdialysis. Antimicrob Agents Chemother. 2009;53:2773–6. doi: 10.1128/AAC.01409-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenberg EJ, Conzentino P, Eickhoff WM, Cundy KC. Pharmacokinetic measurement of drugs in lung epithelial lining fluid by microdialysis: aminoglycoside antibiotics in rat bronchi. J Pharmacol Toxicol Methods. 1993;29:93–8. doi: 10.1016/1056-8719(93)90056-k. [DOI] [PubMed] [Google Scholar]
- 14.Stolle LB, Plock N, Joukhadar C, Arpi M, Emmertsen KJ, Buerger C, et al. Pharmacokinetics of linezolid in bone tissue investigated by in vivo microdialysis. Scand J Infect Dis. 2008;40:24–9. doi: 10.1080/00365540701509873. [DOI] [PubMed] [Google Scholar]
- 15.Tegeder I, Schmidtko A, Brautigam L, Kirschbaum A, Geisslinger G, Lotsch J. Tissue distribution of imipenem in critically ill patients. Clin Pharmacol Ther. 2002;71:325–33. doi: 10.1067/mcp.2002.122526. [DOI] [PubMed] [Google Scholar]
- 16.Thallinger C, Buerger C, Plock N, Kljucar S, Wuenscher S, Sauermann R, et al. Effect of severity of sepsis on tissue concentrations of linezolid. J Antimicrob Chemother. 2008;61:173–6. doi: 10.1093/jac/dkm431. [DOI] [PubMed] [Google Scholar]
- 17.US Food and Drug Administration. Challenge and opportunity on the critical path to new medical products. Silver Spring, MD: FDA; 2004. [Google Scholar]
- 18.European Medicines Agency. Points to consider on pharmacokinetics and pharmacodynamics in the development of antibacterial medicinal products. London, UK: Committee for Proprietary Medicinal Products (CPMP); 2000. [Google Scholar]
- 19.Prokocimer P, Bien P, Surber J, Mehra P, DeAnda C, Bulitta JB, et al. Phase 2, randomized, double-blind, dose-ranging study evaluating the safety, tolerability, population pharmacokinetics, and efficacy of oral torezolid phosphate in patients with complicated skin and skin structure infections. Antimicrob Agents Chemother. 2011;55:583–92. doi: 10.1128/AAC.00076-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ståhle L, Arner P, Ungerstedt U. Drug distribution studies with microdialysis. III: Extracellular concentration of caffeine in adipose tissue in man. Life Sci. 1991;49:1853–8. doi: 10.1016/0024-3205(91)90488-w. [DOI] [PubMed] [Google Scholar]
- 21.Bien P, Munoz KA, Bohn J, Wright R, Bethune C, Prokocimer P. Human pharmacokinetics of TR-700 after ascending single oral doses of the prodrug TR-701, a novel oxazolidinone antibiotic. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); 25–28 October 2008; Washington, DC. Washington, DC: ASM Press; 2008. [Google Scholar]
- 22.Bien P, Prokocimer P, Munoz KA, Bethune C. Absolute bioavailability of TR-701 FA and pharmacokinetics after single and multiple dose intravenous administration in healthy adult subjects. 50th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); 12–15 September 2010; Boston, MA. Washington, DC: ASM Press; 2010. [Google Scholar]
- 23.Stalker DJ, Jungbluth GL, Hopkins NK, Batts DH. Pharmacokinetics and tolerance of single- and multiple-dose oral or intravenous linezolid, an oxazolidinone antibiotic, in healthy volunteers. J Antimicrob Chemother. 2003;51:1239–46. doi: 10.1093/jac/dkg180. [DOI] [PubMed] [Google Scholar]
- 24.Gresham HD, Lowrance JH, Caver TE, Wilson BS, Cheung AL, Lindberg FP. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J Immunol. 2000;164:3713–22. doi: 10.4049/jimmunol.164.7.3713. [DOI] [PubMed] [Google Scholar]
- 25.Lemaire S, Van Bambeke F, Appelbaum PC, Tulkens PM. Cellular pharmacokinetics and intracellular activity of torezolid (TR-700): studies with human macrophage (THP-1) and endothelial (HUVEC) cell lines. J Antimicrob Chemother. 2009;64:1035–43. doi: 10.1093/jac/dkp267. [DOI] [PubMed] [Google Scholar]
- 26.Bien P, DeAnda C, Prokocimer P. Microbiological efficacy of torezolid in patients with complicated skin and skin structure infections. European Congress of Clinical Microbiology and Infectious Diseases (ECCMID); 10–13 April 2010; Vienna, Austria. Basel, Switzerland: European Society of Clinical Microbiology and Infectious Diseases; 2010. p. Abstract P1592. [Google Scholar]
- 27.Nicasio AM, Bulitta JB, Lodise TP, D’Hondt RE, Kulawy R, Louie A, et al. Evaluation of once-daily vancomycin against methicillin-resistant Staphylococcus aureus in a hollow-fiber infection model. Antimicrob Agents Chemother. 2012;56:682–6. doi: 10.1128/AAC.05664-11. [DOI] [PMC free article] [PubMed] [Google Scholar]