Abstract
Modulation of Ca2+ within cells is tightly regulated through complex and dynamic interactions between the plasma membrane and internal compartments. In this study, we exploit in vivo imaging strategies based on genetically encoded Ca2+ indicators to define changes in perikaryal Ca2+ concentration of intact photoreceptors. We developed double-transgenic zebrafish larvae expressing GCaMP3 in all cones and tdTomato in long-wavelength cones to test the hypothesis that photoreceptor degeneration induced by mutations in the phosphodiesterase-6 (Pde6) gene is driven by excessive [Ca2+]i levels within the cell body. Arguing against Ca2+ overload in Pde6 mutant photoreceptors, simultaneous analysis of cone photoreceptor morphology and Ca2+ fluxes revealed that degeneration of pde6cw59 mutant cones, which lack the cone-specific cGMP phosphodiesterase, is not associated with sustained increases in perikaryal [Ca2+]i. Analysis of [Ca2+]i in dissociated Pde6βrd1mouse rods shows conservation of this finding across vertebrates. In vivo, transient and Pde6-independent Ca2+ elevations (‘flashes') were detected throughout the inner segment and the synapse. As the mutant cells proceeded to degenerate, these Ca2+ fluxes diminished. This study thus provides insight into Ca2+ dynamics in a common form of inherited blindness and uncovers a dramatic, light-independent modulation of [Ca2+]i that occurs in normal cones.
Keywords: calcium, zebrafish, neurodegenerative disease, photoreceptors, phosphodiesterases
Photoreceptor degeneration is a devastating disease that affects millions of people worldwide. Mutations that alter cyclic guanosine-mono-phosphate (cGMP) levels are a common cause of photoreceptor degeneration; mutations in guanylate cyclase, guanylate cyclase-activating proteins, phosphodiesterase-6 (Pde6) and the Pde6 chaperone, aryl hydrocarbon receptor interacting protein-like 1, all cause loss of rods and/or cones (Retinal Information Network, https://sph.uth.edu/retnet/home.htm). The precise mechanism of cGMP-mediated cell death is poorly defined, and thus animal models are particularly relevant to study as a tool to help cure and treat this disease.
The most studied animal model for photoreceptor degeneration due to elevated cGMP is the rd1 mouse, identified more than 90 years ago.1, 2 rd1 mice have a mutation in the beta subunit of rod cGMP Pde6, a key mediator of the light response.3, 4 Normally, light activates a molecular cascade that causes Pde6 to hydrolyze cGMP, resulting in closure of cation channels within the photoreceptor outer segment (OS). In the absence of Pde6 beta in rd1 mice, cGMP levels remain elevated and rod photoreceptors start to degenerate after their differentiation and before eye opening.3 Cone photoreceptors lacking Pde6c also have elevated cGMP in their OSs.5 Elevated cGMP is proposed to result in sustained increases in OS Ca2+, triggering death. Consistently, elevated OS Ca2+ was measured in hypomorphic Pde6 mutant rods6 and rod viability was recently shown to improve in Cngb1(−/−)× Pde6βrd1double-mutant mice.7 However, the degeneration processes take place within the perikaryon, not the OS. Thus, a key challenge has been to determine the signaling pathways through which elevated cGMP and Ca2+ within OSs of Pde6 mutant cells affect photoreceptor cell biology downstream from the OS.
Although events associated with non-apoptotic cell death pathways such as poly-ADP-ribose-polymerase and calpain activation occur in rods dying due to elevated cGMP,8, 9 the molecular cascade activated in degenerating Pde6 mutant photoreceptors is unknown. Specifically, involvement of Ca2+ in the rd1 pathology is a major unsolved question.10 It is often assumed that elevated Ca2+ within OSs triggers cell death by activating Ca2+-dependent mechanisms within the cell body.11 However, cGMP and Ca2+ diffusion from the OS to the inner segment are likely to be limited by anatomical constraints (the ciliary bottleneck), buffering and the ellipsoid mitochondrial barrier.
To ascertain the role of Ca2+ in photoreceptor degeneration caused by loss of Pde6, it is necessary to measure this ion within the inner segment. We therefore generated here a double-transgenic zebrafish, transgenic (Tg; Trß2:tdTomato;TαCP:GCaMP3), in which the Ca2+ indicator GCaMP3 is selectively expressed in the photoreceptor cell body, not the OS. We recorded Ca2+ dynamics within intact cones from wild type (WT) and mutant (pde6cw59) retinas using multiphoton time-lapse imaging. pde6cw59 cones degenerate starting immediately after their differentiation at 4 days post fertilization (d.p.f.), and are mostly lost by 7 d.p.f.12, 13 This mutation is thus analogous to the well-characterized rod Pde6βrd1mutation.3, 4 To confirm our results in the mammalian model, we extended our approach to record intracellular Ca2+ ([Ca2+]i) levels in rd1 mouse rods.
By combining the power of zebrafish genetics with live, noninvasive imaging, we were able to follow Ca2+ signaling within degenerating vertebrate cones in real-time. Surprisingly, we did not detect sustained [Ca2+]i increases in pde6cw59 mutant cones nor were abnormalities in baseline [Ca2+]i observed in degenerating rd1 mouse rods. Ca2+ signals in non-mutant and pde6cw59 mutant zebrafish cones include recurring transient [Ca2+]i increases (‘flashes'), consistent with complex regulation of the Ca2+ homeostatic apparatus within the photoreceptor regions downstream from the OS. Our results thus challenge the prevailing view that rd1 degeneration is driven by global Ca2+ elevations that overload the endogenous Ca2+ buffering and clearance mechanisms within the cell body, and thus have implications for the development of neuroprotection strategies in retinitis pigmentosa (RP) models of retinal degeneration.
Results
We constructed a transgenic line expressing GCaMP3 specifically in zebrafish cones using the cone transducin promoter (TαCP) that we previously isolated.14 GCaMP3 is a fluorescent Ca2+ indicator consisting of circularly permuted green fluorescent protein (GFP), calmodulin, and the Ca2+-calmodulin target peptide M13.15 GCaMP3 has increased fold fluorescence change (increased Fmax/Fmin) and higher Ca2+ affinity (660±19 nm) than many other genetically encoded indicators.15 It is widely used as an indicator of in vivo changes in intracellular Ca2+.16, 17, 18 In zebrafish cones, GCaMP3 localizes throughout the cone cytoplasm, but is less abundant in the OS (Figures 1b–e). We then generated double transgenics by crossing the Tg(TαCP:GCaMP3) strain with a transgenic fish line expressing the fluorescent protein tdTomato selectively in long-wavelength cone photoreceptors using the thyroid hormone receptor β2 promoter, Tg(Trβ2:tdTomato) (Williams et al.19 and Figures 1a and c–e). The tdTomato fluorescence in long-wavelength cones has several uses. Uniform cytoplasmic distribution of tdTomato facilitates visualization of the overall cell shape so that morphological changes during cell death can be analyzed. Further, its expression in a subset of discretely spaced cones defines these cells for visual analysis, and serves as a photobleaching control. Double-transgenic fish (Tg(Trβ2:tdTomato;TαCP:GCaMP3)) were used for live imaging experiments, where we directed our observation of Ca2+ dynamics to the cone photoreceptor cell body and synaptic terminal.
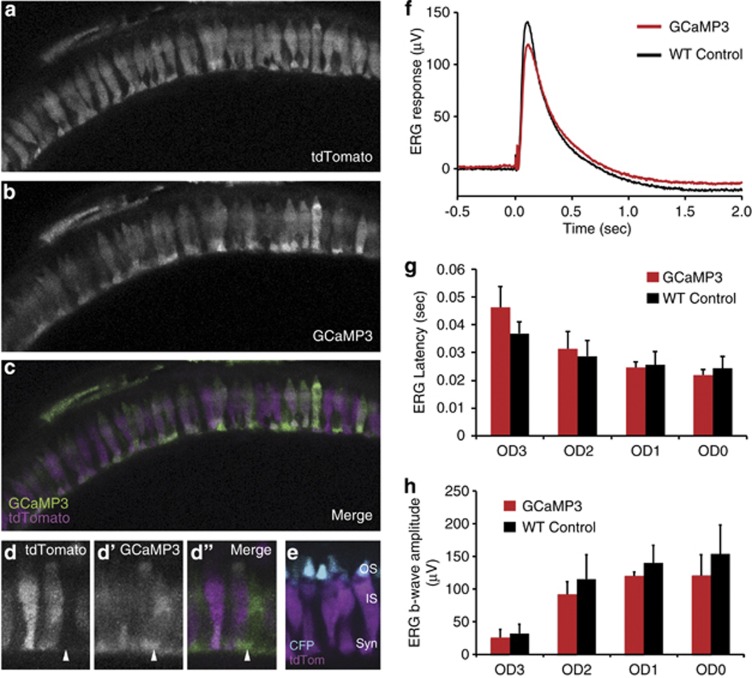
Figure 1.
GCaMP3 is expressed in all cone photoreceptors and does not interfere with light responses. (a–c) Retinal slice projections from a representative 5 d.p.f. Tg(Trß2:tdTomato;TαCP:GCaMP3) double-transgenic pde6cw59 mutant zebrafish larvae displaying intact photoreceptors prior to their degeneration. The tdTomato (a) is expressed only in long-wavelength cones, whereas the GCaMP3 (b) is expressed in all cone photoreceptors. Panel c shows both tdTomato (magenta) and GCaMP3 (green) channels merged. (d) Magnified view of cone photoreceptors in the Tg(Trß2:tdTomato;TαCP:GCaMP3) double transgenic. Arrow indicates a cell expressing GCaMP3 (green) but not tdTomato (magenta). Less expression of both GCaMP3 and tdTomato is observed in the cone outer segments (OS), whose location within the photoreceptor layer is clearly defined by the TαCP:membrane CFP (membrane cyan fluorescent protein (mCFP); cyan) transgene (e) Retinal section from a Tg(Trß2:tdTomato; TαCP:mCFP) double-transgenic fish expressing tdTomato in long-wavelength cones (magenta) and membrane CFP (cyan) in all cones. The outer segments (OS), inner segments (IS), and synapses (syn) are as indicated. (f–h) ERG waveforms (f), b-wave latencies (g), and b-wave amplitudes (h) for 5 d.p.f. WT non-transgenic versus Tg(TαCP:GCaMP3) larvae show no significant differences in their light responses over three orders of magnitude. n=3 fish per condition; bars=S.D.
ERG analysis of transgenic GCaMP3 cones
A potential problem when expressing fluorescent indicators of intracellular Ca2+ is the disruption of Ca2+ homeostasis due to abnormal buffering. To ensure that GCaMP3 expressed in cones does not alter normal fluxes in Ca2+, we measured electroretinogram (ERG) responses from our transgenic line and compared these with WT ERG responses. ERGs were recorded from excised eyes as previously described.20 If introduction of excess buffer affected the photoreceptor [Ca2+]i, the delay and/or reduction in synaptic signaling would be detected as a diminished and/or delayed ERG b-wave. We found that the ERG waveforms appeared similar, and no significant differences in b-wave amplitude or latency between transgenic and non-transgenic WT cones were observed (Figures 1f–h). These results indicate that the GCaMP3 transgene does not significantly alter the visual responses of photoreceptors.
Ca2+ dynamics in dying zebrafish cones
Our previous characterization of the pde6cw59 mutant used multiphoton imaging to analyze morphological changes associated with cone photoreceptor cell death that occur in vivo.12 Mutant cells differentiate normally and appear morphologically normal, with small OSs and extended synaptic processes, until ∼4 d.p.f. The mutant cells then proceed through a series of morphological changes prior to their clearance from the retina. Typically, the synapse is first retracted, followed by the contraction of the OS and the rounding of the cell body. These morphological changes occur asynchronously across the retina over 2–3 days; by 7 d.p.f. the vast majority of cones are gone in the mutant retina. In an individual cell, morphological changes occur over ∼8 h (Lewis et al.12 and Figure 2b). Based on recent studies in the rd1 mouse, morphological changes associated with cell death in pde6cw59 cones are likely late in the cell death cascade.21
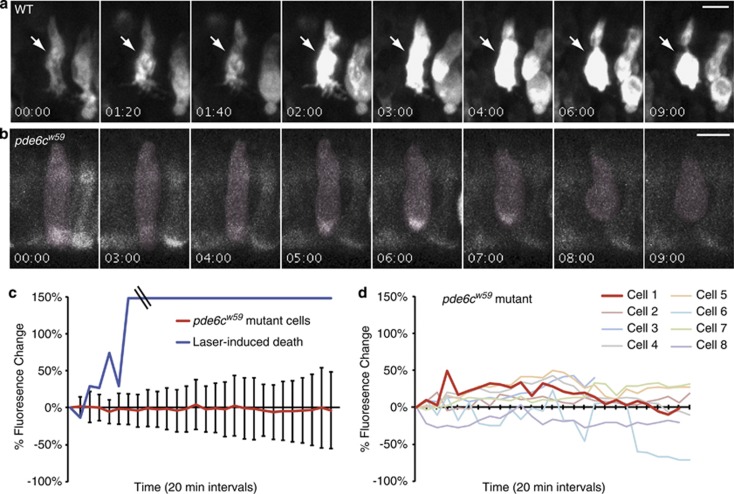
Figure 2.
Modest and variable changes in Ca2+ occur during the death of pde6cw59 cones. Photoreceptors from 5—6 d.p.f. larvae expressing TαCP:GCaMP3 were imaged every 20 min for 9 h. (a, b) Montages of representative cells at select time points show GCaMP3 fluoresence and morphology changes in dying photoreceptors for both pde6cw59 heterozygous (a) and pde6cw59 homozygous (b) photoreceptors. Time=(h:min). (c) Graph showing mean percentage of fluorescence change of GCaMP3 from 19 pde6cw59 mutant cells undergoing cGMP-mediated death (bars=S.D.) and the single representative laser-induced dying cell shown in a. Laser power and time of exposure was increased to stimulate laser-induced death of non-mutant cones. In addition, these cells were more sensitive than the homozygous pde6cw59 cones to prolonged laser exposure. For homozygous pde6cw59mutants, laser conditions were optimized to not cause morphological defects during the extended time-lapses. (d) Graph showing variations in percentage of GCaMP3 fluorescence changes in eight individual cones from a single pde6cw59 mutant fish. The red line (cell 1) indicates the percentage of change in GCaMP3 fluorescence of the representative dying pde6cw59 mutant cone shown in b
To examine Ca2+ dynamics during cell death, we monitored both changes in GCaMP3 fluorescence and in cell shape at 20 min intervals in time-lapse experiments that extended up to 9 h. We then quantified changes in GCaMP3 fluorescence in dying cells that initially appeared morphologically normal at the beginning of the time-lapse, but had completely rounded up by the end of the experiment. As a proof of principle for this study, we initially caused death of non-mutant cells by intense infrared laser illumination via multiphoton time-lapse imaging. This method led to profound increases in GCaMP3 fluorescence that saturated our detectors (Figures 2a and c). During the dying process of these laser-damaged cells, dramatic localized increases in GCaMP3 fluorescence typically around the nucleus were observed. These initial GCaMP3 changes were either transient or sustained, but were consistently followed by dramatic persistent increases that extended throughout the entire cell body as the photoreceptor rounded up (Figure 2a).
We used optimized multiphoton imaging conditions that do not damage homozygous pde6cw59 cones12 to monitor GCaMP3 fluorescence changes in mutant cells that degenerate during the 9 h time-lapse. In contrast to the dramatic changes observed with laser-induced death, we did not detect pronounced intracellular increases in GCaMP3 fluorescence in the pde6cw59 mutant dying photoreceptors (Figure 2b). We quantified intracellular fluorescent changes in 19 individual cells from four different fish, and none of these cells showed sustained increases in GCaMP3 fluorescence over time (Figure 2c). Even though the average change in cell body fluorescence centered at zero for these 19 cells, the specific changes in individual cells were unique. Some cells showed very little change, some decreased in overall fluorescence, whereas others showed recurring small transient increases in GCaMP3 fluorescence (Figure 2d and Figure 4).
Ca2+ dynamics in dying mouse rods
To determine whether our finding represented a general feature of RP-like photoreceptor degeneration across vertebrates, we extended our measurements to intracellular Ca2+ in rod photoreceptors from rd1 mice, arguably the most widely used model of Pde6 dysfunction.7, 8, 9, 10, 11 Intracellular Ca2+ was assessed in acutely isolated rd1 rods and age-matched WT cells at the start of degeneration (postnatal day 8–10), and at the peak of degeneration after eye opening (postnatal day 14–21). The cells were identified by shape, size and/or expression of GFP (Figure 3b).22 Figure 3a illustrates averaged data from 19 simultaneously recorded rd1 rods loaded with the Ca2+ indicator Fura-2. The calibration protocol shows baseline ratio values for dissociated light-adapted rods, followed by decreased ratio in Ca2+ free saline (possibly mediated by the closure of store-operated channels)22 and then ionomycin-induced Ca2+ release from internal stores. The inset displays calibrated [Ca2+]i with stable baseline levels maintained at ∼50 nM. [Ca2+]i levels in developing rods were comparable to concentrations measured in adult WT cells.22 Consistent with in vivo measurements in intact teleost cones, baseline [Ca2+]i in dissociated rd1 rod somata was not significantly elevated before or during cGMP-driven rod degeneration even at ages P14–21 during massive rod loss (Figure 3c).
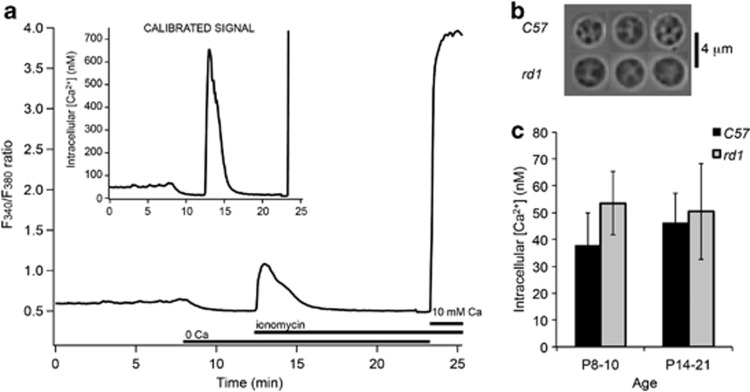
Figure 3.
Ca2+ measurements in dying mouse rods. (a) Average (n=19) fluorescence ratio and calibrated signal during baseline calcium measurements from acutely isolated Pde6βrd1 (rd1) mouse rod perikarya at P10. Ionomycin was used to clamp intracellular calcium levels to external zero calcium (containing 1 mM EGTA to obtain Rmin) or 10 mM calcium level (to obtain Rmax), allowing for a cell-by-cell calibration of the fluorescent signal. (b) Typical morphology of C57Bl6J (C57) or rd1 mouse rod photoreceptors. (c) No significant differences in absolute baseline Ca2+ concentration of acutely isolated C57Bl6J (C57) or Pde6βrd1 (rd1) mouse rod perikarya at ages between 8 and 10 days and 14 and 21 days were observed. Shown are group averages±S.E.M. p8–10: 37.7±12.2 (C57), 53.5±11.8 (rd1); p14–21: 46.2±11.0 (C57), 50.4±17.8 (rd1)
Ca2+ oscillations in pde6cw59 zebrafish cone photoreceptors
We next analyzed the Ca2+ oscillations that we detected in zebrafish cones to determine how these related to the cell death process. We found that 27% of pde6cw59 mutant cones (n=84) exhibited oscillations in GCaMP3 fluorescence during our 9-hr time-lapse experiments. The intensity of oscillations varied from cell to cell and even within cells, thus we used a change in intensity of ∼18% as a lower limit for our definition of an increase. GCaMP3 increases occurred both simultaneously throughout the cell body and synapse, or were often localized only to the synaptic region (Figure 4a). Both morphologically normal cells (Figures 4a and c) and those undergoing morphological changes (Figures 4b and c) exhibited Ca2+ oscillations. For the dying cells that eventually rounded up, the most significant increase(s) in [Ca2+]i occurred at the beginning of the time-lapse before any major cell shape changes were observed (Figures 4b and c).
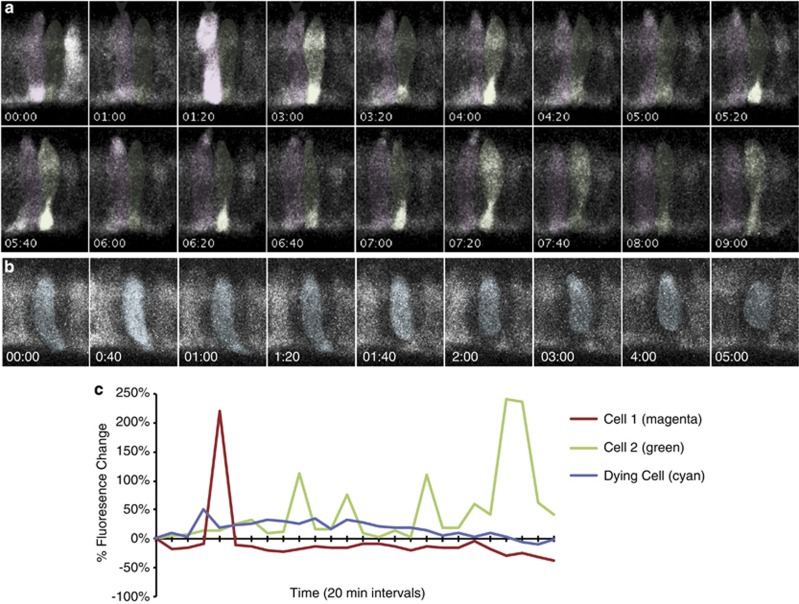
Figure 4.
Ca2+ oscillations occur in morphologically normal and dying pde6cw59 cones. Photoreceptors from homozygous pde6cw59 double-transgenic animals Tg(Trß2:tdTomato;TαCP:GCaMP3) were imaged every 20 min. Only the GCaMP3 channel is shown. (a, b) Montages of representative morphologically normal (a) and degenerating (b) pde6cw59 cones show transient increases in GCaMP3 fluorescence. Time=(h:min). (c) Graph of percentage change in GCaMP3 fluorescence intensity for outlined cells shown in a and b. Oscillations in GCaMP3 fluorescence occur at random intervals throughout the time-lapse in morphologically normal cones, whereas transient increases in dying cells usually occur near the beginning of the time-lapses (c and Figure 2d)
To analyze the temporal and spatial characteristics of these recurring transient GCaMP3 increases in greater detail, we conducted shorter time-lapse experiments (ranging from 15–30 min) and acquired images every 5 s. Image acquisition every 5 s allowed sampling of a single retinal plane containing ∼30–50 cells. As the overall experiment was short, we focused our analysis on the pde6cw59 mutant cone cells that appeared morphologically normal throughout the experiment. A representative experiment and subsequent data analysis is shown in Figure 5. Using this rapid imaging paradigm, rapid recurring Ca2+ increases (‘flashes') were observed in many cells within this imaged population (see Supplemental Movie). For example, in the representative cell shown in Figure 5a, a bright increase is observed in the synapse at 0905 hours, but is not seen in the time point before (0900 hours) nor after (0910 hours). Another synaptic increase is seen in the same cell at 0915 hours, is gone at 0920 hours, and reoccurs at 0925 hours. This analysis indicates that these particular flashes lasted for 5 s or less. To optimize our analysis of multiple pde6cw59photoreceptor cells in a single retinal population, we straightened our retinal images (Figure 5b) and generated kymographs (Figure 5c). Two kymographs were generated for each time-lapse; one for the synapse and one for the cell body. These kymographs are overlaid in two different colors in Figure 5c. Green shows GCaMP3 fluorescence increases localized to the synapse regions and magenta shows Ca2+ changes throughout the cell body (Figure 5c).
Figure 5.
Recurrent Ca2+ flashes are variable, rapid, asynchronous, and prevalent. Photoreceptors from homozygous double-transgenic Tg(Trß2:tdTomato;TαCP:GCaMP3) pde6cw59 fish were rapidly imaged every 5 s for 15–30 min. Only the GCaMP3 channel is shown. (a) Montage of a representative cell (cell 13; see b and c) at select time points show rapid transient increases in GCaMP3 fluorescence occurring in both the cell body and synapse. Time=(min:s). (b) Representative image (time) projection from a straightened Tg(Trß2:tdTomato;TαCP:GCaMP3) pde6cw59 retina shows GCaMP3 fluorescence in all cones. The shaded areas represent the areas used to generate the kymograph (c) for the cell body (magenta) and for the synapse (syn; green). All identifiable cells are numbered below and correspond to a column on the kymograph in c. (c) Representative kymograph of GCaMP3 signal across all the time points for both the cell body (magenta) and synapse (green). The boxed areas on both the kymograph (c) and the straightened retina (b) represent the cell (cell 13) and time period shown in the montage in a
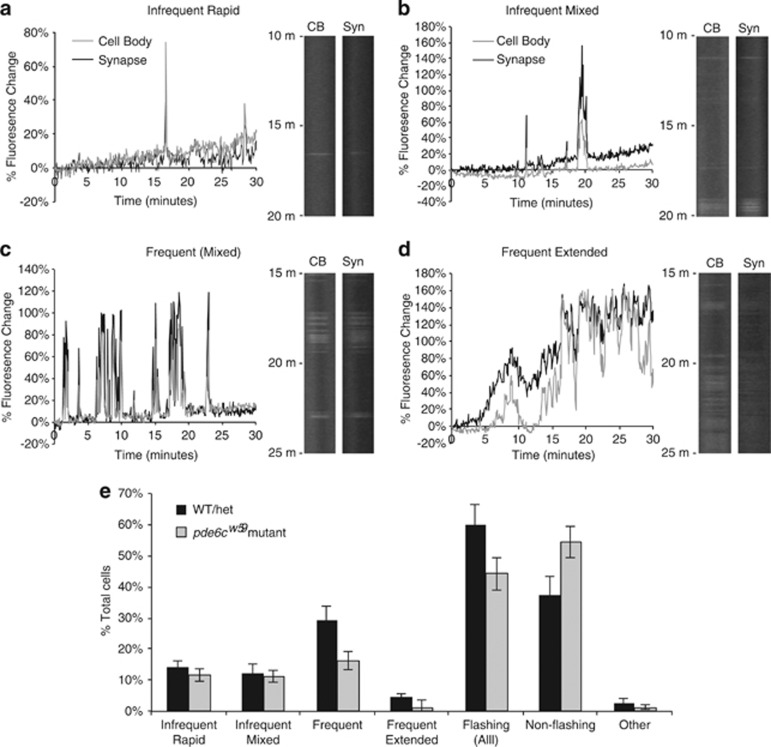
Using our kymographs, we graphed the GCaMP3 signal intensity over time for each photoreceptor and established general categories describing the types of flashes we detected. Cells that flashed fewer than 10 times over 30 min were labeled as ‘infrequent,' whereas those flashing more than 10 times were categorized as ‘frequent'. Cells that flashed infrequently were also subdivided based upon the duration of the flashes. Cells whose infrequent flashes lasted for only 1–3 time points were deemed as ‘infrequent rapid', whereas ‘infrequent mixed' cells exhibit flashes that were sustained over several time points (Figures 6a and b). Cells with frequent flashes exhibited transient GCaMP3 increases of mixed duration (Figure 6c), but a few cells showed recurring transient increases that lasted over an extended period of time (categorized as ‘frequent extended'), often with varying intensities within the elevated GCaMP3 signal (Figure 6d). A few cells also showed a change in fluorescence, either increasing or decreasing, that persisted throughout the time-lapse. This small number of cells was categorized in the ‘other' category. From our analysis of 447 cells from 11 pde6cw59fish, we determined that 46% of cells flash during the 15–30 min time-lapse experiments, and that there were roughly equal number of cells that flash infrequently (both rapid and mixed) and those that flash frequently. Consistent with our longer time-lapse experiments, extended increases in fluorescence were detected in only a few cells (Figure 6e).
Figure 6.
Characterization of heterogeneous Ca2+ flashes show fewer pde6cw59 mutant cones with frequent flash patterns. Individual cones from kymographs of the rapid 5 s time-lapse imaging experiments were analyzed and categorized according to their pattern of GCaMP3 flashes. (a–d) Representative graphs and kymograph segments of GCaMP3 flash patterns in both the cell body (CB) and synapse (syn) of cones. Flashing cells were categorized into four main categories: ‘infrequent rapid' (a), ‘infrequent mixed' (b), ‘frequent' (c), and ‘frequent extended' (d). See text for more details regarding flash categorization. (e) Graph showing the percentage of various categories of flashing cells in both pde6cw59 mutant and non-mutant (WT/het) cones. The ‘other' category consists of a small number of non-flashing cells that showed persistent increasing or decreasing GCaMP3 fluorescence throughout the duration of the time-lapse (e). Overall, fewer cells exhibiting Ca2+ flashes were observed in pde6cw59 mutants than in WT/het larval retinas (‘Flashing (All)'), largely due to a reduction in the ‘frequent' category. No significant difference in mutant and non-mutant cones was observed in the other categories. For pde6cw59mutants: n=447 cells; 11 fish. For WT/het: n=263 cells; six fish. Bars=S.E.M.
Ca2+ dynamics in non-mutant cone photoreceptors
As Ca2+ flashes within photoreceptors have not been reported previously, we speculated that elevated cGMP and consequent metabolic changes in pde6cw59cones disrupt Ca2+ homeostasis, causing transient increases in [Ca2+]i. To examine this possibility, we analyzed flashes in photoreceptors from both WT and pde6cw59 heterozygous fish, siblings of the pde6cw59 mutants previously analyzed, and imaged these photoreceptors in vivo at 5 s intervals for 15–30 min. We noted that the light-sensitive cones from WT and pde6cw59 heterozygous fish were more sensitive to laser exposure than the light-insensitive homozygous pde6cw59photoreceptors. Slight laser-induced morphological abnormalities were detected in some photoreceptors during imaging, and data were not analyzed from these cells.
To our surprise, kymograph analysis of cone photoreceptors from WT and pde6cw59 heterozygous fish (n=263 cells; 6 fish) revealed the same categories of flashes as observed in the pde6cw59mutant photoreceptors. Further, our results indicated that altered metabolism due to elevated cGMP did not increase the number of cells flashing or the flash frequency of individual cells, but rather decreased these; 44% of mutant cells flashed versus 60% of non-mutant cones (P=0.09), and this reduction in flashing pde6cw59mutant cones was primarily cells categorized as ‘frequent' (16% mutant versus 29% non-mutant, P=0.03, Figure 6e).
Taken together, these data show that dynamic modulation of [Ca2+]i occurs in WT cone photoreceptors and that this modulation is mostly retained in light-insensitive pde6cw59 mutant cones prior to their degeneration, although fewer frequently flashing cells were observed. However, at late stages of death when mutant cells degenerate, Ca2+ transients are substantially reduced or cease and catastrophic Ca2+ release into the cytoplasm does not occur.
Discussion
In this study, we report an in vivo analysis of Ca2+ dynamics in cone photoreceptors within a host animal. We exploit a double-transgenic zebrafish that enables simultaneous analysis of cytoplasmic changes in Ca2+ and of overall changes in cell morphology, and extend our analysis of Ca2+ regulation in pde6cw59 cones to the commonly studied rd1 mouse model. We discovered that (1) pde6cw59 mutant cone photoreceptors and rd1 rods do not exhibit sustained intracellular Ca2+ increases during degeneration; (2) transient Ca2+ increases are prevalent in pde6cw59 mutant and non-mutant cone photoreceptors; (3) these Ca2+ flashes occur throughout the cell body and synapse, or are only localized to the synapse; (4) Ca2+ transients vary in frequency and duration; and (5) fewer cells in the pde6cw59 mutant retinas show a pattern of frequent recurring Ca2+ flashes compared with non-mutant retinas. Our findings shed light on the mechanism of cell death occurring in pde6cw59 mutant cones and rd1 rods, and highlight a potentially functionally important measure of normal cone photoreceptor physiology.
Pde6 is a key mediator of the well-established vertebrate phototransduction cascade. Activated by GTP-bound α-transducin, it hydrolyzes cGMP, resulting in closure of cation channels in the OS plasma membrane. Loss of Pde6 activity increases OS Ca2+ levels6 possibly causing cell death, a conjecture supported by the observation that reducing Ca2+ influx by knocking out cGMP-gated cation channels (Cngb1(−/−) × Pde6βrd1) improves viability of rd1 rods.7 However, to understand the physiological coupling between [Ca2+]i and the degeneration process, it is necessary to ascertain Ca2+ dynamics within the inner segment. We therefore developed a novel zebrafish transgenic line, Tg(Trß2:tdTomato;TαCP:GCaMP3), and compared Ca2+ signals obtained in degenerating pde6cw59cones with [Ca2+]i levels within the Pde6βrd1 rods. To avoid interference from signals emanating from over-activation of Ca2+ fluxes through cGMP channels,7 pde6cw59mutants were designed to express the genetically encoded Ca2+ indicator GCaMP3 within cell compartments downstream from the OS.
One possible mechanism for pde6cw59and rd1 photoreceptor degeneration was mitochondrial Ca2+ overload and a consequent increase in cytosolic [Ca2+]i within the perikaryon.23 Using live imaging, we detected modest changes in intracellular Ca2+ that varied between individual dying cells; some cells displayed decreases in overall GCaMP3 fluorescence and several displayed small Ca2+ oscillations. Nonetheless, sustained increases in Ca2+ in cell bodies of pde6cw59 cones undergoing morphological changes of cell death were not detected during our in vivo time-lapse experiments nor were elevated perikaryal [Ca2+]i levels observed in Pde6βrd1 mouse rods. This finding is in stark contrast to the pronounced [Ca2+]i increases elicited in non-mutant cones by excessive laser exposure (Figure 2). Hence, our observations argue against a major role of depolarization, voltage-activated Ca2+ influx, mitochondrial overload and Ca2+ release from internal stores in cone and rod death driven by Pde6 mutations. Consistent with this, elimination of Ca2+ influx through L-type channels slightly delayed, but did not prevent, rd1 degeneration.24
We observed dramatic transient increases in [Ca2+]i in WT and in pre-degeneration pde6cw59 cones. This finding indicates that Ca2+ homeostasis in photoreceptors is dynamically regulated and similar in light-adapted and unstimulated photoreceptors. Thus, the Ca2+ changes that we report appear independent of phototransduction. Although future studies will explore possible sources for such Ca2+ flashes, potential sources include voltage-gated Ca2+ channels, receptor-mediated and/or store-operated Ca2+ influx from the extracellular space channels, as well as Ca2+ release from internal stores gated by ryanodine/inositol triphosphate release Ca2+ channels.22 Numerous studies focusing on non-excitable and excitable cells25 suggest that internal compartments represent major contributors to spontaneous elevations in [Ca2+]i. ‘Sparks' or ‘puffs' have been proposed to regulate cardiac and skeletal muscle excitation–contraction coupling, vascular tone regulation, membrane excitability and neuronal secretion, but were also implicated in regulation of developmental changes, plasticity or cell to cell communication.25 Ryanodine-sensitive Ca2+ stores are present in photoreceptors and have been proposed to help maintain Ca2+ levels within a physiological range optimal for normal function under dim lights,26 whereas store-operated calcium entry and transient receptor potential channels might have a more significant function under bright and prolonged illumination.22, 27
In conclusion, we here document new features of Ca2+ signaling in cone photoreceptors and address the long-standing question whether Pde6 mutations trigger photoreceptor degeneration through Ca2+ -dependent activation of proapoptotic pathways driven by cGMP-induced Ca2+ overloads within the cell body. We found that, surprisingly, Pde6 loss does not result in sustained increases in [Ca2+]i in either mouse rods or zebrafish cones. Our data show that in vivo analysis of photoreceptor physiology represents a powerful new tool to study function in normal and diseased retinal tissue.
Materials and Methods
Zebrafish maintenance and mutant isolation
Adult fish and larvae were maintained at 28.5 °C in reverse-osmosis distilled water reconstituted for fish compatibility by addition of salts and vitamins28 on a 10/14 h dark/light cycle. Unless otherwise noted, all zebrafish strains are homozygous for the roy orbison (roy) mutation.29 Fish used in live imaging experiments were maintained in embryo media28 containing 0.003% 1-phenyl 2-thiourea (PTU) starting at 8–24 h post fertilization (h.p.f.). The pde6cw59 mutant was originally isolated in a screen of mutagenized-zebrafish using the optokinetic response behavioral assay as described previously.13 The pde6cw59 mutation is maintained as heterozygotes and intercrossed to generate homozygous mutants.
Construction of transgenic zebrafish
To express GCaMP3 specifically in cones, we used the Gateway-based Tol2kit30 and inserted GCaMP3 downstream of the 3.2 Kb of the TαCP.14 The resulting construct was injected into WT zebrafish embryos at the 1–2-cell stage together with Tol2 transposase mRNA.31 Mosaics were identified at 4 d.p.f. and raised to adulthood. Germline carriers were identified in the F1 generation. To visualize cone morphology, we generated a transgenic line Tg(Trβ2:tdTomato) expressing tdTomato in the long-wavelength cones (this paper and Suzuki et al., in preparation). The Trβ2 promoter isolation, clone construction, and the general expression were described previously.19 Germline carriers were identified from mosaic fish injected with this construct. Both Tg(TαCP:GCaMP3) and Tg(Trβ2:tdTomato) were crossed into the pde6cw59 mutant lines. The transgenic line Tg(TαCP:membrane CFP) was described previously.12
Pde6cw59 genotyping
After imaging, zebrafish larval genotypes were verified by PCR analysis followed by restriction enzyme digest, as previously described.28 Primers were used to create a restriction site for BsaXI in only the mutant locus (forward: 5′-TTGGCCTCTGGAATACTGGCT-3′ reverse: 5′-GTTTGACCAGAACCCGGAAG-3′). PCR products were digested with BsaX1 and genotyped according to its restriction profile.
ERG recordings
ERGs were recorded as described previously.32 Briefly, 5 and 6 d.p.f. larvae were anesthetized in 0.02% tricaine, and eyes were removed using a fine tungsten wire loop. Excised eyes were then placed in an oxygenated Ringer's solution (in mM; 130 NaCl, 2.5 KCl, 20 NaHCO3, 0.7 CaCl2, 1.0 MgCl2, and 20 glucose), and a glass electrode was positioned directly onto the cornea. After 3 min (min) of dark adaptation, eyes were exposed to white light flashes, and their electrical responses were recorded. Data were acquired and processed as described previously.33 Peak values are listed as the mean±S.D. from three animals.
Live time-lapse imaging in zebrafish
For time-lapse imaging, transparent PTU-treated 5–6 d.p.f. larvae were embedded in a drop of 1% low melting point agarose in embryo media. After solidifying, the agarose was covered with embryo media containing 0.003% PTU and 0.02% (w/v) tricaine. Live time-lapse imaging experiments were performed on an Olympus FV1000MPE multiphoton laser scanning microscope equipped with a × 25 (NA 1.0) long working distance water objective. Images of the Tg(Trβ2:tdTomato; TαCP:GCaMP3) double transgenics were acquired using a red/green filter cube at 890 nm wavelength. For the extended time-lapse experiments, images stacks of 30–40 μm (1 μm per slice) were taken every 20 min for 9 h. Single image slices were taken for the shorter time-lapse experiments, which require rapid sampling every 5 s for 15–30 min.
Image processing and analysis
Image processing and analysis was carried out using the ImageJ (Fiji; http://fiji.sc/Fiji) software.34 Dying pde6cw59 cells were identified by examining images collected from the extended 9-hour time-lapse experiments. Images were Z-projected using 7–10 stacks at 1 μm depth, for a total of 7–10 μm depth depending on the shape of the cell. The Z-projected images were adjusted to correct for xy drift using the ‘stack reg' function, and further corrected for bleaching using the EMBL tools ‘bleach correction' function with the tdTomato image selected as a reference (using the ‘simple ratio' setting with background set to 0.0). These adjustments were done using the entire image prior to cropping to focus on the dying cell(s). Regions of interest (ROIs) were placed on the cell body of the dying cell and the mean intensity (gray value) measurements were taken. Due to the shape changes these cells undergo, we used the center of the cell body to ensure that the cell continued to be inside the ROI throughout the dying process. Percent change in fluorescence is calculated as ((Fx–F0)/F0 × 100%) where Fx is the mean florescence intensity at time point x and F0 is the starting fluorescence at time=0.
For generating kymographs, retina time stacks were first straightened out using the ‘Straighten' function in Fiji (Edit>Selection>Straighten) on a segmented line that followed the retina curvature. The synapse and the cell body layers were then separately selected, resliced (Image>Stack>Reslice), and Z-projected (Sum slices) to generate two separate kymographs for each time-lapse. These two kymographs were then combined in two different channels (represented in magenta and green in Figure 5c) and analyzed for flashes. Individual flashing cells, represented as striped columns in the kymographs, were analyzed using the ‘Plot Profile' function in Fiji (Analyze>Plot Profile) with options set to vertical profile. Adobe Photoshop CS5 (San Jose, CA, USA) was used to process the images further. Final figures were assembled using Adobe Illustrator CS5.
Ca2+ measurements in mouse rods
[Ca2+]i in acutely isolated mouse rods was measured as described previously.22 Briefly, postnatal day P8–P10 and P14–P21, C57Bl6J (C57) and Pde6βrd1 (rd1) mice (Jackson Laboratories, Bar Harbor, ME, USA) were killed, enucleated and the retinas were treated with papain (30–50 U/ml; Worthington, Freehold, NJ, USA; 1 h at room temperature). Acutely isolated photoreceptor cells with intact somas were identified based on their size and morphology (Figure 3b;22).The number of mice (N) and cells (n) in each group was: C57 (P8–10): N=3, n=122; rd1 (P8–10): N=7, n=186; C57 (P14–21): N=3, n=126; rd1 (P14–21): N=3, n=27. Following trituration, cells were plated on concanavalin A-coated (0.2 mg/ml; Sigma, St. Louis, MO, USA) coverslips, loaded with fura-2 AM (1–5 μM; Invitrogen, Life Technologies, Grand Island, NY, USA) for 30 min, and washed for 30 min in dye-free L-15 medium at room temperature. Fluorescence was measured using 340 and 380 nm excitation filters (Chroma, Brattleboro, VT, USA), whereas emission was high-pass filtered at 510 nm and captured with cooled digital CCD cameras (HQ2, Photometrics, Tucson, AZ, USA). Data acquisition (10 pairs of images/min) and fluorescence ratio calculations with background subtraction in ROIs encompassing the rod perikaryon were performed with NIS Elements (Melville, NY, USA). Calibration of cytosolic free [Ca2+]i was carried out by using 10 μM ionomycin in combination with 0 Ca2+/1 mM EGTA followed by 10 mM Ca2+ (Figure 3a;35). The apparent free [Ca2+]i was determined from the equation [Ca2+]i=((R—Rmin)/(Rmax—R)) × β × Kd, where R is the ratio of emission intensity at 510 nm evoked by 340 nm excitation versus emission intensity at 510 nm evoked by 380 nm excitation; Rmin is the ratio at zero free Ca2+; Rmax is the ratio at saturating Ca2+; Kd the dissociation constant for Ca2+ binding to fura-2 in the presence of millimolar Mg2+ was taken from the literature (224 nM;35, 36); β=(F380max/F380min). R, Rmin, Rmax, and β were measured and calculated for each ROI (cell) separately. Baseline values were established for each cell by averaging baseline [Ca2+]i over a 5 min period. Group baseline values are averages of individual cell data±S.E.M.
Acknowledgments
Research reported in this manuscript was supported by NEI of the National Institutes of Health under award numbers R01 EY018814 (SEB), Core Grant P30EY001730 (UW), RO1 EY14358 (SS), R01 EY13870 (DEK), EY022076 (DEK), Core Grant EY014800 (Moran Eye Center) and also Knights Templar Foundation and International Retina Research Foundation (PB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Ashley George, and Drs. Gordon Fain, Jim Hurley and Whitney Cleghorn for suggestions on the manuscript.
Author Contributions
EM, AL, and GS conducted zebrafish experiments. PB made calcium measurements from mouse rods. SS generated the Trβ2:tdTomato transgenic zebrafish. SEB conceived and directed research. EM, AL, PB, DK and SEB analyzed data. Paper was written by SEB with major contributions from EM, DK, AL, and PB.
Glossary
- cGMP
cyclic guanosine-mono-phosphate
- Pde6
phosphodiesterase-6
- OS
outer segment
- d.p.f.
days post fertilization
- WT
wild type
- RP
retinitis pigmentosa
- PTU
1-phenyl 2-thiourea
- ERG
electroretinogram
- TαCP
cone α-transducin promoter
- [Ca2+]i
intracellular calcium
- ROI
region of interest
- Tg
transgenic
- Trβ2
thyroid hormone receptor β2
- GFP
green flourescent protein
- mCFP
membrane cyan fluorescent protein
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by A Stephanou
Supplementary Material
References
- Keeler CE. The inheritance of a retinal abnormality in white mice. Proc Natl Acad Sci USA. 1924;10:329–333. doi: 10.1073/pnas.10.7.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittler SJ, Keeler CE, Sidman RL, Baehr W. PCR analysis of DNA from 70-year-old sections of rodless retina demonstrates identity with the mouse rd defect. Proc Natl Acad Sci USA. 1993;90:9616–9619. doi: 10.1073/pnas.90.20.9616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes C, Li T, Danciger M, Baxter LC, Applebury ML, Farber DB. Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature. 1990;347:677–680. doi: 10.1038/347677a0. [DOI] [PubMed] [Google Scholar]
- Pittler SJ, Baehr W. Identification of a nonsense mutation in the rod photoreceptor cGMP phosphodiesterase beta-subunit gene of the rd mouse. Proc Natl Acad Sci USA. 1991;88:8322–8326. doi: 10.1073/pnas.88.19.8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifunovic D, Dengler K, Michalakis S, Zrenner E, Wissinger B, Paquet-Durand F. cGMP-dependent cone photoreceptor degeneration in the cpfl1 mouse retina. J Comp Neurol. 2010;518:3604–3617. doi: 10.1002/cne.22416. [DOI] [PubMed] [Google Scholar]
- Davis RJ, Tosi J, Janisch KM, Kasanuki JM, Wang NK, Kong J, et al. Functional rescue of degenerating photoreceptors in mice homozygous for a hypomorphic cGMP phosphodiesterase 6 b allele (Pde6bH620Q) Invest Ophthalmol Vis Sci. 2008;49:5067–5076. doi: 10.1167/iovs.07-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquet-Durand F, Beck S, Michalakis S, Goldmann T, Huber G, Muhlfriedel R, et al. A key role for cyclic nucleotide gated (CNG) channels in cGMP-related retinitis pigmentosa. Hum Mol Genet. 2011;20:941–947. doi: 10.1093/hmg/ddq539. [DOI] [PubMed] [Google Scholar]
- Paquet-Durand F, Silva J, Talukdar T, Johnson LE, Azadi S, van Veen T, et al. Excessive activation of poly(ADP-ribose) polymerase contributes to inherited photoreceptor degeneration in the retinal degeneration 1 mouse. J Neurosci. 2007;27:10311–10319. doi: 10.1523/JNEUROSCI.1514-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquet-Durand F, Azadi S, Hauck SM, Ueffing M, van Veen T, Ekstrom P. Calpain is activated in degenerating photoreceptors in the rd1 mouse. J Neurochem. 2006;96:802–814. doi: 10.1111/j.1471-4159.2005.03628.x. [DOI] [PubMed] [Google Scholar]
- Barabas P, Cutler Peck C, Krizaj D. Do calcium channel blockers rescue dying photoreceptors in the Pde6b ( rd1 ) mouse. Adv Exp Med Biol. 2010;664:491–499. doi: 10.1007/978-1-4419-1399-9_56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doonan F, Donovan M, Cotter TG. Activation of multiple pathways during photoreceptor apoptosis in the rd mouse. Invest Ophthalmol Vis Sci. 2005;46:3530–3538. doi: 10.1167/iovs.05-0248. [DOI] [PubMed] [Google Scholar]
- Lewis A, Williams P, Lawrence O, Wong RO, Brockerhoff SE. Wild-type cone photoreceptors persist despite neighboring mutant cone degeneration. J Neurosci. 2010;30:382–389. doi: 10.1523/JNEUROSCI.5019-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns G, Evangelista M, Fadool JM, Brockerhoff SE. A mutation in the cone-specific pde6 gene causes rapid cone photoreceptor degeneration in zebrafish. J Neurosci. 2007;27:13866–13874. doi: 10.1523/JNEUROSCI.3136-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BN, Alvarez Y, Brockerhoff SE, Stearns GW, Sapetto-Rebow B, Taylor MR, et al. Identification of a zebrafish cone photoreceptor-specific promoter and genetic rescue of achromatopsia in the nof mutant. Invest Ophthalmol Vis Sci. 2007;48:522–529. doi: 10.1167/iovs.06-0975. [DOI] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghuis BG, Tian L, Xu Y, Nikonov SS, Vardi N, Zemelman BV, et al. Imaging light responses of targeted neuron populations in the rodent retina. J Neurosci. 2011;31:2855–2867. doi: 10.1523/JNEUROSCI.6064-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterberg R, Hailey DW, Coffin AB, Raible DW, Rubel EW. Disruption of intracellular calcium regulation is integral to aminoglycoside-induced hair cell death. J Neurosci. 2013;33:7513–7525. doi: 10.1523/JNEUROSCI.4559-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitz AC, Behrend MR, Lee NS, Klein RL, Chiodo VA, Hauswirth WW, et al. Imaging the response of the retina to electrical stimulation with genetically encoded calcium indicators. J Neurophysiol. 2013;109:1979–1988. doi: 10.1152/jn.00852.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PR, Suzuki SC, Yoshimatsu T, Lawrence OT, Waldron SJ, Parsons MJ, et al. In vivo development of outer retinal synapses in the absence of glial contact. J Neurosci. 2010;30:11951–11961. doi: 10.1523/JNEUROSCI.3391-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A, Wilson N, Stearns G, Johnson N, Nelson R, Brockerhoff SE. Celsr3 is required for normal development of GABA circuits in the inner retina. PLoS Genet. 2011;7:e1002239. doi: 10.1371/journal.pgen.1002239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahaboglu A, Paquet-Durand O, Dietter J, Dengler K, Bernhard-Kurz S, Ekstrom PA, et al. Retinitis pigmentosa: rapid neurodegeneration is governed by slow cell death mechanisms. Cell Death Dis. 2013;4:e488. doi: 10.1038/cddis.2013.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar T, Barabas P, Birnbaumer L, Punzo C, Kefalov V, Krizaj D. Store-operated channels regulate intracellular calcium in mammalian rods. J Physiol. 2012;590 (Pt 15:3465–3481. doi: 10.1113/jphysiol.2012.234641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol. 2012;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- Read DS, McCall MA, Gregg RG. Absence of voltage-dependent calcium channels delays photoreceptor degeneration in rd mice. Exp Eye Res. 2002;75:415–420. [PubMed] [Google Scholar]
- Ross WN. Understanding calcium waves and sparks in central neurons. Nat Rev Neurosci. 2012;13:157–168. doi: 10.1038/nrn3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizaj D, Lai FA, Copenhagen DR. Ryanodine stores and calcium regulation in the inner segments of salamander rods and cones. J Physiol. 2003;547 (Pt 3:761–774. doi: 10.1113/jphysiol.2002.035683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizaj D. Calcium stores in vertebrate photoreceptors. Adv Exp Med Biol. 2012;740:873–889. doi: 10.1007/978-94-007-2888-2_39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Brachydanio rerio) University of Oregon Press: Eugene, Orgeon, USA; 1995. [Google Scholar]
- Ren JQ, McCarthy WR, Zhang H, Adolph AR, Li L. Behavioral visual responses of wild-type and hypopigmented zebrafish. Vision Res. 2002;42:293–299. doi: 10.1016/s0042-6989(01)00284-x. [DOI] [PubMed] [Google Scholar]
- Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, et al. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn. 2007;236:3088–3099. doi: 10.1002/dvdy.21343. [DOI] [PubMed] [Google Scholar]
- Kawakami K, Shima A, Kawakami N. Identification of a functional transposase of the Tol2 element, an Ac-like element from the Japanese medaka fish, and its transposition in the zebrafish germ lineage. Proc Natl Acad Sci USA. 2000;97:11403–11408. doi: 10.1073/pnas.97.21.11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KY, Gray J, Hayward CJ, Adolph AR, Dowling JE. Glutamatergic mechanisms in the outer retina of larval zebrafish: analysis of electroretinogram b- and d-waves using a novel preparation. Zebrafish. 2004;1:121–131. doi: 10.1089/zeb.2004.1.121. [DOI] [PubMed] [Google Scholar]
- Van Epps HA, Yim CM, Hurley JB, Brockerhoff SE. Investigations of photoreceptor synaptic transmission and light adaptation in the zebrafish visual mutant nrc. Invest Ophthalmol Vis Sci. 2001;42:868–874. [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Neher E. The use of fura-2 for estimating Ca buffers and Ca fluxes. Neuropharmacology. 1995;34:1423–1442. doi: 10.1016/0028-3908(95)00144-u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.