Abstract
Prostate cancer is one of the most frequently diagnosed cancers among men. Dietary intake of nutrients is considered crucial for preventing the initiation of events leading to the development of carcinoma. Many dietary compounds have been considered to contribute to cancer prevention including zinc, which has a pivotal role in modulating apoptosis. However, the mechanism for zinc-mediated prostate cancer chemoprevention remains enigmatic. In this study, we investigated the therapeutic effect of zinc in prostate cancer chemoprevention for the first time. Exposure to zinc induced apoptosis and resulted in transactivation of p21WAF1/Cip1 in a Smad-dependent and p53-independent manner in prostate cancer cells. Smad2 and PIAS1 proteins were significantly upregulated resulting in dramatically increased interactions between Smad2/4 and PIAS1 in the presence of zinc in LNCaP cells. Furthermore, it was found that the zinc-induced Smad4/2/PIAS1 transcriptional complex is responsible for Smad4 binding to SBE1 and SBE3 regions within the p21WAF1/Cip1 promoter. Exogenous expression of Smad2/4 and PIAS1 promotes zinc-induced apoptosis concomitant with Smad4 nuclear translocation, whereas endogenous Smad2/4 silencing inhibited zinc-induced apoptosis accompanying apparent p21WAF1/Cip1 reduction. Moreover, the knockdown of PIAS1 expression attenuated the zinc-induced recruitment of Smad4 on the p21WAF1/Cip1 promoter. The colony formation experiments demonstrate that PIAS1 and Smad2/4 silencing could attenuate zinc apoptotic effects, with a proliferation of promoting effects. We further demonstrate the correlation of apoptotic sensitivity to zinc and Smad4 and PIAS1 in multiple cancer cell lines, demonstrating that the important roles of PIAS1, Smad2, and Smad4 in zinc-induced cell death and p21WAF1/Cip1 transactivation were common biological events in different cancer cell lines. Our results suggest a new avenue for regulation of zinc-induced apoptosis, and provide a model that demonstrates zinc endorses the Smad2/4/PIAS1 complex to activate the p21WAF1/Cip1 gene that mediates apoptosis.
Keywords: PIAS1, Smad2, Smad4, zinc, prostate cancer
Prostate cancer is the most common malignancy in men in the west world and has markedly increased in the last two decades in Asian countries.1, 2 Increasing clinical and experimental evidence suggest a potential role of zinc in human malignancies, including prostate cancer.3 Normal human prostate glands accumulate almost 10 times more zinc than other soft tissues, such as liver and kidney, and significantly decreased zinc levels are shown in malignant prostate tissues.3, 4, 5, 6, 7 Most human prostate cancer develops from the peripheral zone of the prostate, where high levels of zinc are identified in the normal epithelium, but dramatically lower levels in the tumor.3, 4, 5, 6, 7 Exogenous zinc showed an anti-proliferation effect in prostate cancer cells via induction of mitochondrial apoptogenesis.3, 8 Increasing evidence has shown a link between the reduction of intracellular zinc concentrations and human tumor development.7 However, the mechanisms underlying the zinc-mediated anti-tumor effect still remain largely unknown.
Zinc affects cell cycle and apoptosis by increasing the ratio of Bax to Bcl-2, which further upregulates the p21WAF1/Cip1 mRNA level in prostate cancer.8, 9, 10 The regulation of the cell cycle through modulation of p21WAF1/Cip1 is considered to be an intrinsic characteristic of many tumor suppressor proteins, including p53, BRAC1, and Smads.9, 11, 12, 13, 14, 15 TGF-β-activating R-Smad/Co-Smad complex directly activates the promoter region of the p21WAF1/Cip1 gene and upregulates cyclin-dependent kinase (CDK) inhibitors to promote G1–S cell cycle arrest.13, 14 Impairment of the Smad pathway causes escape from growth inhibition and leads to the promotion of cell proliferation, thereby contributing to carcinogenesis.16, 17, 18, 19 The re-establishment of the Smad4-involved complexes may reverse tumor cell development and shed light into therapeutic strategies for cancer treatment.20
It has been shown that protein inhibitors of activated signal transducers and activators of transcription (PIAS) proteins interact with the TGF-β pathway and regulate Smad-mediated transcriptional activity.21, 22, 23 The PIAS proteins are implicated in apoptotic pathways, such as Smad, p53, and AR signaling.24, 25, 26 PIAS1 is shown to be the downregulated factor screened from 16 AR coactivators in hormone-refractory prostate tumors as compared with benign prostatic hyperplasia.27 Moreover, substantially reduced expression of PIAS1 is indicated to be associated with the development of both colon cancer and gastric cancer, suggesting its important roles in cancer.28, 29, 30 Notably, PIAS proteins contain a RING finger-like zinc-binding domain; however, the roles of PIAS proteins in zinc-induced apoptosis have not been addressed yet. The increased p21WAF1/Cip1 expression by zinc treatment in LNCaP (androgen-dependent) and PC3 cells (androgen-independent) has been well documented.8, 9, 31, 32 However, their associated pathways are still unclear. Therefore, this study was conducted to determine the potential contribution of the PIAS-Smad signaling in zinc-induced apoptosis.
Results
Zinc treatment resulted in the overexpression of Smad and PIAS in prostate cancer cells
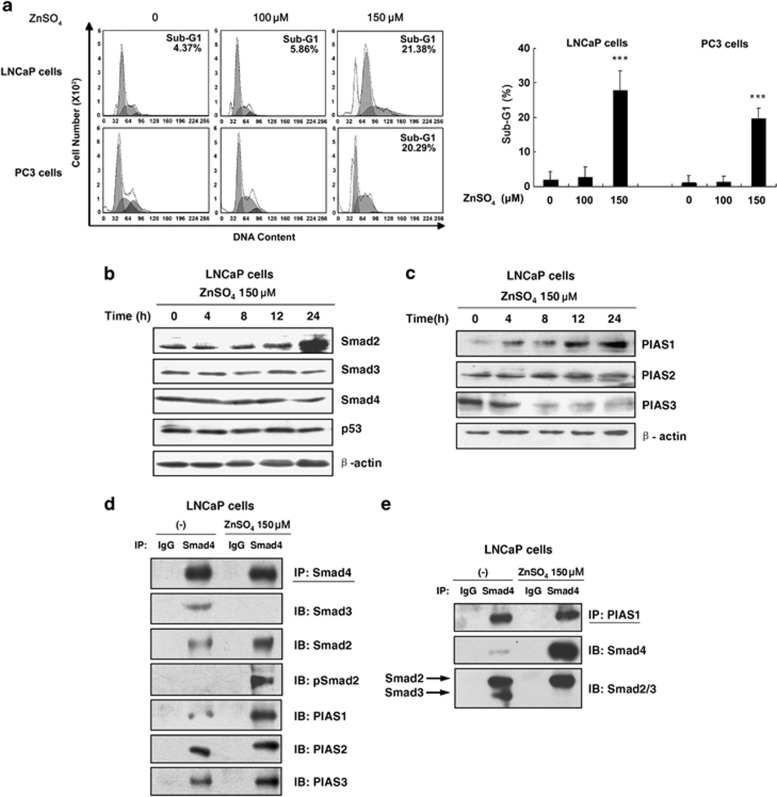
To examine the apoptotic effect of zinc on human prostate cancer cells, flow cytometric analyses were performed. Figure 1a demonstrates that with ZnSO4 (150 μM) treatment, an estimated 20% of cells progressed to sub-G1 cell fraction in both LNCaP and PC3 cells. To determine whether PIASs, as RING zinc-finger proteins and Smad-interacting proteins,21, 22, 23 are involved in the zinc-induced apoptosis, we next examined the expression of Smad and PIAS proteins in zinc (150 μM)-treated LNCaP cells. As shown in Figures 1b and c, there was a significant increase in the expression of Smad2 and PIAS1 in zinc (150 μM)-treated LNCaP cells in a time-dependent manner. We also examined the effect of zinc (150 μM) on Smad3, Smad4, PIAS2 and p53, and observed that zinc did not alter the expression of these proteins within this time frame (Figures 1b and c). These results indicated the possible role of Smad2 and PIAS1 in zinc-induced apoptosis.
Figure 1.
The induction of PIAS1 and Smad2 expression and the formation of PIAS1/Smad2/4 complex induced by zinc. (a) Zinc promoted cell apoptosis of either androgen-dependent LNCaP or androgen-independent PC3 prostate cancer cells. LNCaP and PC3 cells were treated with indicated concentrations of ZnSO4 for 24 h. Sub-G1 populations were detected using flow cytometry. Columns, mean (n=3); bars, S.D.; ***P<0.001 (one-way ANOVA). (b) Zinc selectively increased Smad2 and PIAS1 expression. LNCaP cells were treated with 150 μM ZnSO4 for indicated periods (0, 4, 8, 12 and 24 h). Immunoblot analyses were performed with antibodies of Smad3, Smad2, Smad4 or β-actin and PIAS1, PIAS2 and (c) PIAS3 or β-actin. (d and e) The immunoprecipitation experiments with anti-Smad4 or anti-PIAS1 were carried out in LNCaP cells with or without 150 μM zinc treatment and then analyzed by immunoblot analysis using the indicated antibodies against Smad4, Smad3, Smad2, phosphorylated Smad2, PIAS1, PIAS2, or PIAS3 (d), or against PIAS1, Smad4, or Smad2/3 (e)
Zinc regulates the Smad2/4 and PIAS1 complex formation
To assess the effect of zinc on the Smad protein complexes formation, co-immunoprecipitation analyses were employed. Figure 1d illustrates that with zinc (150 μM) treatment, interaction between Smad3 and Smad4 was significantly reduced in LNCaP cells. However, dramatically increased Smad2 or phosphorylated Smad2 and Smad4 interactions were observed in the presence of zinc. In addition, it appears that PIAS1, but not PIAS2 and PIAS3, strongly increased interaction with Smad4 by zinc treatment, although Smad4 interacted with all of them in the absence of zinc. These results suggested that zinc (150 μM) promoted Smad4, Smad2 and PIAS1 ternary complex formation, which is consistent with the increase of Smad2 and PIAS1 levels in response to zinc.
To confirm our observation, reverse co-immunoprecipitation analyses were performed using the specific PIAS1 antibody (Figure 1e). A dramatically increased interaction of Smad4 binding to PIAS1 was detected in the zinc-treated LNCaP cells. Meanwhile, in the absence of zinc, PIAS1 exhibited interactions with both Smad2 and Smad3. In contrast, in the presence of zinc, PIAS1 displayed the interaction only with Smad2, but not with Smad3. We repeated the above experiments in PC3 cells and similar results were observed (data not shown). The above data demonstrated that zinc regulates the Smad2/4 and PIAS1 involved complex formation.
Zinc enhances the recruitment of Smad2/4/PIAS complex on the p21 WAF1/Cip1 promoter
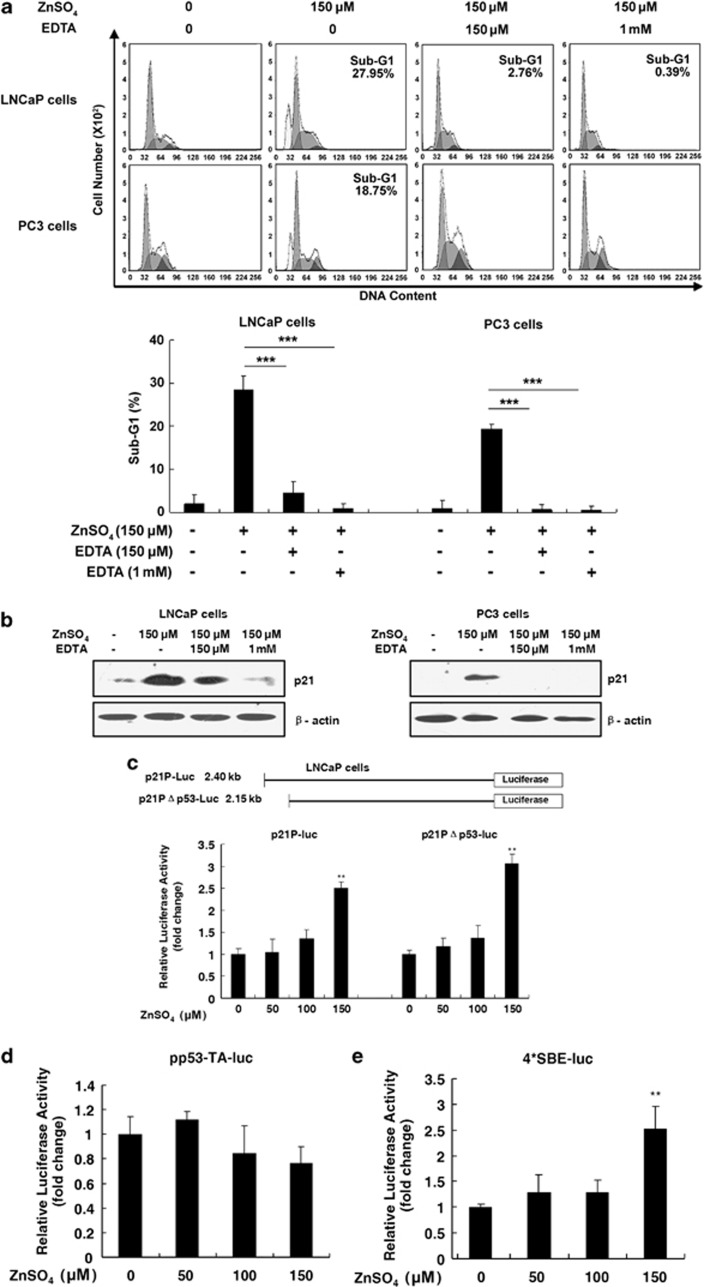
We further used a zinc ion chelating agent, EDTA (CaEDTA) (Figure 2a),33, 34 to validate the specificity for zinc-induced cell apoptosis. In both LNCaP and PC3 cell lines, the apoptotic sub-G1 cell fractions induced by exogenous zinc (150 μM) could be blocked by EDTA (either 150 μM or 1 mM), suggesting the reduction of zinc level is related to the loss of apoptotic ability in prostate cancer cells.
Figure 2.
The p21Waf1/Cip1 upregulation and activation corresponding to apoptotic process induced by zinc: (a) zinc-augmented p21WAF1/Cip1 expression. (a) Zinc ion chelating agents blocked zinc-induced apoptotic effect. LNCaP and PC3 cells were exposed to 150 μM ZnSO4 in the presence or absence of zinc inhibitor of 150 μM or 1 mM CaEDTA for 24 h. Sub-G1 populations were detected using flow cytometry. Columns, mean (n=3); bars, S.D.; ***P<0.001 (one-way ANOVA). (b) Zinc-augmented p21WAF1/Cip1 expression. LNCaP and PC3 cells were exposed to 150 μM ZnSO4 in the presence or absence of zinc inhibitor of 150 μM or 1 mM CaEDTA for 24 h. The p21WAF1/Cip1 levels were examined by immunoblot analysis with either p21WAF1/Cip1 or β-actin antibody. (c) Zinc increased p21WAF1/Cip1 transactivation in a Smad-dependent manner. LNCaP cells were cotransfected with 25 ng of Renilla luciferase reporter and 100 ng of luciferase reporter, p21P-luc or p21PΔp53-luc (c), pp53-luc (d) or 4*SBE-luc (e) as indicated, and then were exposed to various concentrations of ZnSO4 for 24 h. Relative luciferase activities were measured and calculated by the ratio of the firefly luciferase activity to Renilla luciferase. Columns, mean (n=5); bars, S.D.; **P<0.01 (one-way ANOVA)
Because p21WAF1/Cip1 is a cyclin-dependent kinase inhibitor and involved in cell growth arrest,11 we further observed the upregulation of p21WAF1/Cip1 levels in the zinc (150 μM) treated LNCaP and PC3 cells (Figure 2b). This enhancement of p21 levels corresponding to the apoptotic process was significantly blocked by the zinc ion chelating agents, EDTA, in a dose-dependent manner, demonstrating that cell growth arrest regulation was significantly dependent on cellular zinc ion levels.
Previous studies have shown that p21WAF1/Cip1 is a potent cell cycle inhibitor downstream of either p53 or Smad tumor suppressor proteins.9, 11, 12, 13, 14, 15 To determine the pathway involved in zinc-induced p21WAF1/Cip1 transactivation, two p21WAF1/Cip1 promoter-driven luciferase reporters were initially adopted for zinc treatment (Figure 2c). There were significant elevations of p21WAF1/Cip1 promoter-driven luciferase activities for both p21P-luc and p21PΔp53-luc reporters in the zinc-treated LNCaP cells in a dose-dependent manner, reaching maximal level, which is about threefold of control after 150 μM zinc concentration treatment, suggesting that the p21WAF1/Cip1 promoter was capable of being activated by zinc, even without p53 binding. To further confirm the involved pathways, a pp53-TA-luc reporter containing p53-binding sites and a 4*SBE-luc reporter, the most frequently used reporter for TGF-β-Smad signaling, were used for the next experiments. The activity of 4*SBE-luc was enhanced to 2.6-fold of control after 150 μM zinc concentration treatment (Figure 2d), whereas pp53-TA-luc was not enhanced by zinc (Figure 2e). These results revealed that zinc could activate p21WAF1/Cip1 transcription in a Smad-dependent manner.
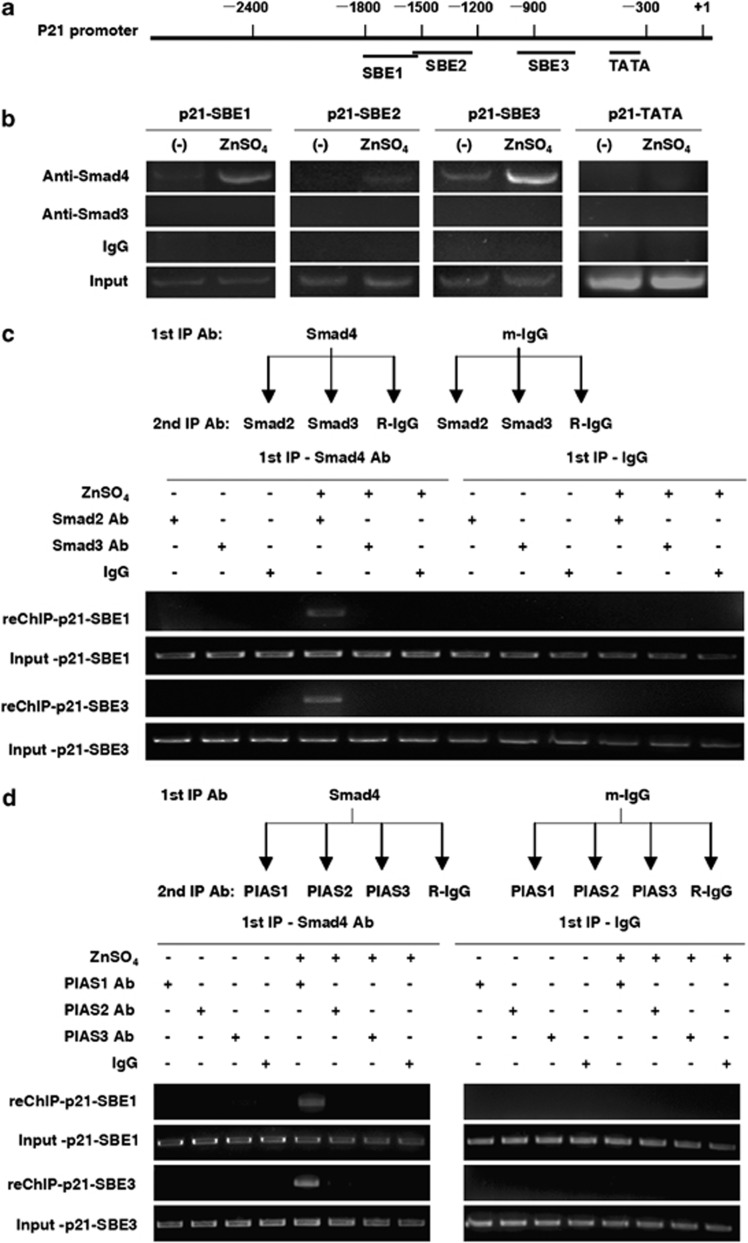
Smad proteins, which include certain R-Smads and Co-Smads, specifically recognize an 8-bp Smad-binding element (SBE) (GTCTAGAC) in downstream gene promoters to activate transcription.13 To ascertain the direct recruitment of the Smad complex on the p21WAF1/Cip1 promoter, chromatin immunoprecipitation (ChIP) assays were performed with Smad4 or Smad3 antibodies in LNCaP cells. Comparison was made among the SBE1 (−1800∼−1500), the SBE2 (−1500∼−1200), the SBE3 (−1000∼−750), and a TATA box fragment of p21WAF1/Cip1 promoter as a negative control (Figure 3a). ChIP results showed that Smad4 occupancy was apparently increased at SBE1/SBE3 in the presence of zinc, whereas no Smad3 recruitments to the p21WAF1/Cip1 promoter were found (Figure 3b). These data suggested the direct increased recruitment of Smad4 on the p21WAF1/Cip1 promoter in response to zinc.
Figure 3.
The recruitment of Smad2/4 and PIAS1 complex to the P21WAF1/Cip1 promoter induced by zinc. (a) Schematic diagram of three SBEs of the p21WAF1/Cip1 promoter. (b) ChIP assays of Smad4 or Smad3 occupancy on the p21WAF1/Cip1 promoter in LNCaP cells with or without zinc treatment. LNCaP cells were used with or without zinc treatment, and anti-Smad4, anti-Smad3 or IgG antibody was added to immunoprecipitate chromatin and the elution was analyzed by p21 promoter-specific primers. (c) Re-ChIP assays of Smad4/Smad2 complex occupancy on the p21WAF1/Cip1 promoter in cells with or without zinc treatment. (d) Re-ChIP assays of Smad4/PIAS1 complex occupancy on the p21WAF1/Cip1 promoter in cells with or without zinc treatment. LNCaP cells were used with or without zinc treatment and mouse anti-Smad4 or IgG antibody was added to immunoprecipitate chromatin. The elution was subjected to a second immunoprecipitation by anti-Smad2, anti-Smad3 or IgG antibody (c), or by anti-PIAS1, PIAS2, PIAS3 or IgG antibody (d). The final elution was analyzed by p21 promoter-specific primers
To probe the relationship between Smad4 and R-Smad (2/3), or between Smad4 and PIAS on the p21WAF1/Cip1 promoter, we performed two sets of re-ChIP assays as described in Materials and methods. As shown in Figure 3c, the presence of Smad2 on SBE1 and SBE3 sites within the p21WAF1/Cip1 promoter was detected in response to the addition of zinc (150 μM) in the immunoprecipitates. Using the PIAS1 antibody, we also detected the presence of Smad4 on SBE1 and SBE3 regions within the p21WAF1/Cip1 promoter (Figure 3d). These results provided a line of evidence demonstrating that zinc can induce the Smad4/2/PIAS1 transcriptional complex, which is responsible for Smad4 binding to SBE1 and SBE3 regions in the p21WAF1/Cip1 promoter.
Exogenous PIAS1 and Smad4 coordinately promote zinc-induced apoptosis and Smad4 nuclear translocation
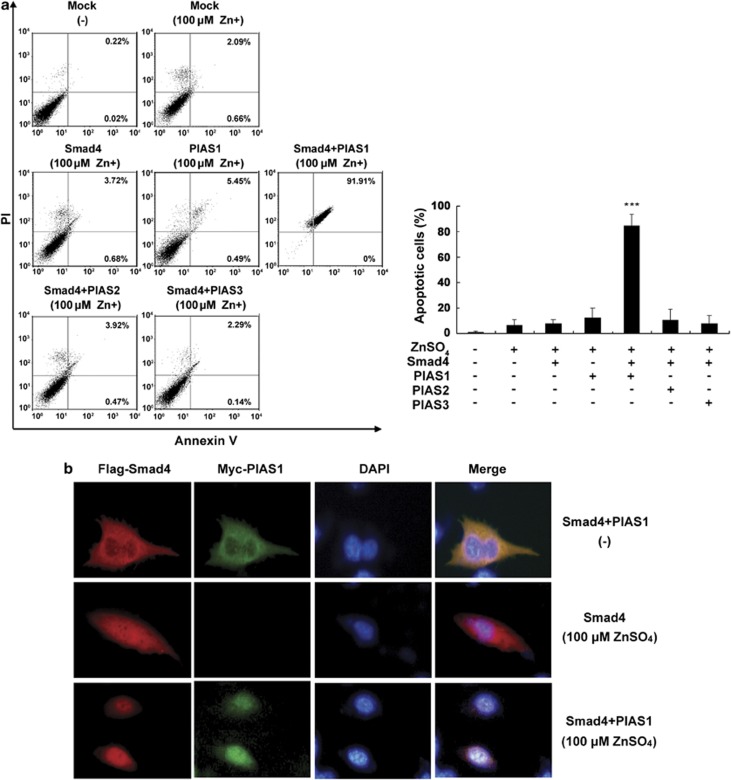
To gain insight into the biological significance of Smad4 and PIAS1 in zinc-induced apoptosis, we tested directly whether the exogenous Smad4 and PIAS1 could sensitize zinc-mediated apoptosis in prostate cancer cells. As 150 μM of zinc is capable of inducing apoptosis in prostate cancer cells via upregulation of Smad4 and PIAS1, we reasoned that exogenous addition of Smad4 and PIAS1 should enhance this effect, thereby sensitizing apoptosis induced by zinc. Therefore, LNCaP cells were cotransfected with plasmid, either Smad4 or PIAS1, by zinc (100 μM of zinc simulation that can only induce less than 5% apoptosis alone) or a combination thereof, and assayed for apoptosis through flow cytometric analysis. Interestingly, the apoptotic rate was dramatically increased to near 90% in the co-expression of Smad4 and PIAS1, but not PIAS2 or PIAS3, suggesting the synergistic effects of Smad4 and PIAS1 on zinc-induced apoptosis (Figure 4a). These results not only further confirmed that Smad4 and PIAS1 have a crucial role in zinc-induced apoptosis but also endorsed our above findings.
Figure 4.
The coordination of PIAS1 and Smad4 in zinc-induced LNCaP cell apoptosis and Smad4 nuclear translocation. (a) PIAS1-coordinated Smad4 promotes zinc-induced cell apoptosis. LNCaP cells transfected with indicated plasmids were treated with 100 μM zinc for 24 h. The apoptotic percentages of cells were measured by flow cytometry. Columns, mean (n=3); bars, S.D. ***P<0.001 versus zinc-treated control group (one-way ANOVA). (b) PIAS1 promotes Smad4 translocation into nucleus in zinc-treated cells. LNCaP cells transiently cotransfected with empty vector, Smad4 alone or with PIAS1 for 24 h were exposed to 100 μM zinc. Cells were fixed and stained with anti-Flag (red) and anti-Myc (green) antibodies
To further investigate the role of Smad4 and PIAS1 in regulating zinc-induced apoptosis, we examined zinc-stimulated cellular localization of Smad4 and PIAS1 proteins in LNCaP cells. Immunostaining analysis revealed that exogenously expressed Smad4 and PIAS1 proteins are distributed in the cytoplasm in the absence of zinc. In contrast, with exposure to zinc (even at low concentration, 100 μM), exogenous expression of Smad4 alone resulted in the partial translocation of Smad4 from the cytoplasm to nucleus, and cotransfected with both PIAS1 and Smad4 plasmids, the significant shift of Smad4 and PIAS1 from the cytoplasm to nucleus was observed, accompanied with the apoptotic condensed phenotype in DAPI staining (Figure 4b). These results suggest that PIAS1 enhances Smad4 nuclear localization in the presence of zinc.
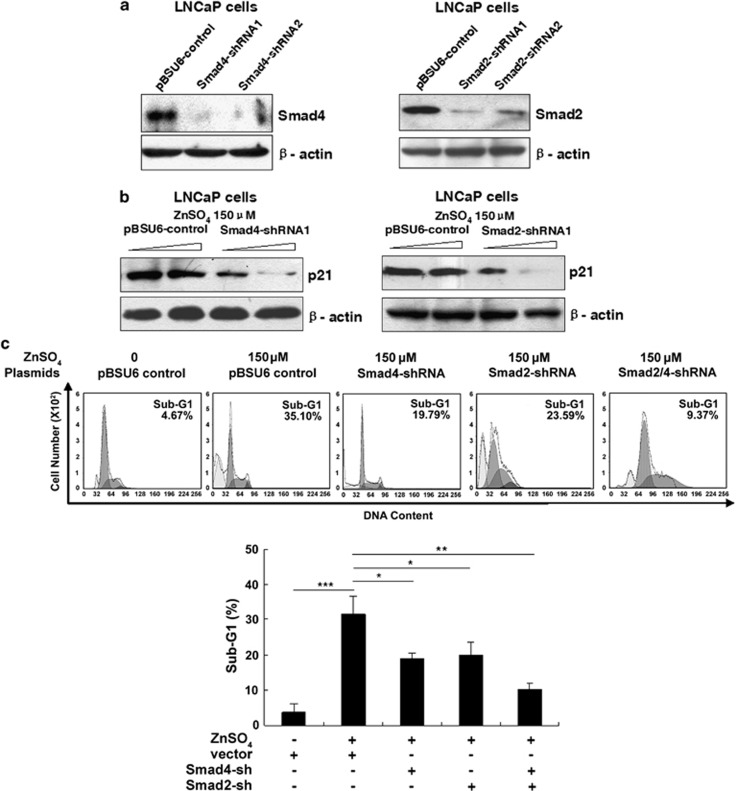
Smad4 and Smad2 are critical for zinc-induced prostate cancer cell apoptosis
Previous studies have demonstrated that both Smad3/4 and Smad2/4 cause high levels of transcriptional activation of the p21WAF1/Cip1 promoter, involved in cell apoptosis.35, 36 These findings prompted us to investigate the involvement of endogenous Smad4 and Smad2 in zinc-induced apoptosis using gene-silencing approaches. The short hairpin RNA (shRNA) constructs for Smad4 or Smad2 were generated and their knockdown effects were tested on ectopically expressed proteins in LNCaP cells. The Smad4-shRNA1 and Smad2-shRNA1, which have been shown to be more effective in Smad expression knockdown (Figure 5a), were selected to examine the attenuation effects on zinc-induced Smad4-mediated p21WAF1/Cip1 transactivation and apoptosis. As presented in Figures 5b and c, disruption of either endogenous Smad4 or Smad2 in zinc-stimulated LNCaP cells resulted in apparent reduction either in zinc-induced p21WAF1/Cip1 induction or in the zinc-mediated proportion of cells in the sub-G1 phase. In addition, the depletion of both Smad4 and Smad2 together causes the most dramatic decline in the sub-G1 phase, suggesting Smad2/4 silencing significantly reduces the cell apoptotic sensitivity to zinc. These data demonstrated that Smad4 and Smad2 have critical roles in zinc-induced cell apoptosis.
Figure 5.
The reduction of LNCaP cell apoptotic sensitivity to zinc with Smad4/Smad2 silencing. (a) LNCaP cells were transfected with two shRNAs targeting the Smad4 or Smad2 gene for 24 h for immunoblot analysis with the specific antibody against Smad4 or Smad2, using pBSU6 vector group as control. (b) LNCaP transfected with increased amounts of Smad4 or Smad2 plasmids for 24 h for immunoblot analysis with p21WAF1/Cip1 antibody. (c) The percentage of cells of sub-G1 region induced by zinc with Smad2 or Smad4 silence in flow cytometry analysis. Columns, mean (n=3); bars, S.D. *P<0.05. **P<0.01. ***P<0.001 (Student's t-test)
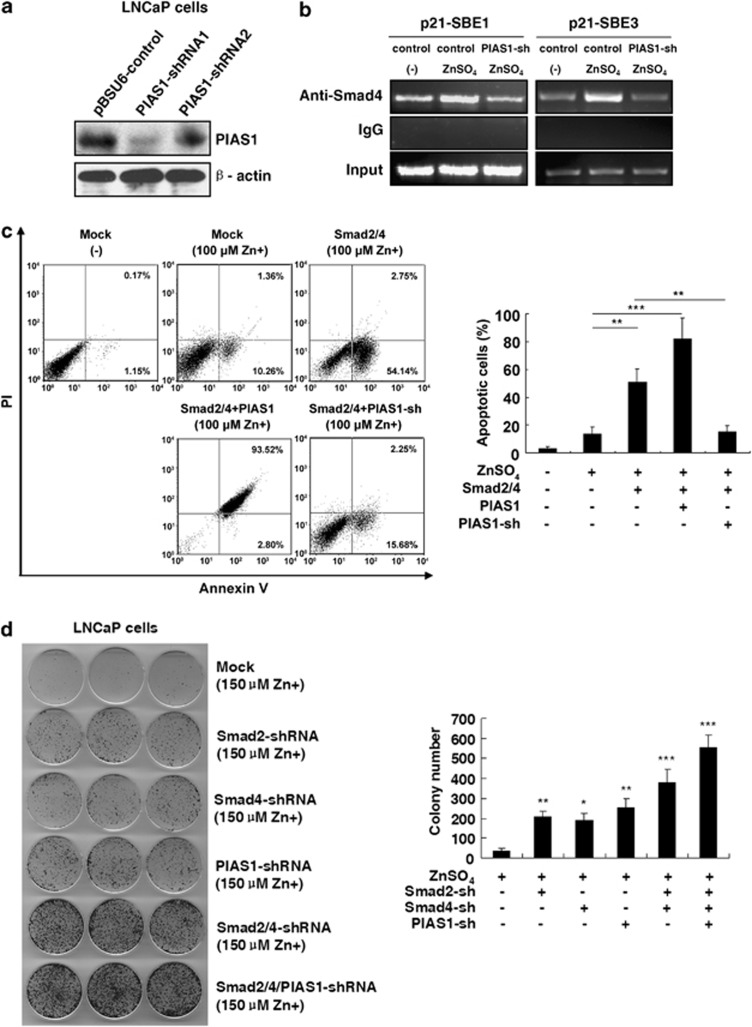
Endogenous PIAS1 is essential for zinc-induced Smad2/4-mediated apoptosis
To determine the role of endogenous PIAS1 in zinc-induced Smad activation and apoptosis, two PIAS1-shRNAs were generated and finally, shRNA1 (most effective) was selected (Figure 6a). Our data reveal that zinc-induced Smad4 recruitment on SBE1 and SBE3 regions of the p21WAF1/Cip1 promoter was significantly reversed by PIAS1-shRNA1 as compared with the control vector (Figure 6b). Moreover, Figure 6c shows that silencing PIAS1 potently inhibited exogenous Smad2/4-mediated zinc-induced apoptosis, indicating endogenous PIAS1 has essential roles in zinc-induced Smad activation and apoptosis.
Figure 6.
The essential roles of PIAS1 for Smad4-mediated p21WAF1/Cip1 promoter binding, cell apoptosis and inhibition of proliferation. (a) LNCaP cells were transfected with PIAS1-shRNAs for immunoblot analysis with PIAS1 antibody. (b) The increased binding of Smad4 to SBE1 and SBE3 regions in the p21WAF1/Cip1 promoter was reversible by PIAS1-shRNA. LNCaP cells transfected with PIAS1-shRNA1 or the control vector were treated with 150 μM ZnSO4 for 24 h. Cross-linked chromatin complexes were immunoprecipitated with anti-Smad4 or IgG antibody. Co-precipitated DNA sequences were amplified using specific primers spanning the SBE1 and SBE3 in the p21WAF1/Cip1 promoter. (c) LNCaP cells were transiently cotransfected with plasmids as indicated, and then treated with 125 μM ZnSO4 for 24 h. The apoptotic percentages of cells were measured by flow cytometry. Columns, mean (n=3); bars, S.D. **P<0.01. ***P<0.001 (Student's t-test). (d) LNCaP cells were transfected with Smad4-shRNA, Smad2-shRNA, or PIAS1-shRNA alone or together, and then cultured in the T medium containing 150 μM ZnSO4 for 12 days. Colonies containing more than 50 cells were counted. Columns, mean (n=3); bars, S.D. *P<0.05. **P<0.01. ***P<0.001. All versus mock-transfected zinc-treated control group (Student's t-test)
Silencing PIAS1 and Smad2/4 attenuates zinc-impeded clonogenic ability in LNCaP cells
To investigate whether zinc affects the clonogenicity either alone or combined with shRNAs of Smad2/4 and PIAS in LNCaP cells, we assessed zinc together with shRNAs and control LNCaP cells in culture using soft agar colony formation assay. As shown in Figure 6d, few colonies were observed in zinc (150 μM)-treated cells after 12 days while introduction of Smad4-shRNA, Smad2-shRNA or PIAS1-shRNA into LNCaP cells increased the colony formation. The number and size of colonies were further increased in cells, which were treated with both Smad2/4-shRNAs and in those which were treated with all the three shRNAs of Smad2/4 and PIAS, compared with the base line cells. These results reveal that with the silencing of PIAS1 and Smad2/4, cell proliferation capacity is upregulated, suggesting a promoting role of Smad2/4 and PIAS1 in zinc-mediated apoptosis.
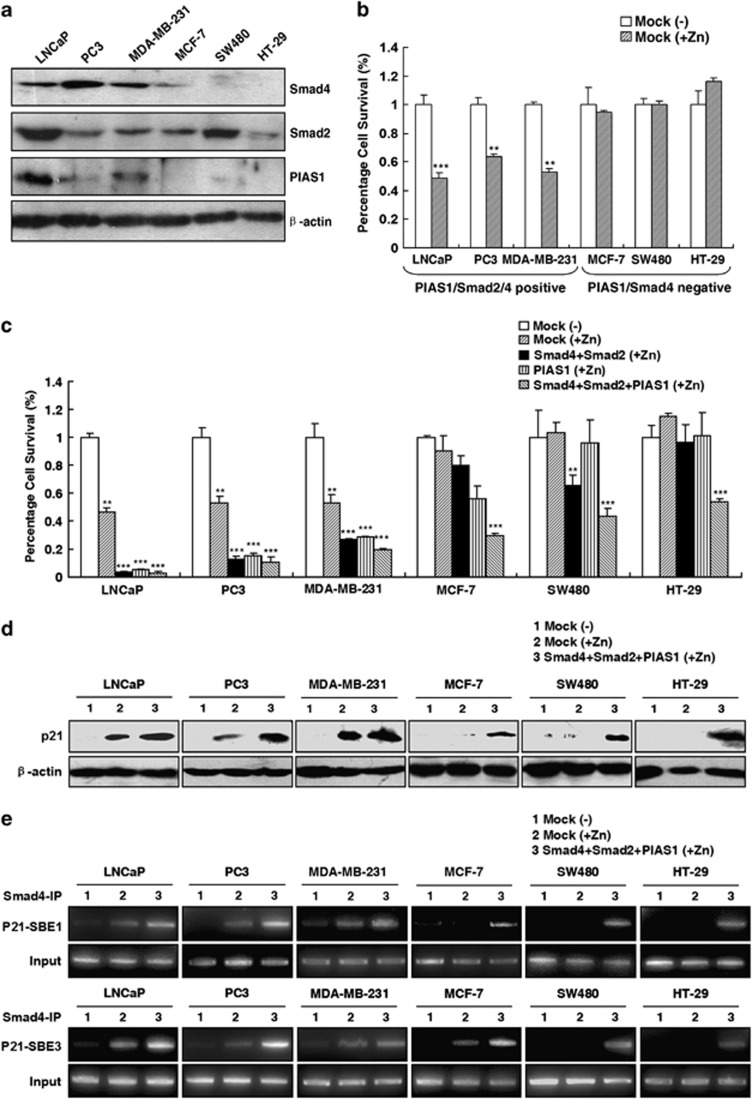
Correlation of apoptotic sensitivity to zinc and Smad4 and PIAS1 in multiple cancer cell lines
Exogenous zinc has been shown to promote apoptosis in various kinds of cancer cells.7, 37 To assess the involvement of the PIAS1/Smad2/4 complex in zinc-induced apoptosis as a common event for other cell types, the correlation between the expression level of PIAS1 or Smad2/4 and cell apoptotic sensitivity response to zinc in six tumor cell lines was examined. As shown in Figures 7a and b, three cells lines, including two prostate cancer (LNCaP and PC3) cells and one breast cancer MDA-MB-231 cell line, all had PIAS1, Smad2 and Smad4-positive expression, and were sensitive to zinc-induced cell growth inhibition. In contrast, the other three cells lines, including one breast cancer MCF-7 cell line, and two colon cancer (SW480 and HT-29) cell lines were deficient in Smad4 or PIAS1 expression, and were significantly insensitive to zinc-induced apoptosis. The three zinc-insensitive cell lines exhibited no p21WAF1/Cip1 expression response to zinc stimulation (line ‘2' in Figures 7d–f). However, the overexpression of Smad2, Smad4 and PIAS1 together remarkably increased zinc apoptotic sensitivities in various cancer cells (Figure 7c). The increase of zinc-induced p21WAF1/Cip1 expression (Figure 7d) and Smad4 complex recruited to the p21WAF1/Cip1 promoter (Figure 7e) attenuated by the overexpression of Smad2/Smad4/PIAS1 could be observed in all six cancer cell lines, especially for the three insensitive cell lines. These data demonstrated the important roles of PIAS1, Smad2, and Smad4 in zinc-induced cell death and p21WAF1/Cip1 transactivation, which were common biological events in different cancer cell lines.
Figure 7.
The universal association of the expression levels of Smad2, Smad4 and PIAS1 with the apoptotic sensitivities in various cancer cells. (a) Immunoblot analysis of Smad4, Smad2 and PIAS1 expression in different cancer cell lines including human prostate cancer cell lines LNCaP and PC3, breast cancer cell lines MDA-MB-231 and MCF-7, and colon cancer cell lines SW480 and HT-29. (b) Cell survival profile in response to 150 μM zinc treatment for 24 h in LNCaP, PC3, MDA-MB-231, MCF-7, SW480, and HT-29 cells. Columns, mean (n=3); bars, S.D. **P<0.01. ***P<0.001, versus mock-transfected group in the absence of zinc for each cell line (Student's t-test). (c) Zinc-induced cell survival inhibition attenuated by the overexpression of Smad2/Smad4 and PIAS1 alone or together. Percentage of surviving cells transfected with empty vector or Smad4/Smad2 and/or PIAS1 plasmids response to zinc treatment is normalized against the cells transfected with the empty vector without zinc treatment for each cell line. Columns, mean (n=3); bars, S.D. **P<0.01. ***P<0.001 (one-way ANOVA). (d) Attenuation of p21WAF1/Cip1 expression by the overexpression of Smad2/Smad4 and PIAS1. Percentage of surviving cells transfected with empty vector or Smad4/Smad2 and/or PIAS1 plasmids response to 150 μM ZnSO4 for 24 h. The p21WAF1/Cip1 levels were examined by immunoblot analysis with either p21WAF1/Cip1 or β-actin antibody. (e) Attenuation of zinc-induced Smad4 occupancy on the p21WAF1/Cip1 promoter by the overexpression of Smad2/Smad4 and PIAS1. ChIP assays of Smad4 occupancy on the p21WAF1/Cip1 promoter were performed with anti-Smad4 or IgG antibody and then analyzed by p21 promoter-specific primers
Discussion
In this study, we have provided compelling evidence that zinc induces apoptosis in prostate cancer with a marked increased in p21WAF1/Cip1 expression, in agreement with previous studies.8, 9, 10 Exposure to zinc resulted in the induction of PIAS1 and Smad2 expression to generate a PIAS1/Smad2/4 complex, which is further recruited to the p21WAF1/Cip1 promoter and transactivates the p21WAF1/Cip1 gene, leading to the apoptosis in cancer cells.
Unlike most other cells in which zinc is sequestered into vesicles and organelles, zinc in cytoplasm of prostate cells comprises ∼35% of the total intracellular zinc content.4 Zinc concentrations used in this study ranged from 100 to 150 μM, which were higher than plasma and serum concentrations (15–20 μM) and lower than zinc concentrations (4–7 mM) in prostate fluid.4, 7 In fact, the concentration of fetal bovine serum (FBS) in the culture medium is an important factor affecting the zinc concentration to induce apoptosis, which is demonstrated to range from 15 μM in the medium without FBS to 150 μM in the medium with 10% FBS supplements.38, 39, 40 Using the regular cell culture condition with 10% FBS, we observed that moderate zinc concentration (150 μM) induces significant apoptosis by promoting p21WAF1/Cip1 expression in both LNCaP and PC3 cells. Moreover, zinc-induced cell apoptosis or p21WAF1/Cip1 augment were blocked by the chelating agents, which provided the evidence for the link between the reduction of intracellular zinc concentration and the development of prostate cancer.
The p21WAF1/Cip1 gene has a key role in cell cycle arrest at the G1 stage by inhibiting CDKs, and was identified as an important transcriptional target of p53 and TGF-β-Smad4 pathways.9, 11, 12, 13, 14, 15 In this study, we showed that the Smad pathway but not the p53 pathway is involved in the zinc-induced apoptosis, in line with other reports.8, 9, 28, 29 Our results demonstrate that zinc induces apoptosis in LNCaP (p53-positive), PC3 (p53-null) cells, indicating that zinc-induced apoptosis is not associated with p53 status, consistent with other studies that zinc leads to IIC9 cell death in a p53-independent manner.41 Furthermore, in our comparative analysis of reporters, p21PΔp53-luc lacking p53-binding sequence, as with p21P-luc or Smad downstream 4*SBE-luc reporter, show significant inductions by zinc, but not the pp53-TA-luc reporter that is only activated by p53, providing evidence that zinc treatment induces p21WAF1/Cip1 transactivation in a Smad-dependent and p53-independent manner.
Smad2 and Smad3 are members of R-Smads, which are activated in response to TGF-β. These proteins associate with receptor kinases and are phosphorylated at an SSXS motif of C-terminus, and then typically bind to the common mediator Smad4. However, Smad2 and Smad3 exhibit different responses to different factors. For example, IGF-I selectively inhibits the TGF-β-triggered activation of Smad3 but not Smad2.42 Our findings reveal that zinc activates Smad2 expression but not Smad3. In particular, the critical suppressor roles of Smad2 have been sufficiently demonstrated in multiple cancers, including skin cancer development and malignant transformation of prostate cancer or breast cancer bone metastasis, whereas Smad3 has inactivating or opposite roles in these processes.19, 43, 44 A recent study demonstrates Smad2 as a critical mediator of TGF-β–induced apoptosis and gene expression by the results that Smad2 silencing alone causes malignant transformation of prostate cancer NRP-152 cells in athymic mice, whereas Smad3 silencing alone did not induce tumors.19 In this study, the important roles of the Smad4/pSmad2 complex in zinc-induced apoptosis is further proved. First, Smad2, but not Smad3, is dramatically increased in response to zinc, and is involved in the formation of a Smad4/Smad2/PIAS1 complex. Second, with zinc stimulation, PIAS1 exhibits the interaction only with Smad2, but not with Smad3 (Figures 1d and e). Moreover, the activated Smad2/4/PIAS1 complex is capable of translocating to the nucleus and being present at the SBE1 and SBE3 regions of the p21WAF1/Cip1 promoter to activate the p21WAF1/Cip1 gene (Figure 3c). Thus, our findings provided the possible mechanisms for the repair of TGF-β/Smad4 proliferation-inhibition signaling in cancer cells by zinc treatment to restore Smad2 expression and activation.
Smad4 has a central role in TGF-β signaling and is associated with the progression of many tumors. A significantly decreased nuclear Smad4 is always shown in many kinds of cancers, suggesting the inactivation of the Smad pathway.13, 20 Here, we demonstrate the critical roles of Smad4 in zinc-induced apoptosis. Although there are no significant changes for the expression levels in response to zinc treatment, Smad4 exhibits increased binding capacity with phosphorylated Smad2 and PIAS1, significant nuclear translocation, and functionally direct binding to SBE1 and SBE3 regions of the p21WAF1/Cip1 promoter. The knockdown of endogenous Smad4 in LNCaP cells resulted in apparent reduction of cell apoptotic sensitivity to zinc and the attenuation of zinc-induced p21WAF1/Cip1 transactivation and apoptosis in zinc-insensitive cell lines by the overexpression of Smad2/Smad4/PIAS1, suggesting the pivotal mediator roles of Smad4 in the zinc-activated Smad pathway.
In this study, we have identified a novel role of PIAS1 in zinc-induced apoptosis. The PIAS family has been proposed to interact with many transcription factors, acting as a transcriptional co-regulator.21, 22, 23, 24, 25, 26 The substantially reduced expression of PIAS1 is reported to be associated with colon cancer, gastric cancer, and hormone-refractory prostate cancers.27, 28, 29, 30 Previous reports suggested PIAS1 physically interacts with Smad4 and enhances the Smad4-dependent transcriptional activation of TGF-β signaling, whereas PIAS3 preferred to activate Smad3.21, 23 Here, we elucidated that PIAS1 is the only member of the PIAS family involved in zinc-induced Smad4 pathway activation. PIAS1 contains conserved SP-RING zinc-finger ring domains as with other PIAS proteins, but several unique sequences are distinguished from other PIAS numbers. Interestingly, zinc stimulation strongly enhanced the PIAS1 interaction with the Smad2/4 complex, with the disassociation of the original PIAS1/Smad3 complex, suggesting the different roles of PIAS1 in Smad3 and Smad2 regulation. In addition, PIAS1 obviously promotes zinc-induced Smad4 nuclear translocation and dramatically increases Smad4 recruitment on the p21WAF1/Cip1 promoter, to further promote Smad2/4 mediated proliferation inhibition. Moreover, PIAS1 contributes to zinc apoptotic sensitivity in all various cancer cells. All our observations supported that PIAS1, the expression of which is restored by zinc, has essential biological regulatory roles in the zinc-induced cell death.
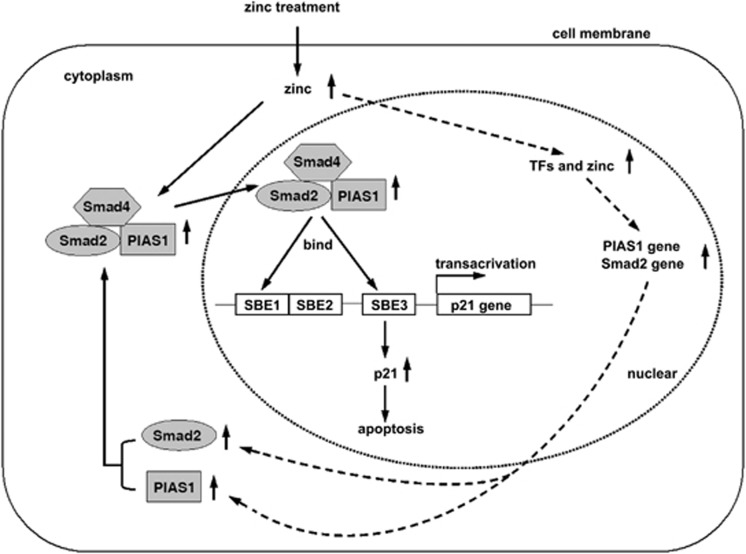
In conclusion, this study demonstrates for the first time that PIAS1, a member of PIAS protein family, augments the transcriptional activity of the Smad2/Smad4 protein complex not only in zinc-induced LNCaP cell apoptosis, but also in various cancer cells. As the deficiency or suppression of Smad2/3/4 is always exhibited in prostate cancer and other cancers, the activation of the Smad pathway is a critical strategy to restore the apoptotic mechanism for cancer therapy. Based on our findings, we provided an overview of possible mechanisms by which zinc induces apoptosis in LNCaP cells in Figure 8. Furthermore, our data provide a novel target for zinc by triggering the Smad2/4/PIAS1 complex to activate the p21WAF1/Cip1 gene, and to further promote apoptosis in cancers, and which may provide interesting avenues for novel therapeutic interventions.
Figure 8.
The model for zinc-induced apoptosis in cancer cells. Zinc increases the expression of Smad2 and PIAS1, and then promotes the formation of the Smad4/Smad2/PIAS1 complex and its subsequent nuclear translocation. In the nucleus, zinc recruits Smad4/Smad2/PIAS1 complex binding onto SBE1 and SBE3 regions of the p21WAF1/Cip1 promoter, to upregulate the transcription and expression of p21, which facilitates the cell apoptosis as a proapoptotic protein
Materials and Methods
DNA plasmids
pRK4-Flag-Smad4 was kindly provided by Dr. Hsiu-Ming Shih (Taipei, China).45 The pcDNA3-FLAG-Smad2 was constructed by subcloning the full-length Smad2 cDNAs into the pcDNA3 vector (Invitrogen, Carlsbad, CA, USA) in-frame with an N-terminal flag epitope tag. The pGADT7-PIAS1, pGADT7-PIASxβ were kindly provided by Dr. Edward E. Schmidt (Bozeman, USA) and PIAS3 by Ehud Razin (Jerusalem, Israel),46, 47 and PIAS family genes were subcloned into pCMV-myc vector (Clontech, Mountain View, CA, USA). The p21WAF1/Cip1 promoter reporter constructs, including one 2.4-kb major portion of the p21WAF1/Cip1 proximal promoter reporter of p21P-luc, and another 2.15 kb of p53-binding site deletion reporter of p21PΔp53-luc, were gifts from Xiao Fang Wang (Duke University, USA).48 The pp53-TA-luc containing just p53-binding sites in the p21WAF1/Cip1 proximal promoter was obtained from BD Clontech. The 4*SBE-Luc reporter containing a synthetic promoter composed of four repeated SBEs, was kindly provided by Dr. Yoichi Kato (Florida State University, USA).49 The small hairpin (sh) RNAs for PIAS1, Smad2 or Smad4 were generated as described previously.50
Cell culture, transient transfection and luciferase assays
The human prostate cancer cell line, LNCaP, was cultured in T medium (GIBCO, Grand Island, NY, USA; custom formula # 02–0056DJ, 80% DMEM, 20% F12K, 3 g/l NaHCO3, 5 μg/ml insulin, 13.6 pg/ml triiodothyronine, 5 μg/ml transferring, 0.25 μg/ml biotin, 25 μg/ml adenine) plus 10% FBS. The prostate cancer cell line, PC3, breast cancer cell lines, MCF-7 and MDA-MB-231 and colon cancer cell lines, SW480 and HT-29, were maintained in DMEM medium with 10% FBS. Transient transfections were carried out using Lipofectamine 2000 (Invitrogen) following the manufacturer's protocol. Cells were seeded into 48-well plates for 16 h and cotransfected with each of four reporter plasmids 100 ng, including two p21WAF1/Cip1 promoter reporters, p21P-luc and p21PΔp53-luc, Smad pathway reporter 4*SBE-luc, and P53 pathway reporter pp53-TA-luc, in the presence of Renilla luciferase control pREP7 vector 25 ng, and then treated with zinc sulfate ranging from 0 to 150 μM for 8 h. Firefly luciferase activities were measured by using the dual-luciferase reporter assay system (Promega, Madison, WI, USA) and the ratio of firefly luciferase activity to Renilla luciferase activity was calculated as relative luciferase activity.
Immunoprecipitation and immunoblot analysis
LNCaP cells were treated with zinc sulfate for 24 h and cross-linked with 1–2 mM dithio-bis succinimidyl propionate for 30 min. Pre-cleared lysates were then incubated with pre-equilibrated protein-A-Sepharose beads with either normal rabbit IgG or PIAS1 polyclonal antibody (Bioworld, Louis Park, MN, USA), or with either normal mouse IgG or Smad4 monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4 °C for 3 h. Eluted proteins were analyzed by immunoblots using Smad4, Smad2/3, PIAS1, PIAS2, PIAS3 (Bioworld), and phosphorylated Smad2 (pSmad2) (Santa Cruz) antibodies at the appropriate dilutions.
Immunofluorescence
LNCaP cells were transfected with Smad4 and/or PIAS1 plasmids and then treated with zinc sulfate for 24 h and then fixed with 3% formaldehyde for 30 min. After being blocked in PBS-BSA buffer for 15 min, the cells were incubated with monoclonal Smad4 and polyclonal PIAS1 antibodies for 2 h, followed by incubation with fluorophore-conjugated secondary antibodies (Proteintech Group, Chicago, IL, USA). DAPI staining for 1 min was carried out after secondary antibody incubation.
Sub-G1 analysis, apoptosis assay and flow cytometry analysis
Cells were transfected with the plasmids and then treated with zinc sulfate for 24 h. The propidium iodide (PI)-Hypotonic lysis buffer was added for a 30-min incubation, and then the cells were analyzed by flow cytometry for sub-G1 analysis as described previously.51 Apoptosis kits (Santa Cruz) containing Annexin-V-FITC and PI were used to determine cell apoptosis according to the manufacturer's instructions.
ChIP and Re-ChIP assays
LNCaP cells were treated with zinc sulfate for 24 h, and then treated with 2% formaldehyde to crosslink protein–DNA. After the cells were lysed by a nucleus lysis buffer, the lysates were sonicated. Sonicated lysates were centrifuged at 14 000 r.p.m. at 4 °C for 15 min to get rid of insoluble fractions. An aliquot of the soluble fraction was set aside and designated as input fraction. The remaining fraction was immunoprecipitated with anti-Smad4 antibody or mouse IgG (Santa Cruz). After immunoprecipitation, the precipitated complexes were collected by adding protein G-agarose beads. For re-ChIP, the immuno-complexes were eluted with re-ChIP elution buffer (10 mM DTT), and the supernatant was diluted 1 : 40 in ChIP dilution buffer. Antibodies against the second proteins of interest were added, incubated at 4 °C overnight, and collected by incubating with protein A beads at 4 °C for 3 h. In both ChIP and re-ChIP assays, the immuno-complexes were eluted from the beads through incubation with 10 × bead volume of elution buffer (1% SDS, 0.1M NaHCO3). Following elution and cross-linking reservation, DNA was recovered by proteinase K digestion, phenol/chloroform extraction and ethanol precipitation. Samples were analyzed by PCR. ChIP primer sequences of SBE1, SBE2, SBE3, and TATA regions in the p21WAF1/Cip1 promoter are described previously.15, 52
Colony formation assay
About 2000 LNCaP cells were transfected with shRNA-Smad4, shRNA-Smad2, and/or shRNA-PIAS1 for 24 h and then 150 μM zinc was supplemented into the medium. After 12 days culture, the cells were stained with 0.05% crystal violet to assess colony staining. Colonies containing more than 50 cells were counted.
Assessment of cell survival by WST-1 assay
Cell viability of LNCaP, PC3, MDA-MB-231, MCF-7, SW480 and HT-29 cells was determined using the WST-1 assay kit (Roche, Indianapolis, IN, USA) in accordance with the manufacturer's recommended protocol. In brief, after zinc treatment for 24 h, the medium was then replaced with fresh growth medium supplemented with 10% WST-1 reagent. This was followed by an additional incubation at 37 °C for 30–60 min. Changes in cell viability were reflected by changes in optical density detected at 450 nm, which was measured using a spectrophotometer microplate reader (SpectraMax 340; Molecular Devices, Sunnyvale, CA, USA).
Statistical analysis
All results are presented as the mean±S.D. Student's t-test was used to compare means of two independent groups. One-way ANOVA was applied to analyze the difference of means of more than two groups. Statistical significance was assumed for a two-tailed P<0.05 using SPSS17.0 (Chicago, IL, USA).
Acknowledgments
This study was supported by Ministry of Science and Technology (No. 2010DFA31430), Ministry of Education of China (NCET-10–0316; 10SSXT147), Jilin Provincial Science & Technology Department (20130521010JH), Changchun Science & Technology Department (No. 2011114-11GH29) and the Program for Introducing Talents to Universities (No. B07017). We thank Mr. Michael Hoyt and Professor Zijie Sun (Stanford University), who critically read and revised our manuscript.
Glossary
- PIAS
protein inhibitor of activated signal
- TGF-β
transforming growth factor-beta
- ZnSO4
zinc sulfate
- EDTA
ethylenediaminetetraacetic acid
- SBE
Smad-binding element
- ChIP
chromatin immunoprecipitation
- shRNA
short hairpin RNA
- FBS
fetal bovine serum
- SDS
sodium dodecyl sulfate
- IGF-I
insulin-like growth factor-I
- Bcl-2
B-cell lymphoma 2
- Bax
Bcl-2-associated X protein
- BRAC1
breast cancer gene 1
- CDKs
cyclin-dependent kinases
- AR
androgen receptor
- PI
propidium iodide
- DTT
dithiothreitol
- DAPI
4′, 6-diamidino-2-phenylindole
The authors declare no conflict of interest.
Footnotes
Edited by A Stephanou
References
- Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- Sim HG, Cheng CW. Changing demography of prostate cancer in Asia. Eur J Cancer. 2005;41:834–845. doi: 10.1016/j.ejca.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Franklin RB, Costello LC. Zinc as an anti-tumor agent in prostate cancer and in other cancers. Arch Biochem Biophys. 2007;463:211–217. doi: 10.1016/j.abb.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello LC, Franklin RB, Feng P, Tan M, Bagasra O. Zinc and prostate cancer: a critical scientific, medical, and public interest issue (United States) Cancer Causes Control. 2005;16:901–915. doi: 10.1007/s10552-005-2367-y. [DOI] [PubMed] [Google Scholar]
- Huang L, Kirschke CP, Zhang Y. Decreased intracellular zinc in human tumorigenic prostate epithelial cells: a possible role in prostate cancer progression. Cancer Cell Int. 2006;6:10. doi: 10.1186/1475-2867-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Kanak MA, Kajdacsy-Balla A, Pestaner JP, Bagasra O. Differential zinc accumulation and expression of human zinc transporter 1 (hZIP1) in prostate glands. Methods. 2010;52:316–321. doi: 10.1016/j.ymeth.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Kelleher SL, McCormick NH, Velasquez V, Lopez V. Zinc in specialized secretory tissues: roles in the pancreas, prostate, and mammary gland. Adv Nutr. 2011;2:101–111. doi: 10.3945/an.110.000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P, Li T, Guan Z, Franklin RB, Costello LC. The involvement of Bax in zinc-induced mitochondrial apoptogenesis in malignant prostate cells. Mol Cancer. 2008;7:25. doi: 10.1186/1476-4598-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P, Li TL, Guan ZX, Franklin RB, Costello LC. Direct effect of zinc on mitochondrial apoptogenesis in prostate cells. Prostate. 2002;52:311–318. doi: 10.1002/pros.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang JY, Liu YY, Zou J, Franklin RB, Costello LC, Feng P. Inhibitory effect of zinc on human prostatic carcinoma cell growth. Prostate. 1999;40:200–207. doi: 10.1002/(sici)1097-0045(19990801)40:3<200::aid-pros8>3.0.co;2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulaire J, Fotedar A, Fotedar R. The functions of the cdk-cyclin kinase inhibitor p21WAF1. Pathol Biol (Paris) 2000;48:190–202. [PubMed] [Google Scholar]
- Chi XZ, Yang JO, Lee KY, Ito K, Sakakura C, Li QL, et al. RUNX3 suppresses gastric epithelial cell growth by inducing p21(WAF1/Cip1) expression in cooperation with transforming growth factor {beta}-activated SMAD. Mol Cell Biol. 2005;25:8097–8107. doi: 10.1128/MCB.25.18.8097-8107.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- Pardali K, Kowanetz M, Heldin CH, Moustakas A. Smad pathway-specific transcriptional regulation of the cell cycle inhibitor p21(WAF1/Cip1) J Cell Physiol. 2005;204:260–272. doi: 10.1002/jcp.20304. [DOI] [PubMed] [Google Scholar]
- Saramaki A, Banwell CM, Campbell MJ, Carlberg C. Regulation of the human p21(waf1/cip1) gene promoter via multiple binding sites for p53 and the vitamin D3 receptor. Nucleic Acids Res. 2006;34:543–554. doi: 10.1093/nar/gkj460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Caestecker MP, Piek E, Roberts AB. Role of transforming growth factor-beta signaling in cancer. J Natl Cancer Inst. 2000;92:1388–1402. doi: 10.1093/jnci/92.17.1388. [DOI] [PubMed] [Google Scholar]
- Perttu MC, Martikainen PM, Huhtala HS, Blauer M, Tammela TL, Tuohimaa PJ, et al. Altered levels of Smad2 and Smad4 are associated with human prostate carcinogenesis. Prostate Cancer Prostatic Dis. 2006;9:185–189. doi: 10.1038/sj.pcan.4500871. [DOI] [PubMed] [Google Scholar]
- Tu WH, Thomas TZ, Masumori N, Bhowmick NA, Gorska AE, Shyr Y, et al. The loss of TGF-beta signaling promotes prostate cancer metastasis. Neoplasia. 2003;5:267–277. doi: 10.1016/S1476-5586(03)80058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Wahdan-Alaswad R, Danielpour D. Critical role of Smad2 in tumor suppression and transforming growth factor-beta-induced apoptosis of prostate epithelial cells. Cancer Res. 2009;69:2185–2190. doi: 10.1158/0008-5472.CAN-08-3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Yang X. Smad4-mediated TGF-beta signaling in tumorigenesis. Int J Biol Sci. 2010;6:1–8. doi: 10.7150/ijbs.6.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M, Melchior F, Feng XH, Lin X. Regulation of Smad4 sumoylation and transforming growth factor-beta signaling by protein inhibitor of activated STAT1. J Biol Chem. 2004;279:22857–22865. doi: 10.1074/jbc.M401554200. [DOI] [PubMed] [Google Scholar]
- Long J, Matsuura I, He D, Wang G, Shuai K, Liu F. Repression of Smad transcriptional activity by PIASy, an inhibitor of activated STAT. Proc Natl Acad Sci USA. 2003;100:9791–9796. doi: 10.1073/pnas.1733973100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J, Wang G, Matsuura I, He D, Liu F. Activation of Smad transcriptional activity by protein inhibitor of activated STAT3 (PIAS3) Proc Natl Acad Sci USA. 2004;101:99–104. doi: 10.1073/pnas.0307598100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M, Liu B, Tan J, French FS, Carey M, Shuai K. Distinct effects of PIAS proteins on androgen-mediated gene activation in prostate cancer cells. Oncogene. 2001;20:3880–3887. doi: 10.1038/sj.onc.1204489. [DOI] [PubMed] [Google Scholar]
- Megidish T, Xu JH, Xu CW. Activation of p53 by protein inhibitor of activated Stat1 (PIAS1) J Biol Chem. 2002;277:8255–8259. doi: 10.1074/jbc.C200001200. [DOI] [PubMed] [Google Scholar]
- Shuai K. Regulation of cytokine signaling pathways by PIAS proteins. Cell Res. 2006;16:196–202. doi: 10.1038/sj.cr.7310027. [DOI] [PubMed] [Google Scholar]
- Linja MJ, Porkka KP, Kang Z, Savinainen KJ, Janne OA, Tammela TL, et al. Expression of androgen receptor coregulators in prostate cancer. Clin Cancer Res. 2004;10:1032–1040. doi: 10.1158/1078-0432.ccr-0990-3. [DOI] [PubMed] [Google Scholar]
- Chen P, Zhao D, Sun Y, Huang L, Zhang S, Yuan Y. Protein inhibitor of activated STAT-1 is downregulated in gastric cancer tissue and involved in cell metastasis. Oncol Rep. 2012;28:2149–2155. doi: 10.3892/or.2012.2030. [DOI] [PubMed] [Google Scholar]
- Coppola D, Parikh V, Boulware D, Blanck G. Substantially reduced expression of PIAS1 is associated with colon cancer development. J Cancer Res Clin Oncol. 2009;135:1287–1291. doi: 10.1007/s00432-009-0570-z. [DOI] [PubMed] [Google Scholar]
- Wei J, Costa C, Ding Y, Zou Z, Yu L, Sanchez JJ, et al. mRNA expression of BRCA1, PIAS1, and PIAS4 and survival after second-line docetaxel in advanced gastric cancer. J Natl Cancer Inst. 2011;103:1552–1556. doi: 10.1093/jnci/djr326. [DOI] [PubMed] [Google Scholar]
- Feng P, Liang JY, Li TL, Guan ZX, Zou J, Franklin R, et al. Zinc induces mitochondria apoptogenesis in prostate cells. Mol Urol. 2000;4:31–36. [PubMed] [Google Scholar]
- Iguchi K, Hamatake M, Ishida R, Usami Y, Adachi T, Yamamoto H, et al. Induction of necrosis by zinc in prostate carcinoma cells and identification of proteins increased in association with this induction. Eur J Biochem. 1998;253:766–770. doi: 10.1046/j.1432-1327.1998.2530766.x. [DOI] [PubMed] [Google Scholar]
- Liang JY, Liu YY, Zou J, Franklin RB, Costello LC, Feng P. Inhibitory effect of zinc on human prostatic carcinoma cell growth. Prostate. 1999;40:200–207. doi: 10.1002/(sici)1097-0045(19990801)40:3<200::aid-pros8>3.0.co;2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeeva O, Nadler JV. Facilitation of granule cell epileptiform activity by mossy fiber-released zinc in the pilocarpine model of temporal lobe epilepsy. Brain Res. 2006;1078:227–234. doi: 10.1016/j.brainres.2006.01.051. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Kardassis D. Regulation of the human p21/WAF1/Cip1 promoter in hepatic cells by functional interactions between Sp1 and Smad family members. Proc Natl Acad Sci USA. 1998;95:6733–6738. doi: 10.1073/pnas.95.12.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang MR, Wang YX, Guo S, Han SY, Wang D. CSMD1 exhibits antitumor activity in A375 melanoma cells through activation of the Smad pathway. Apoptosis. 2012;17:927–937. doi: 10.1007/s10495-012-0727-0. [DOI] [PubMed] [Google Scholar]
- Franklin RB, Costello LC. The important role of the apoptotic effects of zinc in the development of cancers. J Cell Biochem. 2009;106:750–757. doi: 10.1002/jcb.22049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello LC, Franklin RB. Novel role of zinc in the regulation of prostate citrate metabolism and its implications in prostate cancer. Prostate. 1998;35:285–296. doi: 10.1002/(sici)1097-0045(19980601)35:4<285::aid-pros8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Yan M, Hardin K, Ho E. Differential response to zinc-induced apoptosis in benign prostate hyperplasia and prostate cancer cells. J Nutr Biochem. 2010;21:687–694. doi: 10.1016/j.jnutbio.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaichick V, Sviridova TV, Zaichick SV. Zinc in the human prostate gland: normal, hyperplastic and cancerous. Int Urol Nephrol. 1997;29:565–574. doi: 10.1007/BF02552202. [DOI] [PubMed] [Google Scholar]
- Klein C, Creach K, Irintcheva V, Hughes KJ, Blackwell PL, Corbett JA, et al. Zinc induces ERK-dependent cell death through a specific Ras isoform. Apoptosis. 2006;11:1933–1944. doi: 10.1007/s10495-006-0089-6. [DOI] [PubMed] [Google Scholar]
- Song K, Cornelius SC, Reiss M, Danielpour D. Insulin-like growth factor-I inhibits transcriptional responses of transforming growth factor-beta by phosphatidylinositol 3-kinase/Akt-dependent suppression of the activation of Smad3 but not Smad2. J Biol Chem. 2003;278:38342–38351. doi: 10.1074/jbc.M304583200. [DOI] [PubMed] [Google Scholar]
- Petersen M, Pardali E, van der Horst G, Cheung H, van den Hoogen C, van der Pluijm G, et al. Smad2 and Smad3 have opposing roles in breast cancer bone metastasis by differentially affecting tumor angiogenesis. Oncogene. 2010;29:1351–1361. doi: 10.1038/onc.2009.426. [DOI] [PubMed] [Google Scholar]
- Tannehill-Gregg SH, Kusewitt DF, Rosol TJ, Weinstein M. The roles of Smad2 and Smad3 in the development of chemically induced skin tumors in mice. Vet Pathol. 2004;41:278–282. doi: 10.1354/vp.41-3-278. [DOI] [PubMed] [Google Scholar]
- Chang CC, Lin DY, Fang HI, Chen RH, Shih HM. Daxx mediates the small ubiquitin-like modifier-dependent transcriptional repression of Smad4. J Biol Chem. 2005;280:10164–10173. doi: 10.1074/jbc.M409161200. [DOI] [PubMed] [Google Scholar]
- Levy C, Sonnenblick A, Razin E. Role played by microphthalmia transcription factor phosphorylation and its Zip domain in its transcriptional inhibition by PIAS3. Mol Cell Biol. 2003;23:9073–9080. doi: 10.1128/MCB.23.24.9073-9080.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge JR, Schmidt EE. Interaction of protein inhibitor of activated STAT (PIAS) proteins with the TATA-binding protein, TBP. J Biol Chem. 2006;281:12260–12269. doi: 10.1074/jbc.M510835200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datto MB, Yu Y, Wang XF. Functional analysis of the transforming growth factor beta responsive elements in the WAF1/Cip1/p21 promoter. J Biol Chem. 1995;270:28623–28628. doi: 10.1074/jbc.270.48.28623. [DOI] [PubMed] [Google Scholar]
- Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier JM. Direct binding of Smad3 and Smad4 to critical TGF beta-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 1998;17:3091–3100. doi: 10.1093/emboj/17.11.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui G, Soohoo C, Affar el B, Gay F, Shi Y, Forrester WC. A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc Natl Acad Sci USA. 2002;99:5515–5520. doi: 10.1073/pnas.082117599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasul A, Ding C, Li X, Khan M, Yi F, Ali M, et al. Dracorhodin perchlorate inhibits PI3K/Akt and NF-kappaB activation, up-regulates the expression of p53, and enhances apoptosis. Apoptosis. 2012;17:1104–1119. doi: 10.1007/s10495-012-0742-1. [DOI] [PubMed] [Google Scholar]
- Mattia M, Gottifredi V, McKinney K, Prives C. p53-Dependent p21 mRNA elongation is impaired when DNA replication is stalled. Mol Cell Biol. 2007;27:1309–1320. doi: 10.1128/MCB.01520-06. [DOI] [PMC free article] [PubMed] [Google Scholar]