Abstract
Developing effective therapies against multiple myeloma (MM) is an unresolved challenge. Phosphatidylinositol-3-kinase (PI3K) activation may be associated with tumor progression and drug resistance, and inhibiting PI3K can induce apoptosis in MM cells. Thus, targeting of PI3K is predicted to increase the susceptibility of MM to anticancer therapy. The lead compound of a novel class of PI3K inhibitors, BAY80-6946 (IC50=0.5 nM against PI3K-α), was highly efficacious in four different MM cell lines, where it induced significant antitumoral effects in a dose-dependent manner. The compound inhibited cell cycle progression and increased apoptosis (P<0.001 compared with controls). Moreover, it abrogated the stimulation conferred by insulin-like growth-factor-1, a mechanism relevant for MM progression. These cellular effects were paralleled by decreased Akt phosphorylation, the main downstream target of PI3K. Likewise, profound antitumoral activity was observed ex vivo, as BAY80-6946 significantly inhibited proliferation of freshly isolated myeloma cells from three patients (P<0.001 compared with vehicle). In addition, BAY80-6946 showed convincing in vivo activity against the human AMO-1 and MOLP-8 myeloma cell lines in a preclinical murine xenograft model, where treatment with 6 mg/kg every other day for 2 weeks reduced the cell numbers by 87.0% and 69.3%, respectively (P<0.001 compared with vehicle), without overt toxicity in treated animals.
Keywords: myeloma, chemoresistance, PI3 kinase
Introduction
Overcoming chemoresistance is arguably the most important challenge in today's cancer therapy. Multiple myeloma (MM), a common hematological malignancy arising from clonal expansion of malignant plasma cells, vividly highlights this notion.1 Despite numerous therapeutic approaches, including high-dose chemotherapy, stem cell transplantation and several innovative compounds or combinations of the above, MM still remains an incurable condition.2, 3, 4, 5 Drug resistance usually leads to the fatal outcome.6
Myeloma cells can exhibit several constitutive or acquired mechanisms of drug resistance, some of which even may be facilitated by chemotherapeutic treatment, such as multidrug resistance gene or P-glycoprotein upregulation.7 In addition, myeloma cell binding to fibronectin can lead to cell adhesion-mediated drug resistance, and various cytokines including insulin-like growth factor type 1 (IGF-1), interleukin-6, vascular endothelial growth factor and tumor necrosis factorα can trigger signaling pathways, supporting proliferation and reducing apoptosis of myeloma cells.8, 9, 10, 11, 12, 13 IGF-1 is of particular importance, as it activates PI3K (phosphatidyl inositol 3-kinase)/Akt signaling, a prototypic survival pathway involved in proliferation, anti-apoptosis, cell invasiveness, angiogenesis and drug efflux in tumors.14
The PI3K family comprises lipid kinases, whose activation generates PI phosphates, mainly PI3, 4P2 (PIP2) and PI3, 4,5P3 (PIP3).15 PIP3functions as a second messenger, which activates multiple downstream targets. The main downstream target of class IA PI3 kinases is Akt, which is recruited to the inner plasma membrane where it becomes phosphorylated on amino acid residues Thr308 and Ser473.16 Akt then leads to activation of downstream substrates involved in cell cycle regulation and apoptosis prevention.16 Akt is directly responsible for the phosphorylation of several components of the cell-death machinery. The latter includes the pro-apoptotic proteins Bad, caspase 9 and FKHR, a member of the Forkhead family of transcription factors, which leads to inhibition of Bim and Fas ligands.17, 18 In addition, Akt is indirectly involved in the regulation of two central modulators of cell death, namely nuclear factor-kappa B (NF-κB) and p53, and it causes cell cycle progression by inhibition of glycogen synthase kinase-3, which inhibits expression of cyclin D1.16
The PI3K/Akt pathway is constitutively active in many malignancies including MM, breast cancer, ovarian cancer, melanoma, acute myelogenous leukemia and gastric carcinomas.19 Akt activation in tumors may be caused by loss of tumor suppressor phosphatase and tensin homolog deleted on chromosome ten (PTEN) function, amplification or mutation of PI3K or growth factor receptors.20, 21, 22 All of them are associated with poor prognosis.23 Blocking of PKC, a treatment that results in Akt inhibition, abrogates tumor growth in some xenograft mouse models, and PKC is a promising therapeutic target in acute myelogenous leukemia and MM.24
Given the pivotal role of the PI3K/Akt pathway in tumor biology and promising results of Akt inhibition in experimental tumor therapy,25 we have investigated the novel highly selective PI3K inhibitor BAY 80-6946 in four different human myeloma cell lines, as well as in freshly isolated myeloma cells from three patients. We demonstrated a profound impact of this compound on Akt phosphorylation, apoptosis induction and reduction of tumor growth in vitro and, using two representative myeloma lines, in a preclinical xenograft model in vivo.
Materials and methods
Cell culture and reagents
The human MM cell lines AMO-1,26 KMS-12-BM,27 MOLP-828 and U-26629 were cultured at 37 °C in a 5% CO2-humidified atmosphere in RPMI 1640 medium supplemented with 10–20% fetal calf serum (FCS), 100 U/ml penicillin and 100 μg/ml streptomycin (PAA Laboratories, Pasching, Austria). All cell cultures were routinely checked for the absence of mycoplasma infection. A 100 μM stock solution of BAY 80-6946 was prepared in vehicle solution (5 mM trifluoroacetic acid in dimethyl sulfoxide). Aliquots were stored at −20 °C. For stimulation with recombinant IGF-1 (ImmunoTools, Friesoythe, Germany), the cells were cultured at 37 °C in RPMI 1640 serum-free culture medium with or without BAY 80-6946 for 16 h, followed by incubation with 100 ng/ml of IGF-1 for 20 min. Vehicle-treated cells served as controls.
Isolation of primary human myeloma cells
The experiments with human cells were approved by the local ethics committee of the University Medical Center Göttingen (protocol 13/3/13). All patients gave their informed consent.
Heparinized blood samples in 50 ml tubes were subjected to density gradient centrifugation without brake for 20 min at a centrifugal force of 600 g using 10 ml Ficoll as the separating phase. The buffy coat containing peripheral blood mononuclear cells was transferred into a fresh tube and washed with PBS. Myeloma cells were isolated by magnetic cell sorting using CD138 MicroBeads (MACS, Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. Samples with a high amount of CD138+ cells were used for proliferation assays.
Preparation of cell extracts and western blot analysis
Cultured human myeloma cells were washed twice with PBS (PAA Laboratories) and solubilized on ice in lysis buffer (100 μl, 1 M Tris, pH 7.0; 200 μl, 10% SDS; 100 μl β-mercaptoethanol; 1314.3 μl dH20, supplemented with 285.7 μl of Complete Protease Inhibitor (Roche Diagnostics, Indianapolis, IN, USA). The protein concentrations in the lysates were determined by Bradford assays (Bio-Rad, Munich, Germany). Equal amounts of protein were separated by SDS-PAGE and transferred onto nitrocellulose membranes. The membranes were incubated with primary antibodies overnight followed by horseradish peroxidase-labeled mouse anti-rabbit immunoglobulin G (IgG) and a chemiluminescence reagent (ECL solution in an equal mix of solution 1 (9 ml dH2O, 1 ml 1 M Tris-HCl, pH 8.5, 45 μl coumaric acid and 100 μl Luminol) and solution 2 (9 ml dH2O, 1 ml 1 M Tris-HCl, pH 8.5, and 6 μl H2O2, 30%)). Phospho-Akt was detected using the Western Breeze chemiluminescent immunodetection system as described in the manual (Invitrogen, Carlsbad, CA, USA). Anti-human actin, clone C4 (Millipore, Temecula, CA, USA), was used at a 1:10 000 dilution, anti-human Akt (Cell Signaling Technology, Danvers, MA, USA) was used at a 1:2000 dilution and anti-human phospho-Akt (Ser473; Cell Signaling Technology) was used at a 1:500 dilution. Anti-PI3 kinases p110α, p110β and p110γ (Cell Signaling Technology) was used at a 1:1000 dilution and anti-PI3 kinase p110δ (Santa Cruz Biotechnology, Heidelberg, Germany) was used at a 1:100 dilution. Secondary anti-mouse IgG horseradish peroxidase conjugate or anti-rabbit IgG horseradish peroxidase conjugate (Promega Corporation, Madison, WI, USA) was used at a 1:2500 dilution.
Flow cytometry
Intracellular staining of phospho-Akt was performed after permeabilization of cells fixed by paraformaldehyde (4%) for 10 min at 37 °C and then stored on ice for 1 min. Ice-cold methanol was slowly added to a final concentration of 90% while gently vortexing the cell suspension. Cells then were incubated on ice for 30 min. Subsequently, 0.5–1.0 × 106 fixed and permeabilized cells were aliquoted in assay tubes, washed twice in incubation buffer (0.5% bovine serum albumin in PBS), blocked in 10% human serum (PAA Laboratories) for 10 min and incubated with Alexa fluor 647-labeled anti-human phospho-Akt antibody (Cell Signaling Technology) at a 1:50 dilution for 1 h. Rabbit IgG isotype (Cell Signaling Technology) served as negative control. Cells were washed in incubation buffer, resuspended in 0.5 ml of PBS and analyzed by flow cytometry using a fluorescence activated cell sorter Canto II (Becton Dickinson, Heidelberg, Germany).
Apoptosis detection (DNA fragmentation assay)
To measure apoptotic responses, we used the Cell Death Detection enzyme-linked immunosorbent assay (ELISA) Plus apoptosis assay according to the manufacturer's instructions (Roche Diagnostics, Mannheim, Germany). Cultured cells were incubated with BAY 80-6946 at the conditions indicated in the text, lysed and centrifuged at 400 g for 10 min. Equal amounts of clear supernatant were added to 96-well microtiter plates coated with streptavidin. Anti-DNA-POD and anti-histone solution of the Kit were added. The plate was incubated for 2 h on a platform rotator at room temperature. Generation of histone-bound DNA fragments resulted in a green color and was quantitated using a plate reader (Thermo Scientific Appliscan, Waltham, MA, USA) at 405 nm. Apoptotic rates of treated cells were calculated as percentage of vehicle controls.
Cell viability assay (MTT)
Triplicates of 25 000 cells per well were seeded into 96-well plates in a total volume per well of 100 μl RPMI containing 10–20% FCS. After 24 h of treatment with BAY 80-6946, the number of viable cells was measured using the Cell Titer 96 Aqueous One Solution Cell Proliferation Assay (Promega, Mannheim, Germany) according to the manual. The assay was quantitated using a plate reader (Thermo Scientific) at 570 nm. A reference wavelength of 630 nm was used to subtract background signals. Viability of treated cells was calculated as percentage of vehicle controls.
Proliferation assay (5-bromo-2'-deoxyuridine incorporation)
To determine the proliferation of myeloma cells, 25 000 cells per well were seeded into 96-well tissue culture plates and treated for 24 h at different conditions indicated in the text. The thymidine analog, 5-bromo-2'-deoxyuridine (BrdU) was added 4 h before the termination of the experiment. The proliferation rate was measured colorimetrically using the Cell Proliferation ELISA (Roche Diagnostics) and a plate reader at 450 nm. Proliferation of myeloma cells was calculated as percentage of vehicle controls.
Cell cycle analysis
Cell cycle analysis was performed by flow cytometry. After 4, 12 and 24 h of culture with BAY 80-6946 or vehicle, the cells were fixed in 70% ice-cold ethanol, washed twice, resuspended in 500 μl cold PBS containing 50 μg/ml propidium iodide and 0.1 mg/ml RNase A (Roth, Karlsruhe, Germany). Cells were incubated at room temperature for 30 min. Thereafter, the cells were analyzed using a fluorescence activated cell sorter Canto II (Becton Dickinson).
Myeloma xenograft mouse model
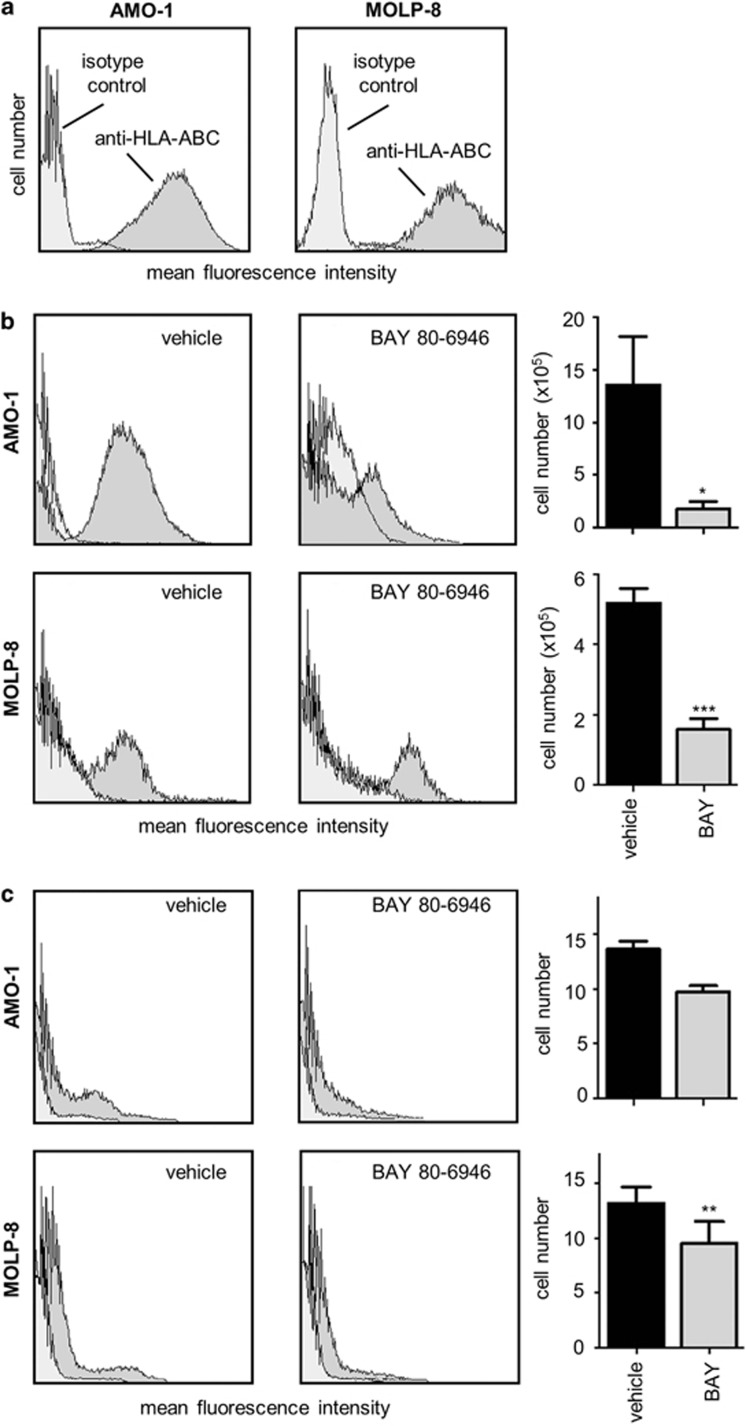
Mouse experiments were approved by the appropriate authorities (Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit, protocol 33.9.42502-04/099/09). Nude mice (NU/J Foxn1nu) were kept in a climate-controlled specific pathogen-free facility of the University Medical Center Göttingen. To evaluate experiments, mice were anaesthetized by isoflurane inhalation and killed by cervical dislocation according to the international guidelines. Age and sex-matched mice were injected into the peritoneum with 5 × 106 human myeloma cells of the cell lines MOLP-8 (n=5 vehicle control mice and n=5 BAY 80-6946-treated mice) or AMO-1 (n=4 vehicle control mice and n=4 BAY 80-6946-treated mice), respectively. Treatments were started 1 day after myeloma cell injection. Treatment regimens were as follows: control mice received i.p. vehicle injections (positive controls), whereas BAY 80-6946-treated mice received i.p. injections of BAY 80-6946 at a dose of 6 mg per kilogram body weight each Monday, Wednesday and Friday for 2 weeks (altogether six injections per mouse). Untreated mice served as negative controls (no myeloma cell injection or treatment). Mice were killed after 2 weeks. Immediately after death, a thorough peritoneal lavage with PBS was performed, the mixed cell suspension obtained by this procedure was stored on ice and the total cell number (containing human myeloma and murine cells) was determined. Human myeloma cells were then identified by fluorescence activated cell sorter analysis. Cells were washed twice, and one sample was stained with anti-human CD138 (Becton Dickinson; 20 μl per test) and another sample was stained with anti-human HLA (human leukocyte antigen)-ABC (Becton Dickinson; 10 μl per test). Isotype-matched control IgG (Becton Dickinson) served as negative control. After the peritoneal lavage, mice were dissected and a piece of each of the following organs was fixed in paraffin, whereas another piece was frozen in liquid nitrogen: spleen, liver, lung, femur, abdominal lymph node and peritoneum. To identify the potential presence of spread tumor cells, spleen cells and bone-marrow cells isolated by rinse cytology were stained with anti-human CD138 and anti-human HLA-ABC and analyzed by flow cytometry. The experiment was performed twice with similar results.
Statistical analysis
Statistical analyses were performed using the GraphPad Prism V5.00 software (GraphPad Software, San Diego, CA, USA). P-values of fluorescence activated cell sorter analyses, proliferation and apoptosis experiments were evaluated using one-way analysis of variance followed by Bonferroni post hoc test. P-values of in vivo experiments were analyzed using the two-tailed t-test. P-values of <0.05 (confidence interval of 95%) were considered statistically significant. Significance levels are given as * when P<0.05, ** when P<0.01 and *** when P<0.001, respectively. All data are represented as mean±s.d.
Results
Differential expression of PI3K isoforms and varying levels of constitutive or IGF-1-induced activation of Akt in human myeloma cells
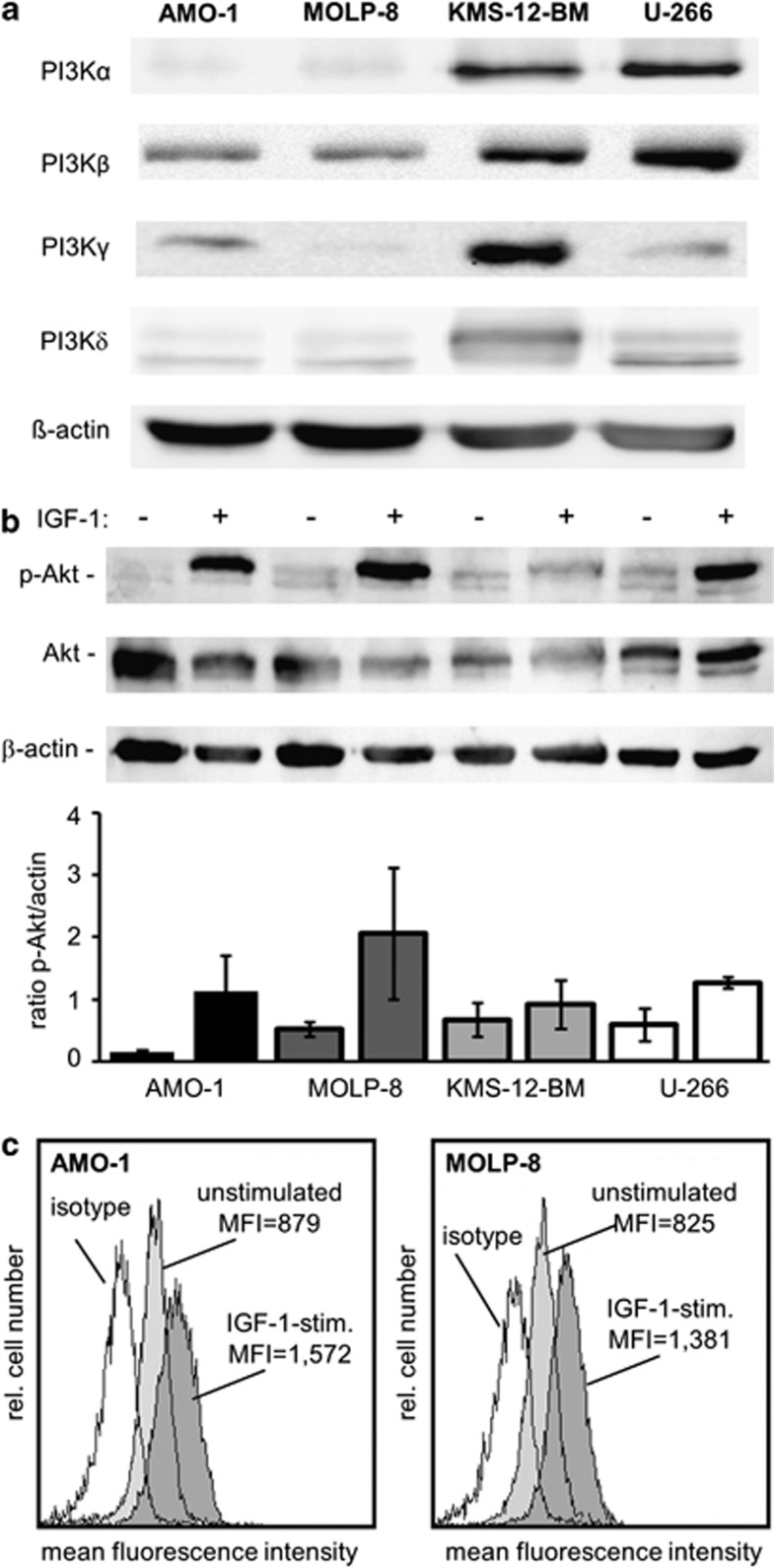
Activation of the PI3K/Akt pathway has been implicated in progression and drug resistance of human MM.25 Indeed, Akt is constitutively active in many types of cancer including MM, but not in nonmalignant hematopoietic cells from the same patients.30, 31, 32, 33 However, the levels of constitutive Akt activation may vary widely. Examination of PI3K expression levels by western blot analysis revealed marked constitutive expression of the PI3K isoforms-α and -β in KMS-12-BM and U-266 myeloma cells compared with the levels detected in AMO-1 and MOLP-8 cells (Figure 1a). Expression of PI3K-γ was low in MOLP-8 cells, whereas AMO-1 and U-266 cells showed intermediate levels of PI3K-γ, and the strongest expression was seen in KMS-12-BM cells, and PI3K-δ showed intermediate expression levels in all four myeloma lines (Figure 1a). Based upon western Blot analyses to examine phosphorylated Akt (indicating activation) in four different human myeloma cell lines, the lowest constitutive activation was found in AMO-1, whereas MOLP-8, U-266 and KMS-12-BM cells showed similar constitutive Akt activity (Figure 1b).
Figure 1.
Different expression levels of PI3K isoforms and constitutive or IGF-1-stimulated activation of Akt in human myeloma cells. (a) Expression of PI3K isoforms-α, -β, -γ and -δ was examined by western blot analysis in whole-cell lysates of four untreated myeloma cell lines, AMO-1, MOLP-8, KMS-12-BM and U-266. (b) To examine activation of Akt, cellular extracts of native and IGF-1-stimulated cells (100 nM for 20 min) were prepared and western Blot analysis was performed. In the bottom panel, the p-Akt/Akt ratio from two independent experiments was determined densitometrically (± s.e.m). (c) Activation (phosphorylation) of Akt was investigated in two myeloma cell lines either under non-stimulated conditions (light gray histograms) or after stimulation with IGF-1 at a concentration of 100 ng/ml for 20 min. Cells were fixed, permeabilized and stained with an anti-human phospho-Akt antibody and analyzed by flow cytometry.
Given that IGF-1 may activate Akt and is a critical growth factor in MM especially in the context of drug resistance,13, 14 we also analyzed IGF-1-stimulated levels of Akt activation in myeloma cells. Stimulation by IGF-1 at a concentration of 100 ng/ml increased the level of phosphorylated Akt in all cell lines. Of note, stimulation of AMO-1 or MOLP-8 cells led to a sharp increase, whereas KMS-12-BM cells only showed a weak response to IGF-1 stimulation (Figure 1b). Flow cytometry analysis was performed as a second independent method and confirmed the results obtained by western blot (Figure 1c). Based on these results, the two cell lines AMO-1 and MOLP-8 showing moderate constitutive and the highest difference between constitutive and induced Akt activation were selected for further functional studies.
BAY 80-6946 is a potent and selective PI3K inhibitor that blocks Akt activation in human myeloma cells
To identify small-molecule compounds that interfered specifically with PI3K and the activation of Akt in human tumor cells, compounds were tested in a large-scale high throughput screening for their ability to inhibit PI3K isoforms.34 BAY 80-6946 (7-methoxy-8-(3-morpholin-4-ylpropoxy)-2,3-dihydroimidazo [1,2-c]quinazolin-5-amine), a small-molecule compound of Mr 359.42 (Figure 2) showed the desired activity: it specifically inhibited several recombinant human PI3K isoforms, with the strongest activity toward PI3K-α (IC50=0.5 nM), followed by PI3K-δ (IC50=0.7 nM), PI3K-β (IC50=3.7 nM) and PI3K-γ (IC50=6.4 nM; Figure 2). The selectivity of the compound was confirmed using a panel of 219 additional cell-free enzyme assays including various other kinases, phosphatases, phospholipases and proteases. There was no inhibitory activity toward any of these enzymes up to a concentration of 1000 nM (Supplementary Table 1), including other kinases of the PI3K/Akt pathway such as PDK-1 and the three Akt isoforms themselves. The only exception was a moderate activity against mTOR (mammalian target of rapamycin), which was lower than that against the PI3K isoforms by one order of magnitude (IC50=45 nM; Figure 2). These results clearly indicated that BAY 80-6946 is a highly selective inhibitor of PI3K.
Figure 2.
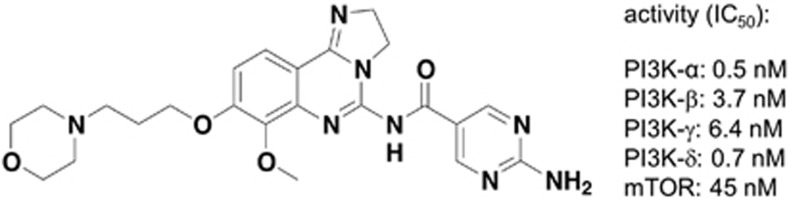
The novel PI3K inhibitor, BAY 80-6946, inhibits all four PI3K isoforms. Chemical structure of BAY 80-6946 (7-methoxy-8-(3-morpholin-4-ylpropoxy)-2,3-dihydroimidazo [1,2-c]quinazolin-5-amine). The right hand side of the panel depicts IC50 values regarding inhibition of PI3K-α, PI3K-β, PI3K-γ, PI3K-δ and mTOR, respectively, as determined by cell-free assays.
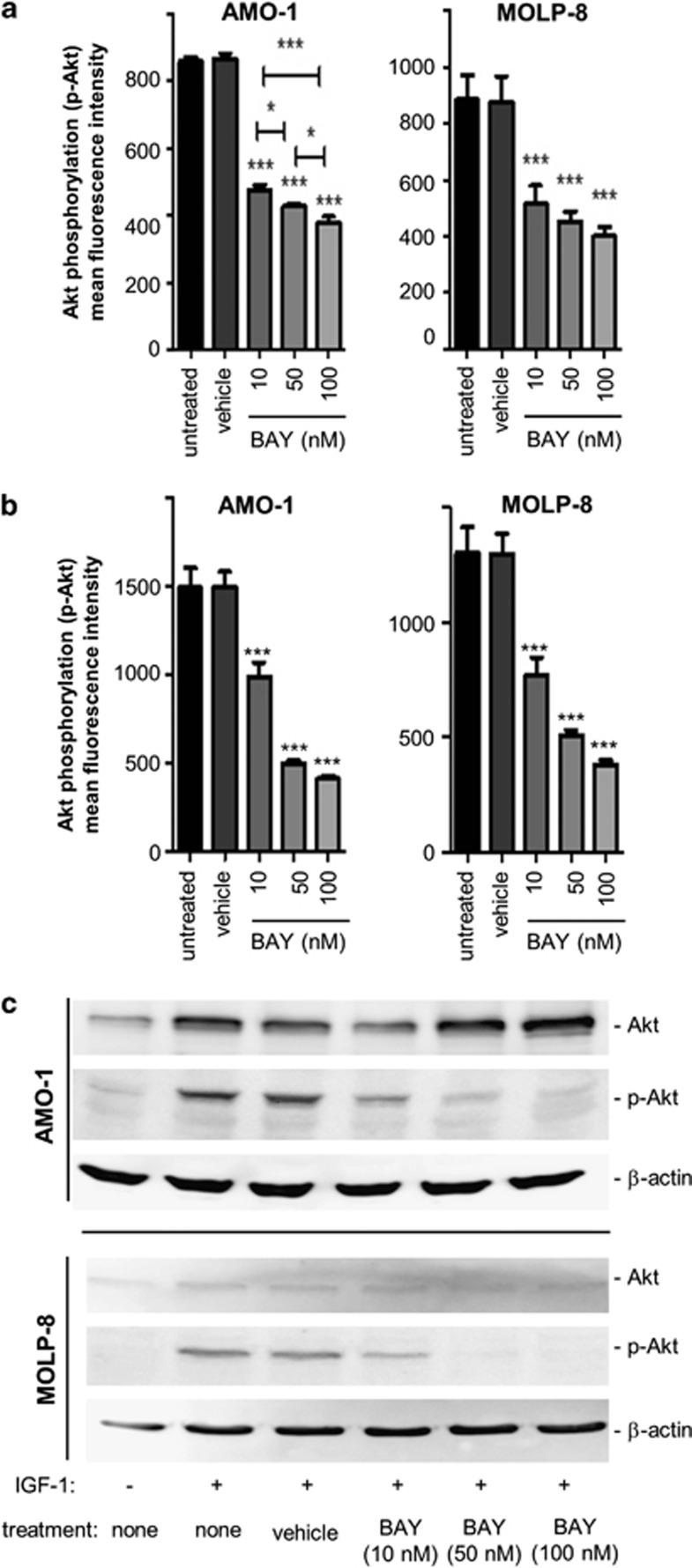
When the impact of different concentrations of BAY 80-6946 (10 nM, 50 nM and 100 nM) against constitutively phosphorylated Akt was assessed on the protein level by flow cytometry, a significant dose-dependent inhibition of Akt activation by up to 50% could be observed in all cell lines examined (cell lines AMO-1 and MOLP-8 depicted in Figure 3a, and KMS-12-BM and U-266 depicted in Supplementary Figure S1a). Indeed, in both AMO-1 and MOLP-8 cells, highly significant inhibition of constitutive Akt phosphorylation (P<0.001 compared with vehicle-treated controls) was observed at concentrations of as low as 10 nM (Figure 3a). In U-266 and KMS-12-BM cells, inhibition of Akt phosphorylation appeared to be somewhat weaker (Supplementary Figure S1a).
Figure 3.
BAY 80-6946 diminishes constitutive and IGF-1-induced phosphorylation of Akt in human myeloma cells. (a) Inhibition of constitutive Akt activation by BAY 80-6946 was examined in the AMO-1 (left) and MOLP-8 (right) human myeloma cell lines. Cells were treated, fixed, permeabilized and stained with an anti-human phosphor-Akt antibody and analyzed by flow cytometry. The values shown represent the averages of three independent experiments (±s.d.). *indicates P<0.05 and ***indicates P<0.001 as compared with vehicle-treated controls. (b) Inhibition of Akt activation by BAY 80-6946 was examined in two IGF-1-stimulated myeloma lines, AMO-1 and MOLP-8. Cells were treated with BAY 80-6946 at the indicated concentrations and then stimulated with 100 ng/ml of IGF-1 for 20 min. Expression of phospho-Akt (p-Akt) was determined by flow cytometry. The values shown represent the average mean fluorescence intensity of three independent experiments (±s.d.). *** indicates P<0.001 as compared with vehicle-treated controls. (c) Inhibition of IGF-1-induced Akt phosphorylation in myeloma cell lines exposed to the indicated concentrations of BAY 80-6946 was confirmed by western blot analysis. The experiment shown is representative of two independent experiments showing similar results.
Given that IGF-1 led to a marked increase of phosphorylated Akt, two myeloma cell lines (AMO-1 and MOLP-8) were exposed to BAY 80-6946 for 16 h and then stimulated by IGF-1 at 100 ng/ml for 20 min. As detected by flow cytometry analysis, the IGF-1-induced increase of Akt phosphorylation was completely abrogated by BAY 80-6946 and was comparable to levels seen in non-stimulated cells (Figure 3b). Western blot analysis used as a second method confirmed the dramatic downregulation of IGF-1-stimulated p-Akt by BAY 80-6046, even at concentrations of as low as 10 nM (Figure 3c). Thus, BAY 80-6946 is a potent PI3K inhibitor in human myeloma cells, which can even overcome stimulatory effects of IGF-1.
Induction of apoptosis, inhibition of proliferation and cell cycle regulation in human myeloma cells through PI3K inhibition by BAY 80-6946
Akt is directly responsible for the phosphorylation of several components of the cell-death machinery such as Bad,35 caspase 917 or FKHR,18 and it is involved indirectly in the regulation of two central modulators of cell fate, NF-κB and p53.17, 36 Therefore, it was reasonable to assume that inhibition of Akt activation by BAY 80-6946 would increase apoptosis and reduce proliferation of myeloma cells.
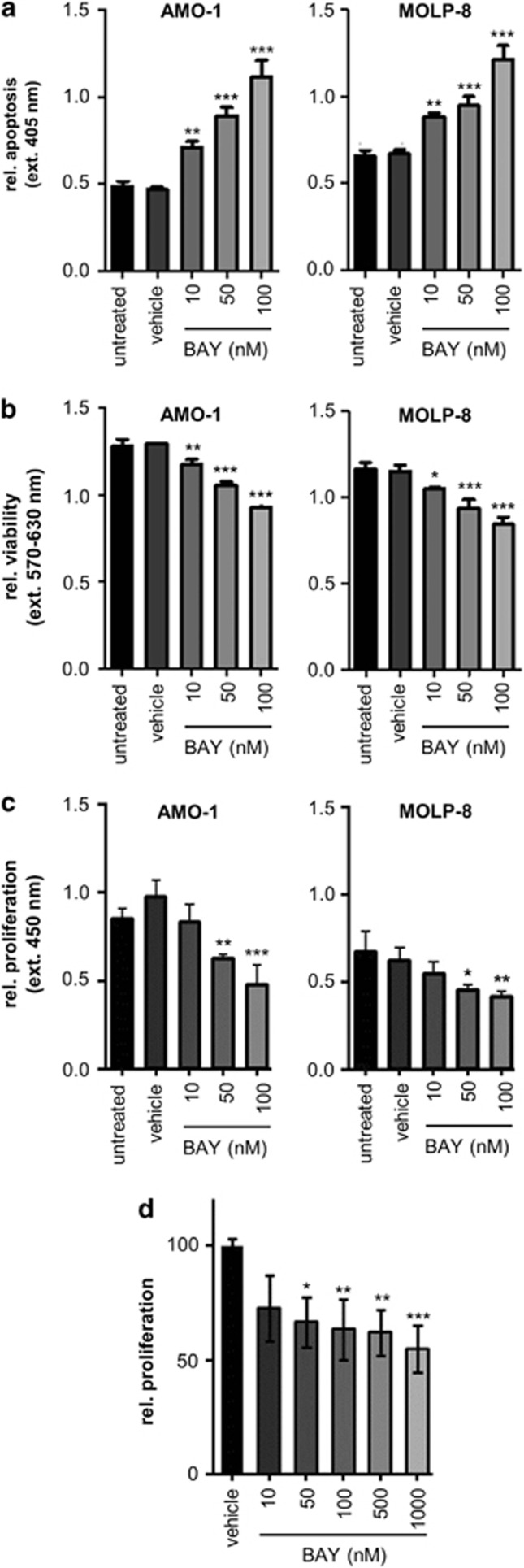
When the generation of histone-bound DNA fragments was used as a parameter to measure apoptotic responses of human myeloma cells to the treatment with BAY 80-6946, a highly significant and dose-dependent increase of apoptosis was observed in all four myeloma lines (Figure 4a and Supplementary Figure S1b). Indeed concentrations as low as 10 nM achieved significant apoptotic responses in all cell lines (P<0.001 in AMO-1 and MOLP-8, and P<0.01 in KMS-12-BM and U-266). At a concentration of 100 nM, BAY 80-6946 doubled the rate of myeloma cell apoptosis (P<0.001 in all cases compared with vehicle-treated cells; Figure 4a and Supplementary Figure S1b).
Figure 4.
Induction of apoptosis and decreased viability and proliferation of human myeloma cells by BAY 80-6946 in vitro and ex vivo. (a) The pro-apoptotic activity of BAY 80-6946 was investigated using the Cell Death Detection ELISA Plus measuring the generation of histone-bound DNA fragments. Cells were treated with BAY 80-6946 for 16 h, lysed and incubated with anti-DNA-POD, and anti-histone solution for 2 h. Apoptotic responses were quantitated using a microplate reader at 405 nm. The values shown represent averages (±s.d.) of triplicate measurements from a representative experiment, which was repeated twice with similar results. (b) Viability of human myeloma cells was assessed using an MTT assay. Cells were treated with BAY 80-6946 for 24 h and the number of viable cells was measured using the Cell Titer 96 Aqueous One Solution Cell Proliferation Assay. The values shown represent the averages (±s.d.) of triplicate measurements from a representative experiment, which was repeated twice with similar results. (c) The impact of BAY80-6946 on myeloma cell proliferation was examined by DNA incorporation of the pyrimidine analog BrdU. Myeloma cells were cultured in FCS-free RPMI medium in the presence of BAY80-6946 for 24 h. After 20 h, the cells were stimulated with 100 ng/ml IGF-1 for 20 min. Subsequently, BrdU was added for 4 h and DNA incorporation was determined colorimetrically using the Cell Proliferation ELISA. The values shown represent the average of triplicate measurements (±s.d.) from a representative experiment. The experiment was repeated twice with similar results. (d) Primary human myeloma cells were isolated from blood samples of three patients with high tumor burden. Myeloma cells were ex vivo cultured in RPMI with 0.5% FCS and exposed to BAY80-6946 for 24 h at the indicated concentrations and with 100 ng/ml IGF-1 for 16 h. The values shown represent the average proliferation as determined by BrdU incorporation of myeloma cells from three individual donors (±s.e.m). In all panels, * indicates P<0.05, ** indicates P<0.01, and *** indicates P<0.001, as compared with vehicle-treated controls.
In addition to its pivotal role in the regulation of apoptosis, PI3K is involved in cell growth regulation. When myeloma cell viability was assessed in response to BAY 80-6946, a moderate, yet consistent and significant reduction was observed in all four myeloma lines in a dose-dependent manner. The viability in the cell lines AMO-1 and MOLP-8 was decreased by up to 30% at a concentration of 100 nM (P<0.001 compared with vehicle-treated cells; Figure 4b), and this decrease was weakest in the U-266 line (P<0.01 only at 100 nM compared with vehicle-treated cells; Supplementary Figure S1c).
The incorporation of BrdU as an indicator of proliferation was reduced likewise in BAY80-6946-treated myeloma cells. Notably, BAY 80-6946 inhibited the proliferation in IGF-1-stimulated myeloma cells in a dose-dependent manner. The strongest decrease was seen in AMO-1 cells, in which proliferation was reduced by 35% at a concentration of 50 nM and by 50% at a concentration of 100 nM (P<0.01 compared with vehicle control; Figure 4c).
To test whether BAY 80-6946 would exert its inhibitory activity against ‘natural' tumor cells as well, we isolated myeloma cells from three patients with a high tumor burden. Indeed, treatment of these freshly isolated CD138+ myeloma cells with BAY 80-6946 resulted in significant inhibition of proliferation in a dose-dependent manner (Figure 4d). Significant inhibition of proliferation was detected at concentrations of as low as 50 nM, and proliferation was reduced by ∼50% when BAY 80-6946 was used at a concentration of 1 μM (P<0.001 compared with vehicle-treated cells; Figure 4d).
The marked apoptosis induction and the growth inhibition by Bay 80-6946 were paralleled by a conspicuous modulation of the cell cycle in myeloma cells. The rationale for these investigations was that the PI3K/Akt pathway profoundly influences cell cycle regulation inasmuch as activation of PI3K phosphorylates and thus inhibits the glycogen synthase kinase-3 protein, which negatively regulates the expression of cyclin D1.40 Increased cyclin D1 expression facilitates the transition from G0/G1 to S phase.48 Thus, it was conceivable that higher cell numbers would remain in the G0/G1 phase after treatment with BAY 80-6946.
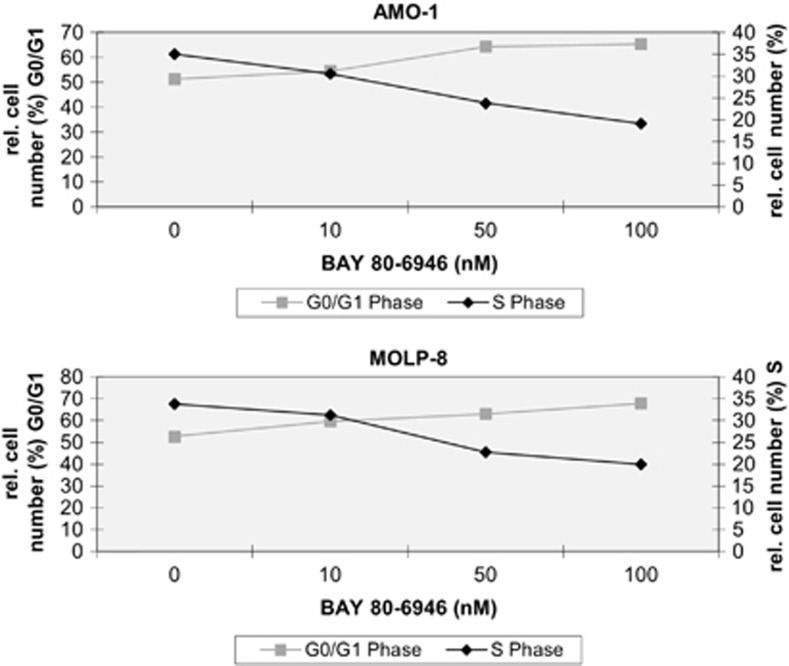
To test the latter hypothesis, the cell cycle phases of two myeloma cell lines, AMO-1 and MOLP-8, were analyzed by flow cytometry. Indeed, a dose-dependent increase of cells in the GO/G1 phase and, conversely, a decrease of cells in the S phase were detected after exposure of the myeloma cells to BAY 80-6946 (Figure 5). After 24 h of treatment with BAY 80-6946 (100 nM), the cell number in the GO/G1 phase increased from 51 to 65% in AMO-1 cultures and from 53 to 68% in MOLP-8 cultures, respectively. Reciprocally, cells in the S phase decreased from 35 to 19% in AMO-1 cultures and from 34 to 20% in MOLP-8 cultures, respectively (Figure 5). Lower concentrations of BAY 80-6946 (10 or 50 nM) showed similar, albeit less pronounced shifts in the same direction indicating dose dependency (Figure 5). Thus, it appears that the mutually synergistic activities of BAY 80-6946 on the cellular level, namely cell cycle arrest, and decreased proliferation on the one hand and apoptosis induction on the other, mount up to the net antitumoral activity exerted on myeloma cells in vitro.
Figure 5.
BAY 80-6946 impairs cell cycle progression of human myeloma cells. The influence of BAY 80-6946 on cell cycle progression was examined in two myeloma cell lines, AMO-1 (upper panel) and MOLP-8 (bottom panel). After 24 h of culture in the presence of BAY 80-6946 at the indicated concentrations, the cells were fixed, washed and resuspended with propidium iodide and RNase A, and analyzed by flow cytometry. The graph depicts the relative cell number in G0/G1 and S phase, depending on the concentration of BAY 80-6946.
Growth of human myeloma cells in vivo and therapeutic effect of BAY 80-6946
Given that no truly reliable preclinical animal model for human myeloma is available, we have used a murine xenograft model system using athymic NU/J Foxn1nu mice transplanted intraperitoneally (i.p.) with the human myeloma cells AMO-1 or MOLP-8 (5 × 106 cells/mouse), respectively. Although this model, similar to others, does not reflect all aspects of human myeloma, it allows the study of systemic effects of antitumoral compounds in an in vivo setting. Human myeloma cells were detected by the expression of HLA-ABC and CD138 (Figure 6a). This method worked reliably when cells derived from the peritoneal cavity were analyzed. In a complete workup, we also assessed myeloma cell spread to other organs such as bone marrow and spleen. Indeed, a small, yet consistent and reproducible number of human cells could be isolated from the spleens, thus allowing an estimation of the systemic spread of myeloma cells. In contrast, only small and varying numbers of human myeloma cells could be detected in the bone marrow, thus precluding a valid assessment of this anatomical site.
Figure 6.
BAY 80-6946 inhibits myeloma progression in a murine model in vivo. (a) To identify human myeloma cells, cultured AMO-1 and MOLP-8 cells were stained using an antibody directed against HLA-ABC and analyzed by flow cytometry (dark gray histograms). Isotype-matched IgG served as negative control (light gray histograms). (b) To assess the in vivo efficacy of BAY 80-6946, a xenograft model using NU/J Foxn1nu mice transplanted i.p. with 5 × 106 human myeloma cells was used. The experiments were performed using AMO-1 and MOLP-8 cells. Recipient mice were injected i.p. three times per week for 2 weeks with BAY 80-6946 at doses of 6 mg/kg body weight. After the experiment was completed, the total cell number was determined by a thorough diagnostic peritoneal lavage. Peritoneal cells were washed, stained with anti-human HLA-ABC and analyzed by flow cytometry to detect human cells. The histograms exemplify typical findings from representative mice, whereas the graphs depict means (±s.d.) from all animals (n=5 mice per group in MOLP-8 recipients, and n=4 mice per group in AMO-1 recipients). * indicates P<0.05 and *** indicates P<0.001, as compared with vehicle-treated controls. (c) To identify human tumor cells in the spleens of the recipient mice, suspensions of splenocytes were prepared, stained with anti-human HLA-ABC and analyzed by flow cytometry. Again, the histograms exemplify typical findings from representative mice, whereas the graphs depict means (±s.d.) from all animals. ** indicates P<0.01 as compared with vehicle-treated controls.
When myeloma-bearing mice were treated systemically for 2 weeks with BAY 80-6946 (i.p., three times/week, 6 mg per kg body weight), flow cytometry analysis of cells obtained by peritoneal lavage revealed a profound therapeutic effect of BAY 80-6946. Compared with vehicle-treated animals, BAY 80-6946 significantly reduced the peritoneal number of MOLP-8 cells by 69.3% and the respective number of AMO-1 cells by 87%, respectively (P<0.001 in both cases; Figure 6b).
In addition, when the systemic spread of human myeloma cells to the spleen was assessed, it was found that BAY 80-6946 did not prevent the systemic spread altogether but significantly reduced the number of human myeloma cells in the spleens of transplanted mice (reduction by ∼30% with both cell lines; P<0.01 compared with vehicle-treated animals; Figure 6c). Thus, even considering the undeniable limitations of this model, it appears that BAY 80-6946 can markedly reduce the overall myeloma tumor burden in vivo and effects a moderate reduction of splenic myeloma cells when administered systemically.
Discussion
Activation (phosphorylation) of Akt in MM cells, a feature that is primarily effected through PI3K signaling, is associated with poor prognosis and reduced survival of affected patients.23 In many instances, constitutive Akt activation in myeloma cells appears to be the result of PTEN deletion, amplification or mutation of PI3K or growth factor receptor activation, resulting in a relative resistance to apoptosis with serious consequences.17, 21, 37 Indeed, triggered by various cytokines, the PI3/Akt pathway activation is one of the most important hallmark features of drug resistance in MM, primarily due to the prevention of apoptosis.6, 38 Furthermore, MM cell migration induced by cytokines, such as vascular endothelial growth factor, depends on PI3K/Akt signaling, whereas it is thought to be independent of mitogen-activated protien kinase signaling.39 In contrast, Akt activation was not detected in nonmalignant hematopoietic cells from the same patients.31, 32 Thus, it appears that constitutive activation of Akt constitutes an important step in the pathogenic cascade of MM. This rationale formed the basis of our investigations using BAY 80-6946, the lead compound of a novel class of small-molecule antitumoral imidazolinoquinazoline compounds. In contrast to other compounds described previously as dual PI3K/mTOR inhibitors such as BEZ-235,40 BAY 80-6946 is a selective inhibitor of PI3K, a trait that may be advantageous when potential side effects are considered. Given that constitutive and IGF-1-induced activation of Akt appear to be a distinctive feature of myeloma cells, it is conceivable that the mode of action of the highly selective pan-class I PI3K inhibitor, BAY 80-6946, or related compounds will primarily affect tumor cells and not other cell types. However, this hypothesis needs to be tested in future large-scale experiments.
Currently used therapeutics, such as thalidomide, bortezomib and lenalidomide, target both myeloma cells and the bone-marrow microenvironment. Although these therapeutic strategies have elicited significant initial responses, only 25–35% of patients with relapsed myeloma respond to these agents.6, 41, 42 Furthermore, several investigations have revealed that treatment with dexamethasone, doxorubicin or bortezomib induces Akt activation and thus leads to myeloma cell resistance.25, 43, 44 In this light, it is reasonable to assume that additional treatment with a compound that specifically targets PI3K/Akt signaling in myeloma cells will be a worthwhile addition to the current therapeutic armamentarium.
Our results are consistent with and extend several previous reports, demonstrating a pivotal role of PI3K/Akt signaling in the (patho)-biology of MM. Regarding the mode of action of BAY 80-6946, the following aspects, which are not mutually exclusive, can be delineated:
First, treatment with BAY 80-6946 at nontoxic doses led to a considerable induction of apoptosis, inhibition of cell cycle progression inasmuch as the G0/G1: S ratio was shifted toward G0/G1, and, albeit to a lesser extent, inhibition of proliferation as well. The profound pro-apoptotic activity of BAY 80-6946 is plausible, because the PI3K/Akt pathway has a great impact on numerous substrates involved in regulation of apoptosis. For instance, Akt is directly responsible for the activation of several components of the cell-death machinery, including Bad, the pro-apoptotic caspase 9 and FKHR (a transcription factor of the forkhead family).17, 18 Furthermore, Akt is indirectly involved in the regulation of NF-κB and p53.17, 18, 35, 38 Although the decrease of Akt phosphorylation and the increase of apoptosis were seen in all four myeloma lines studied, the anti-viability activity of BAY 80-6946 against U-266 cells appeared to be less pronounced compared with the AMO-1, KMS-12-BM and MOLP-8 cell lines, respectively. Although there is no obvious explanation for this apparent discrepancy, one may speculate that compensatory activation of other proliferation promoting pathways in U-266 cells may contribute to the somewhat differential responses of the human myeloma lines to BAY 80-6946. Of note, BAY 80-6946 significantly inhibited proliferation ex vivo of ‘natural' myeloma cells isolated from three patients. In any case, it is reasonable to assume that the negative effects of BAY 80-6946 on myeloma proliferation and cell cycle regulation, as well as the induction of apoptosis, add up to a profound overall antitumoral activity, presumably with a predominant role of the pro-apoptotic activity. Given that the acquisition of (drug-induced) apoptosis deficiency is arguably the single most important trait conferring drug resistance in myeloma cells,45 the activity of BAY 80-6946 may contribute to overcoming drug resistance.
Second, BAY 80-6946 even overcame the growth advantages conferred by IGF-1. IGF-1 is one of the most important growth factors within the bone marrow, protecting myeloma cells from glucocorticoid-induced apoptosis through activation of the PI3K/Akt pathway.13 There have been attempts to use the inhibition of IGF-1 receptors for anti-myeloma therapy.11 It is worth noting that IGF-1 is not only secreted in the bone marrow, but is also synthesized in the liver and found throughout the circulation.13 As a result, myeloma cells are constantly exposed to IGF-1 stimulation. Thus, this growth factor is a general promoter of systemic myeloma cell proliferation and survival, and antagonizing its biological effects is, therefore, predicted to aid antitumoral therapies at many sites of the body. In our study, IGF-1 failed to protect the myeloma cells against BAY 80-6946-induced inhibition of proliferation, indicating that BAY 80-6946 can overcome the protective effects of this cytokine.
Third, in parallel to the in vitro effects, BAY 80-6946 also showed convincing anti-myeloma activity in vivo in a murine xenograft model using human myeloma cells. Research into the pathophysiology of myeloma is largely hampered by the lack of an appropriate animal model. In most of the recent studies, myeloma cells were simply inoculated subcutaneously and the local tumor volume was monitored.26, 46 On the one hand, the strength of such models is the ease to directly assess local tumor growth. On the other hand, such approaches are limited by difficulties to determine the overall tumor cell load (that is, the number of tumor cells within a potentially mixed infiltrate or nodule) as well as the distant spread of tumor cells. Most of the current models suffer from the lack of an accurate reflection of the relevant bone-marrow environment, although first attempts to design such new mouse models have been made.47 In this less-than-optimal situation, we have developed an easy-to-use murine xenograft model in which human myeloma cells were transplanted i.p. into NU/J Foxn1nu mice. Bearing in mind said limitations, our model is an approximation that reflects some, but not all aspects of human myeloma. Using a short-term treatment regimen including vehicle-treated controls, BAY 80-6946 dramatically reduced the total myeloma cell count by 87.0% when AMO-1 cells were grafted, and by 69.3% in the case of MOLP-8 cells. Of note, BAY 80-6946 could also reduce systemic tumor progression, as exemplified by our analysis of the spleens of the recipient animals. It is also conceivable that BAY 80-6946 prevented myeloma cells from spreading to the spleen. In any case, this notion may be of particular interest, as mechanisms of myeloma cell spread are not yet fully understood, although extramedullary manifestations occur regularly.48
In conclusion, this study provides strong circumstantial evidence that the novel PI3K inhibitor, BAY 80-6946, carries the potential to impede the growth of MM in vivo and in vitro. We have primarily addressed its direct activity against myeloma cells themselves. However, within the complex “natural” environment in human patients, multiple interactions of myeloma cells within the bone marrow and other anatomical sites may trigger activation not only of PI3K/Akt, but also of MEK/ERK, JAK/STAT3, NF-κB or other signaling cascades.45, 49 All of them may contribute to tumor cell survival, drug resistance and/or migration.6 Thus, although the activity of BAY 80-6946 certainly will have to be explored in additional settings, such as rationale-based combinations with targeted and chemo therapeutics, our data provide the first experimental evidence that such future studies are a worthwhile path to pursue.
Acknowledgments
We thank K Zachmann and B Messerschmidt for their excellent technical assistance, T Stühmer (University of Würzburg) for providing myeloma cell lines and T Aung for the recruitment of myeloma patients. This work was supported in part by a grant from the Deutsche Krebshilfe to MS and MPS.
NL and KZ are employees of Bayer HealthCare, the manufacturer of Bay 80-6946. All the remaining authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Blood Cancer Journal website (http://www.nature.com/bcj)
JG, NP and MS performed most in vitro and in vivo experiments, interpreted data and drafted the manuscript, PS performed some western blot experiments, NL and KZ generated BAY 80-6946 and conducted cell-free enzyme assays, GG W provided the blood samples of myeloma patients and supported the establishment of myeloma cell isolation, SE contributed to study design and cell cycle analysis, and MPS designed the study, interpreted the data and wrote the manuscript. All authors have critically revised the manuscript.
Supplementary Material
References
- Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. Multiple myeloma. Lancet. 2009;374:324–339. doi: 10.1016/S0140-6736(09)60221-X. [DOI] [PubMed] [Google Scholar]
- Attal M, Harousseau J-L, Facon T, Guilhot F, Doyen C, Fuzibet JG, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495–2505. doi: 10.1056/NEJMoa032290. [DOI] [PubMed] [Google Scholar]
- Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351:1860–1873. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]
- Lenhoff S, Hjorth M, Holmberg E, Turesson I, Westin J, Nielsen JL, et al. Impact on survival of high-dose therapy with autologous stem cell support in patients younger than 60 years with newly diagnosed multiple myeloma: a population-based study. Nordic Myeloma Study Group. Blood. 2000;95:7–11. [PubMed] [Google Scholar]
- Richardson P, Hideshima T, Anderson KC. An update of novel therapeutic approaches for multiple myeloma. Curr Treat Options Oncol. 2004;5:227–238. doi: 10.1007/s11864-004-0014-6. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Anderson KC. Molecular mechanisms of novel therapeutic approaches for multiple myeloma. Nat Rev Cancer. 2002;2:927–937. doi: 10.1038/nrc952. [DOI] [PubMed] [Google Scholar]
- Schwarzenbach H. Expression of MDR1/P-glycoprotein, the multidrug resistance protein MRP, and the lung-resistance protein LRP in multiple myeloma. Med Oncol. 2002;19:87–104. doi: 10.1385/MO:19:2:87. [DOI] [PubMed] [Google Scholar]
- Hazlehurst LA, Damiano JS, Buyuksal I, Pledger WJ, Dalton WS. Adhesion to fibronectin via beta1 integrins regulates p27kip1 levels and contributes to cell adhesion mediated drug resistance (CAM-DR) Oncogene. 2000;19:4319–4327. doi: 10.1038/sj.onc.1203782. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Chauhan D, Schlossman R, Richardson P, Anderson KC. The role of tumor necrosis factor alpha in the pathophysiology of human multiple myeloma: therapeutic applications. Oncogene. 2001;20:4519–4527. doi: 10.1038/sj.onc.1204623. [DOI] [PubMed] [Google Scholar]
- Landowski TH, Olashaw NE, Agrawal D, Dalton WS. Cell adhesion-mediated drug resistance (CAM-DR) is associated with activation of NF-kappa B (RelB/p50) in myeloma cells. Oncogene. 2003;22:2417–2421. doi: 10.1038/sj.onc.1206315. [DOI] [PubMed] [Google Scholar]
- Mitsiades CS, Mitsiades N, Munshi NC, Anderson KC. Focus on multiple myeloma. Cancer Cell. 2004;6:439–444. doi: 10.1016/j.ccr.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Shringarpure R, Akiyama M, et al. Inhibition of the insulin-like growth factor receptor-1 tyrosine kinase activity as a therapeutic strategy for multiple myeloma, other hematologic malignancies, and solid tumors. Cancer Cell. 2004;5:221–230. doi: 10.1016/s1535-6108(04)00050-9. [DOI] [PubMed] [Google Scholar]
- Sprynski AC, Hose D, Caillot L, Réme T, Shaughnessy JDJ, Barlogie B, et al. The role of IGF-1 as a major growth factor for myeloma cell lines and the prognostic relevance of the expression of its receptor. Blood. 2009;113:4614–4626. doi: 10.1182/blood-2008-07-170464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12:159–169. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- Toker A, Cantley LC. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- Martelli AM, Evangelisti C, Chappell W, Abrams SL, Bäsecke J, Stivala F, et al. Targeting the translational apparatus to improve leukemia therapy: roles of the PI3K/PTEN/Akt/mTOR pathway. Leukemia. 2011;25:1064–1079. doi: 10.1038/leu.2011.46. [DOI] [PubMed] [Google Scholar]
- Downward J. PI 3-kinase, Akt and cell survival. Semin Cell Dev Biol. 2004;15:177–182. doi: 10.1016/j.semcdb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Ghobrial IM, Witzig TE, Adjei AA. Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin. 2005;55:178–194. doi: 10.3322/canjclin.55.3.178. [DOI] [PubMed] [Google Scholar]
- Bartholomeusz C, Gonzalez-Angulo AM. Targeting the PI3K signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16:121–130. doi: 10.1517/14728222.2011.644788. [DOI] [PubMed] [Google Scholar]
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- Maehama T. PTEN: its deregulation and tumorigenesis. Biol Pharm Bull. 2007;30:1624–1627. doi: 10.1248/bpb.30.1624. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- Saal LH, Johansson P, Holm K, Gruvberger-Saal SK, She QB, Maurer M, et al. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci USA. 2007;104:7564–7569. doi: 10.1073/pnas.0702507104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri A, Marmiroli S, Tassone P, Lombardi L, Nobili L, Verdelli D, et al. The oral protein-kinase C beta inhibitor enzastaurin (LY317615) suppresses signalling through the AKT pathway, inhibits proliferation and induces apoptosis in multiple myeloma cell lines. Leuk Lymphoma. 2008;49:1374–1383. doi: 10.1080/10428190802078289. [DOI] [PubMed] [Google Scholar]
- Pene F, Claessens YE, Muller O, Viguie F, Mayeux P, Dreyfus F, et al. Role of the phosphatidylinositol 3-kinase/Akt and mTOR/P70S6-kinase pathways in the proliferation and apoptosis in multiple myeloma. Oncogene. 2002;21:6587–6597. doi: 10.1038/sj.onc.1205923. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Catley L, Yasui H, Ishitsuka K, Raje N, Mitsiades C, et al. Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood. 2006;107:4053–4062. doi: 10.1182/blood-2005-08-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Takiguchi T, Fukutoku M, Yoshioka R, Hirose Y, Fukuhara S, et al. Establishment of a CD4-positive plasmacytoma cell line (AMO-1) Leukemia. 1993;7:274–280. [PubMed] [Google Scholar]
- Ohtsuki T, Yawata Y, Wada H, Sugihara T, Mori M, Namba M. Two human myeloma cell lines, amylase-producing KMS-12-PE and amylase-non-producing KMS-12-BM, were established from a patient, having the same chromosome marker, t(11;14)(q13;q32) Br J Haematol. 1989;73:199–204. doi: 10.1111/j.1365-2141.1989.tb00252.x. [DOI] [PubMed] [Google Scholar]
- Matsuo Y, Drexler HG, Harashima A, Okochi A, Hasegawa A, Kojima K, et al. Induction of CD28 on the new myeloma cell line MOLP-8 with t(11;14)(q13;q32) expressing delta/lambda type immunoglobulin. Leuk Res. 2004;28:869–877. doi: 10.1016/j.leukres.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Schwab G, Siegall CB, Aarden LA, Neckers LM, Nordan RP. Characterization of an interleukin-6-mediated autocrine growth loop in the human multiple myeloma cell line, U-266. Blood. 1991;77:587–593. [PubMed] [Google Scholar]
- Alkan S, Izban KF. Immunohistochemical localization of phosphorylated AKT in multiple myeloma. Blood. 2002;99:2278–2279. doi: 10.1182/blood-2001-01-0317. [DOI] [PubMed] [Google Scholar]
- Hsu J, Shi Y, Krajewski S, Renner S, Fisher M, Reed JC, et al. The AKT kinase is activated in multiple myeloma tumor cells. Blood. 2001;98:2853–2855. doi: 10.1182/blood.v98.9.2853. [DOI] [PubMed] [Google Scholar]
- Tu Y, Gardner A, Lichtenstein A. The phosphatidylinositol 3-kinase/AKT kinase pathway in multiple myeloma plasma cells: roles in cytokine-dependent survival and proliferative responses. J Biomol Screen. 2002;7:441–450. [PubMed] [Google Scholar]
- Fuchikami K, Togame H, Sagara A, Satho T, et al. A versatile high-throughput screen for inhibitors of lipid kinase activity: development of an immobilized phospholipid plate assay for phosphoinositide 3-kinase gamma. Cancer Res. 2000;60:6763–6770. doi: 10.1177/108705702237676. [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Hemmings BA. Akt signaling: linking membrane events to life and death decisions. Science. 1997;275:628–630. doi: 10.1126/science.275.5300.628. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Chauhan D, Richardson P, Anderson KC. Identification and validation of novel therapeutic targets for multiple myeloma. J Clin Oncol. 2005;23:6345–6350. doi: 10.1200/JCO.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22:8983–8998. doi: 10.1038/sj.onc.1207115. [DOI] [PubMed] [Google Scholar]
- Podar K, Tai YT, Davies FE, Lentzsch S, Sattler M, Hideshima T, et al. Vascular endothelial growth factor triggers signaling cascades mediating multiple myeloma cell growth and migration. Blood. 2001;98:428–435. doi: 10.1182/blood.v98.2.428. [DOI] [PubMed] [Google Scholar]
- Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3- kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- Ghobrial IM, Leleu X, Hatjiharissi E, Hideshima T, Mitsiades C, Schlossman R, et al. Emerging drugs in multiple myeloma. Expert Opin Emerg Drugs. 2007;12:155–163. doi: 10.1517/14728214.12.1.155. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Nakamura N, Chauhan D, Anderson KC. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene. 2001;20:5991–6000. doi: 10.1038/sj.onc.1204833. [DOI] [PubMed] [Google Scholar]
- Mitsiades CS, Mitsiades N, Poulaki V, Schlossman R, Akiyama M, Chauhan D, et al. Activation of NF-kappaB and upregulation of intracellular anti-apoptotic proteins via the IGF-1/Akt signaling in human multiple myeloma cells: therapeutic implications. Oncogene. 2002;21:5673–5683. doi: 10.1038/sj.onc.1205664. [DOI] [PubMed] [Google Scholar]
- van de Donk NWCJ, Lokhorst HM, Bloem AC. Growth factors and antiapoptotic signaling pathways in multiple myeloma. Leukemia. 2005;19:2177–2185. doi: 10.1038/sj.leu.2403970. [DOI] [PubMed] [Google Scholar]
- Podar K, Raab MS, Zhang J, McMillin D, Breitkreutz I, Tai YT, et al. Targeting PKC in multiple myeloma: in vitro and in vivo effects of the novel, orally available small-molecule inhibitor enzastaurin (LY317615.HCl) Blood. 2007;109:1669–1677. doi: 10.1182/blood-2006-08-042747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urashima M, Chen BP, Chen S, Pinkus GS, Bronson RT, Dedera DA, et al. The development of a model for the homing of multiple myeloma cells to human bone marrow. Blood. 1997;90:754–765. [PubMed] [Google Scholar]
- Varettoni M, Corso A, Pica G, Mangiacavalli S, Pascutto C, Lazzarino M. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Ann Oncol. 2010;21:325–330. doi: 10.1093/annonc/mdp329. [DOI] [PubMed] [Google Scholar]
- Lentzsch S, Chatterjee M, Gries M, Bommert K, Gollasch H, Dörken B, et al. PI3-K/AKT/FKHR and MAPK signaling cascades are redundantly stimulated by a variety of cytokines and contribute independently to proliferation and survival of multiple myeloma cells. Leukemia. 2004;18:1883–1890. doi: 10.1038/sj.leu.2403486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.