Imatinib (IM) resistance occurs in cells from chronic myelogenous leukemia (CML) patients. Resistance can be caused by mutations in the Abl kinase domain (for example, T315)1 within Bcr-Abl or by overexpression of Bcr-Abl. Other forms of resistance to tyrosine kinase inhibitors (TKIs) exist. For example, Donato and colleagues2 found that IM resistance can be caused by the expression of high amounts of tyrosine-phosphorylated Lyn, a member of the Src kinase family. IM resistance is attributed to the inability of IM to inhibit Lyn kinase and other Src kinase family members. The overexpression of activated Lyn kinase was also observed in the K562R cell line2 and in cells from blast crisis (BC) CML patients.3 Knockdown of Lyn kinase induced cell death in BC CML cells.3 Our previous findings also showed that Jak2 kinase inhibitors induced apoptosis in BC CML cells.4, 5
Our studies indicate that Jak2 is a major signaling kinase in Bcr-Abl+ cells.4, 5 Jak2 drives the activation of the Ras and PI-3 kinase signaling pathways,4 and maintains the stability of Bcr-Abl thereby indirectly controlling other pathways requiring Bcr-Abl activity, such as the STAT5 and STAT3 pathways.5
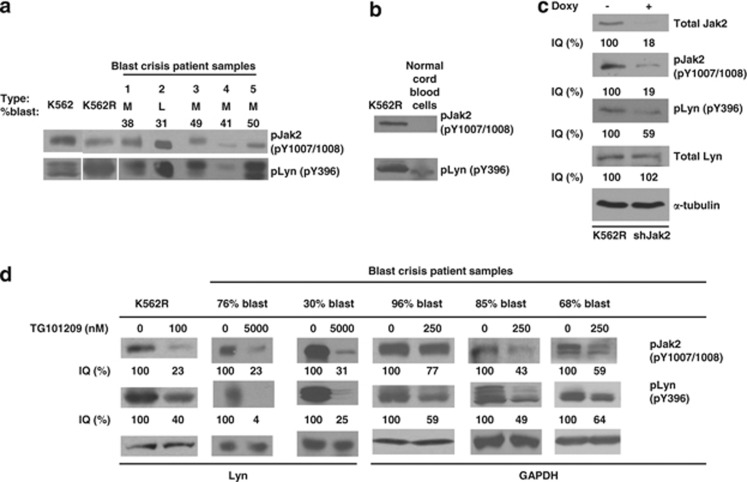
We examined SDS extracts from blood cells obtained from five patients with BC CML by western blotting for activated Jak2, and found that antibodies to Jak2 pTyr 1007, an indicator of activated Jak2, were positive for Jak2 activity in these BC cell extracts (Figure 1a). These extracts also contained active tyrosine phosphorylated 396 Lyn kinase, which is recognized by Src anti-Y416 antibody. Viable cells from BC CML also contained activated Jak2 and activated Lyn kinase (Figure 1d). Treatment of these BC cells with Jak2 inhibitor TG101209 with doses ranging from 250 nM to 5 μM for 16 h inhibited Jak2 kinase as expected, and also inhibited the phosphorylation of Lyn kinase (Figure 1d). Importantly, normal cord blood cells lacked activated Jak2 and had only trace amounts of activated Lyn kinase (Figure 1b).
Figure 1.
Jak2 inhibition led to the inactivation of Lyn kinase in blast crisis CML cells. (a) Activated Jak2 and activated Lyn kinase as detected by western blotting are expressed in blast crisis CML cells from either myeloid (M) or lymphoid (L) blasts ranging from 31 to 50% blasts; (b) normal cord blood cells lack activated Jak2 and Lyn; (c) inhibition of Jak2 activities by expressing Jak2 shRNA in K562R cells decreased the activation of Lyn kinase. (d) Treatment of K562R cells with a low dose of Jak2 inhibitor (100 nM) decreased the activation of Jak2 and activation of Lyn kinase. Levels of Lyn protein were unchanged. In BC CML cells, Jak2 inhibition in cultured BC CML cells strongly reduced the levels of activated Jak2 and activated Lyn, but had no effect on total Lyn protein. Protein lysates (50 μg) were analyzed for pY1007/1008 Jak2, pTyr 396 Lyn, and total Lyn by western blotting. Image quant (IQ) % of the intensity of the blots is shown.
We also examined K562R cells for the presence of activated Jak2. Activated Jak2 was detected in K562R cells, and we also detected activated Lyn kinase as expected. Treatment of these cells with low doses TG101209 (100 nM) for 16 h inhibited Jak2 and Lyn kinase activation, but did not reduce Lyn protein levels (Figure 1d). Knockdown of human Jak2 was performed using an inducible human Jak2 short hairpin RNA (shRNA) as described.4 The Jak2 knockdown cells had reduced levels of both activated Jak2 and activated Lyn kinase (Figure 1c). Thus, inhibition of the Jak2 kinase and severe reduction of Jak2 protein by knockdown with Jak2-specific shRNA decreased the activation of Jak2 as expected, and caused inactivation of Lyn kinase. The amount of the Lyn protein was not reduced by Jak2 inhibition (Figures 1c and d). These findings provide more evidence for the important role of Jak2 in CML (Supplementary Information).
Our past findings indicate that Jak2 inhibition induced cell death in BC CML cells, which are resistant to IM.4, 5, 6 Our current findings provide an explanation for these results, as BC CML cells contain activated Jak2 which in turn leads to the activation of Lyn kinase. Therefore, inhibition of Jak2 would strongly reduce levels of activated Lyn kinase ultimately leading to cell death as described.3 The mechanism of Lyn kinase activation could be direct, as there are several consensus Jak2 phosphorylation sites in Lyn kinase.5 However, the effects of Jak2 on Lyn kinase activation may be indirect. The indirect mechanism may involve the ability of Jak2 to regulate SET.5 Reduction of Lyn kinase activity would induce cell death in BC CML cells, as it is known that knockdown of Lyn kinase causes apoptosis induction in BC CML cells.3 Therefore, our findings explain why Jak2 inhibition is a potent inducer of cell death in BC CML cells,4, 5, 6 namely Jak2 drives the activation of Lyn kinase and inhibition of Jak2 will depress activation of Lyn kinase leading to cell death.
In previous studies, Bcr-Abl+ cells and CML cell lines were shown to have high levels of activated Jak2.4, 5 Moreover, Jak2 activation in Bcr-Abl+ cells has a critical role in maintaining the viability of Bcr-Abl+ cells because of the following events. Knockdown of Jak2 either by Jak2 short interfering RNA or by Jak2 shRNA drastically reduces the level of Bcr-Abl protein in cells, reduces the phosphorylation of tyrosine 177, prevents activation of SET and thus allows PP2A to be active allowing Shp1 tyrosine phosphatase to dephosphorylate Bcr-Abl, Jak2 and Lyn kinase, and possibly other signaling proteins in Bcr-Abl+ cells.4, 5 It is not surprising that Bcr-Abl+ cells, which are resistant to IM (for example, T315I mutants of Bcr-Abl), are readily induced to undergo cell death by the treatment of cells with selective Jak2 inhibitors such as TG101209 as measured by Annexin/propidium iodide analyses.4, 5 These findings raise the possibility that Jak2 inhibitors may be useful for the treatment of patients with CML in BC.
Acknowledgments
We thank Dr Steven Kornblau, Director of the P01 tissue bank, for supplying SDS lysates from CML patients in BC. This research was supported by P01 CA49639, project 5, directed by RBA.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Blood Cancer Journal website (http://www.nature.com/bcj)
Supplementary Material
References
- Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- Donato NJ, Wu JY, Stapley J, Lin H, Arlinghaus R, Aggarwal BB, et al. Imatinib mesylate resistance through BCR-ABL independence in chronic myelogenous leukemia. Cancer Res. 2004;64:672–677. doi: 10.1158/0008-5472.can-03-1484. [DOI] [PubMed] [Google Scholar]
- Ptasznik A, Nakata Y, Kalota A, Emerson SG, Gewirtz AM. Short interfering RNA (siRNA) targeting the Lyn kinase induces apoptosis in primary, and drug-resistant, BCR-ABL1(+) leukemia cells. Nat Med. 2004;10:1187–1189. doi: 10.1038/nm1127. [DOI] [PubMed] [Google Scholar]
- Samanta A, Perazzona B, Chakraborty S, Sun X, Modi H, Bhatia R, et al. Janus kinase 2 regulates Bcr-Abl signaling in chronic myeloid leukemia. Leukemia. 2011;25:463–472. doi: 10.1038/leu.2010.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta AK, Chakraborty S, Wang Y, Kantarjian H, Sun X, Hood J, et al. Jak2 inhibition deactivates Lyn kinase through the SET-PP2A-SHP1 pathway, causing apoptosis in drug-resistant cells from chronic myelogenous leukemia patients. Oncogene. 2009;28:1669–1681. doi: 10.1038/onc.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta AK, Lin H, Sun T, Kantarjian H, Arlinghaus RB. Janus kinase 2: a critical target in chronic myelogenous leukemia. Cancer Res. 2006;66:6468–6472. doi: 10.1158/0008-5472.CAN-06-0025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.