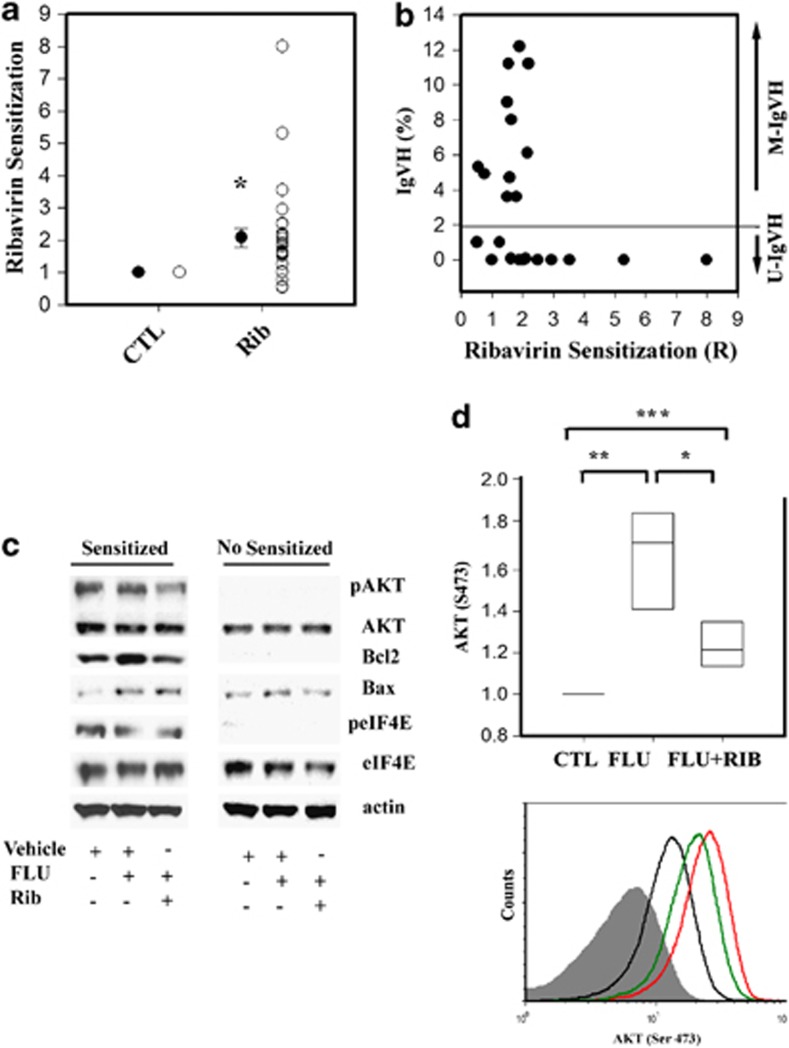
Eukaryotic translation initiation factor 4E (eIF4E) is a rate-limiting factor for cap-dependent protein synthesis regulated by the PI3K/AKT/mTOR signaling pathway as well as MNK1/2-mediated phosphorylation.1, 2 In addition to its cytoplasmic functions in translation, nuclear eIF4E aids in the cytoplasmic export of specific mRNAs.1, 3 eIF4E is overexpressed in many cancers and has been reported to have important roles in the development and progression of hematological malignancies in animal models.2 However, the role of eIF4E in drug resistance in primary human cancer cells is less well documented.4, 5, 6 In this study, we sought to assess the contribution of eIF4E to fludarabine (FLU) resistance in primary chronic lymphocytic leukemia (CLL) lymphocytes as this nucleoside analog is used as a first-line treatment for the disease.7 To this end, we used a panel of primary CLL samples from 26 affected patients (Supplementary Table). To interfere with eIF4E function, we used Ribavirin, a well-characterized antiviral drug that has also been shown to target eIF4E in a variety of systems, including patients with acute myeloid leukemia (AML).6, 8, 9 A clinically achievable concentration of Ribavirin (10 μM), which was not cytotoxic to primary CLL lymphocytes in culture, significantly sensitized 76% of the samples tested to FLU with the sensitization index (R) ranging from 1.25 to eightfold (Figures 1a, P<0.001 and Supplementary Table). Sensitization was observed in 50% of the CD38-positive samples, 60% of the del17-positive samples, 50% of the del11-positive samples and samples from clinically resistant patients (Supplementary Table). Notably, the effect of Ribavirin was significantly associated with IgVH status, as revealed by the non-parametric Spearman Rank Order Correlation (r=−0.45, P=0.02); that is, better sensitization in high-risk U-IgVH samples, (Figure 1b).
Figure 1.
Ribavirin sensitization to FLU is associated with IgVH mutational status and abrogation of FLU-induced AKT phosphorylation and BCL2 expression in vitro. (a) The open circles represent the R-value calculated as the ratio between (FLU IC50)/(IC50 of FLU+Ribavirin (Rib) and vehicle (CTL) in each sample tested. The close circles represent the median values (±s.e.) (*P<0.001, Mann–Whitney U-statistic). (b) Graphical representation of the percentage of mutations at the IgVH locus (y axis) and Ribavirin-mediated sensitization to FLU (R-values, x axis). The horizontal line on the y axis (2%) indicates the cutoff used to define IgVH status. (c) The expression and phosphorylation of the indicated targets was assessed by western blot analysis in two representative samples 12 h after ex vivo treatment with vehicle, the FLU IC50 concentration of each sample in combination with vehicle or with 10 μM Ribavirin. Sensitized: R=5; not sensitized: R=1. (d) The y axis represents the changes in AKT phosphorylation (S473) with respect to vehicle (CTL)-treated lymphocytes 12 h after the treatment as indicated (x axis). The bars represent the median values and the 25th/75th percentile intervals (n=7). The Krustal–Wallis analysis of variance (***P<0.001) followed by paired t-test (**P=0.014 and *P=0.035). A representative sample showing AKT phosphorylation (S473) after treatment with vehicle (black line), FLU alone (IC50 concentration, red line) or in combination with 10 μM Ribavirin (FLU+Rib; green line). The gray area represents the negative control (control isotype antibody).
Because Ribavirin was not cytotoxic when used alone (up to 60 μM), we hypothesized that FLU might induce changes in signaling events targeted by Ribavirin. To begin to assess this hypothesis, we monitored the effect of FLU or the combination of FLU and 10 μM Ribavirin on the expression or phosphorylation of eIF4E and AKT and the expression of Bcl2 and BAX in two samples with R values of five (highly sensitized) and one (not sensitized), respectively (Figure 1c). FLU treatment did not effect eIF4E expression or phosphorylation in either sample, and this was confirmed in six additional samples by flow cytometry (data not shown). In contrast, FLU ex vivo treatment increased the expression of both Bcl2 and the proapoptotic protein Bax, and the induction of Bcl2 was abrogated by co-treatment with Ribavirin in the sensitized sample (Figure 1c) suggesting that Ribavirin may alter the balance of pro- and anti-apoptotic proteins, thus favoring cell death in FLU-treated cells. Similarly, Ribavirin suppressed FLU-induced AKT phosphorylation in the sensitized sample. FLU-induced AKT phosphorylation, which has not previously been reported, was confirmed in four additional samples by flow cytometry (P<0.001, Figure 1d), and Ribavirin consistently suppressed this induction. Of interest, our preliminary results also suggest that 10 μM Ribavirin can decrease AKT phosphorylation and survival clues provided by a stromal feeder layer in a similar manner to the PI3K inhibitor BKM-120 (Supplementary Figure 1a and b).10, 11
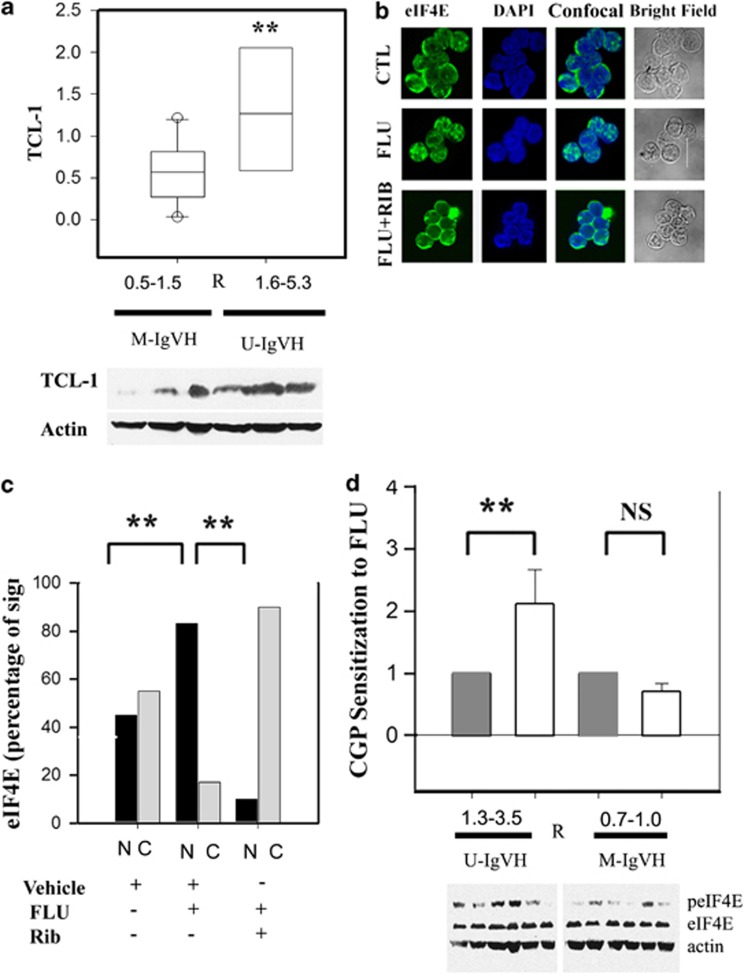
Although Ribavirin sensitization to FLU may involve suppression of AKT signaling, western blot analysis in available samples revealed no significant association between ribavirin sensitization and basal AKT phosphorylation (data not shown). However, an association between sensitization and basal expression of the TCL-1 oncogene was observed (P<0.001, Figure 2a). TCL-1 is an AKT co-activator that, when overexpressed in mice, results in the development of a leukemia phenotypically similar to U-IgVH human CLL.12 Interestingly, consistent with a potential role for TCL-1 in Ribavirin-mediated sensitization to FLU, abrogation of FLU-induced AKT phosphorylation and Bcl2 expression were observed in a TCL-1-positive CLL cell line (MEC-2; Supplementary Figure 1A) but not in a TCL-1-negative CLL cell line (MEC-1; data not shown).13
Figure 2.
Ribavirin-mediated sensitization is associated with TCl-1 expression and abrogation of FLU-induced eIF4E nuclear localization. (a) TCL-1 expression is significantly higher in primary CLL samples showing better Ribavirin-mediated sensitization to FLU. TCL-1 expression was assessed in protein extracts of six available samples by western blot. TCL-1 expression was compared using the Mann–Whitney U-statistics (P<0.001). (b) Primary CLL lymphocytes were treated with vehicle (CTL) or FLU IC50 in combination with vehicle (FLU) or 10 μM Ribavirin (FLU+Rib) for 24 h followed by eIF4E staining (green). Nuclear counterstaining was performed with 4',6-diamidino-2-phenylindole (DAPI, blue). Subcellular localization of eIF4E was imaged using a confocal microscope. Bright-field images of the CLL lymphocytes analyzed are shown in the right panel. (c) Nuclear (N, black bars) or cytoplasmic (C, gray bars) eIF4E localization after treatment with vehicle, FLU IC50 or FLU IC50 plus 10 μM Ribavirin is represented as percentage of the fluorescent signal calculated from 15 to 25 cells per condition. The Fisher test indicates that there is a significant difference between the eIF4E nuclear staining patterns after the treatments (**P<0.01, Fisher test). (d) The bars represent the mean sensitization value of CGP57380 on FLU sensitivity in seven M-IgVH and seven U-IgVH samples (open bars) with respect to paired vehicle-treated samples (gray bars) (y axis). eIF4E phosphorylation is shown in representative samples from each group.
In AML patients treated with Ribavirin as single agent, clinical response correlated with drug-induced eIF4E re-localization from the nucleus to the cytoplasm.8 We therefore assessed the cellular localization of eIF4E in untreated and treated CLL lymphocytes (Figure 2b). Interestingly, we found that FLU treatment resulted in a marked change in eIF4E localization. Although untreated cells showed approximately equal eIF4E staining in the cytoplasm vs the nucleus, cells treated with FLU IC50 for 12 h showed about 80% nuclear eIF4E staining. Importantly, this effect was negated by co-treatment with FLU IC50 and 10 μM Ribavirin, which instead caused eIF4E to accumulate in the cytoplasm (Figure 2c).
In addition to promote tumor growth, MNK-mediated phosphorylation of eIF4E has been reported to mediate drug resistance.14, 15, 16 Thus, we tested whether an inhibitor of MNK1/2, CGP57380, could also enhance FLU cytotoxicity. Indeed, a non-toxic concentration of CGP57380 (5 μM), sufficient to decrease basal eIF4E phosphorylation (Supplementary Figure 1d) increased FLU sensitivity by 1.3- to 3.5-fold, specifically in U-IgVH CLL samples (Figure 2d). Consistently, U-IgVH samples displayed a trend toward higher basal eIF4E phosphorylation than M-IgVH CLL samples (P=0.058; Figure 2d). It has been previously reported in AML cells that the effect of CGP57380 on drug resistance is mediated by the abrogation of chemotherapeutically induced eIF4E phosphorylation. In contrast, our results indicate that, albeit FLU does not affect eIF4E phosphorylation status (Figure 1c), MNK inhibition can sensitize primary CLL samples to FLU, thus suggesting that basal phosphorylation of eIF4E (in contrast to drug-induced phosphorylation) contributes to FLU resistance in U-IgVH samples.
In summary, we report that eIF4E is highly expressed and variably phosphorylated in primary CLL lymphocytes and that pharmacological targeting of eIF4E function or phosphorylation can increase FLU sensitivity ex vivo, preferentially in primary U-IgVH CLL lymphocytes. Our results further suggest that signaling differences in eIF4E-regulated processes exist between U-IgVH and M-IgVH CLL cells. Interestingly, our data show that sensitization to FLU by Ribavirin coincides with suppression of two cellular responses that may limit the effectiveness of FLU; activation of AKT, bcl2 expression and nuclear localization of eIF4E. Biologically, our results thus underscore the contribution of eIF4E in the survival of quiescent primary human malignant cells and future studies elucidating the exact mechanisms involved are warranted.
Acknowledgments
This work was supported by a Canadian Institute of Heath Research (CIHR) Operating Grant to RA (MOP 106528).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Blood Cancer Journal website (http://www.nature.com/bcj)
Supplementary Material
References
- Sonenberg N, Gingras AC. The mRNA 5′ cap-binding protein eIF4E and control of cell growth. Curr Opin Cell Biol. 1998;10:268–275. doi: 10.1016/s0955-0674(98)80150-6. [DOI] [PubMed] [Google Scholar]
- Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, et al. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–337. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- Strudwick S, Borden KL. The emerging roles of translation factor eIF4E in the nucleus. Differentiation. 2002;70:10–22. doi: 10.1046/j.1432-0436.2002.700102.x. [DOI] [PubMed] [Google Scholar]
- Kraljacic BC, Arguello M, Amri A, Cormack G, Borden K. Inhibition of eIF4E with ribavirin cooperates with common chemotherapies in primary acute myeloid leukemia specimens. Leukemia. 2011;25:1197–1200. doi: 10.1038/leu.2011.57. [DOI] [PubMed] [Google Scholar]
- Graff JR, Konicek BW, Carter JH, Marcusson EG. Targeting the eukaryotic translation initiation factor 4E for cancer therapy. Cancer Res. 2008;68:631–634. doi: 10.1158/0008-5472.CAN-07-5635. [DOI] [PubMed] [Google Scholar]
- Kentsis A, Topisirovic I, Culjkovic B, Shao L, Borden KL. Ribavirin suppresses eIF4E-mediated oncogenic transformation by physical mimicry of the 7-methyl guanosine mRNA cap. Proc Natl Acad Sci USA. 2004;101:18105–18110. doi: 10.1073/pnas.0406927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robak T, Kasznicki M. Alkylating agents and nucleoside analogues in the treatment of B cell chronic lymphocytic leukemia. Leukemia. 2002;16:1015–1027. doi: 10.1038/sj.leu.2402531. [DOI] [PubMed] [Google Scholar]
- Assouline S, Culjkovic B, Cocolakis E, Rousseau C, Beslu N, Amri A, et al. Molecular targeting of the oncogene eIF4E in acute myeloid leukemia (AML): a proof-of-principle clinical trial with ribavirin. Blood. 2009;114:257–260. doi: 10.1182/blood-2009-02-205153. [DOI] [PubMed] [Google Scholar]
- Pettersson F, Yau C, Dobocan MC, Culjkovic-Kraljacic B, Retrouvay H, Puckett R, et al. Ribavirin treatment effects on breast cancers overexpressing eIF4E, a biomarker with prognostic specificity for luminal B-type breast cancer. Clin Cancer Res. 2011;17:2874–2884. doi: 10.1158/1078-0432.CCR-10-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoellenriegel J, Meadows SA, Sivina M, Wierda WG, Kantarjian H, Keating MJ, et al. The phosphoinositide 3'-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118:3603. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrein L, Shawi M, Grenier J, Aloyz R, Panasci L. The phosphatidylinositol-3 kinase I inhibitor BKM120 induces cell death in B-chronic lymphocytic leukemia cells in-vitro. Int J Cancer. 2012;133:247–252. doi: 10.1002/ijc.27989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AJ, Lucas DM, Muthusamy N, Smith LL, Edwards RB, De Lay MD, et al. Characterization of the TCL-1 transgenic mouse as a preclinical drug development tool for human chronic lymphocytic leukemia. Blood. 2006;108:1334–1338. doi: 10.1182/blood-2005-12-011213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer SW, Pinon JD, Brachtl G, Haginger L, Wang W, Johrer K, et al. Modifying akt signaling in B-cell chronic lymphocytic leukemia cells. Cancer Res. 2010;70:7336–7344. doi: 10.1158/0008-5472.CAN-09-4411. [DOI] [PubMed] [Google Scholar]
- Altman JK, Szilard A, Konicek BW, Iversen PW, Kroczynska B, Glaser H, et al. Inhibition of Mnk kinase activity by cercosporamide and suppressive effects on acute myeloid leukemia precursors. Blood. 2013;121:3675–3681. doi: 10.1182/blood-2013-01-477216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman JK, Glaser H, Sassano A, Joshi S, Ueda T, Watanabe-Fukunaga R, et al. Negative regulatory effects of Mnk kinases in the generation of chemotherapy-induced antileukemic responses. Mol Pharmacol. 2010;78:778–784. doi: 10.1124/mol.110.064642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J, Lam F, Proud C, Wang S. Targeting Mnks for cancer therapy. Oncotarget. 2012;3:118–131. doi: 10.18632/oncotarget.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.