Abstract
The discovery that estrogen receptors (ERs) are involved in bone cells' responses to mechanical strain offered the prospect of establishing the link between declining levels of circulating estrogen and the progressive failure of the mechanically adaptive mechanisms that should maintain structurally appropriate levels of bone mass in age-related and post-menopausal osteoporosis. Such clarification remains elusive but studies have confirmed ligand-independent involvement of ERs as facilitators in a number of the pathways by which mechanical strain stimulates osteoblast proliferation and bone formation. The presence of α and β forms of ER that oppose, supplement or replace one another has complicated interpretation of studies to identify their individual roles when both are present in normal amounts. However, it appears that, in mice at least, ERα promotes cortical bone mass in both males and females through its effects in early members of the osteoblast lineage, but enhances loading-related cortical bone gain only in females. In addition to its role as a potential replacement for ERα, and modifier of ERα activity, the less well-studied ERβ appears to facilitate rapid early effects of strain including activation of extracellular signal-regulated kinase and downregulation of Sost in well-differentiated cells of the osteoblast lineage including osteocytes. If these different roles are substantiated by further studies, it would appear that under normal circumstances ERα contributes primarily to the size and extent of bones' osteogenic response to load bearing through facilitating anabolic influences in osteoblasts and osteoblast progenitors, whereas ERβ is more involved in the strain-related responses generated within resident cells including osteocytes.
ERs' potential role in failure of the mechanostat with age and declining estrogen
It has long been recognized that age-related decline in estrogen levels, notably but not exclusively in women after the menopause, is associated with loss of bones' ability to continue to adjust their mass and architecture to maintain their resistance to fracture. The mechanisms by which bone architecture is normally matched to customary loading is commonly referred to as the mechanostat. The initial in vitro finding that blocking ER activity not only reduces osteoblasts' proliferative response to estradiol, but also their similar response to mechanical strain, potentially offered a mechanistic explanation for the involvement of estrogen signaling in the mechanostat.1 This possibility was reinforced by in vivo confirmation2 that the absence of ERα activity in ERα knockout mice was associated with a reduced osteogenic response to loading.
The hypothesis derived from these studies3 envisaged that it was not any effect of estrogen itself, but ligand-independent ER activity within bone cells' responses to strain that influenced the efficacy of bone cells' adaptive response to load bearing. ER levels are substantially regulated by estrogens,4 the availability of ERα in resident bone cells is normally low compared with that in other estrogen responsive cells,5,6 and strain-related responses are increased by transfection of additional ERα.7 Thus, reduced ER levels as a result of reduced circulating estrogens, could become a limiting factor in bone cells' responses to load-induced mechanical strain in their surrounding tissue. Reduction in the effectiveness of these responses could account for the progressive ineffectiveness of bone cells to maintain structurally appropriate bone mass and architecture in post-menopausal osteoporosis in women, and age-related osteoporosis in both men and women.
The importance of osteoporosis, and the therapeutic potential of being able to modify ER function with one of the many selective estrogen receptor modulators, has encouraged investigation of the roles of the ERs in bone biology and the processes by which strain in bone tissue is transduced into stimuli for adaptive (re)modeling. These have ranged from association studies in humans, through study of the normal and adaptive phenotype of a number of genetically modified mice, to a larger number of cell-based studies in vitro.
In vivo studies in humans and animals: the confounding effects of ERα and ERβ
A critical role for ERα in human skeletal biology was first demonstrated by the report of a male patient harboring a homozygous loss-of-function mutation of ERα who developed tall stature and osteopenia with a large endosteal circumference and low trabecular bone mass.8,9 Studies on the effects of the ERα and ERβ isoforms in humans at the population level have relied on establishing associations between genetic polymorphisms and measurable phenotypes such as decreased bone mineral density and fracture risk.10 ERα polymorphisms influence bone gain during exercise intervention11,12 and genetic studies suggest that polymorphisms in the two ERs interact with each other, and with polymorphisms in the insulin-like growth factor-1 (IGF1) gene, to modify the risk of fracture,13 a finding consistent with well-established molecular cross-talk between these receptors.14 Nevertheless, the few studies on ER dynamics associated with age and declining estrogen in bone cells in humans15 are insufficient to draw any substantial conclusions. Most of the work in this area relies instead on animal models.
Interactions between the effects of ERα and ERβ, together with ‘incomplete' deletion of each ER in early knockout models, have confounded studies attempting to establish separate and unambiguous roles for ERα and ERβ, as reviewed in detail elsewhere.16,17 The continued expression of truncated ERs in early knockout models rendered them incomplete. Thus, sufficient expression of the ERα ligand-binding activation function (AF)-2 domain was present in double ERα/ERβ knockout mice to enable estradiol to stimulate similar increases in their cortical, but not trabecular, bone mass to wild type (WT).18 Nevertheless, this early finding is consistent with the recent demonstration that mutation of the ERα AF-2 domain in female mice prevents estradiol-induced cortical and trabecular bone gain, whereas deletion of the AF-1 domain only prevents cortical bone gain following treatment with a high dose of estradiol.19 In studies using ‘complete' global knockout mice, deletion of both ERα and ERβ prevented any significant effect of estradiol treatment on trabecular bone mass or turnover, whereas estradiol did exert effects when either receptor was present.20 These analyses of the effects of estradiol treatment in ovariectomized mice lacking one of both ER isoforms suggest that in female mice ERβ mediates inhibitory effects of estradiol on bone turnover and facilitates ERα-mediated responses to estradiol treatment.20
A further complication in studies involving knockout of the ER receptor has been the resulting high levels of circulating estrogen in ERα, but not ERβ, knockout mice.21 Similarly, whereas IGF1 is reduced in ERα−/− female mice it is elevated in those lacking ERβ,18 potentially exerting estrogen-independent osteogenic effects including the role of IGF1 in the mechanostat, which is facilitated in osteoblastic cells subjected to mechanical strain through non-genomic activity of ERα.22 The importance of non-genomic ER signaling in bone has been extensively studied, in part thanks to the development of the global ‘non-classical ER knock-in' mouse, which express an ERα unable to bind DNA. This ‘knock-in' is associated with lower cortical and trabecular bone mass in the tibia of 3-month-old female mice.23 In a different model, also investigating non-genomic ER signaling, treatment of female mice with an estradiol-dendrimer complex, which selectively activates membrane-initiated signaling, was as effective as estradiol at inhibiting ovariectomy-induced bone loss in cortical, but not cancellous bone.24 These findings suggest that, at least in female mice, bone mass is influenced by a balance between classical and non-classical ERα signaling.
This complexity of ERα signaling is derived not only from variable interaction with ERβ, but also by the expression in bone cells of various different ERα isoforms. In addition to the classical 66-kDa full-length ERα (ERα66), osteoblast-like cells also express ERα46 and ERα36. ERα46 lacks the AF-1 domain and strongly inhibits the genomic action of ERα66 in osteoblastic cells,25 potentially promoting non-classical ER knock-in-like signaling. ERα36 lacks both the complete AF-1 and part of the AF-2 domains so it only contains a ligand-binding domain, the DNA-binding domain and a short sequence unique to this ERα isoform. Unlike ERα66, which is predominantly localized in the nucleus, ERα36 is targeted to the cell membrane from where it initiates non-genomic signaling.26 The roles of these isoforms of ERα have been most extensively studied in breast cancer, in which ERα36 and ERα46 appear to have opposite effects. High ERα36 expression has been associated with more proliferative phenotypes and resistance to tamoxifen therapy, whereas ERα46 expression conveys sensitivity to tamoxifen.26,27
In bone, ERα36 expression has been found to be lower in osteoblasts, and osteoclasts from osteoporotic than similarly aged non-osteoporotic postmenopausal women.28 In cultures from non-osteoporotic postmenopausal women, low-dose estradiol triggered osteoblastic cell proliferation while promoting osteoclastic cell apoptosis. These responses were not observed in similarly derived cultures from osteoporotic women. However, transfection of exogenous ERα36 rescued both osteoblast proliferation and osteoclast apoptosis in response to low-dose estradiol treatment in cultures from osteoporotic women.28 Studies such as these are at present too few, and their results too ambiguous, for any firm conclusions to be drawn regarding contribution of the ERs to clinical osteoporosis.
ERα promotes cortical bone mass through its action in early progenitors
Notwithstanding, the potential for different ERα isoforms to exert different effects, complete deletion of ERα selectively at specific stages of the osteoblast lineage and in osteoclasts, has provided a detailed map of its action in different bone compartments. Such a map is not yet available for ERβ. Two different models (Cathepsin K-cre and LysM-cre) have established that ERα expression in osteoclasts mediates the protective effects of estrogens in female trabecular bone, but not in male trabecular bone or in cortical bone of either sex.29,30 A recent report by Almeida et al.31 provides a detailed analysis of bone phenotypes of mice in which ERα was deleted at different stages of the osteoblast lineage including mesenchymal progenitors using Prx-cre, early osteoblasts/chondrocytes using osterix-cre or committed osteoblasts using Col1a1-cre. Prx-cre-mediated deletion of ERα results in lower cortical thickness in both male and female mice at 6–8 weeks of age. Eight-week-old Prx-cre female mice have reduced bone formation rate at the periosteal, but not the endocortical surface. This effect persists in female, but not in male, mice up to 28 weeks, the latest time point reported. Trabecular bone mass is also reduced in female Prx-cre mice because of a reduction in trabecular number at 12 weeks of age, but not at other time points tested.31
Deletion of ERα later in the osteoblast lineage using an osterix-cre reduces cortical thickness in 24-week-old female mice with floxed ERα, whereas Col1A1-cre does not alter cancellous or cortical bone mass in 12- or 26-week-old female mice.31 These findings suggest that the role of ERα in the attainment of cortical bone mass in female mice involves its activity in early members of the osteoblast lineage. This is consistent with the finding that loss of ERα in osteocytes using DMP1-cre does not alter female bone mass in any compartment.32 In contrast, deletion of floxed ERα in mature osteoblasts using an osteocalcin-cre model reduces both cortical and trabecular bone mass in female mice.33 These differences are more evident in young adult female mice; whereas the conditional knockout mice have lower tibial cortical bone volume and bone volume per tissue volume at 3.5 and 6 months neither of these parameters are significantly different from WT by 12 months because of greater loss of bone mass in the WTs.33 Histomorphometric analysis of bone formation and resorption in vertebral trabecular bone indicates that both processes are reduced in the osteoblast ERα-deleted mice.33
In males, osteocalcin-cre-mediated ERα deletion only significantly reduces trabecular bone mass at 6 months of age, not earlier,33 although in a separate report DMP1-targeted deletion reduces trabecular bone volume at 11 weeks of age.32 It is puzzling that ERα deletion in osteocytes is associated with lower trabecular bone mass in male mice,32 whereas its deletion earlier in the lineage (therefore, presumably including deletion in osteocytes)31,33 has no detectable effect on this compartment. Taken together, these studies suggest that ER expression in osteocytes in males appears also to contribute to trabecular bone mass, whereas the osteogenic effects of ERα expression on cortical bone accrual in female mice relate to its actions in early osteoblasts, not terminally differentiated osteocytes (Figure 1). This is consistent with in vitro findings that ERα can promote osteoblast differentiation31 and facilitates proliferation following estradiol,1 Wnt331 and mechanical strain.1,34
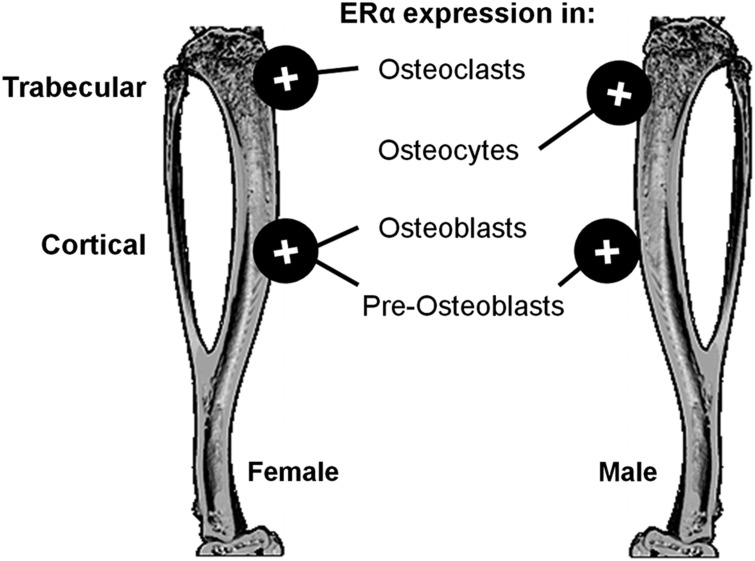
Figure 1.
Estrogen receptor α (ERα) expression increases bone mass in different bone compartments through its action in different cell types. ERα deletion in osteoclasts (Cathepsin K-cre and LysM-cre) reduced trabecular bone mass in female, but not in male, mice, whereas its deletion in osteocytes (DMP1-cre) reduced trabecular bone mass in male but not in female mice. Cortical bone in female mice was reduced by deletion of ERα in osteoblasts (Osteoclacin-cre, osterix-cre) and pre-osteoblasts (Prx-cre). In male mice, deletion of ERα in osteoblasts (osteoclacin-cre, Col1A1-cre) had no effect on male cortical bone, whereas its deletion in pre-osteoblasts (Prx-cre) reduced cortical thickness.
The ligand-independent role of contribution of ER to the mechanostat
The important role of ERα in the control of cortical bone, in female mice at least, is consistent with the initial finding by Lee et al.2 that the absence of ERα blunts the increase in cortical bone formation associated with short periods of artificial loading.35,36,37 In a subsequent study, incomplete deletion of ERβ was found to reduce the osteogenic response in the ulna of female mice similarly to ERα deletion,37 suggesting that the two ERs complemented each other's function in the mechanostat. However, studies using more complete ERβ knockout mice suggest an enhanced response to loading in the cortical bone of both male and female mice36 (Figure 2).
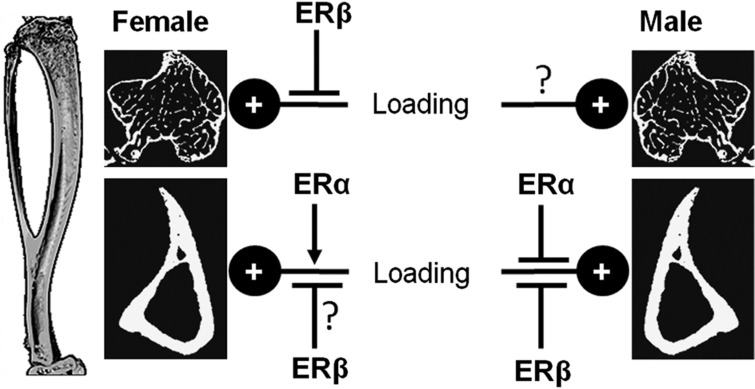
Figure 2.
Schematic representation of the influences of estrogen receptor α (ERα) and ERβ on loading-related bone gain in cortical and trabecular bone of male and female mice. Global ERα deletion has been associated with reduced cortical bone gain in female mice but increased cortical bone gain in males following loading.35,36,37 Complete deletion of ERβ increased trabecular bone gain in females and cortical bone gain in both males and females following loading,36 whereas in an earlier study incomplete ERβ deletion impaired cortical bone gain following loading in the ulna of female mice.37 Deletion of either ER does not alter trabecular bone gain following loading in male mice,36 but the effects of double ER deletion or cell type-specific deletions have not been investigated.
In trabecular bone, global deletion of ERα does not impair the osteogenic response to loading in female mice and, paradoxically, increases cortical and trabecular bone gain following loading in male mice relative to WT controls.36 In such experiments, inferences of adaptive responses to artificial loading in trabecular bone are limited because current techniques are unable to accurately measure or assess the strain magnitudes in the trabeculae of bones subject to either experimental or natural loading. This may be a significant deficiency as deletion of ERα or ERβ increases trabecular bone mass in female mice,36 such that strains engendered in this compartment may be lower than those generated by similar loads in WT controls.
In female mice, differences in serum estradiol between WT and transgenics can also alter ER-mediated effects in response of bone to loading. Windahl et al.35 investigated this by ovariectomizing mice before subjecting their tibiae to loading. Ovariectomy did not alter the loading-related increase in bone formation at either the periosteal or endosteal surface, whereas global deletion of ERα significantly reduced the increase in formation on both surfaces.35 This ligand independency of contribution of ERα to the mechanostat is further substantiated by analyzing the response to loading in mice expressing a truncated ERα lacking specific receptor domains. The AF-2 domain of ERα contains the ligand-binding site, whereas its AF-1 domain mediates interactions with other proteins. Relative to WT, female mice lacking AF-1 show a lower increase in bone formation at both periosteal and endosteal surfaces in response to loading. However, mutation of the AF-2 domain did not significantly reduce periosteal or endosteal bone formation relative to the response in WT littermates (although the percentage increase in bone area in WT mice of the AF-2 colony was lower than that observed in the AF-1 colony).35
Involvement of the AF-1 domain of ERα in osteogenesis stimulated by loading is the opposite from that seen following activation of the ERs with their endogenous ligand, estradiol. The increase in cortical thickness following high-dose estradiol administration to ovariectomized mice requires the AF-2 domain of ERα but not its AF-1 domain, whereas the increase in trabecular bone following estradiol treatment requires a fully functional ERα containing both AF-1 and AF-2.19 However, experimental studies investigating responses of bone to high doses of estradiol must be interpreted with caution, and it remains to be determined how relevant they are for determining the role of ERs in osteoporosis. Notwithstanding, taken together, these findings support an important role for ERα acting through its ligand-independent AF-1 domain to facilitate functional adaptation to loading in the cortical bone of female mice (Figure 3).
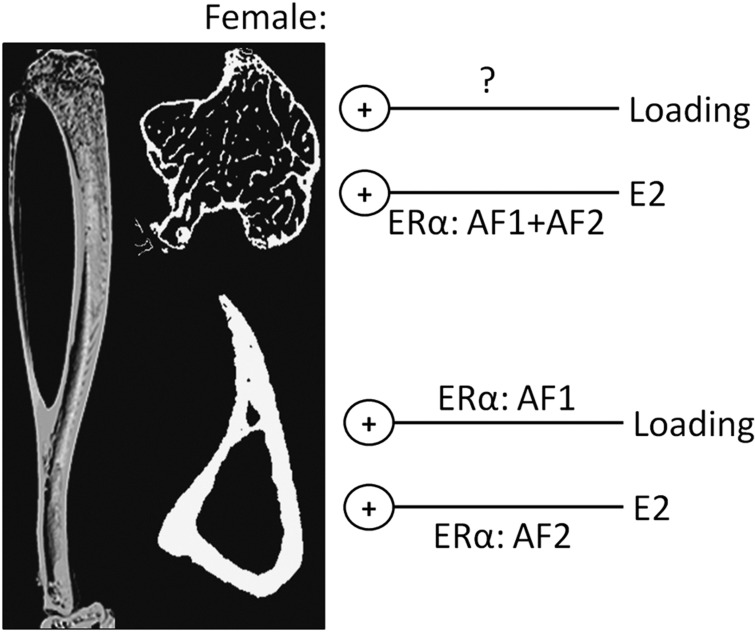
Figure 3.
The osteogenic effects of loading and estrogen (E2) on trabecular and cortical bone of female mice are differentially mediated by its activation function 1 (AF1) and AF2 domains. Deletion of either the AF1 or AF2 domains of estrogen receptor α (ERα), or complete ERα deletion, does not impair the osteogenic response of loading on female trabecular bone, whereas deletion of either domain reduces trabecular bone gain following treatment with estrogen. In cortical bone, deletion of the AF1 domain impaired the osteogenic response to loading, whereas only deletion of the AF2 domain impaired the increase in cortical bone mass following treatment with estrogen.
Does contribution of ER to the mechanostat extend to mature osteocytes?
Ligand-dependent contributions of ER to the effects of estrogen are systemic, whereas its ligand-independent contributions to the mechanostat are local and site specific. In vitro, in rat osteoblastic cells subjected to strain ERα can be ligand independently activated through AF-1 phosphorylation at S122 involving protein kinase A and ERK.38 ERα activation may lead to activation of genomic estrogen response elements,7 promote interleukin-11 upregulation,35 and facilitate local strain-induced osteoblast proliferation.34,39 Osteoblast proliferation is initiated very rapidly, within 30 min following exposure to strain,34 which is consistent with ERα phosphorylation increasing by this time.38 Proliferation of osteoblastic cells has been assumed to be a necessary prelude to the increased new bone formation normally associated with bone gain following in vivo loading.40,41 Increasing the expression of ERα in osteoblastic cells increases their proliferation following strain,7 suggesting that the availability of ERα may normally limit this response.
Osteoblastic proliferation following strain involves a number of signaling pathways including IGF1 and canonical Wnt signaling.34 ERα interacts with both these pathways, sensitizing the IGF receptor to ambient IGFs leading to activation of AKT signaling and stabilizing the Wnt secondary mediator β-catenin,22 as well as facilitating β-catenin translocation to the nucleus.42 These, and other functions of the ERs in osteoblastic cells' responses to strain, are represented in Figure 4. Even in the absence of strain, there is increasing evidence that ERα modulates Wnt signaling. Osteoblastic cells lacking ERα are less proliferative basally and do not proliferate as vigorously following treatment with canonical Wnts as cells derived from WT mice.31 Almeida et al. also observe that knockdown of ERα greatly reduces the induction of TCF-luciferase reporter construct activation by Wnt3,31 which is consistent with the previous report that ERα interacts with TCF-4 in osteoblastic cells.43 Furthermore, the increase in alkaline phosphatase activity stimulated by Wnt3 in cells from WT mice is absent in cells from ERα-deleted mice,31 suggesting that ERα is an intrinsic component of Wnt signaling involved in regulatory processes unrelated to mechanical strain. However, strain-related effects operate within a context of non-strain derived influences (such as growth44) that may have a profound effect on the outcome. This may represent another influence of ERα on the mechanostat as Wnt signaling is a key determinant of bone mass involved in the adaptation of both cortical and trabecular bone to loading in both male and female mice.45
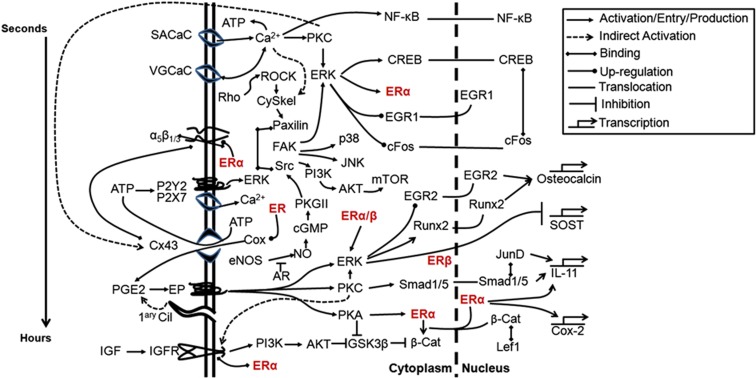
Figure 4.
Schematic representation of the involvement of estrogen receptor (ER) in signaling pathways activated by mechanical stimulation of osteoblastic cells. Membrane-initiated signaling events are grouped temporally from stretch-activated calcium channels (SACaC) activated within seconds, to voltage-gated calcium channels (VGCaC), integrins including α5β1/3, ATP's P2 receptors, connexion (Cx)43 hemichannels, PGE2's EP receptors, the primary cilium (1ary Cil) and the insulin-like growth factor (IGF) receptor. The ERs (red) are emphasized to illustrate their contribution to several pathways. Timing of intracellular signaling is difficult to dissect because of cross-talk between different pathways but a general timeframe is indicated (left). AKT, protein kinase B; AR, androgen receptor; cGMP, cyclic guanosine monophosphate; CREB, cAMP response element binding protein; eNOS, endothelial nitric oxide synthase; EP, E series prostaglandin receptors; FAK, focal adhesion kinase; GSK3β, glycogen synthase kinase 3β; IL, interleukin; JNK, c-Jun N-terminus kinase; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor κB; PGE2, prostaglandin E2; PI3K, phosphoinositide 3-kinase; PKC, protein kinase C.
Although the findings referred to indicate a potentially important role for ERα in the early anabolic responses of osteoblasts to strain-related stimulation, they do little to clarify the question of which processes ERα ligand independently facilitates in cortical osteoblasts rather than in osteocytes. Osteocytes are the most numerous resident strain-sensitive cells in the skeleton and are regarded by many as being the primary orchestrators of functional (re)modeling. If contributions of ERα to the mechanostat predominate in members of the osteoblast lineage able to proliferate and/or differentiate, this would explain why its deletion in osteocytes using DMP1-cre does not alter the osteogenic response to loading in the cortical bone of female mice.32 However, if the role of ERα is confined to the signaling processes involved in converting resident bone cells' strain-related experience into an osteogenic (re)modeling response, does either ERα or ERβ have a role in the earliest processes of strain transduction by which exposure to strain initiates the signaling cascades, which culminate in mechanically appropriate (re)modeling?
Separate roles for ERβ in the mechanostat?
Mechanistically, ERβ expression in bone suppresses the expression of a set of genes following estrogen treatment when ERα is present, but promotes expression of a subset of genes when ERα is deleted.46 This is consistent with the finding that ERβ suppresses basal osteoblastic cell proliferation mediated by ERα34 and that osteoblastic cells from ERβ knockout mice show a greater ERα-mediated proliferative response following exposure to strain than cells similarly derived from their WT counterparts.47 Thus, in proliferative osteoblasts in which roles of ERα are facilitatory, the influence of ERβ on the mechanostat may be to suppresses bone gain triggered by loading.
This inhibitory role alone cannot explain the repeated association between polymorphisms in ERβ with lower bone mass in humans,10,13 and the reduced osteogenic response to loading initially observed in ERβ−/− female mice.37 Although global ERβ−/− models have not shown low-bone-mass phenotypes, findings in these models must be interpreted with caution given increased circulating levels of IGF118 and potentially compensatory upregulation of ERα.48
Determining the potentially separate roles of ERα and ERβ in osteocytes has been complicated by the lack of a suitable cell model system expressing markers of both late osteocyte differentiation and strain sensitivity. ERα and ERβ independently mediate strain-induced activation of ERK signaling in osteocytic MLO-Y4 cells, potentially related to the anti-apoptotic effect of strain in these cells.49 ERK signaling in Saos-2 human osteoblastic cells, which both express and secrete the Wnt antagonist Sost/sclerostin, mediates Sost downregulation following strain.34 The requirement in these cells for ERβ, and not ERα, to enable strain to downregulate Sost34 is consistent with osteocyte ERα being dispensable for loading-related cortical bone gain in female mice32 and suggests a separate role for ERβ. This needs to be substantiated in normal mature osteocytes in situ.
It is clear that the role of ERβ in adaptation of bone to loading remains controversial and, compared with ERα, under-studied. The realization that ERβ could have a separate role from that of ERα, as well as potentially modulating ERα activity in different cell types (Figure 5), implies that it is necessary to review carefully all previous studies that did not specifically distinguish between such potential separate effects to ensure that the findings are not over or misinterpreted.
Figure 5.
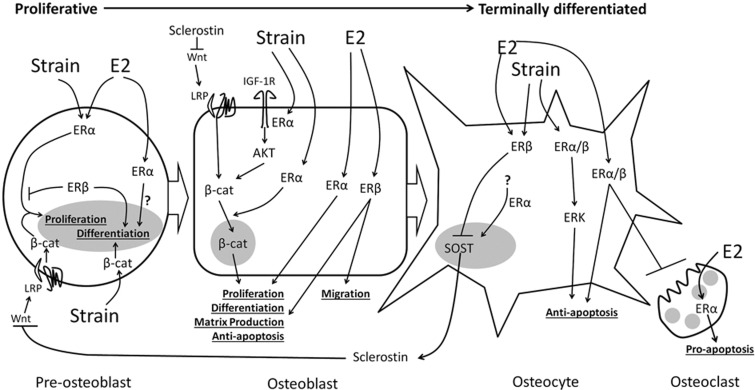
Schematic illustrating the roles of estrogen receptor α (ERα) and ERβ at different stages of the osteoblastic lineage. Early osteoblasts can proliferate or differentiate. ERα promotes their proliferation28,39 and either promotes31 or suppresses differentiation.50 There is evidence that ERβ promotes differentiation while inhibiting proliferation. ERβ and ERα both contribute to matrix production, and ERβ also selectively regulates genes associated with cell migration. In osteoblastic cells exposed to mechanical strain, ERα facilitates other osteogenic signaling pathways, specifically insulin-like growth factor (IGF), ERK and Wnt/β-catenin signaling. ERα and ERβ also contribute to anti-apoptotic signaling. Both ERs may also influence osteoclastogenic cytokine expression by osteoblastic cells. This diagram is based on our previous report.34 β-cat, beta-catenin; LRP, low-density lipoprotein receptor related protein.
Summary
Taken together, the studies reviewed here confirm that both ERα and ERβ make significant contributions to the mechanisms involved in the regulation of bone mass and architecture. These contributions are site and gender specific and include those by which mechanical strain in bone tissue influences the adaptive (re)modeling involved in loading-related control of bone mass and architecture.
Work by Almeida et al.31 has shown that ERα promotes cortical bone mass through the action of early osteoblast progenitors. This action may be reproduced in responses of bone cells to mechanical strain as it is fairly clear from a number of studies that ERα facilitates a number of the early strain-related pathways in osteoblastic cells, and the proliferation of osteoblasts in their response to mechanical strain. As elegantly confirmed by Windahl et al.,35 these contributions of ERα do not require the presence of either estrogens or the ERα's AF-2 ligand-binding domain. Absence or deficiency of ERα activity in humans would be expected to downregulate the anabolic response to strain-related stimulation, possibly contributing to osteopenia and bone fragility.
ERβ has been far less well studied than ERα and its role has been assumed to be primarily to either oppose actions of ERα, act together with ERα, or substitute for ERα in its absence. It may, however, be that ERβ makes a separate contribution to the mechanostat in the earliest strain-related responses of more differentiated bone cells including osteocytes. The actions of ERβ clearly require further study.
The most obvious, and most important, deficiency in the study of the effect of the estrogen receptors on the control of bone architecture is the absence of good data from humans. Without such data the potential implications of ER activity in ensuring that bone structure matches bone loading will go un-investigated and the therapeutic potential of ER modification by selective estrogen receptor modulators unexplored.
Footnotes
The authors declare no conflict of interest.
References
- Cheng MZ, Rawlinson SC, Pitsillides AA, Zaman G, Mohan S, Baylink DJ et al. Human osteoblasts' proliferative responses to strain and 17beta-estradiol are mediated by the estrogen receptor and the receptor for insulin-like growth factor I. J Bone Miner Res 2002;17:593–602. [DOI] [PubMed] [Google Scholar]
- Lee K, Jessop H, Suswillo R, Zaman G, Lanyon L. Endocrinology: bone adaptation requires oestrogen receptor-alpha. Nature 2003;424:389. [DOI] [PubMed] [Google Scholar]
- Lanyon L, Skerry T. Postmenopausal osteoporosis as a failure of bone's adaptation to functional loading: a hypothesis. J Bone Miner Res 2001;16:1937–1947. [DOI] [PubMed] [Google Scholar]
- Zaman G, Jessop HL, Muzylak M, De Souza RL, Pitsillides AA, Price JS et al. Osteocytes use estrogen receptor alpha to respond to strain but their ER alpha content is regulated by estrogen. J Bone Miner Res 2006;21:1297–1306. [DOI] [PubMed] [Google Scholar]
- Eriksen EF, Colvard DS, Berg NJ, Graham ML, Mann KG, Spelsberg TC et al. Evidence of estrogen receptors in normal human osteoblast-like cells. Science 1988;241:84–86. [DOI] [PubMed] [Google Scholar]
- Lim SK, Won YJ, Lee HC, Huh KB, Park YS. A PCR analysis of ERalpha and ERbeta mRNA abundance in rats and the effect of ovariectomy. J Bone Miner Res 1999;14:1189–1196. [DOI] [PubMed] [Google Scholar]
- Zaman G, Cheng MZ, Jessop HL, White R, Lanyon LE. Mechanical strain activates estrogen response elements in bone cells. Bone 2000;27:233–239. [DOI] [PubMed] [Google Scholar]
- Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 1994;331:1056–1061. [DOI] [PubMed] [Google Scholar]
- Smith EP, Specker B, Bachrach BE, Kimbro KS, Li XJ, Young MF et al. Impact on bone of an estrogen receptor-alpha gene loss of function mutation. J Clin Endocrinol Metab 2008;93:3088–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennari L, Merlotti D, De Paola V, Calabro A, Becherini L, Martini G et al. Estrogen receptor gene polymorphisms and the genetics of osteoporosis: a HuGE review. Am J Epidemiol 2005;161:307–320. [DOI] [PubMed] [Google Scholar]
- Suuriniemi M, Mahonen A, Kovanen V, Alen M, Lyytikainen A, Wang Q et al. Association between exercise and pubertal BMD is modulated by estrogen receptor alpha genotype. J Bone Miner Res 2004;19:1758–1765. [DOI] [PubMed] [Google Scholar]
- Remes T, Vaisanen SB, Mahonen A, Huuskonen J, Kroger H, Jurvelin JS et al. Aerobic exercise and bone mineral density in middle-aged finnish men: a controlled randomized trial with reference to androgen receptor, aromatase, and estrogen receptor alpha gene polymorphisms small star, filled. Bone 2003;32:412–420. [DOI] [PubMed] [Google Scholar]
- Rivadeneira F, van Meurs JB, Kant J, Zillikens MC, Stolk L, Beck TJ et al. Estrogen receptor beta (ESR2) polymorphisms in interaction with estrogen receptor alpha (ESR1) and insulin-like growth factor I (IGF1) variants influence the risk of fracture in postmenopausal women. J Bone Miner Res 2006;21:1443–1456. [DOI] [PubMed] [Google Scholar]
- Pettersson K, Delaunay F, Gustafsson JA. Estrogen receptor beta acts as a dominant regulator of estrogen signaling. Oncogene 2000;19:4970–4978. [DOI] [PubMed] [Google Scholar]
- Braidman IP, Baris C, Selby PL, Adams JE, Freemont AJ, Hoyland JA. Preliminary report of impaired oestrogen receptor-alpha expression in bone, but no involvement of androgen receptor, in male idiopathic osteoporosis. J Pathol 2000;192:90–96. [DOI] [PubMed] [Google Scholar]
- Windahl SH, Andersson G, Gustafsson JA. Elucidation of estrogen receptor function in bone with the use of mouse models. Trends Endocrinol Metab 2002;13:195–200. [DOI] [PubMed] [Google Scholar]
- Borjesson AE, Lagerquist MK, Windahl SH, Ohlsson C. The role of estrogen receptor alpha in the regulation of bone and growth plate cartilage. Cell Mol Life Sci 2013; ( http://www.ncbi.nlm.nih.gov/pubmed/?term=23516016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg MK, Weihua Z, Andersson N, Moverare S, Gao H, Vidal O et al. Estrogen receptor specificity for the effects of estrogen in ovariectomized mice. J Endocrinol 2002;174:167–178. [DOI] [PubMed] [Google Scholar]
- Borjesson AE, Windahl SH, Lagerquist MK, Engdahl C, Frenkel B, Moverare-Skrtic S et al. Roles of transactivating functions 1 and 2 of estrogen receptor-alpha in bone. Proc Natl Acad Sci USA 2011;108:6288–6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims NA, Clement-Lacroix P, Minet D, Fraslon-Vanhulle C, Gaillard-Kelly M, Resche-Rigon M et al. A functional androgen receptor is not sufficient to allow estradiol to protect bone after gonadectomy in estradiol receptor-deficient mice. J Clin Invest 2003;111:1319–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims NA, Dupont S, Krust A, Clement-Lacroix P, Minet D, Resche-Rigon M et al. Deletion of estrogen receptors reveals a regulatory role for estrogen receptors-beta in bone remodeling in females but not in males. Bone 2002;30:18–25. [DOI] [PubMed] [Google Scholar]
- Sunters A, Armstrong VJ, Zaman G, Kypta RM, Kawano Y, Lanyon LE et al. Mechano-transduction in osteoblastic cells involves strain-regulated estrogen receptor alpha-mediated control of insulin-like growth factor (IGF) I receptor sensitivity to Ambient IGF, leading to phosphatidylinositol 3-kinase/AKT-dependent Wnt/LRP5 receptor-independent activation of beta-catenin signaling. J Biol Chem 2010;285:8743–8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed FA, Fraser DG, Monroe DG, Khosla S. Distinct effects of loss of classical estrogen receptor signaling versus complete deletion of estrogen receptor alpha on bone. Bone 2011;49:208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell SM, Han L, Kim HN, Kim SH, Katzenellenbogen JA, Katzenellenbogen BS et al. Non-nuclear-initiated actions of the estrogen receptor protect cortical bone mass. Mol Endocrinol 2013;27:649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denger S, Reid G, Kos M, Flouriot G, Parsch D, Brand H et al. ERalpha gene expression in human primary osteoblasts: evidence for the expression of two receptor proteins. Mol Endocrinol 2001;15:2064–2077. [DOI] [PubMed] [Google Scholar]
- Rao J, Jiang X, Wang Y, Chen B. Advances in the understanding of the structure and function of ER-alpha36,a novel variant of human estrogen receptor-alpha. J Steroid Biochem Mol Biol 2011;127:231–237. [DOI] [PubMed] [Google Scholar]
- Klinge CM, Riggs KA, Wickramasinghe NS, Emberts CG, McConda DB, Barry PN et al. Estrogen receptor alpha 46 is reduced in tamoxifen resistant breast cancer cells and re-expression inhibits cell proliferation and estrogen receptor alpha 66-regulated target gene transcription. Mol Cell Endocrinol 2010;323:268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Sun M, Liao XB, Yuan LQ, Sheng ZF, Meng JC et al. Estrogen receptor alpha36 mediates a bone-sparing effect of 17beta-estrodiol in postmenopausal women. J Bone Miner Res 2011;26:156–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell 2007;130:811–823. [DOI] [PubMed] [Google Scholar]
- Martin-Millan M, Almeida M, Ambrogini E, Han L, Zhao H, Weinstein RS et al. The estrogen receptor-alpha in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone. Mol Endocrinol 2010;24:323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida M, Iyer S, Martin-Millan M, Bartell SM, Han L, Ambrogini E et al. Estrogen receptor-alpha signaling in osteoblast progenitors stimulates cortical bone accrual. J Clin Invest 2013;123:394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windahl SH, Borjesson AE, Farman HH, Engdahl C, Moverare-Skrtic S, Sjogren K et al. Estrogen receptor-alpha in osteocytes is important for trabecular bone formation in male mice. Proc Natl Acad Sci USA 2013;110:2294–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maatta JA, Buki KG, Gu G, Alanne MH, Vaaraniemi J, Liljenback H et al. Inactivation of estrogen receptor alpha in bone-forming cells induces bone loss in female mice. FASEB J 2013;27:478–488. [DOI] [PubMed] [Google Scholar]
- Galea GL, Meakin LB, Sugiyama T, Zebda N, Sunters A, Taipaleenmaki H et al. Estrogen receptor alpha mediates proliferation of osteoblastic cells stimulated by estrogen and mechanical strain, but their acute down regulation of the Wnt antagonist Sost is mediated by Estrogen Receptor beta. J Biol Chem 2013;288:9035–9048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windahl S, Saxon L, Borjesson A, Lagerquist M, Frenkel B, Henning P et al. Estrogen receptor-alpha is required for the osteogenic response to mechanical loading in a ligand-independent manner involving its activation function 1 but not 2. J Bone Miner Res 2013;28:291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon LK, Galea G, Meakin L, Price J, Lanyon LE. Estrogen receptors alpha and beta have different gender-dependent effects on the adaptive responses to load bearing in cancellous and cortical bone. Endocrinology 2012;153:2254.-–2266. [DOI] [PubMed] [Google Scholar]
- Lee KCL, Jessop H, Suswillo R, Zaman G, Lanyon LE. The adaptive response of bone to mechanical loading in female transgenic mice is deficient in the absence of oestrogen receptor-alpha and -beta. J Endocrinol 2004;182:193–201. [DOI] [PubMed] [Google Scholar]
- Jessop HL, Sjoberg M, Cheng MZ, Zaman G, Wheeler-Jones CP, Lanyon LE. Mechanical strain and estrogen activate estrogen receptor alpha in bone cells. J Bone Miner Res 2001;16:1045–1055. [DOI] [PubMed] [Google Scholar]
- Damien E, Price JS, Lanyon LE. The estrogen receptor's involvement in osteoblasts' adaptive response to mechanical strain. J Bone Miner Res 1998;13:1275–1282. [DOI] [PubMed] [Google Scholar]
- Sakai D, Kii I, Nakagawa K, Matsumoto HN, Takahashi M, Yoshida S et al. Remodeling of actin cytoskeleton in mouse periosteal cells under mechanical loading induces periosteal cell proliferation during bone formation. PLoS One 2011;6:e24847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barou O, Palle S, Vico L, Alexandre C, Lafage-Proust MH. Hindlimb unloading in rat decreases preosteoblast proliferation assessed in vivo with BrdU incorporation. Am J Physiol 1998;274:E108–E114. [DOI] [PubMed] [Google Scholar]
- Armstrong VJ, Muzylak M, Sunters A, Zaman G, Saxon LK, Price JS et al. Wnt/beta-catenin signaling is a component of osteoblastic bone cell early responses to load-bearing and requires estrogen receptor alpha. J Biol Chem 2007;282:20715–20727. [DOI] [PubMed] [Google Scholar]
- McCarthy TL, Kallen CB, Centrella M. beta-Catenin independent cross-control between the estradiol and Wnt pathways in osteoblasts. Gene 2011;479:16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley JR, Lanyon LE. Growth rate rather than gender determines the size of the adaptive response of the growing skeleton to mechanical strain. Bone 2002;30:314–319. [DOI] [PubMed] [Google Scholar]
- Saxon LK, Jackson BF, Sugiyama T, Lanyon LE, Price JS. Analysis of multiple bone responses to graded strains above functional levels, and to disuse, in mice in vivo show that the human Lrp5 G171V High Bone Mass mutation increases the osteogenic response to loading but that lack of Lrp5 activity reduces it. Bone 2011;49:184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg MK, Moverare S, Skrtic S, Gao H, Dahlman-Wright K, Gustafsson JA et al. Estrogen receptor (ER)-beta reduces ERalpha-regulated gene transcription, supporting a "ying yang" relationship between ERalpha and ERbeta in mice. Mol Endocrinol 2003;17:203–208. [DOI] [PubMed] [Google Scholar]
- Jessop HL, Suswillo RF, Rawlinson SC, Zaman G, Lee K, Das-Gupta V et al. Osteoblast-like cells from estrogen receptor alpha knockout mice have deficient responses to mechanical strain. J Bone Miner Res 2004;19:938–946. [DOI] [PubMed] [Google Scholar]
- Windahl SH, Hollberg K, Vidal O, Gustafsson JA, Ohlsson C, Andersson G. Female estrogen receptor beta−/− mice are partially protected against age-related trabecular bone loss. J Bone Miner Res 2001;16:1388–1398. [DOI] [PubMed] [Google Scholar]
- Aguirre JI, Plotkin LI, Gortazar AR, Millan MM, O'Brien CA, Manolagas SC et al. A novel ligand-independent function of the estrogen receptor is essential for osteocyte and osteoblast mechanotransduction. J Biol Chem 2007;282:25501–25508. [DOI] [PubMed] [Google Scholar]
- Leskela HV, Olkku A, Lehtonen S, Mahonen A, Koivunen J, Turpeinen M et al. Estrogen receptor alpha genotype confers interindividual variability of response to estrogen and testosterone in mesenchymal-stem-cell-derived osteoblasts. Bone 2006;39:1026–1034. [DOI] [PubMed] [Google Scholar]